Abstract
Background
The study on bacteremia helps empirically select the proper antibiotics before the results of culture test about causative pathogen. The purpose of this study is to investigate causative pathogen in bloodstream infection, changing aspects based on elapsed time after burn, relationship with other sites and resistance of important causative pathogen against antibiotics through analysis on bacteria isolated from blood culture of patients hospitalized in burn intensive care unit (BICU).
Materials and Methods
A retrospective study was conducted targeting patients hospitalized in BICU from January 2007 to June 2011. Changes of causative pathogen in bloodstream infection based on elapsed time after injury were analyzed. We would like to examine the relationship between bloodstream infection and infection on other body parts by comparing results of cultures in burn wound site, sputum, urine and catheter tip. Antibiotics resistance patterns of Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, Enterococcus species, and Klebsiella pneumoniae were studied.
Results
A total of 2,337 burn patients were hospitalized in BICU for 54 months. Causative pathogen was cultured in blood cultures from 397 patients (17.0%). P. aeruginosa (169, 30.1%) was the most cultured and A. baumannii (107, 19.0%) and S. aureus (81, 14.4%) were followed. It was confirmed that the relative frequency of A. baumannii tended to get lower as the period got longer after injury, but the relative frequency of K. pneumoniae got higher as the period got longer after injury. With comparison without bacteremia, P. aeruginosa bacteremia showed high probability in which the same bacteria were cultured in wound site, sputum and cathether tip, and A. baumannii bacteremia and candida bacteremia had high probability in sputum, and urine and catheter tip, respectively. 95.9% of P. aeruginosa and 95.3% of A. baumannii showed the resistance against carbapenem. 96.3% of S. aureus was methicillin resistant and 36.2% of Enterococcus species were vancomycin resistant. 75.0% of K. pneumonia were extended-spectrum beta-lactamase (ESBL)-producing bacteria.
Conclusions
Since the highly antibiotic resistant microorganisms were isolated from the patients hospitalized in BICU during early phase, the empirical selection of antibiotics targeting these pathogens should be considered before the results of microbiologic culture test. In addition, use of empirical antifungal agent after 1 week of injury can be considered for patients who have risk factor of fungal infection.
Keywords: Blood stream infection, Burn, ICU
Introduction
The mortality of severe burn patients has been reduced rapidly since the pathophysiologic mechanism has been established followed by the availability of effective treatments, yet infections are the leading cause of death among burn patients [1, 2]. Studies on bacteremia help to select the proper empirical antibiotics prior to receiving the results of the culture test for the causative pathogen, which in turn improve the therapeutic effect and decrease mortality in sepsis patients [3].
In this study, we investigated the causative pathogens of severe burn patients from a different perspective based on the elapsed time after the burn injury and the antibiotic resistance of important causative pathogens identified from blood cultures among the admitted patients in the burn intensive care unit (BICU). The results were compared to the results of surveillance cultures of other parts of the body which were conducted simultaneously when isolating microorganisms from the blood cultures, so that this study could determine which infections in other parts of the body are related to bacteremia.
Materials and Methods
1. Study subjects and methods
This study was conducted in the BICU with 32 beds. Surveillance cultures were done once weekly for all admitted patients from burn wound, sputum, urine, and blood. Catheter tip cultures were conducted for the cases with fever. In addition, most of the patients went through escharectomy to remove the damaged tissue of the burn wound site. The study subjects consisted of patients admitted to the BICU between January 2007 and June 30, 2011 for a period of 4 years and 6 months who had microorganisms isolated from their blood cultures, and their patient medical records and culture test results were retrospectively analyzed.
2. Definitions
Among the patients who had coagulase-negative staphylococci (CNS) cultured from the blood cultures, the patients who had the same culture two or more times were included in the study. The blood culture results were subjected to the analysis only if they included the results from the BICU hospitalization period and did not include the results after transfer to the general ward. In the case the same causative microorganism was isolated from a patient more than once, the initial isolated microorganism was included in the analysis, and when different types of microorganisms were isolated, each microorganism was included in the analysis. The above definitions of duplicated cases were based on other published studies [4, 5]. The date of the microorganism culture in reference to the date of the burn was analyzed and results isolated after day 60 of the burn were excluded from the analysis. To determine the change of causative pathogen in blood stream infections in the BICU based on the change in time, the cultured microorganisms were analyzed for the following periods; within 1 week, within 2 weeks and within 3 weeks of the burn injury, and 3 weeks after the burn injury. To determine the relationship between blood stream infection and other site infections, a comparison was done for the positive results of the surveillance cultures, which were conducted 1 week prior/after the date of the positive blood culture. Resistance to carbapenem antibiotics was assessed in Pseudomonas aeruginosa and Acinetobacter baumannii, and resistance to methicillin and vancomycin antibiotics was assessed in Staphylococcus species and Enterococcus species, respectively. The presence of ESBL (extended-spectrum beta-lactamase) among Klebsiella pneumonia was assessed, too.
3. Microbiologic analysis
VITEK II (bio-Meríeux, Hazelwood, MO, USA) was used for microorganism isolation and antibiotic susceptibility test.
4. Statistical analysis
The analysis of covariance (ANCOVA) was done to adjust for the effect of age on the difference between the mean number of days for the isolation of microorganisms after the burn injury, and Chi-square trend test was done to analyze the association of the frequency of the cultured microorganisms after the burn. For the analysis of the relationship between bacteremia and other parts of the body, the presence of bacteremia was set as an independent variable and each culture site on the body was set as the dependent variable. Then, conditional logistic regression analysis with matched age group and sex was conducted, and the odds ratio was presented adjusting for the effect of age group and sex. A total of 9 age groups were divided as follows: 0-9 years old /10-19 years old /20-29 years old /30-39 years old /40-49 years old /50-59 years old /60-69 years old /70-79 years old and 80 years old or above. Then, the statistical test was done. Chi-square trend test was done to determine the association between the resistance and the elapsed time after the burn injury. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) program was used for the statistical analysis with two-tailed test, and the statistical significance was defined as P-value < 0.05.
Results
1. Characteristics of blood culture positive patients
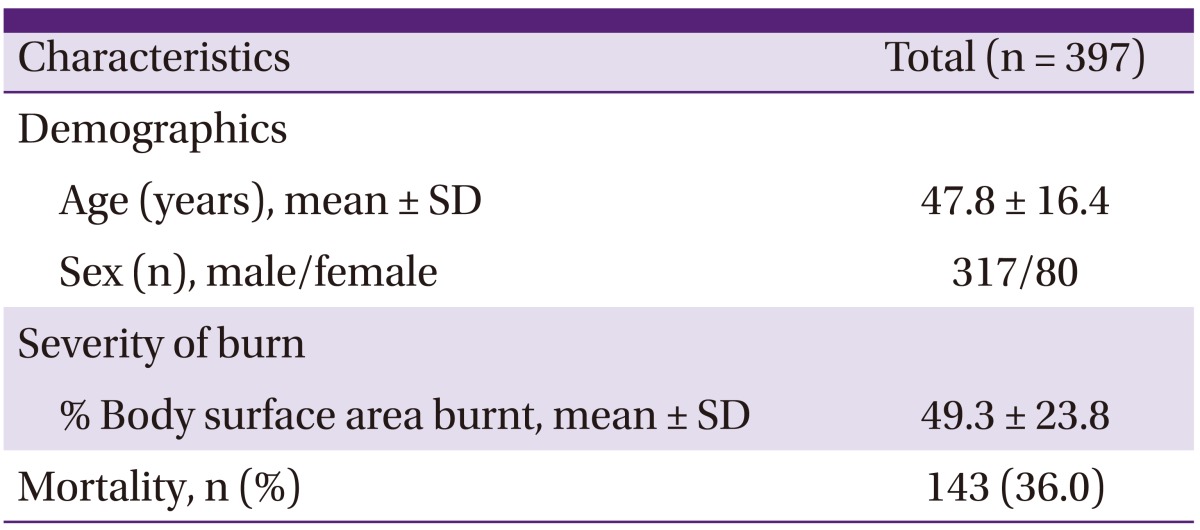
A total of 2,337 burn patients were admitted to the BICU from January 2007-July 2011 for a period of 4 years and 6 months. During this period, a total of 4,333 blood cultures were done from burn patients in the BICU, and 562 causative pathogens were cultured from 397 patients. Among the blood culture positive patients, 317 (79.8%) patients were men. The mean body surface area burnt was 49.3% and mortality during the hospitalization period was 36.0% (Table 1).
Table 1.
Characteristics of patients admitted to the BICU
2. Microorganisms from blood cultures
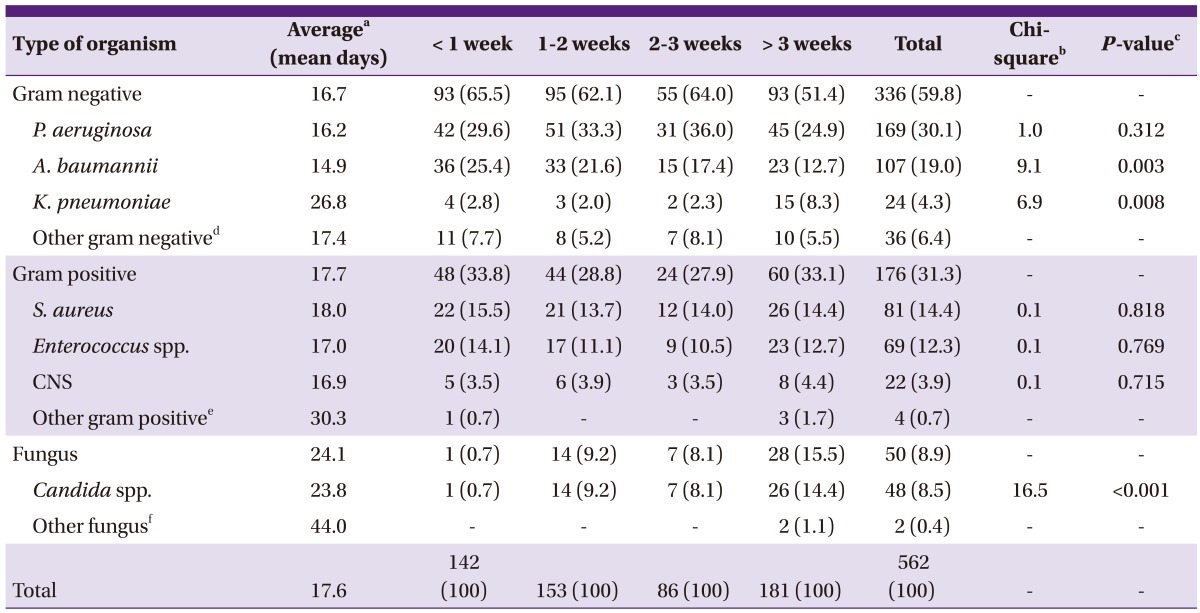
From culture isolates, gram negative organisms accounted for 59.8%, gram positive organisms accounted for 31.3% and fungi accounted for 8.9%. P. aeruginosa was the most common with 169 cases (30.1%), followed by 107 cases (19.0%) of A. baumannii, 81 cases (14.4%) of Staphylococcus aureus, and 69 cases (12.3%) of Enterococcus species (Table 2). The fungi mostly consisted of the Candida species. Seventeen cases of C. albicans, 12 cases of C. parapsilosis, 11 cases of C. tropicalis, 6 cases of C. glabrata, 1 case of C. famata and 1 case of C. guilliermondii. The mean elapsed days from the time of burn injury to the isolation of microorganisms was 17.6 days. From the day of the burn injury, A. baumannii (mean 14.9 days) was isolated the earliest, followed by the Candida species (23.8 days) and then K. pneumoniae (26.8 days). As a result of ANCOVA, there was a difference in the mean days to isolate microorganisms between each causative microorganism, and the difference in each mean number of days was statistically significant (P = 0.047).
Table 2.
Microorganism isolated from blood culture in the different period after burn (N, %)
CNS, coagulase-negative staphylococci.
aElapsed days from burn injury by ANCOVA (analysis of covariance).
b.cP-values were obtained using the Chi-square test for trend.
dOther gram negative: Alcaligenes xylosoxidans, Burkholderia cephacia, Citrobacter freundii, Cryseobacterium indologenes, Enterobacter spp., Escherichia coli, Proteus mirabilis, Serratia marcescens, Sphingomonas paucimobilis, Stenotrophomonas maltophilia.
eOther gram positive: Micrococcus luteus, Streptococcus ovis, Streptococcus parasanguinis, Streptococcus pneumoniae.
fOther fungus: Trichosporon asahii, Yarrowia lipolytica.
3. Changing aspect of causative pathogen based on hospitalization period
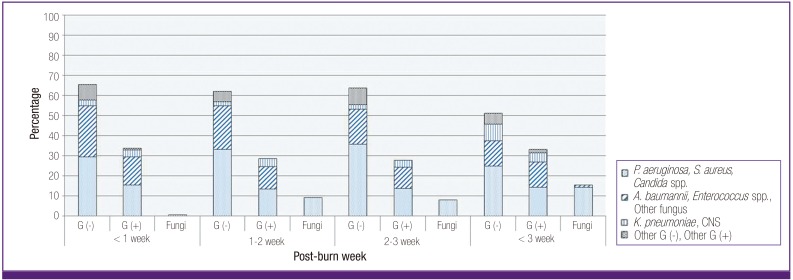
P. aeruginosa accounted for major microorganism in every period, regardless of the period after the burn injury (Fig. 1), and there was no significant difference between the frequency based on the period after the burn injury (χ2 = 1.0, P = 0.312). A gradual decrease of the relative frequency along with a longer period after the burn injury was observed in A. baumannii (χ2 = 9.1, P = 0.003). The relative frequency gradually increased with a longer period after the burn injury in Candida species (χ2 = 16.5, P < 0.001). In addition, the relative frequency gradually increased with a longer period after the burn injury in K. pneumonia (χ2 = 6.9, P = 0.008).
Figure 1.
Percentage (%) of microorganisms isolated from blood culture after burn is different according to time elapsed from burn injury.
4. Relationship between blood stream infection and cultures from other parts of the body
The following results were from the analysis of wound site, sputum, catheter tip, and urine culture result which were conducted 1 week prior/after the positive blood culture result.
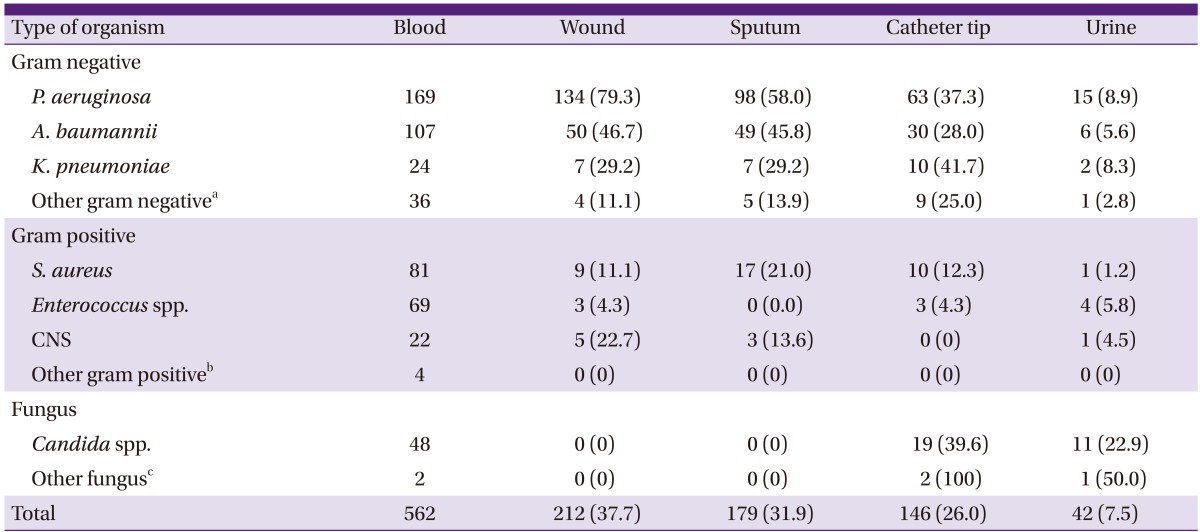
The sites accounting for the highest rates of concordance with blood stream infection were as follows: wound (37.7%) followed by catheter tip (26.0%) and urine (7.5%) (Table 3). A high rate of concordance with the wound culture (79.3%), sputum culture (58.0%) and catheter tip culture (37.3%) was seen in P. aeruginosa bacteremia, and a high rate of concordance with the sputum culture (45.8%) was seen in A. baumannii bacteremia. A high rate of concordance with the catheter tip culture (39.6%), urine culture (22.9%) was seen in candidemia.
Table 3.
Concordance of culture positivity (N, %) of wound, sputum, catheter tip and urine among bacteremic cases
CNS, coagulase-negative staphylococci.
aOther gram negative: Alcaligenes xylosoxidans, Burkholderia cephacia, Citrobacter freundii, Cryseobacterium indologenes, Enterobacter spp., Escherichia coli, Proteus mirabilis, Serratia marcescens, Sphingomonas paucimobilis, Stenotrophomonas maltophilia.
bOther gram positive: Micrococcus luteus, Streptococcus ovis, Streptococcus parasanguinis, Streptococcus pneumoniae.
cOther fungus: Trichosporon asahii, Yarrowia lipolytica.
Compared with cases without bacteremia, in the case of P. aeruginosa bacteremia, the probability of the same bacteria being cultured was high from the wound (odds ratio [OR], 16.5, 95% confidence interval [CI]: 10.4 to 26.1), sputum (OR: 5.6, CI: 3.7 to 8.3), and catheter tip (OR: 2.2, CI: 1.5 to 3.3) (Table 4). Compared to the cases without bacteremia, in the case of A. baumannii bacteremia, the probability of the same bacteria being cultured was high from the sputum (OR: 2.0, CI: 1.3 to 3.2). In the case of candidemia, the probability of the candida being cultured was high in the catheter tip (OR: 2.0, CI: 1.0 to 3.7) and urine (OR: 5.0, CI: 2.3 to 11.2). Each odd ratio was adjusted for the effect of the age group and sex.
Table 4.
Relative risk of bacteremia or fungemia according to positive culture results from the other sites
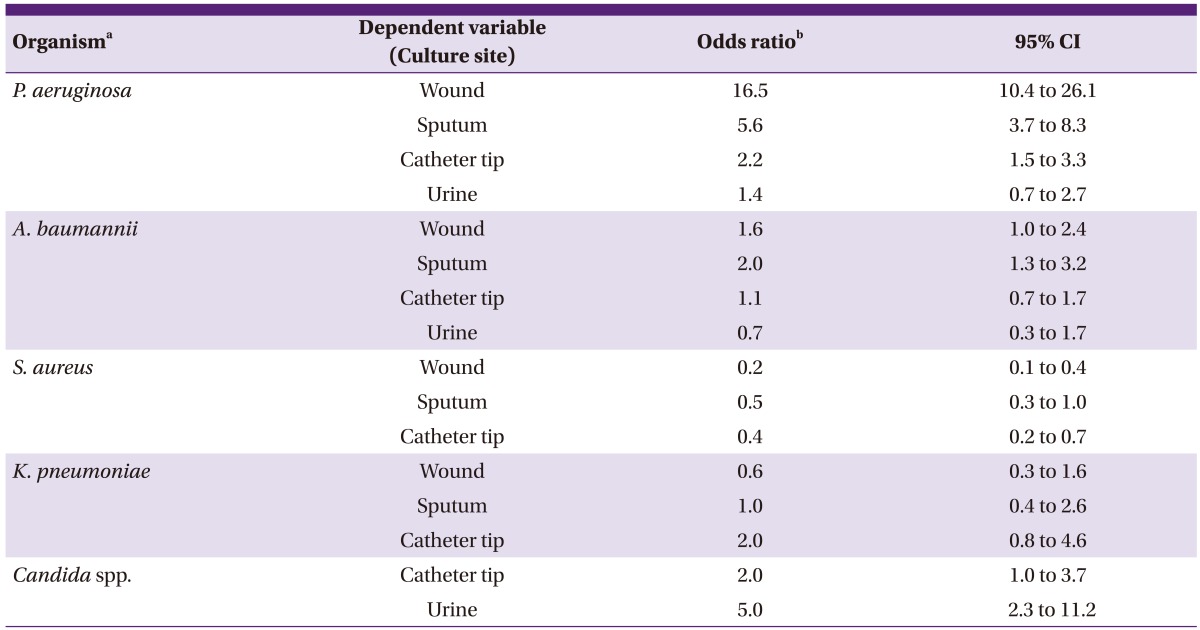
aOrganism which cultured at each local site and blood concomitantly.
bIndependent variable is the bacteremia with the same organism. Odds ratio were adjusted by age and sex. The odds ratio means the increased (when OR > 1) or decreased risk of culture positive for local samples in bacteremia cases compared with non-bacteremia cases by each cultured organism.
5. Proportion of antibiotic resistance in major isolates
One hundred sixty-two cases (95.9%) out of a total of 169 cases of P. aeruginosa bacteremia and 102 cases (95.3%) out of a total of 107 cases of A. baumannii bacteremia showed resistance to the carbapenem such as imipenem and meropenem. Seventy-eight cases (96.3%) out of 81 cases in S. aureus bacteremia were methicillin resistant Staphylococcus aureus (MRSA), and 25 (36.2%) out of 69 cases in enterococcal bacteremia were vancomycin resistant enterococcus (VRE), and 39 cases (56.5%) showed resistance to ampicillin/sulbactam. Eighteen cases (75.0%) out of a total of 24 cases in K. pneumoniae bacteremia were ESBL-producing bacteria (Table 5).
Table 5.
Ratio of antibiotics resistance in major causative microorganism
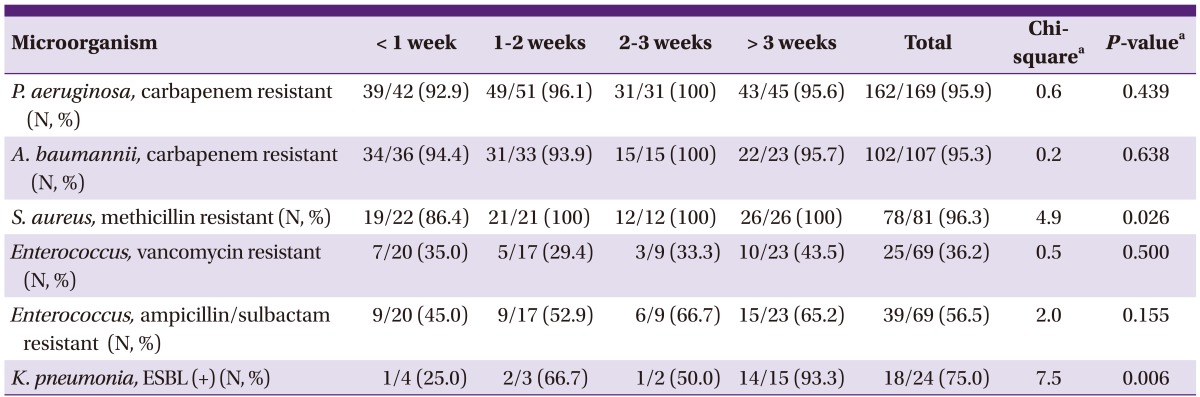
P. aeruginosa, C arbapenem resistant (N, %) methicillin resistant (N, %)
aP-values were obtained using the Chi-square test for trend.
For the resistance to methicillin in S. aureus, the relative frequency gradually increased statistically significantly with a longer period elapsing after the burn injury (χ2 = 4.9, P = 0.026). For the proportion of ESBL-producing K. pneumoniae, the relative frequency gradually increased significantly with a longer period elapsing after the burn injury (χ2 = 7.5, P = 0.006).
Discussion
In this study, the mortality of bacteremia patients in BICU was 36.0% overall, and it was similar to the mortality of 30.0% in another study conducted in 2011 [6]. The mean number of day to the occurrence of bacteremia was 17.6 days after burn injury in this study, which was similar to the 16 days that was reported by Vostrugina et al. [7].
Among the patients admitted to the BICU with burn injuries, gram negative organisms accounted for 59.8% of the causative pathogens for blood stream infections, which was almost twice the proportion of gram positive organisms with 31.3%, and P. aeruginosa was the most common causative pathogen from the date of admission to the occurrence of bacteremia, regardless of the period. However, existing study results reported that infections by gram positive organisms are predominant in the early period of burn injuries, and those are quickly replaced by gram negative organisms thereafter. Then, with delayed wound closure and an increase in the need for broad-spectrum antibiotics, further replacement of fungi and antibiotic-resistant bacteria takes place [2]. Other studies showed the same concordant result of this study that P. aeruginosa was the most common pathogen causing wound infection and bacteremia in the burn center [1]. A. baumannii was the second most isolated organism, which accounted for 19.0% of all the microorganisms, and it was isolated more frequently in the early stage of the burn injury (mean 14.9 days) and the frequency gradually decreased over time.
Among the gram positive organism, S. aureus accounted for 14.4% which was the third most common isolate; furthermore, S. aureus is known to be the major causative pathogen for wound infection and septicemia in other burn center studies, and Gang et al. reported that 74% of septicemic patients were confirmed to have Staphylococcus species in their blood [8]. Enterococcus species accounted for 12.3% of all the causative pathogens as the fourth most common isolate. In another study, Enterococcus species was the causative pathogen for 11% of the bacterial infections among 1,146 burn patients [9].
Candida species was the fifth most common isolate (48 cases, 8.5%) in this study. The frequency of candida isolates increased with a longer period after the burn injury, and it was confirmed in other studies as well [10-11]. C. albicans was the most common isolate with 17 cases followed by C. parapsilosis (12), C. tropicalis (11), and C. glabrata (6). Recent studies reported that Candida species are the most common fungal isolates in burn patients [12-13]. In a study on candidemia in burn patients by Pedrosa et al., C. parapsilosis was the second most frequent isolate (25.6% of all fungi) subsequent to C. albicans in Spanish & Canadian hospitals, and a study from Switzerland reported that C. glabrata, which accounted for 14% of all cases of candidemia, was the second most frequent isolate [12]. In the case of burn patients, generally, the rate of candida infections caused by species other than C. albicans is increasing. Some centers administer fluconazole as a prophylaxis, and the possibility of fluconazole-resistant candida infection is increasing. In this study, C. glabrata, which is highly resistant to fluconazole, comprised 12.5% of all candida infections, and C. parapsilosis, which is highly associated with central line infection, was 25.0%. The aggressive nature of treating burn patients such as the use of a central venous line, urine catheter, endotracheal tube, total parenteral nutrition (TPN), prolonged mechanical ventilation, systemic steroid therapy, and broad-spectrum antibiotics increase the risk of fungal infections in burn patients [12].
In cases where the presumed primary focus was secondary bacteremia, burn wound had the highest rate of concordant cultures at 37.7% followed by sputum 31.9%, catheter tip 26.0%, and urine 7.5% which was relatively low.
P. aeruginosa bacteremia had a high rate of concordant cultures for the burn wound (79.3%), sputum (58.0%) and catheter tip (37.3%), and candida had a high rate of concordant cultures for the urine (22.9%) and catheter tip (39.6%). Therefore, a primary focus of bacteremia would be easier to assume based on the types of the microorganisms identified from the blood cultures.
Most of P. aeruginosa (95.9%) cultured in this study showed resistance to carbapenem. An increase in carbapenem-resistant bacteria has been continuously reported in other studies [14]. In addition, most of A. baumannii (95.3%) were carbapenem-resistant bacteria. Those bacteria exhibited high carbapenem resistance even in isolates from less than 1 week after burn injury. The above cases are believed to be due to not keeping the patients isolated from each other in the BICU. There is a high risk of cross contamination of causative pathogens through the skin barrier when 40% or greater of the body's surface area is open to infection in patients. Although completely preventing the occurrence of resistance from cross contamination would be difficult, changing the burn wound dressings in designated places such as a wound dressing room would help to minimize the risk.
Fortunately, P. aeruginosa and A. baumannii, which are resistant to colistin, were not found; this finding is identical to the results of another study [15]. The effectiveness of colistin was shown for the treatment of nosocomial infections caused by multi-drug resistant (MDR) P. aeruginosa and A. baumannii [16], and the use of colistin for severe gram negative infections in the BICU should be prioritized in real practice. The proportion of MRSA was very high accounting for 96.3% of all the S. aureus, and 56.5% of all the enterococci exhibiting resistance to ampicillin/sulbactam, and 36.2% of Enterococcus species were VRE. In another study, 28% of enterococcus bacteremia was confirmed to be VRE [9].
There were limitations in this study. First, we excluded duplicate cases that were defined in other studies [4, 5], and this exclusion may have caused a distortion in the bacteremia results. Second, it was a retrospective study which was conducted after the patients' data such as the isolated microorganisms from blood cultures were obtained. This may have caused difficulty in finding the rate of at least one or more blood cultures and the rate of regular weekly blood cultures without an exception, among all the admitted patients. Therefore, the representativeness of the study could be reduced. In addition, with this study being a retrospective study, the difficulty in determining the incidence of blood stream infections based on the duration of the ICU stay in the hospital made it difficult to more accurately calculate the statistical data which was another limitation of this study.
In conclusion, gram-negative bacteria such as multi-drug resistant P. aeruginosa and A. baumannii, MRSA, resistant enterococci were isolated at high rates from the early period of the burn injury in severe patients admitted to the BICU; hence, the selection of empirical antibiotics that target those highly antibiotic resistant bacteria should be considered in severe burn patients prior to receiving the results of the microbiological culture test. Specifically, in the early period of the burn injury, de-escalation therapy can be applied where broad-spectrum antibiotics are administered targeting multi-drug resistant gram-negative bacteria and gram-positive bacteria. Then, the antibiotics are changed to narrow spectrum antibiotics based on the test results of the blood cultures. In addition, the use of antifungal agents should be considered by 1 week after the burn injury when the relative frequency of candidemia is high, and the use of empirical antifungal agents should be considered more in patients without any clinical improvement despite the administration of broad-spectrum antibiotics and patients with risk factors of candidemia.
References
- 1.Lari AR, Alaghehbandan R. Nosocomial infections in an Iranian burn care center. Burns. 2000;26:737–740. doi: 10.1016/s0305-4179(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 2.Weber J, McManus A Nursing Committee of the International Society for Burn Injuries. Infection control in burn patients. Burns. 2004;30:A16–A24. doi: 10.1016/j.burns.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Altoparlak U, Erol S, Akcay MN, Celebi F, Kadanali A. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns. 2004;30:660–664. doi: 10.1016/j.burns.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Suljagic V, Jevtic M, Djordjevic B, Romic P, Ilic R, Stankovic N, Milovic N, Novakovic M, Kozarski J, Roganovic Z, Popovic Z, Jovelic A. Epidemiology of nosocomial colonization/infection caused by Acinetobacter spp. in patients of six surgical clinics in war and peacetime. Vojnosanit Pregl. 2011;68:661–668. doi: 10.2298/vsp1108661s. [DOI] [PubMed] [Google Scholar]
- 5.Henwood CJ, Livermore DM, Johnson AP, James D, Warner M, Gardiner A The Linezolid Study Group. Susceptibility of gram-positive cocci from 25 UK hospitals to antimicrobial agents including linezolid. J Antimicrob Chemother. 2000;46:931–940. doi: 10.1093/jac/46.6.931. [DOI] [PubMed] [Google Scholar]
- 6.Chong SJ, Ahmed S, Tay JM, Song C, Tan TT. 5 year analysis of bacteriology culture in a tropical burns ICU. Burns. 2011;37:1349–1353. doi: 10.1016/j.burns.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Vostrugina K, Gudaviciene D, Vitkauskiene A. Bacteremias in patients with severe burn trauma. Medicina (Kaunas) 2006;42(7):576–579. [PubMed] [Google Scholar]
- 8.Gang RK, Sanyal SC, Bang RL, Mokaddas E, Lari AR. Staphylococcal septicaemia in burns. Burns. 2000;26:359–366. doi: 10.1016/s0305-4179(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 9.Still J, Law E, Friedman B, Fuhrman S, Newton T. Vancomycin-resistant organisms on a burn unit. South Med J. 2001;94:810–812. [PubMed] [Google Scholar]
- 10.Nasser S, Mabrouk A, Maher A. Colonization of burn wounds in Ain Shams University Burn Unit. Burns. 2003;29:229–233. doi: 10.1016/s0305-4179(02)00285-1. [DOI] [PubMed] [Google Scholar]
- 11.Vindenes H, Bjerknes R. Microbial colonization of large wounds. Burns. 1995;21:575–579. doi: 10.1016/0305-4179(95)00047-f. [DOI] [PubMed] [Google Scholar]
- 12.Pedrosa AF, Rodrigues AG. Candidemia in burn patients: figures and facts. J Trauma. 2011;70:498–506. doi: 10.1097/TA.0b013e3181f2d4fb. [DOI] [PubMed] [Google Scholar]
- 13.Ballard J, Edelman L, Saffle J, Sheridan R, Kagan R, Bracco D, Cancio L, Cairns B, Baker R, Fillari P, Wibbenmeyer L, Voight D, Palmieri T, Greenhalgh D, Kemalyan N, Caruso D Multicenter Trials Group, American Burn Association. Positive fungal cultures in burn patients: a multicenter review. J Burn Care Res. 2008;29:213–221. doi: 10.1097/BCR.0b013e31815f6ecb. [DOI] [PubMed] [Google Scholar]
- 14.Ozkurt Z, Ertek M, Erol S, Altoparlak U, Akcay MN. The risk factors for acquisition of imipenem-resistant Pseudomonas aeruginosa in the burn unit. Burns. 2005;31:870–873. doi: 10.1016/j.burns.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Trottier V, Segura PG, Namias N, King D, Pizano LR, Schulman CI. Outcomes of Acinetobacter baumannii infection in critically ill burned patients. J Burn Care Res. 2007;28:248–254. doi: 10.1097/BCR.0B013E318031A20F. [DOI] [PubMed] [Google Scholar]
- 16.Levin AS, Barone AA, Penço J, Santos MV, Marinho IS, Arruda EA, Manrique EI, Costa SF. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008–1011. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]