Abstract
Thalidomide and procarbazine have demonstrated single agent activity against malignant gliomas (MG). We evaluated the combination of thalidomide and procarbazine with a single arm phase II trial in adults with recurrent or progressive MG. Procarbazine was given at a dose of 250 mg/m2/d × 5day q 28 days. Thalidomide was administered at a dose of 200 mg/day continuously. Intrapatient dose escalation of thalidomide was attempted (increase by 100 mg/day weekly as tolerated) to a maximum of 800 mg/day. The primary outcome was tumor response, assessed by MRI and CT. Secondary outcomes were progression free survival (PFS), overall survival (OS) and toxicity. In addition, quality of life questionnaires were performed at baseline and prior to each odd cycle in all treated patients. Eighteen patients (median age of 50) were accrued and received a total of 36 cycles (median 2) of therapy. The median maximum thalidomide dose achieved was 400 mg (range 0–800). No complete or partial responses were seen. One patient (6%) experienced stable disease, fourteen (78%) progressed as best response and three (17%) were not evaluable for response. Median time to progression was 2.1 months (95% CI, 1.5–2.5). Seventeen patients have died (one patient lost to follow-up after progression); median survival from enrollment was 7.6 months (95% CI, 3.5–9.4). Grade 3/4 drug related toxicity was minimal. Quality of life diminished over time. The combination of thalidomide and procarbazine demonstrated no efficacy in this trial.
Keywords: Chemotherapy, Clinical trial, High grade astrocytoma, Glioblastoma multiforme, Thalidomide, Procarbazine
Introduction
Malignant gliomas (MG) account for the majority of primary brain tumors in adults with an estimated annual incidence of 15,000 cases in the United States [1, 2]. Despite aggressive therapy with maximal surgical resection, radiotherapy, and chemotherapy with temozolomide (TMZ), nearly all patients with malignant glioma will die of their disease. The prognosis remains poor for patients with recurrent disease with a median survival of only 25 weeks for recurrent glioblastoma multiforme (GBM) and 40 weeks for recurrent anaplastic gliomas (grade III) [3]. The 3-year survival rate for GBM is only 4–15% [2, 4]. Surgery followed by cranial irradiation remains the standard treatment for malignant gliomas, with an established survival benefit gained through the use of systemic chemotherapy in patients with glioblastoma multiforme [5]. In the pre-temozolomide era, a published meta-analysis confirmed that systemic chemotherapy with nitrosourea-based regimens was a marginally useful adjunctive therapy to surgery followed by cranial irradiation in patients with high-grade gliomas [6]. Following publication of the EORTC-NCIC study of temozolomide (TMZ) in patients with newly diagnosed glioblastoma multiforme in 2005, concurrent radiation with TMZ followed by adjuvant monthly TMZ became the “gold standard” first line therapy for patients with newly diagnosed glioblastoma [7]. Although TMZ has significantly prolonged survival when combined with radiation, disease progression ultimately occurs in the majority of patients. Unfortunately, few effective salvage regimens are available for these patients at the time of recurrence. A number of studies have failed to demonstrate particular efficacy for nitrosoureas at progression or recurrence including BCNU (Carmustine), ACNU (Nimustine), Procarbazine, and Procarbazine, lomustine, and vincristine (PCV). Bevacizumab, a monoclonal antibody that inhibits the activity of vascular endothelial growth factor, has more recently demonstrated clinical activity in patients with recurrent MG in phase II studies, both as a single agent and when given in combination with other chemotherapeutic drugs such as irinotecan. It was approved as a single agent for this indication in 2009 [8, 9]. During the past few years, retreatment with temozolomide using alternative or so-called metronomic treatment schedules has also been studied and shown to have activity in selected patients with recurrent glioma [10].
At the time this study was initiated in 2003, there were no effective salvage therapies for patients with recurrent MG. It was hypothesized that combining cytotoxic and cytostatic agents without overlapping toxicities might enhance antitumor effects with an acceptable side effect profile of the regimen.
The highly vascular glioblastoma multiforme was an early candidate tumor in which the efficacy of antiangiogenic therapies could be evaluated, and the beneficial results seen with bevacizumab appears to validate the utility of this approach in affected patients [8, 9]. Thalidomide (Thalomid®), the first clinically available oral agent with antiangiogenic properties, exhibited activity against a variety of malignancies, including myeloma, renal cell carcinoma, Kaposi's sarcoma, and glioblastoma multiforme [11–16]. It has been used as a single agent and in combination with carmustine in the setting of recurrent malignant glioma with a modest benefit [13, 17].
Procarbazine (Matulane®), an oral alkylating agent that readily crosses the intact blood–brain barrier, is FDA approved for use in patients with high grade astrocytomas. Procarbazine has been used as a single agent in patients with MG at doses that range from 60 mg/m2/day for 14 days every month (total 1680 mg/m2 in 2 months) to 150 mg/m2/day for 30 days every 60 days (total 4500 mg/m2 in 2 months). It is a critical component of the “PCV” regimen (procarbazine, CCNU, and vincristine) which has been used in clinical trials in Europe and North America in patients with glioblastoma multiforme, anaplastic astrocytoma, and anaplastic oligodendroglioma [18–27].
Based on the established modest efficacy of each agent in patients with gliomas and their mostly non-overlapping toxicities, we conducted a phase II trial of the combination of procarbazine and thalidomide in patients with progressive or recurrent MG.
Methods
Patient characteristics
Eligible patients included adults with progressive or recurrent, histologically confirmed high grade glioma (glioblastoma multiforme, anaplastic astrocytoma, anaplastic oligodendroglioma or anaplastic mixed oligoastrocytoma) who received prior radiation therapy with or without chemotherapy. All patients had measurable evidence of tumor progression or recurrence on MRI or CT imaging performed within 2 weeks of starting treatment. Patients were required to have a complete recovery from the toxicity of prior therapy. An interval of at least 3 months must have elapsed since the completion of the most recent course of radiation therapy. At least 3 weeks had elapsed since the completion of a non-nitrosourea containing chemotherapy regimen and at least 6 weeks must have elapsed since the completion of a nitrosourea containing regimen. Eligible patients could have received up to two prior chemotherapy regimens for their malignant glioma. Additional eligibility criteria included an ECOG performance status of ≥2; adequate hematologic (absolute neutrophil count ≥ 1500/mm3, platelets ≥ 100,000/mm3), renal (creatinine ≤ 1.7 mg/dl), and hepatic (total bilirubin ≤ 1.5 mg/dl, transaminases ≤ 4 times above the upper limits of norm) functions. Women of child-bearing potential were required to have a negative pregnancy test prior to treatment and contraceptive measures were required as outlined in the FDA-mandated System for Thalidomide Education and Prescription Safety (S.T.E.P.S.®) Program. The research protocol was approved by the Wake Forest University Institutional Review Board. Patients provided written informed consent.
Treatment
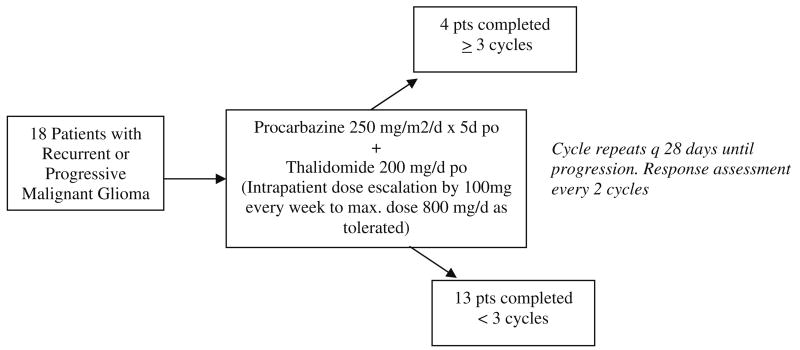
Treatment was initiated with procarbazine given at a dose of 250 mg/m2 daily for 5 days, and repeated every 28 days until tumor progression. This dose and schedule were based on a New Approaches to Brain Tumor Therapy (NABTT) consortium dose escalation trial of procarbazine ongoing at the time this study was initiated. From this trial, it was known that the dose of 250 mg/m2 daily for 5 days was below the maximum tolerated single agent procarbazine dose for patients who were and those who were not taking enzyme inducing antiepileptic drugs [27]. Thalidomide was administered at a dose of 200 mg/day continuously beginning on the first day of procarbazine administration. Intrapatient dose escalation of Thalidomide was attempted (increase by 100 mg/day weekly as tolerated) to a maximum of 800 mg/day. A single cycle of therapy consisted of 1 month of treatment (i.e., 5 days of procarbazine and 28 days of thalidomide) (Fig. 1).
Fig. 1. Treatment plan with distribution of patients enrolled.
Patient evaluations
Patients were reevaluated with history, physical, and laboratory studies at the completion of each 4 week cycle. Repeat brain imaging studies were obtained after every two completed cycles of therapy. Quality of life questionnaires including the FACT-Br, the “Additional Concerns” module of the FACIT-F (Fatigue) and the Mini-Mental Status Exam (MMSE) were performed at baseline and prior to each odd cycle in all treated patients. All patients were followed until death for disease-free and overall survival endpoints. Toxicity was described as per version 2.0 of the NCI Common Toxicity Criteria (CTC) for Toxicity and Adverse Event Reporting.
Treatment response evaluations
Response to therapy was described as complete, partial, or stable using the standardized Macdonald response criteria [28]. Contrast enhanced MRI/CT scans and neurologic examinations were used to determine response to therapy. A complete response was defined as MRI/CT demonstration of complete disappearance of all enhancing tumor off all glucocorticoids with a stable or improving neurologic examination, for a minimum of 8 weeks. A partial response required greater than or equal to 50% reduction in enhancing tumor volume on contrast enhanced MRI/CT scan [i.e., ≥50% decrease in the sum of the products of the longest perpendicular diameters of indicator lesions(s)], on a stable or decreasing dose of glucocorticoids, with a stable or improving neurologic examination, for a minimum of 8 weeks. Progressive disease was defined as progressive neurologic abnormalities not explained by causes unrelated to tumor progression or a greater than 25% increase in enhancing tumor volume by MRI/CT scan [i.e., ≥25% increase in the sum of the products of the longest perpendicular diameters of indicator lesions(s)]. Mixed response of regression and progression was considered progressive disease as was the development of a new lesion. Stable disease was defined on MRI/CT volumetrics that did not meet the criteria for complete response, partial response or progressive disease.
Statistical methods
This study was originally designed as a two-stage phase II trial to evaluate objective tumor response rate (estimated as the proportion of partial and complete responders among all evaluable patients). Toxicity, time to progression, survival and quality of life were secondary outcome measures. Toxicity was assessed throughout the study. Quality of life was quantified using the FACT-Br, FACIT-F (additional concerns module) and MMSE forms at baseline and every 2 months. A maximum sample size of 55 evaluable patients was needed to test the null hypothesis that the response rate was ≤15% versus the alternative hypothesis that the response rate was ≥30%. Twenty-three patients were targeted for accrual in the first stage, and an additional 32 patients were to be accrued if more than 3 of the first 23 evaluable patients responded to therapy. The study was ultimately stopped early for futility after none of the first 18 patients accrued showed evidence of tumor response and statistical modeling suggested that it was highly unlikely that further accruals would result in a need to progress to a second stage.
Descriptive statistics (means, standard deviations, frequencies, etc.,) are used to summarize pretreatment patient characteristics and the outcome measures mentioned above. Kaplan–Meier methods were used to estimate the time to progression and survival distributions. Previous studies had shown that the median time to progression and survival in these patients was approximately 10 and 30 weeks, respectively. Median estimates with 95% confidence intervals were calculated for descriptive comparison with these values.
Results
Patients and treatment
A total of 18 patients (11 men) with recurrent or progressive malignant glioma were enrolled (median age 50 years, range 27–63, accrual from December 2003 to October 2006). Patient characteristics are listed in Table 1. Histologic diagnoses of enrolled patients included glioblastoma multiforme (14), anaplastic astrocytoma (3) and anaplastic oligoastrocytoma (1). Sixteen patients had a PS of 1 or less, with two patients with a PS of 2. All of the patients had previously received radiation and chemotherapy. Following enrollment on this trial, 1 patient (6%) became sick after registration and did not receive any chemotherapy, 16 (89%) received 1–3 courses of treatment, and 1 (6%) received 9 courses (Fig. 1). The median number of courses received was 2. Twelve patients (67%) were able to have a Thalidomide dose escalation; the median maximum Thalidomide dose achieved was 400 mg (range 0–800).
Table 1. Patient characteristics.
| Characteristic | # (%) |
|---|---|
| Total | 18 (100) |
| Age | |
| Median (range) | 50 (27–63) |
| ≥60 years | 4 (22) |
| Diagnosis | |
| Glioblastoma multiforme | 14 (78) |
| Anaplastic astrocytoma | 3 (17) |
| Anaplastic oligoastrocytoma | 1 (5) |
| Gender | |
| Female | 7 (39) |
| Male | 11 (61) |
| Race | |
| Black | 3 (17) |
| White | 15 (83) |
| Performance status | |
| 0 | 2 (11) |
| 1 | 14 (78) |
| 2 | 2 (11) |
| Prior treatment | |
| Chemotherapy | 17 (94) |
| RT | 18 (100) |
| Courses received | |
| 0 | 1 (6) |
| 1 | 8 (44) |
| 2 | 5 (28) |
| 3 | 3 (17) |
| 9 | 1 (6) |
Treatment efficacy
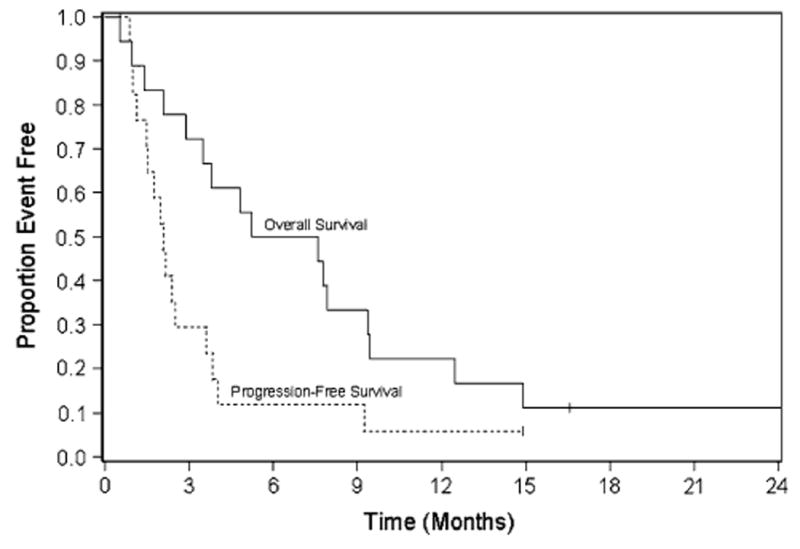
No complete or partial responses were seen. One patient (6%) experienced stable disease, fourteen (78%) progressed as best response, and three (17%) were not evaluable for response. (One patient did not receive any therapy and two went off study before reevaluation.) Median time to progression was 2.1 months (95% CI, 1.1–3.6) with median survival of 6.4 months (95% CI, 2.9–9.4) (Fig. 2).
Fig. 2. Kaplan–Meier estimates of overall survival and progression-free survival.
Toxicity
Few Grade 3 and 4 toxicities were observed (Table 2). Two patients (12%) developed grade 3–4 leukopenia with one episode of neutropenia. Other SAEs include 4 patients (24%) with grade 3–4 fatigue/weakness. Grade 3 neurotoxicity was documented in 4 (24%) patients. Neurologic deterioration included patients that had vision changes, aphasia, and mental slowing. Drug-related toxicities in this treatment cohort were without clinical consequence; no patient withdrew from the trial as a result of drug toxicity.
Table 2. Serious adverse events (N = 17).
| Toxicity | Grade 3 | Grade 4 |
|---|---|---|
| Weakness | 2 | 1 |
| Deep venous thrombosis | 0 | 1 |
| Neurologic deterioration | 4 | 0 |
| Constipation | 1 | 0 |
| Depression | 1 | 0 |
| Somnolence/fatigue | 4 | 0 |
| Neuropathy | 1 | 0 |
| Neutropenia | 1 | 1 |
Quality of life and cognitive outcomes are summarized in Table 3. At baseline, 17 patients completed the FACT-G with a mean score of 80.1 (SD = 17.6). This score is similar to that reported by Cella and colleagues in the standardization sample for the FACT [29]. There was a significant attrition rate from baseline to 2 months (40–80 days following baseline) at which time only nine patients completed the QOL instruments or MMSE as a result of disease progression. As seen in Table 3, the MMSE and almost all QOL scores declined in patients with serial measurements during the first 2 months. The brain and fatigue subscales had the greatest declines, decreasing by over 20% from baseline. It is unclear whether these changes were due to progressive disease or the impact of treatment.
Table 3. Quality of life and cognitive measures at baseline and follow-up.
| QoL/cognitive measure | All patients—baseline\ | Patients with baseline and follow-up data | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | Mean (SD) | N | Baseline mean (SD) | Follow-up mean (SD) | P value | |
| Physical | 17 | 21.0 (6.7) | 8 | 19.8 (7.7) | 15.9 (6.9) | 0.16 |
| Social | 17 | 24.1 (3.4) | 7 | 24.3 (3.2) | 24.2 (4.3) | 0.96 |
| Emotional | 17 | 18.5 (4.4) | 7 | 18.6 (3.5) | 15.9 (6.0) | 0.24 |
| Functional | 17 | 16.5 (7.5) | 7 | 13.6 (6.3) | 13.4 (7.3) | 0.93 |
| FACT-G | 17 | 80.1 (17.6) | 7 | 75.6 (17.8) | 70.5 (21.9) | 0.45 |
| Brain subscale | 16 | 47.1 (12.7) | 6 | 46.0 (5.1) | 36.3 (14.8) | 0.13 |
| Fatigue subscale | 17 | 32.6 (13.0) | 7 | 33.6 (14.4) | 23.9 (15.7) | 0.05 |
| MMSE | 18 | 25.0 (6.1) | 8 | 25.0 (5.8) | 21.8 (12.1) | 0.33 |
Follow-up measures were assessed between 40 and 80 days following baseline
Discussion
This was an open-label, single institution study of thalidomide and procarbazine in patients with histologically proven progressive or recurrent malignant glioma conducted prior to the availability of bevacizumab. The results of this study suggest that the combination of thalidomide and procarbazine has no clinical activity in recurrent malignant glioma when given at the dose and schedule tested in this trial.
Procarbazine and thalidomide were selected for evaluation based on their modest single agent activity and mostly non-overlapping toxicities in prior studies in patients with malignant brain tumors. In a phase II study, Fine et al. treated 36 patients with recurrent high-grade glioma with thalidomide escalated by 200 mg/day every 2 weeks to a final daily dose of 1200 mg; there were two partial responders, two minor responders, and 12 patients with stable disease. Eight patients were alive greater than 1 year after starting thalidomide, although the majority had tumor progression [13]. Similarly, Marx et al. [30] started patients at a dose of 100 mg/day and escalated to 500 mg/day. Of 38 evaluable patients, two achieved response and 16 had transient disease stabilization.
Procarbazine has been used in combination as part of the “standard treatment” arm in several large brain tumor studies. The Radiation Therapy Oncology Group (RTOG) protocol 9402 used PCV in patients with low grade glioma and reported an improved PFS in WHO grade II LGG who received RT + PCV versus RT alone. After 2 years, the addition of PCV to RT conferred both a significant OS and PFS advantage, and reduced the risk of death by 48% and progression by 55% [31]. The NABTT CNS consortium performed a two-arm dose escalation study of single agent procarbazine in patients who were receiving and those who were not receiving enzyme inducing antiepileptic drugs (EIAED) [27]. Forty-nine adult patients with recurrent high-grade glioma were enrolled and received procarbazine orally for 5 consecutive days each month. Dose escalation between cohorts utilized the continual reassessment method for determining subsequent dose levels. The maximum tolerated dose was 393 mg/m2/day on the +EIAED arm and 334 mg/m2/day on the −EIAED arm. Four (8%) partial responses were seen and the median time to progression was only 2 months. The median overall survival for all patients accrued was 6.5 months.
The use of an angiogenesis inhibitor and cytotoxic chemotherapy was hypothesized to be of potential benefit for patients with GBM. One of the first trials to use thalidomide in combination with a cytotoxic agent utilized carmustine. Fine et al. [17] enrolled 38 patients receiving the combination treatment and reported a 24% radiographic objective response rate. Glass et al. performed a phase I/II study of carboplatin and thalidomide in patients with recurrent glioblastoma multiforme. Five of 46 evaluable patients demonstrated partial responses with this therapy while 26 patients had stable disease. The mean survival of patients on this study was 40 weeks [15]. Baumann et al. used thalidomide in combination with temozolomide versus single agent thalidomide alone. They found that the combination was more effective than thalidomide alone with a median survival of 103 weeks for the combination therapy versus 63 weeks for single agent thalidomide [32]. Groves et al. [33] tested the combination of thalidomide with TMZ in recurrent GBM with minimal improvement in outcome versus TMZ alone. Puduvalli et al. [34] reported a phase II trial with the use of irinotecan and thalidomide in recurrent GBM and found a PFS-6 rate that was superior to that observed in historical controls, with 8 of 32 being progression-free at 6 months. However, other studies have not shown promising results with combination based thalidomide therapy. Fadul et al. [35] reported a study of irinotecan and thalidomide that showed limited activity while Chang et al. [36] found no survival advantage with TMZ and thalidomide added to XRT when compared with historical studies of XRT in combination with nitrosourea-based chemotherapy.
Since the completion of our study, another thalidomide analogue was introduced into clinical trials. Lenalidomide was used in a 28 patient phase I study by Fine et al. where there was an observed increased risk of thromboembolic disease. There were no objective radiographic responses seen in any of the treated patients. The median time to tumor progression was less than 2 months and only 12.5% of patients were progression-free at 6 months [37]. In a pediatric phase I study of lenalidomide for recurrent, refractory, or progressive CNS tumors, Warren et al. found both objective responses and long-term stable disease, primarily in low grade gliomas.
Overall, our study reports no activity with the use of procarbazine and thalidomide in patients with recurrent glioma. A prolonged cytostatic effect was not apparent as all patients had early evidence of progressive disease. In addition, MMSE and QOL scores declined in patients with serial measurements during the study period. It is unclear whether these changes were due to progressive disease or the impact of treatment.
Unfortunately, these results provide no evidence that the combination of procarbazine and thalidomide tested provides any advantage over the limited efficacy seen when these drugs are used as single agents in patients with recurrent glioma. Although alternate prolonged dosing strategies of procarbazine could potentially be used in combination with daily thalidomide, concerns about increased toxicity—particularly fatigue, peripheral neurotoxicity and potentially myelosuppression—led us to use a shorter duration of procarbazine administration in this trial. This approach was also felt to minimize the dietary restrictions (and subsequent quality of life impact) required while patients are taking procarbazine. Furthermore, following the development of bevacizumab and other selectively targeted therapies with effective antiangiogenic activity in patients with malignant brain tumors, there appears to be little, if any, reason to further evaluate thalidomide—a drug with multiple potential mechanisms of action including modest antiangiogenic effects—in this patient population. In light of these findings, it is unlikely that any dose or schedule of the combination of thalidomide and procarbazine will provide enough of a benefit to patients with recurrent GBM to merit further clinical evaluation.
Acknowledgments
This study was supported by National Cancer Institute grant 3 U10 CA81851-08-12.
Footnotes
Conflict of interest There is no conflict of interest for all authors.
Contributor Information
Jimmy Ruiz, Section on Hematology and Oncology, Department of Internal Medicine, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA.
Doug Case, Biostatistical Science, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA.
Gina Enevold, Radiation Oncology, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA.
Robin Rosdhal, Radiation Oncology, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA.
Stephen B. Tatter, Neurosurgery, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA
Thomas L. Ellis, Neurosurgery, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA
Richard P. McQuellon, Section on Hematology and Oncology, Department of Internal Medicine, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA
Kevin P. McMullen, Radiation Oncology, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA
Volker W. Stiebr, Radiation Oncology, Forsyth Medical Center, Winston-Salem, NC 27157, USA
Edward G. Shaw, Radiation Oncology, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA
Glenn J. Lesser, Radiation Oncology, Wake Forest School of Medicine, Medical Center Boulevard, Winston Salem, NC 27157, USA
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Central brain tumor registry of the United States (2004–2006) Statistical report: primary brain tumors in the United States [Google Scholar]
- 3.Wu W, Lamborn KR, Buckner JC, et al. Joint NCCTG and NABTC prognostic factors analysis for high-grade recurrent glioma. Neuro Oncol. 2010;12:164–172. doi: 10.1093/neuonc/nop019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 5.Fine HA, Dear KBG, Loeffler JS, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, Mason WP, van den Bent MJ, et al. Radiothearpy plus concomitant and adjuvant temozololmide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 8.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 10.Perry JR, Bélanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 11.Singhal S, Mehta J, Eddlemon P, et al. Marked anti-tumor effect from anti-angiogenesis therapy with Thalidomide in high risk refractory multiple myeloma (Abst) Blood. 1998;92(Suppl 1: part 1 of 2):318a. [Google Scholar]
- 12.Figg WD, Bergan R, Brawley O, et al. Randomized phase II study of thalidomide in androgen independent prostate cancer (Abst) Proc Am Soc Clin Oncol. 1997;16:333a. [Google Scholar]
- 13.Fine HA, Figg WD, Jaeckle K, et al. Phase II trial of antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J Clin Oncol. 2000;18:708–715. doi: 10.1200/JCO.2000.18.4.708. [DOI] [PubMed] [Google Scholar]
- 14.Politi P, Reboredo G, Losso M, et al. Phase I trial of thalidomide in AIDS-related Kaposi Sarcoma (Abst) Proc Am Soc Clin Oncol. 1998;17:41a. [Google Scholar]
- 15.Glass J, Gruber ML, Nirenberg A. Phase I/II study of carboplatin and thalidomide in recurrent glioblastoma multiforme (Abst) Proc Am Soc Clin Oncol. 1999;18:144a. [Google Scholar]
- 16.Durie BGM, Stepan DE. Efficacy of low dose thalidomide in multiple myeloma. VII international multiple myeloma workshop, Stockholm 1999 [Google Scholar]
- 17.Fine HA, Maher EA, Wen PY, et al. Phase II trial of thalidomide and carmustine for patients with recurrent high-grade gliomas. J Clin Oncol. 2003;21(12):2305–2311. doi: 10.1200/JCO.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 18.Chabner BA, Sponzi R, Hubbard S, et al. High-dose intermittent intravenous infusion of procarbazine. Cancer Chemother Rep. 1973;57:361–363. [PubMed] [Google Scholar]
- 19.Coyle T, Bushunow P, Winfield J, et al. Hypersensitivity reactions to procarbazine with mechlorethamine, vincristine, and procarbazine chemotherapy in the treatment of glioma. Cancer. 1992;69:2523–2540. doi: 10.1002/1097-0142(19920515)69:10<2532::aid-cncr2820691024>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann DF, Hurteau TE, Newman N, et al. Anticonvulsant usage is associated with an increased risk of procarbazine hypersensitivity reactions in patients with brain tumors. Clin Pharmacol Ther. 1997;62:225–229. doi: 10.1016/S0009-9236(97)90071-0. [DOI] [PubMed] [Google Scholar]
- 21.Newton HB, Bromberg J, Junck L, et al. Comparison between BCNU and procarbazine chemotherapy for treatment of gliomas. J Neurooncol. 1986;15:257–263. doi: 10.1007/BF01050072. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez LA, Prados M, Silver P, et al. Reevaluation of procarbazine for the treatment of recurrent malignant central nervous system tumors. Cancer. 1989;64:2420–2423. doi: 10.1002/1097-0142(19891215)64:12<2420::aid-cncr2820641204>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Newton HB, Junck L, Bronberg J, et al. Procarbazine chemotherapy in the treatment of recurrent malignant astrocytomas after radiation and nitrosourea failure. Neurology. 1990;40:1743–1746. doi: 10.1212/wnl.40.11.1743. [DOI] [PubMed] [Google Scholar]
- 24.Brandes AA, Ermani M, Turazzi S, et al. Procarbazine and high-dose tamoxifen as a second-line regimen in recurrent high-grade gliomas: a phase II study. J Clin Oncol. 1999;17:645–650. doi: 10.1200/JCO.1999.17.2.645. [DOI] [PubMed] [Google Scholar]
- 25.Gundersen S, Lote K, Watne K. A retrospective study of the value of chemotherapy as adjuvant therapy to surgery and radiotherapy in grade 3 and 4 gliomas. Eur J Cancer. 1998;34:1565–1569. doi: 10.1016/s0959-8049(98)00146-4. [DOI] [PubMed] [Google Scholar]
- 26.Yung A, Albright R, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossman S, Carson KA, Batchelor TT, et al. The effect of enzyme inducing anti-seizure drugs on the pharmacokinetics and tolerability of procarbazine hydrochloride. Clin Cancer Res. 2006;12:5174–5181. doi: 10.1158/1078-0432.CCR-06-0932. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald D, Cascino T, Schold SJ, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 29.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy (FACT) scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 30.Marx GM, Pavlakis N, McCowatt S, et al. Phase II study of thalidomide in the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2001;54(1):31–38. doi: 10.1023/a:1012554328801. [DOI] [PubMed] [Google Scholar]
- 31.Shaw EG, Wang M, Coons S, et al. Final report of Radiation Therapy Oncology Group (RTOG) protocol 9802: radiation therapy (RT) versus RT + procarbazine, CCNU, and vincristine (PCV) chemotherapy for adult low-grade glioma (LGG) J Clin Oncol. 2008 May 20;26(suppl) abstr 2006. [Google Scholar]
- 32.Baumann F, Kollias SS, et al. Combined thalidomide and temozolomide treatment in patients with glioblastoma multiforme. J Neurooncol. 2004;67(1–2):191–200. doi: 10.1023/b:neon.0000021803.01170.03. [DOI] [PubMed] [Google Scholar]
- 33.Groves MD, Puduvalli VK, Chang SM, et al. A North American brain tumor consortium (NABTC 99-04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007;81(3):271–277. doi: 10.1007/s11060-006-9225-y. [DOI] [PubMed] [Google Scholar]
- 34.Puduvalli VK, Giglio P, Groves MD, et al. Phase II trial of irinotecan and thalidomide in adults with recurrent glioblastoma multiforme. Neuro Oncol. 2008;10(2):216–222. doi: 10.1215/15228517-2007-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fadul CE, Kingman LS, Meyer LP, et al. A phase II study of thalidomide and irinotecan for treatment of glioblastoma multiforme. J Neurooncol. 2008;90(2):229–235. doi: 10.1007/s11060-008-9655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang SM, Lamborn KR, Malec M, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;60(2):353–357. doi: 10.1016/j.ijrobp.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Fine HA, Kim L, Albert PS, et al. A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin Cancer Res. 2007;13(23):7101–7106. doi: 10.1158/1078-0432.CCR-07-1546. [DOI] [PubMed] [Google Scholar]