Abstract
Tracheal agenesis/atresia (TA) is a rare but fatal congenital disease in which the breathing tube fails to grow. The etiology of this serious condition remains largely unknown. We found that Bmp signaling is prominently present in the anterior foregut where the tracheal primordium originates and targeted ablation of Bmp4 (Bmp4cko) resulted in a loss-of-trachea phenotype that closely resembles the Floyd type II pathology, the most common form of TA in humans. In Bmp4cko embryos, tracheal specification was not affected, however, its outgrowth was severely impaired due to reduced epithelial and mesenchymal proliferation. In agreement, we also observed significant reduction in the expression of Cyclin D1, a key cell cycle regulator associated with cellular proliferation. However, the proliferative effect of Bmp signaling appears to be independent of Wnt signaling. Interestingly, we found significantly reduced expression of activated extracellular signal-regulated kinase (Erk) in the Bmp4cko ventral foregut, suggesting that Bmp signaling promotes Erk phosphorylation which has been associated with cellular proliferation. This study provides the first evidence linking Bmp signaling to tracheal formation by regulating the proliferative response of the anterior ventral foregut. Our finding sheds light on human tracheal malformations by providing a novel mouse model implicating Bmp signaling, non-canonical Erk activation and cellular proliferation.
INTRODUCTION
The trachea/lung and esophagus, respiratory and digestive organs respectively, originate from a common foregut endodermal tube surrounded by splanchnic mesoderm. Ventral outpocketing of the respiratory primordium from the anterior foregut endoderm, clearly discernible at E9.5 in the mouse embryo, gives rise to the tracheal diverticulum with a pair of lung buds at its distal end while the dorsal foregut domain develops into the esophagus (Cardoso and Lu, 2006; Shannon and Hyatt, 2004; Warburton et al., 2000). Defective development of these organs results in congenital malformations in humans such as esophageal atresia and tracheoesophageal fistula (EA/TEF) and a spectrum of tracheal defects known as tracheal agenesis or atresia (TA).
TA has been diagnosed in neonates with cyanosis and severe respiratory distress and at an incidence of less than 1 in 50,000 births, TA is considered a rare congenital malformation but uniformly fatal (Felix et al., 2006; Manschot et al., 1994; van Veenendaal et al., 2000). It is often associated with a wide variety of other congenital anomalies of the heart, genitourinary, gastrointestinal, pulmonary and central nervous system but specific etiologies have not been identified (Evans et al., 1999; Faro et al., 1979; Kerschner and Klotch, 1997; Lander et al., 2004; Saleeby et al., 2003; Sparey et al., 2000; Wei et al., 2003). The Floyd anatomical classification describes three types of TA (Fig. 1A). In Type I, there is atresia of part of the trachea with a normal but short distal trachea, normal bronchi and tracheoesophageal fistula. In Type II, the most common type in humans reported at around 60% of TA cases, there is complete tracheal agenesis but with normal bifurcation and bronchi. Type III has no trachea and the bronchi arise directly from the esophagus (Diaz et al., 1989; Faro et al., 1979; Floyd et al., 1962; Fraser et al., 2005; Heimann et al., 2007; Manschot et al., 1994; van Veenendaal et al., 2000; Wei et al., 2003).
Figure 1A.
Classification scheme for tracheal agenesis of Floyd et al. (14). 1B. Expression of Bmp4-lacZ and Foxg1CreXRosa26R in the anterior foregut at different stages. a-d: Bmp4-lacZ expression is restricted to the ventral foregut during tracheal morphogenesis. e-f”: Foxg1 expression at E8.5 (e, e’, e”) and E9.5 (f, f’, f”) by lacZ staining of embryos from Foxg1CreXRosa26R. Lines in e and f denote the cross-section planes of e’, e”, f’ and f”. Foxg1 starts to be expressed in the foregut endoderm as early as E8.5 (e-e”), and its expression becomes more uniform in both the endoderm and the mesenchyme by E9.5 (f-f”). fg-foregut; es-esophagus; tr-trachea. Magnification: B: a-c-200X; d-100X; e-400X; f-200X; e’,e”,f’,f”-200X. Scale bar: 50 μm.
Elucidating the molecular pathogenesis of foregut anomalies will add significantly to our progress in understanding these congenital malformations of unknown etiology. Recent studies by us and others have elucidated a key role of Bmp signaling in the generation of EA/TEF (Li et al., 2007; Que et al., 2006). Therefore, we asked whether Bmp signaling could also have a critical role in tracheal morphogenesis. We found that the distribution of phosphorylated-Smad1/5/8 (p-Smad1), indicative of Bmp signaling, is restricted to the ventral foregut endoderm and mesenchyme (this work). In agreement, a previous study has shown that Bmp4 is expressed in the ventral foregut mesenchyme surrounding the lung primordium and the future trachea (Weaver et al., 1999). These observations raised the possibility that Bmp signaling may be important in tracheal morphogenesis.
During development of the foregut tube, inductive signals emanating from the ventral foregut mesoderm, such as Bmps, are thought to pattern the underlying foregut endoderm resulting in outgrowth of primitive buds that give rise to structures such as the lung, liver and ventral rudiment of the pancreas during an active period of organogenesis (Hogan, 1996; Rossi et al., 2001; Zaret, 2002). Bmp 2, 4 and 7 are by far the most studied members of the Bmp family. Signaling by Bmps is mediated by a receptor complex consisting of type 1 (Bmpr-1a/Alk3, Bmpr-1b/Alk6) and type 2 (Bmpr2) transmembrane receptor serine-threonine kinases. Type 1 receptors for Bmps phosphorylate downstream targets, Smad1/5/8, which then form heteromeric complexes with Smad4 followed by translocation to the nucleus where they bind cis elements associated with specific gene expressions which regulate diverse cellular processes such as proliferation, apoptosis, growth arrest or cell migration (Aubin et al., 2004; Chen et al., 2004; Kishigami and Mishina, 2005; Massague, 2000; Mishina, 2003). Besides Smad-mediated transcription, Bmp-induced receptor complex can also activate p38 MAPK (mitogen-activated protein kinase) pathway to modulate apoptosis (Kendall et al., 2005; Kimura et al., 2000; Moustakas and Heldin, 2005). Several reports also indicate activation of Ras and ERK (Extracellular-Signal Regulated Kinase, i.e. p44/42 MAPK) by Bmps under physiological and pathological conditions (Haynes et al., 2007; Moustakas and Heldin, 2005; Zhou et al., 2007).
In this study, we address the role of Bmp4 signaling in tracheal morphogenesis, by conditionally ablating Bmp4 using Foxg1Cre (Bmp4cko) since early embryonic lethality of Bmp4 null mutants precludes analysis of Bmp function at a stage when the trachea begins to develop (Fujiwara et al., 2002; Fujiwara et al., 2001; Lawson et al., 1999). We found that Bmp signaling is essential to tracheal formation and the Bmp4cko foregut displayed loss of trachea resembling the Floyd type II pathology.
RESULTS
Conditional ablation of Bmp4 in the ventral foregut by Foxg1Cre transgene
To follow Bmp4 transcript during anterior foregut patterning, we used a well established Bmp4-lacZ reporter mouse line (Jiao et al., 2003; Lawson et al., 1999). As shown in Fig. 1B-a, expression of Bmp4lacZ in the embryonic foregut is restricted to the ventral mesenchyme as early as E8.5. Such a ventrally-restricted pattern is maintained at later stages when the trachea and esophagus are completely separated (Fig. 1B b-d). Similarly, immunostaining of phosphorylated-Smad1/5/8 (p-Smad1/5/8), a read-out of activated Bmp signaling (Knosp et al., 2007), also appeared to be restricted to the ventral mesoderm and endoderm in the anterior foregut (Fig. 2A-a and c). The specific role of this ventrally-restricted Bmp4 expression and signaling in anterior foregut growth and patterning remains to be elucidated. We reasoned that morphogenesis of the trachea, a ventral endoderm derivative, would likely involve epithelial-mesenchymal interactions involving Bmp signaling; therefore, to investigate the role of Bmp signaling in tracheal morphogenesis, we took advantage of a Foxg1-Cre transgenic mouse line to specifically delete Bmp4 function in the ventral foregut domain. Foxg1 is expressed in the foregut endoderm at E8.5 (Fig. 1B-e, e’,and e”), which is earlier than previously reported (Hebert and McConnell, 2000). Foxg1 expression appeared to be more uniform and robust in both the foregut endoderm and mesoderm by E9.5, as highlighted by lacZ staining of embryos generated by crossing Foxg1-Cre with ROSA26R (Fig.1B-f, f’ and f”). Therefore, we generated conditional deletion of Bmp4 in the ventral foregut by crossing Bmp4lacZ/+;Foxg1Cre mice with Bmp4loxp/loxp mice (Kulessa and Hogan, 2002). Based on the early pattern of Bmp4 expression in the ventral mesoderm (Fig.1B-a), forxg1cre-mediatedBmp4 ablation in this tissue would likely affect Bmp4-mediated signaling in the ventral foregut mesoderm and endoderm. Indeed, we found almost complete loss of Bmp signaling as demonstrated by p-Smad1/5/8 immunostaining in the anterior ventral foregut endoderm and mesoderm corresponding to the tracheal rudiment in Bmp4cko embryos compared with wildtype at E9.0, prior to the emergence of the respiratory primordium (Fig. 2A a and b). We observed limited residual pSmad1/5/8 expression in the more posterior ventral foregut, close to the emerging lung bud region, either due to incomplete Bmp4 excision or presence of another Bmp (Bmp7) (data not shown). Based on pSmad1/5/8 staining of all embryos examined (n=3), it appeared that Foxg1Cre-mediated Bmp4 deletion occurred effectively in the anterior ventral foregut.
Figure 2A.
Expression of p-Smad1/5/8, indicative of activated Bmp signaling, is almost completely lost in E9.0 Bmp4cko foregut compared with wildtype. (a, c) sections of wildtype embryos show ventrally restricted p-Smad1/5/8 expression (green) throughout the anterior foregut. (b, d) p-Smad1/5/8 immunostaining is substantially reduced in the anterior ventral foregut endoderm and mesoderm corresponding to tracheal rudiment in Bmp4cko embryos compared with wildtype at E9.0, prior to the emergence of the respiratory primordium (b). Limited pSmad1/5/8 activity is observed in the more posterior ventral foregut close to the emerging lung bud region (d), consistent with lung development, albeit severely hypoplastic, in Bmp4cko embryos (see figure 3F). 2B. Expression of Id1, Id2 and Id3. All Id gene expressions are significantly downregulated in the ventral foregut epithelium and mesenchyme of Bmp4cko (b, d, f) compared with wildtype (a, c, e). fg-foregut. Magnification: A-400X. B-200X. Scale bar: 25 μm.
Expression of Id genes is significantly downregulated in Bmp4cko foregut
We also examined the expression of inhibitor of differentiation (Id) genes, putative Bmp targets, in Bmp4cko ventral foreguts. Bmp signaling can transcriptionally regulate expression of Id genes which encode negative regulators of basic helix-loop-helix (bHLH) transcription factors (Hollnagel et al., 1999; Miyazono and Miyazawa, 2002; ten Dijke et al., 2003; Ying et al., 2003). Id proteins have distinct functions in development and disease, and have been implicated in cell cycle regulation, G1 progression and the control of growth induction [Lyden, 1999 #509; Benezra, 2001 #507; Barone, 1994 #501; Lasorella, 2001 #519]. We found that all the Id genes (Id1, 2, 3) were expressed in both the ventral foregut mesoderm and endoderm while Id3 expression is relatively higher in the ventral foregut mesoderm compared to the endoderm (Fig.2B a, c, e). In Bmp4cko embryos, expression of all three Ids was significantly downregulated in the ventral foregut (Fig.2B b, d, f), consistent with downregulation of Bmp signaling detected by pSmad1/5/8 (Fig.2A).
Bmp4-deficient foregut displays loss of trachea
To determine the functional significance of this specific ablation, we examined the gross foregut morphologies of Bmp4cko embryos by Foxa2 immunohistochemistry to highlight the foregut endoderm (Litingtung et al., 1998). While there was no apparent morphological differences between wildtype and Bmp4cko foregut at E9.0 (Fig. 3A and B), a stage prior to the appearance of the tracheal primordium, all Bmp4cko embryos displayed a single endodermal tube at E10.5 (n=3, Fig. 3D) and E11.5 (n=3, Fig. 3F) compared with wildtype littermates which displayed two distinct gut tube derivatives, the esophagus and trachea (Fig. 3C and E). In addition, we also observed hypoplastic lungs in all the Bmp4cko embryos examined (Fig. 3E and F), indicating an additional role of Bmp4 signaling during early lung bud growth.
Molecular analysis of E12.5 embryos showed that the single endodermal tube in the Bmp4cko embryos did not stain with respiratory/tracheal specific marker Nkx2.1 (n=3, Fig. 3I and J), whereas Nkx2.1 expression could still be detected in the underdeveloped lung epithelium (data not shown) (Minoo et al., 1999). In contrast, Pax9, an esophageal specific marker, labeled this endodermal tube (n=3, Fig. 3G and H). We checked expression of the cartilage marker collagen type IIa (Col2a) which normally surrounds the ventral part of the tracheal epithelium and found that Col2a expression was also lost in the ventral mesenchyme in Bmp4cko foregut compared to wildtype foregut (Fig. 3K and L). Taken together, these findings indicate that the single endodermal tube in Bmp4cko embryos has an esophageal characteristic and completely lacks tracheal identity, thus manifesting tracheal agenesis (TA). We found that the foregut phenotype in Bmp4cko mutant embryos more closely resembles Floyd Type II tracheal agenesis (Diaz et al., 1989; Faro et al., 1979; Felix et al., 2006; van Veenendaal et al., 2000)(Fig. 1), as the bronchi arising from the midline of the esophageal tube/ fistula are formed between the esophageal tube and carina (Fig. 3F and inset).
Specification of the tracheal primordium appears normal in Bmp4cko embryos
To address whether loss of trachea is due to an early defect in respiratory endodermal cell specification, we first examined the ventral foregut endoderm in Bmp4cko embryos by immunostaining Nkx2.1, a respiratory/tracheal specific marker. At E9.25 (20-22 somites), a stage prior to the appearance of the respiratory rudiment and when no apparent abnormal morphology was evident in Bmp4cko foregut, Nkx2.1 expression level and distribution in the Bmp4cko foregut was comparable with wildtype (Fig. 4A and B). We also examined Nkx2.1 expression at later stages (E9.5-E10.5) and found its level to be comparable between Bmp4cko and wildtype foreguts (Fig.4C and D). The Nkx2.1 expression domain appeared reduced in Bmp4cko foregut (compare bracket width in Fig.4 C and D), likely due to the reduced Bmp4cko foregut size. Taken together, the presence of Nkx2.1-positive cells in the Bmp4cko foregut endoderm at E9.25 suggests that Bmp4-mediated signaling is required for the outgrowth, but not the initial specification, of the tracheal primordium. In line with this notion, it has been proposed that TA might occur due to failure of the tracheal tube to elongate (Effmann et al., 1975; Lander et al., 2004).
Figure 4.
Specification of tracheal primordium appears normal in Bmp4cko embryos. (A, B) Nkx2.1 immunostaining of embryos at E9.25 (20-22 somites), a stage just before the emergence of the respiratory bud, reveals that the extent of Nkx2.1 expression is comparable in Bmp4cko and wildtype foregut, suggesting that the initial specification of the tracheal primordium is not affected in Bmp4cko embryos. (C, D) Nkx2.1 expression at E9.5 (23-25s). Nkx2.1 expression domain is reduced in Bmp4cko foregut (compare length of white brackets in Fig.4 C and D), likely due to reduced foregut size in Bmp4cko embryos apparent at this later stage. fg-foregut. Magnification: 400X. Scale bar: 25 μm.
Bmp4cko foregut displays decreased cell proliferation but no significant cell death.
To further investigate the decrease in growth potential of the Bmp4cko foregut, we determined the proliferative capacity of E9.5 wildtype and mutant foreguts by in vivo pulse labeling with 5’-Bromodeoxyuridine (BrdU), a nucleotide analog that incorporates into replicating DNA. Taking into account the reduced Bmp4cko foregut size, we determined the BrdU-positive cells as percentage of total number of cells in Bmp4cko embryonic foreguts compared with wildtype littermates on five sequential sections of the upper foregut in each of three pairs of mutant or wildtype embryos; wildtype and mutant embryos were paired based on somite number within litters. We found significant difference in cell proliferation in the Bmp4cko foregut epithelium (p<0.001) and mesenchyme (p<0.01-0.001). BrdU pulse-labeling revealed a relatively lower percentage of proliferating epithelial cells in the Bmp4cko foregut (28.2-37.7%) compared with wildtype foregut (53.3-56.9%). Mesenchymal cells in the Bmp4cko foregut also displayed a lower proliferative capacity (25.0-41.6%) compared with wildtype foregut (48.8-52.2%) (Fig. 5A).
Figure 5.
Bmp4cko foregut displays reduced cell proliferation and Cyclin D1 compared with wildtype. (A) Histograms showing the percentage of BrdU-labeled cells in the epithelium and mesenchyme of E9.5 Bmp4cko foregut compared with wildtype foregut and Cyclin D1 in the epithelium. (B) Immunohistochemistry of foregut sections showing Brdu and Cyclin D1 labelings. All the compared values are statistically significant (p<0.05). Magnification in B: 400X. Scale bar: 25 μm.
Cyclins are evolutionarily conserved proteins that are essential for cell cycle control and Cyclin D1 is a critical component for G1 to S progression (Sanchez and Dynlacht, 2005; Sherr and Roberts, 1999). By immunohistochemical analysis, we found that the expression level and distribution of cyclin D1 was reduced in both the epithelium and mesenchyme of Bmp4cko foregut during respiratory bud emergence at E9.5 compared with wildtype. Cell counting indicates a significant decrease in the distribution of cyclinD1-positive cells in the Bmp4cko foregut epithelium (36.9%) compared with wildtype (58.5%) which is consistent with the impaired growth capacity of the Bmp4cko trachea (Fig. 5A).
We also examined Bmp4cko foregut for alterations in the level of cell death by TUNEL assay. We did not observe significant apoptotic cells in the ventral foregut endoderm of Bmp4cko and wildtype embryos at E9.0-10.5 (data not shown).
Wnt/β-catenin signaling is required for foregut endoderm development but does not appear to act downstream of Bmp signaling.
As several studies have suggested that Bmp signaling may regulate cell proliferation/cell cycle progression via cross talk with Wnt signaling (Burstyn-Cohen et al., 2004; Marcelle et al., 1997; Ovchinnikov et al., 2006), and Cyclin D1 is a known target of the Wnt canonical pathway (Civenni et al., 2003; Tetsu and McCormick, 1999; Willert et al., 2002), we next examined potential alteration of the Wnt pathway in Bmp4cko foreguts using transgenic Top-Gal reporter mice which express β-galactosidase under the control of multimerized LEF/TCF consensus binding sites (DasGupta and Fuchs, 1999). Top-Gal expression can be detected at E9.5 in the developing anterior foregut endoderm at the level of the laryngotracheal groove and Wnt activity persists in foregut endoderm derivatives at later stages (Okubo and Hogan, 2004; Shu et al., 2005), suggesting that canonical Wnt signaling may play a key role in anterior foregut morphogenesis. We therefore introduced Top-Gal transgene into Bmp4lacZ/+; Foxg1Cre/+ mice and mated them with Bmp4flox/flox to obtain Bmp4cko embryos harboring Top-Gal. E9.25 (20-22 somites) embryos expressing both Bmp4-lacZ and Top-Gal upon lacZ staining were selected, based on specific Bmp4-lacZ expression in the limb mesoderm and mid-hind gut (red arrows in Fig. 6A-a, b) and distinct Top-Gal expression in the midbrain ectoderm (black arrowheads in Fig. 6A-a, b). Bmp4cko embryos were identified by Cre PCR. At E9.25, while Bmp4-lacZ was restricted in the ventral foregut mesenchyme (also refer to Fig. 1B-b), Top-Gal expression was observed only in the ventral endodermal layer, consistent with a previous report (Okubo and Hogan, 2004) (Fig. 6A-d, f). We found that Top-Gal expression in the Bmp4cko foregut (Fig. 6A-e, g) was largely comparable to control (Bmp4lacZ/flox) littermates (Fig. 6A-d, f), suggesting that Wnt/β-catenin signaling was not affected in the Bmp4cko foreguts. This finding does not support a role of Wnt signaling aberration in the Bmp4cko tracheal phenotype.
Figure 6A.
Wnt/β-catenin signaling remains unaffected in Bmp4cko embryos. (A-C) Whole-mount view of lacZ stained E9.0-9.25 (20-22 somites) Bmp4-lacZ, Top-gal and Bmp4-lacZ+Top-gal embryos. Note specific expression of Top-gal in the mid-brain ectoderm (black arrowheads in a, b) and Bmp4-lacZ in the limb mesoderm and midhindgut (red arrows in a, b). Lines in c denote the cross-section planes of d-g. (d-g) Top-gal expression level (see lacZ expression in the foregut endoderm) remains unaltered in the Bmp4cko compared to control (Bmp4lacZ) littermates. The lacZ staining in the ventral mesenchyme is from Bmp4-lacZ allele (see Fig.1B), which is present in both the control and Bmp4cko embryos. 6B. Targeted deletion of β-catenin in the ventral foregut. β-Catenincko (by Foxg1Cre) foregut (b, b’) displays an overall reduced growth including tracheal atresia compared with staged wildtype foregut (a, a’), as revealed by whole-mount immunostaining of E11.5 embryos with Foxa2. Both lateral (a’, b’) and ventral (a, b) views of embryos are shown. fg-foregut; es-esophagus; tr-trachea; lu-lung; st-stomach. Magnification: A: a to c-250X; d to g-200X; B: 500X. Scale bars: 50 μm in A; 20 μm in B.
Interestingly, however, when we conditionally ablated β-catenin function using Foxg1Cre, we observed an overall reduction in anterior foregut growth including shortened trachea and esophagus (Fig. 6B). This phenotype is distinct from Bmp4cko mutants where the primary defects lie in the trachea/lung, highlighting the unique role of Bmp signaling in tracheal morphogenesis.
Activation of Erk1/2 is severely impaired in Bmp4cko foreguts
In light of accumulating evidence suggesting that Bmp can alternatively signal through Ras/ErK pathway in regulating cell proliferation/regeneration and morphogenetic processes (Derynck and Zhang, 2003; Haynes et al., 2007; Yang et al., 2005; Zhou et al., 2007), we examined whether the Erk pathway is affected in Bmp4cko foreguts. While activated Erk (p-Erk) was detected in the ventral foregut endoderm and mesoderm in E9.25 wildtype embryos (n=2, Fig. 7, A, C, E), its expression was almost completely lost in the Bmp4cko ventral foreguts (n=2, Fig. 7, B, D, F). We note that p-Erk expression in the vessel endothelial cells remains unaffected in the mutant (Fig. 7, arrow). This result indicates that loss of Bmp signaling has profound effects on Erk pathway activation, which may regulate tracheal proliferation and outgrowth.
Figure 7.
Erk1/2 pathway is severely impaired in Bmp4cko foregut. While phosphorylated-Erk (p44/42 MAPK) is detected in the ventral endoderm and mesoderm of E9.25 wildtype embryos (A, C, E), its expression is almost completely lost in the Bmp4cko ventral foreguts (B, D, F). Note that p-Erk is detected in both wildtype and mutant vessel endothelial cells (arrow). fg, foregut. Magnification: 200X. Scale bar: 50 μm.
DISCUSSION
Tracheal atresia/agenesis (TA) is a rare but fatal foregut anomaly of unknown etiology (Diaz et al., 1989; Kerschner and Klotch, 1997; Manschot et al., 1994). We found that mouse embryos with conditional ablation of Bmp4 in the ventral foregut domain by a Foxg1Cre transgene displayed TA with a fistula between the esophagus and carina which divides to form the bronchi. Therefore, this study reveals a critical role of Bmp signaling in tracheal morphogenesis. Further analysis of Bmp4cko embryos indicated that respiratory specification was unaffected based on Nkx2.1 expression. Therefore, Bmp4 is not required for the initial specification of the respiratory bud; rather, it plays a vital role in tracheal outgrowth and elongation which was severely impaired. This finding is consistent with significantly reduced tracheal epithelial and mesenchymal proliferation indicative of impaired G1-S cell cycle progression but without significant alteration in cell death. Bmp signaling is involved in epithelial growth and tissue morphogenesis of the lung and kidney (Cardoso and Lu, 2006; Eblaghie et al., 2006; Miyazaki et al., 2000). This finding contrasts with the role of Bmp signaling in liver development, where it appears to be essential for both initial hepatic fate specification and subsequent outgrowth of hepatic endoderm into liver buds (Rossi et al., 2001; Zaret, 2000; Zaret, 2001)
Like lung development, tracheal morphogenesis is also governed by a complex process of inductive interactions between the endoderm and its surrounding mesoderm. We observed reduction in Bmp signaling in both the ventral foregut endoderm and mesenchyme, suggesting that Bmp4 signals not only function in an autocrine but also paracrine fashion to regulate the growth of the underlying foregut endoderm. In addition, n Bmp4 signaling could act indirectly on the endoderm by regulating mesenchymal signals that are critical for epithelial growth.
Several signaling pathways notably Wnt and Shh have been shown to play a critical role during endoderm growth and patterning. In the chick anterior foregut, Wnt5a and Wnt11 are expressed in the early tracheal mesenchyme, the laryngotracheal groove, suggesting that they may be involved in tracheal morphogenesis (Sakiyama et al., 2000). Transgenic Top-Gal reporter expression in the mouse can be detected as early as E9.5 in the developing anterior foregut endoderm suggesting that Wnt signaling may play a key role in anterior foregut morphogenesis (Okubo and Hogan, 2004; Shu et al., 2005). Wnt5a null embryos displayed defects in late lung maturation and tracheal length was significantly reduced (Li et al., 2002). Likewise Wnt11 has been implicated in the proliferation of intestinal epithelial cells and morphogenesis of the chick respiratory tract (Ouko et al., 2004; Sakiyama et al., 2000). Furthermore, Bmp has also been shown to regulate the cell cycle by controling Cyclin D1 expression and G1/S transition via activation of canonical Wnt signaling during neural crest delamination (Burstyn-Cohen et al., 2004; Marcelle et al., 1997). Conditional inactivation of Bmp receptor, BMPR-IA, in the developing limb bud mesenchyme resulted in reduced levels of Wnt5a and cyclin D1, reduced cell proliferation and severe defect in distal limb outgrowth (Ovchinnikov et al., 2006). Conversely, canonical Wnt signaling has been shown to transcriptionally regulate the expression of Bmp4 in the developing lung epithelium (Shu et al., 2005). Hence, the coexistence of these two conserved signaling systems appears to be important during embryogenesis in promoting tissue growth and patterning in certain context. However, using the Top-Gal reporter mouse line (DasGupta and Fuchs, 1999), we did not observe changes in Wnt signaling in Bmp4cko foregut. In addition, targeted deletion of •-catenin in the foregut resulted in tracheal defects distinct from that of Bmp4cko, suggesting that Bmp signaling likely does not act via Wnt/•-catenin signaling during tracheal morphogenesis.
Embryos with Shh loss-of-function displayed severe esophageal and tracheal defects suggesting a critical role of Shh signaling in foregut morphogenesis (Litingtung et al., 1998; Motoyama et al., 1998; Pepicelli et al., 1998). Since it has been suggested that Bmp signaling can cross-talk with Shh during palatogenesis and tooth germ development (Chen et al., 1996; Zhang et al., 2000; Zhang et al., 2002), we also examined the potential role played by Shh in the Bmp4cko tracheal phenotype. However, the expression of Shh and its downstream target gene Gli1 in Bmp4cko foregut appeared comparable to wildtype at E9.0-E9.5 (Supplemental Fig. 1A-D, data not shown). Furthermore, constitutive activation of Shh pathway using SmoM2 allele in the ventral foregut did not rescue the Bmp4cko TA phenotype. Thus, Shh signaling does not appear to mediate the loss-of-trachea phenotype in Bmp4cko embryos.
Besides the canonical pathway mediated by Smads, Bmp/TGFbeta signaling has also been shown to activate the mitogen-activated protein kinase (MAPK) pathway via ERK, JNK or p38 (Derynck and Zhang, 2003; Hamada et al., 2007; Haynes et al., 2007; Miyazono et al., 2005; Moustakas and Heldin, 2005; Nohe et al., 2002; Piek et al., 1999; Yang et al., 2005; Zhou et al., 2007). Here, we found that pErk1/2 was almost completely lost in Bmp4cko ventral foregut suggesting that Erk signaling may be the underlying effector of Bmp4 pathway in regulating tracheal proliferation and outgrowth. It has been shown that during the initial stage of retinal regeneration, Bmp signaling directs FGF-dependent activation of Erk resulting in proliferation and differentiation (Haynes et al., 2007). Although Fgf signaling is active during foregut morphogenesis, no mutants in Fgf signaling have been shown, sofar, that display a tracheal phenotype (Celli et al., 1998; De Moerlooze et al., 2000; Min et al., 1998; Sekine et al., 1999). Therefore, Bmp signaling in the anterior ventral foregut regulates activation of Erk pathway possibly via a more direct mechanism. In a recent finding, ERK activation was shown as a central regulator for BMP-4 dependent capillary sprouting in human umbilical vein endothelial cells (HUVECs) and Smad6 (I-Smad) can inhibit phosphorylation of ERK1/2 and endothelial cell sprouting (Zhou et al., 2007). Interestingly, Smad4 (co-Smad) siRNA knockdown in HUVECs did not affect BMP-4 induced sprout length, suggesting that the ERK, but not Smad pathway, is necessary for endothelial cell sprouting. In our study, we detected expression of activated Smad1/5/8 and Erk1/2 in the ventral foregut, both of which are substantially lost in the Bmp4cko foregut; how or whether these two pathways cross talk during tracheal formation remains to be investigated.
Bmp-induced receptor complexes can also phosphorylate and activate p38 MAPK pathway via TAK1 (TGFbeta-activated kinase 1) to modulate apoptosis (Kendall et al., 2005; Kimura et al., 2000; Moustakas and Heldin, 2005). We did not detect activated/phosphorylated p38 MAPK staining in wildtype foregut at E9.25-E9.5, nor did we find alteration in phosphorylated-p38 staining in Bmp4cko foreguts (data not shown), suggesting that Bmp4 does not directly activate the p38 MAPK pathway during tracheal formation. This is consistent with our finding that there was no significant apoptotic cells in the ventral foregut endoderm of Bmp4cko and wildtype embryos at E9.0-10.5 (data not shown).
Tracheal agenesis is considered a rare congenital malformation but uniformly fatal (Felix et al., 2006; Manschot et al., 1994; van Veenendaal et al., 2000). However, the cellular and molecular mechanisms underlying this serious congenital defect are poorly understood. Our finding that Bmpcko mutants resemble the most common form of tracheal agenesis observed in human underscores the critical role of Bmp signaling in tracheal morphogenesis and provides a unique animal model to further elucidate the pathogenesis of this disease. Since familial patterns are uncommon, external factors have been implicated in foregut malformations such as EA/TEF and TA (van Veenendaal et al., 2000). Taken together, our findings implicate Bmp signaling as a potential teratogenic target at a critical period during human foregut development.
MATERIALS AND METHODS
Animals
Bmp4loxp/loxp and Bmp4lacZ/+ mice were kindly provided by Dr. Brigid Hogan and Dr. Holger Kulessa. β-cateninflox/flox mice were obtained from Dr. Kemler. Foxg1Cre, ROSA26R, SmoM2 and Top-Gal transgenics were obtained from the Jackson Laboratory. All mouse strains were maintained in a C57BL/6 background, except for ROSA26R, which was maintained in a mixed background. Bmp4 conditional knockout (Bmp4cko) embryos were generated by crossing Bmp4flox/flox mice with Bmp4lacZ/+; Foxg1Cre mice, and identified by Cre and Bmp4-lacZ PCRs using the following primers: Cre(f): 5’-TCGATGCAACGAGTGATGAG-3’; Cre(r): 5’-TTCGGCTATACGTAACAGGG-3’; Bmp4-lacZ(f):5’-CAGGGCGATTCTTACTTTCG-3’; Bmp4-lacZ(r):5’-AGCTTGGCGTAATCATG GTC-3’. Conditions for PCRs were: 94°C for 4minutes; 32 cycles of (94°C for 30 seconds, 55°C for 30 seconds, 72°C for 40 seconds); 72°C for 10 minutes. Amplifications of Cre and Bmp4-lacZ allele generate a 480-bp product and a 339-bp product, respectively. To characterize Foxg1 expression, Foxg1Cre was crossed with ROSA26R. To study the effect of Bmp4 ablation on Wnt signaling, the Top-Gal transgene was introduced into Bmp4lacZ/+; Foxg1Cre before mating with Bmp4flox/flox homozygotes. Bmp4cko embryos containing Top-gal were determined by lacZ staining. To evaluate the role of Wnt/β–catenin signaling in tracheal outgrowth, β-cateninflox/flox mouse was first bred with Sox2Cre transgene to generate a β-catenin null allele (Hayashi et al., 2002). Foxg1Cre was then introduced into β-catenin+/− mouse before mating with the β-cateninflox/flox homozygote. Genotyping of β-catenin wild type (wildtype), floxed, and floxed deleted alleles were performed as previously described (Brault et al., 2001).
Immunohistochemistry and IacZ staining
Immunohistochemistry on paraffin-embedded sections and lacZ staining were performed as previously described (Li et al., 2004). Briefly, embryos were collected in cold PBS, fixed in 4% PFA for 1 hour at 4°C, dehydrated in a series of methanol washes (25%, 50%, 75% methanol/PBS+0.1%Tween, and 2X 100% methanol), and embedded in paraffin. Embryos used for phospho-ERK (p44/42) immunostaining were fixed in EFA solution and processed as previously described (Li et al., 2006). Immunostaining using Nkx2.1 on embryos younger than E10 was performed on cryosections. Briefly, embryos were fixed in 4% PFA at 4°C for 40 minutes, washed in PBS 3 times, 10 minutes each, and embedded in OCT. Cryosections were incubated incubation with primary antibody in blocking solution at 4°C overnight. Primary antibodies were used at the following dilutions: rabbit anti-phospho-Smad1/5/8 (gift of Dr. Laufer 1:1500); rabbit anti-Foxa2 (1:50); mouse anti-Nkx2.1 (Lab Vision, 1:200); rabbit anti-Shh (Santa Cruz H-160, 1:200); rat anti-E-cadherin (Zymed, 1:200); mouse anti-CylinD1 (BD pharmingen, 1:100); rabbit anti-CyclinD2 (Santa Cruz, M20, 1:150); and mouse anti-CyclinD3 (Lab Vision, 1:200); rabbit anti-phosphorylated ERK (p44/42) (Cell Signaling, 20G11, 1:200 ); rabbit anti-phosphorylated p38 MAPK (Cell Signaling, 12F8, 1:200 ).
Analysis of cell proliferation and cell death
Pregnant female mice at E9.5 were injected intraperitoneally with 5-Bromodeoxyuridine (BrdU; 50mg per kg body weight) for 30 min before embryo collection and processing for BrdU immunodetection as described in our previous studies (Li et al., 2004; Litingtung et al., 1998). Briefly, samples were incubated with mouse anti-BrdU antibody (Roche, 1:15 diluted in PBS+10% goat serum) overnight at 4°C. Slides were washed in PBTw (PBS+0.1% Tween20) 3 times for 10 minutes each, and incubated with goat antimouse horseradish peroxidase (HRP) conjugated secondary antibody (Jackson ImmunoResearch, 1:300) for 1.5 hours. Slides were then incubated with chromogenic substrate DAB (Invitrogen) and counterstained with hematoxylin. TUNEL assay (ApopTag Apoptosis Detection Kit, Chemicon) was used for detection of apoptotic cells in embryonic sections as previously described (Li et al., 2007).
In situ hybridization
Cryosection in situ hybridizations were performed as previously described (Li et al., 2004). The following cDNAs were used as templates for synthesizing digoxygenin-labeled riboprobes: Id1-3 (R. Benezra), Pax9 (R. Balling) and mCol2a (Y. Yamada).
Statistical analysis
Sections processed for BrdU detection were photographed at 200X magnification. Total number and percentage of BrdU-positive cells in Bmp4cko embryonic foreguts compared with wildtype littermates on five sequential sections of the upper foregut (anterior to lung primordium) per mutant or wildtype embryo were counted, with three pairs of embryos. All values were represented as means +/- standard error of mean (SEM). Student’s t-test was applied to determine statistical significance of differences between wildtype and Bmp4cko embryos. Statistical significance was defined as p<0.05.
Supplementary Material
Activation of Shh pathway does not rescue the Bmp4cko TA phenotype. (A-D) Shh expression (green) in wildtype and Bmpcko mutants at E9.25 and E9.5. (E, F) Note that Shh expression is not significantly affected in Bmpcko mutant foregut. Constitutive activation of Shh signaling in the ventral foregut generated by Foxg1Cre;SmoM2 transgenes in wildtype (E) and Bmp4cko mutant background (F). Note that constitutive Shh pathway activation in the ventral foregut did not significantly alter tracheal and esophageal development, although it had a significant effect on the forebrain (data not shown). Magnification: A to D-200X; E, F-500X. Scale bars: 50 μm in A-D; 20 μm in E, F.
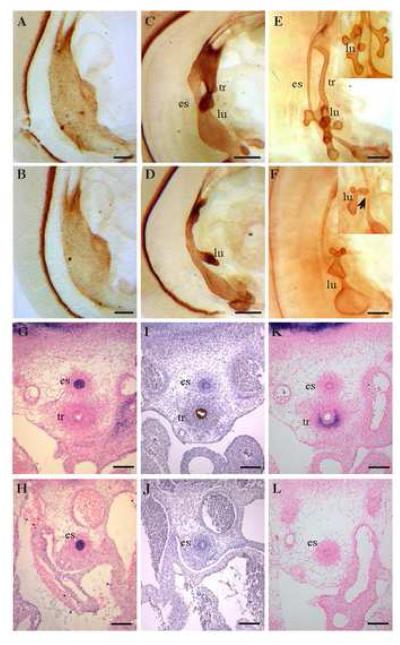
Figure 3.
Bmp4-deficient foregut displays loss of trachea. Bmp4cko embryos display a single tube and hypoplastic lungs, at E10.5 (D) and E11.5 (F) while wildtype embryos display two distinct gut tube derivatives, the esophagus and trachea (C, E). Notably, no obvious defect is observed in Bmp4cko foregut at E9.0 (A, B). Molecular analysis of E12.5 embryos indicates that the single tube in Bmp4cko embryos adopts an esophageal fate and completely lacks the trachea identity, as it stains negatively for the respiratory/tracheal marker Nkx2.1 (brown, I and J) but is positively-labeled with Pax9, an esophageal specific marker, (purple, G and H). Col2a expression, which normally surrounds the tracheal epithelium, is also lost in the ventral mesenchyme in Bmp4cko foregut compared to wildtype foregut (purple, K and L). tr-trachea; es-esophagus; lu-lung. Magnification: A,B-900X; C, D-630X; E, F-500X; G to L-100X. Scale bars: 10 μm in A, B; 20 μm in C-F; 100 μm in G-L.
ACKNOWLEDGMENTS
We thank Dr. Brigid Hogan and Dr. Holger Kulessa for providing the Bmp4cko mice, Dr. Robert Benezra for providing the Id1 and Id3 mutant mice and Dr. Kemler for providing the β-cateninflox/flox mice. We also thank Drs. Tom Jessell and Ed Laufer for providing us with the pSmad1/5/8 antibody. We thank Frank Revetta for providing assistance in pERK and phospho-p38 immunostainings. This work was supported by the March of Dimes grant (C. C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aubin J, et al. In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004;18:1482–94. doi: 10.1101/gad.1202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, et al. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development. 2004;131:5327–39. doi: 10.1242/dev.01424. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–24. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Celli G, et al. Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. Embo J. 1998;17:1642–55. doi: 10.1093/emboj/17.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, et al. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–44. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- Civenni G, et al. Wnt1 and Wnt5a induce cyclin D1 expression through ErbB1 transactivation in HC11 mammary epithelial cells. EMBO Rep. 2003;4:166–71. doi: 10.1038/sj.embor.embor735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, et al. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Diaz EM, Jr., et al. Tracheal agenesis. A case report and literature review. Arch Otolaryngol Head Neck Surg. 1989;115:741–5. doi: 10.1001/archotol.1989.01860300095025. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, et al. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Effmann EL, et al. Tracheal agenesis. Am J Roentgenol Radium Ther Nucl Med. 1975;125:767–81. doi: 10.2214/ajr.125.4.767. [DOI] [PubMed] [Google Scholar]
- Evans JA, et al. Tracheal agenesis revisited: analysis of associated anomalies. Am J Med Genet. 1999;82:415–22. [PubMed] [Google Scholar]
- Faro RS, et al. Tracheal agenesis. Ann Thorac Surg. 1979;28:295–9. doi: 10.1016/s0003-4975(10)63123-2. [DOI] [PubMed] [Google Scholar]
- Felix JF, et al. Agenesis of the trachea: phenotypic expression of a rare cause of fatal neonatal respiratory insufficiency in six patients. Int J Pediatr Otorhinolaryngol. 2006;70:365–70. doi: 10.1016/j.ijporl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Floyd J, et al. Agenesis of the trachea. Am Rev Respir Dis. 1962;86:557–60. doi: 10.1164/arrd.1962.86.4.557. [DOI] [PubMed] [Google Scholar]
- Fraser N, et al. Tracheal agenesis with unique anatomy. J Pediatr Surg. 2005;40:e7–10. doi: 10.1016/j.jpedsurg.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, et al. Distinct requirements for extra-embryonic and embryonic bone morphogenetic protein 4 in the formation of the node and primitive streak and coordination of left-right asymmetry in the mouse. Development. 2002;129:4685–96. doi: 10.1242/dev.129.20.4685. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, et al. Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc Natl Acad Sci U S A. 2001;98:13739–44. doi: 10.1073/pnas.241508898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, et al. Bone morphogenetic protein 4 induces epithelial-mesenchymal transition through MSX2 induction on pancreatic cancer cell line. J Cell Physiol. 2007;213:768–74. doi: 10.1002/jcp.21148. [DOI] [PubMed] [Google Scholar]
- Hayashi S, et al. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns. 2002;2:93–7. doi: 10.1016/s0925-4773(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Haynes T, et al. BMP signaling mediates stem/progenitor cell-induced retina regeneration. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0707202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Heimann K, et al. Three new cases of congenital agenesis of the trachea. Eur J Pediatr. 2007;166:79–82. doi: 10.1007/s00431-006-0210-4. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Current Opinion in Genetics & Development. 1996;6:432–8. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hollnagel A, et al. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–45. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- Jiao K, et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–7. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall SE, et al. NRAGE mediates p38 activation and neural progenitor apoptosis via the bone morphogenetic protein signaling cascade. Mol Cell Biol. 2005;25:7711–24. doi: 10.1128/MCB.25.17.7711-7724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschner J, Klotch DW. Tracheal agenesis: a case report and review of the literature. Otolaryngol Head Neck Surg. 1997;116:123–8. doi: 10.1016/s0194-5998(97)70364-4. [DOI] [PubMed] [Google Scholar]
- Kimura N, et al. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275:17647–52. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–78. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Knosp WM, et al. Elucidation, quantitative refinement, and in vivo utilization of the HOXA13 DNA binding site. J Biol Chem. 2007;282:6843–53. doi: 10.1074/jbc.M610775200. [DOI] [PubMed] [Google Scholar]
- Kulessa H, Hogan BL. Generation of a loxP flanked bmp4loxP-lacZ allele marked by conditional lacZ expression. Genesis. 2002;32:66–8. doi: 10.1002/gene.10032.abs. [DOI] [PubMed] [Google Scholar]
- Lander TA, et al. Tracheal agenesis in newborns. Laryngoscope. 2004;114:1633–6. doi: 10.1097/00005537-200409000-00024. [DOI] [PubMed] [Google Scholar]
- Lawson KA, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–36. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, et al. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Aberrant Bmp signaling and notochord delamination in the pathogenesis of esophageal atresia. Dev Dyn. 2007;236:746–54. doi: 10.1002/dvdy.21075. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Sonic hedgehog signaling regulates Gli3 processing, mesenchymal proliferation, and differentiation during mouse lung organogenesis. Dev Biol. 2004;270:214–31. doi: 10.1016/j.ydbio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc Natl Acad Sci U S A. 2006;103:6548–53. doi: 10.1073/pnas.0600124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, et al. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Manschot HJ, et al. Tracheal agenesis. Anaesthesia. 1994;49:788–90. doi: 10.1111/j.1365-2044.1994.tb04453.x. [DOI] [PubMed] [Google Scholar]
- Marcelle C, et al. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development. 1997;124:3955–63. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Min H, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–61. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P, et al. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(-/-) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Mishina Y. Function of bone morphogenetic protein signaling during mouse development. Front Biosci. 2003;8:d855–69. doi: 10.2741/1097. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, et al. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–73. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, et al. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–63. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Miyazawa K. 2002. Id: a target of BMP signaling. Sci STKE. 2002:PE40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- Motoyama J, et al. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–7. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–84. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Nohe A, et al. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277:5330–8. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouko L, et al. Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem. 2004;279:26707–15. doi: 10.1074/jbc.M402877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov DA, et al. BMP receptor type IA in limb bud mesenchyme regulates distal outgrowth and patterning. Dev Biol. 2006;295:103–15. doi: 10.1016/j.ydbio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, et al. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–6. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Piek E, et al. Specificity, diversity, and regulation in TGF-beta superfamily signaling. Faseb J. 1999;13:2105–24. [PubMed] [Google Scholar]
- Que J, et al. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–37. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- Rossi JM, et al. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama J, et al. Coordinated expression of Hoxb genes and signaling molecules during development of the chick respiratory tract. Dev Biol. 2000;227:12–27. doi: 10.1006/dbio.2000.9880. [DOI] [PubMed] [Google Scholar]
- Saleeby MG, et al. Tracheal agenesis: a rare disease with unique airway considerations. Anesth Analg. 2003;97:50–2. doi: 10.1213/01.ane.0000066358.67483.4d. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Dynlacht BD. New insights into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol. 2005;16:311–21. doi: 10.1016/j.semcdb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Sekine K, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–41. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–45. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shu W, et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–39. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Sparey C, et al. Esophageal atresia in the Northern Region Congenital Anomaly Survey, 1985-1997: prenatal diagnosis and outcome. Am J Obstet Gynecol. 2000;182:427–31. doi: 10.1016/s0002-9378(00)70234-1. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, et al. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–13. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- van Veenendaal MB, et al. Congenital absence of the trachea. Eur J Pediatr. 2000;159:8–13. doi: 10.1007/s004310050002. [DOI] [PubMed] [Google Scholar]
- Warburton D, et al. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Weaver M, et al. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–15. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- Wei JL, et al. Tracheal agenesis with anomalies found in both VACTERL and TACRD associations. Int J Pediatr Otorhinolaryngol. 2003;67:1013–7. doi: 10.1016/s0165-5876(03)00180-0. [DOI] [PubMed] [Google Scholar]
- Willert J, et al. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, et al. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–63. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- Ying QL, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–92. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Zaret KS. Liver specification and early morphogenesis. Mech Dev. 2000;92:83–8. doi: 10.1016/s0925-4773(99)00326-3. [DOI] [PubMed] [Google Scholar]
- Zaret KS. Hepatocyte differentiation: from the endoderm and beyond. Curr Opin Genet Dev. 2001;11:568–74. doi: 10.1016/s0959-437x(00)00234-3. [DOI] [PubMed] [Google Scholar]
- Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. A new function of BMP4: dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development. 2000;127:1431–43. doi: 10.1242/dev.127.7.1431. [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129:4135–46. doi: 10.1242/dev.129.17.4135. [DOI] [PubMed] [Google Scholar]
- Zhou Q, et al. ERK signaling is a central regulator for BMP-4 dependent capillary sprouting. Cardiovasc Res. 2007;76:390–9. doi: 10.1016/j.cardiores.2007.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activation of Shh pathway does not rescue the Bmp4cko TA phenotype. (A-D) Shh expression (green) in wildtype and Bmpcko mutants at E9.25 and E9.5. (E, F) Note that Shh expression is not significantly affected in Bmpcko mutant foregut. Constitutive activation of Shh signaling in the ventral foregut generated by Foxg1Cre;SmoM2 transgenes in wildtype (E) and Bmp4cko mutant background (F). Note that constitutive Shh pathway activation in the ventral foregut did not significantly alter tracheal and esophageal development, although it had a significant effect on the forebrain (data not shown). Magnification: A to D-200X; E, F-500X. Scale bars: 50 μm in A-D; 20 μm in E, F.