Abstract
Objectives
Recent studies demonstrated that prolactin has beneficial effects on β-cells for islet transplantation. We examined the effect of human recombinant prolactin (rhPRL) supplementation to the culture media to determine its potential use in the context of clinical islet transplantation.
Materials and Methods
Each human islet isolated from 14 deceased multi-organ donors was cultured in Miami modified media-1 supplemented with or without rhPRL (500μg/L) for 48 hours. β-cell survival and proliferation (BrdU and Ki-67) were determined by laser-scanning cytometry. The cytoprotective effects of rhPRL against noxious stimuli were assessed by flow cytometry (tetramethylrhodamine ethyl ester). Cytokine/chemokine and tissue factor (TF) production were measured in vitro and islet potency was assessed in vivo into diabetic immunodeficient mice.
Results
β-cell survival during culture was 37% higher in the rhPRL group than in control (p=0.029). rhPRL protected β-cells in vitro from cytokines, Nitric Oxide donor and H2O2. The exposure to rhPRL did not affect human beta-cell proliferation with our protocol. rhPRL treatment did not alter cytokine/chemokine and TF production in vitro nor affected human islet functionality in vivo: recipient mice achieved normoglycemia with a comparable tempo, while loss of graft function was observed in 2/7 mice in the control group and in none of the rhPRL group (p=n.s.).
Conclusion
rhPRL supplementation to islet culture media improved human β-cell-specific survival without altering islet quality. Addition of rhPRL to cultured islets may grant a more viable β-cell mass in culture. The development of β-cell cytoprotective strategies will be of assistance in improving islet transplantation outcomes.
Keywords: islet, transplantation, prolactin, cytokine, chemokine
Introduction
Islet transplantation is considered an optional treatment for selected patients with type 1 diabetes (1, 2). Most islet transplant centers perform transplantation of cultured islets (3–6). The advantages of culturing islet preparations prior to transplantation over freshly isolated islets include (i) allowing the time for arranging the logistics of patient admission to the hospital and (ii) implementation of pre-conditioning therapy and (iii) providing adequate time to assess the safety of the cell product by microbiological (i.e., mycoplasma, aerobes and anaerobs cultures) and pyrogenic (endotoxin) tests. In addition, the development of a culturing system has allowed for the successful shipment of clinical human islet cell products to remote centers for transplantation (7, 8). The metabolic success measured after islet transplantation greatly depends upon the mass of islets transplanted into the recipient (>13,000 IEQ/recipient kg in recent trials) and insulin independence is achieved generally after transplantation of more than one human preparation per recipient (1–8). Unfortunately, loss of islet mass during pre-transplant culture (~20–30 %) is one of the major issues requiring improvements to maximize the number of transplants and improve clinical outcomes (4). Implementation of improved culture conditions may be of assistance in preventing/reducing the loss of islet mass during pre-transplant culture (9).
Pancreatic islet cells, especially β-cells, undergo structural and functional alterations during pregnancy in order to cope with the increased insulin demand. The relationship between pregnancy and β-cell proliferation has been investigated to elucidate the mechanism of these changes (10–15). It has become clear that lactogens including prolactin (PRL), growth hormone (GH) and placental lactogens (PLs) are involved in the phenomenon of β-cell proliferation. In addition, several recent studies demonstrated cytoprotective effects of PRL on insulin-producing cell lines and rodent islets treated by streptozotocin (STZ) in vitro (16–18) and in vivo (18). Treatment with GH and PRL protects the rat insulin-producing INS-1 cells from cytokine-induced apoptosis (19). Furthermore, in vivo studies in mice showed that PRL treatment significantly reduced the elevation of blood glucose levels in serum and the degree of insulitis in a model of streptozotocin-induced diabetes (20). These results suggest that lactogen hormones may protect β-cells against the noxious stimuli occurring during pancreas preservation, islet isolation and culture for clinical transplantation.
The purpose of the present study was to investigate the effects on human β-cells of recombinant human prolactin (rhPRL) supplementation to the culture media for clinical islet transplantation. Our study shows that rhPRL resulted in a significant improvement in β-cell survival during culture and also in protection of β-cells against noxious stimuli in vitro. Moreover, surviving β-cells demonstrated good functionality when transplanted into chemically-induced diabetic immunodeficient mice. PRL supplementation to the culture media did not alter the production of pro-inflammatory mediators (cytokine/chemokine and tissue factor) during culture of islet preparations. These results suggest that PRL supplementation to culture media may represent a beneficial strategy in minimizing β-cell loss during pre-transplant culture, which in turn could lead to an increase of successful islet transplantations.
Materials and Methods
Human islet isolation and culture
Pancreata were recovered from deceased multi-organ donors and then immediately placed in either pre-oxygenated (30-min) two-layer perfluorocarbon/University of Wisconsin solution (PFC/UW) (21, 22) or with UW solution alone. The donor characteristics of 14 human pancreata used for this study are shown in Table 1. Islets were isolated using the modified automated method (23) at the Human Cell Processing Facility of the Diabetes Research Institute’s Cell Transplant Center at the University of Miami Miller School of Medicine. Islet yield and purity were determined by dithizone staining. Islet aliquots (3,000 islet equivalent (IEQ) were cultured in Miami defined culture medium (MM1, Mediatech-Cellgro, VA) (7) with or without 500μg/L of rhPRL (Sigma-Aldrich, St. Louis, MO) at 37°C for 2 days in 5% CO2 humidified incubator. Following culture with or without rhPRL, islet samples were collected to count IEQ recovered and expressed a percent of IEQ recovered over plated on day 0.
Table 1.
Donor and islet characteristics
| Donor | Age | Gender | BMI | CIT(min) | Preservation method | FDA/PI(%) | SI |
|---|---|---|---|---|---|---|---|
| 1 | 15 | M | 22.0 | 564 | UW | 95.0 | 1.19 |
| 2 | 44 | M | 25.1 | 855 | UW | 93.4 | 1.83 |
| 3 | 37 | M | 28.9 | 568 | UW | 97.8 | 1.57 |
| 4 | 45 | F | 27.0 | 265 | TLM | 99.7 | N/A |
| 5 | 48 | F | 25.5 | 842 | UW | 95.8 | 2.89 |
| 6 | 53 | F | 22.0 | 679 | TLM | 93.2 | 1.62 |
| 7 | 48 | M | 31.0 | 895 | TLM | 85.6 | 1.21 |
| 8 | 47 | F | 33.4 | 346 | UW | 96.0 | 2.70 |
| 9 | 25 | M | 29.1 | 468 | UW | 94.5 | 1.97 |
| 10 | 46 | M | 33.0 | 740 | UW | 96.9 | N/A |
| 11 | 33 | M | 37.9 | 893 | UW | 88.5 | 0.74 |
| 12 | 28 | M | 23.1 | 650 | UW | 96.1 | 0.99 |
| 13 | 22 | M | 34.0 | 1230 | UW | 80.8 | 0.56 |
| 14 | 45 | F | 31.8 | 688 | UW | 74.3 | N/A |
| Average ±SEM | 48.3±3.1 | M:F=9:5 | 28.8±1.3 | 692±67 | UW:TLM=11:3 | 92.0±1.93 | 1.57±0.20 |
BMI : body mass index, CIT: cold ischemic time, UW: University of Wisconsin solution, TLM: Two layer method, FDA/PI: Fluorescein diacetate / propidium iodide, SI: static incubation insulin release test.
Assessment of cellular composition
As previously described (24), human islets were dissociated into single-cell suspensions using Accutase (Innovative Cell Technologies, San Diego, CA) for 10 min at 37°C. Dispersed cells were fixed on glass slides with 2.5% paraformaldehyde (Electron Microscopy Sciences, Washington, PA). To reduce non-specific antibody binding, fixed cells were incubated overnight at 4°C with non-diluted Protein Block (BioGenex, San Ramon, CA). Subsequently, the cells were incubated for 2 hrs at room temperature (RT) with primary antibodies: monoclonal mouse anti-C-peptide (1:100; Abcam Inc., Cambridge, MA); monoclonal mouse anti-glucagon (1:500; Sigma-Aldrich); polyclonal rabbit anti-somatostatin (1:500; Dako, Carpinteria, CA). After washing, samples were incubated at RT for 1 hr with AlexaFluor-488 goat anti-mouse IgG (1:200 Molecular Probes Eugene, OR), Alexa Fluor 647 goat anti-rabbit IgG (1:200 Molecular Probes Eugene, OR) and the nuclear-binding dye 4′, 6-diamidino-2-phenylindole (DAPI; 1:300). Slides were analyzed using a LSC/iCys (CompuCyte, Cambridge, MA) (24, 25).
Absolute β-, α-, and δ-cell mass after culture with or without rhPRL were calculated with following formulas (24–26):
F1: Absolute α-cell mass = α-cell content (%) × total protein content of islet aliquots (μg).
F2: Absolute β-cell mass = β-cell content (%) × total protein content of islet aliquots (μg).
F3: Absolute δ-cell mass = δ-cell content (%) × total protein content of islet aliquots (μg).
Assessment of fractional β-cell viability
After dissociation of islet aliquots using Accutase (see above), islet cell suspensions were incubated with 1μM Newport Green PDX acetoxymethylether (NG; Molecular Probes) and 100 ng/mL of tetramethylrhodamine ethyl ester (TMRE; Molecular Probes) for 30 minutes at 37°C in PBS. After washing, cells were stained with 7-aminoactinomycin D (7-AAD; Molecular Probes) and then analyzed using a FACScan cytometer (Becton Dickinson, Mountain View, CA) with the CellQuest software, as described (24).
Assessment of β-cell proliferation
Erk2 phosphorylation
Aliquots of islets cultured with or without rhPRL (500μg/L) were collected and frozen (−80°C) until assayed for Erk2 phosphorylation using fluorescence-based quantitative measurement on a BioPlex® system (BioRad, Hercules, CA), as described (27). Lysate protein concentration was determined by BioRad DC protein assay. Quantitative determination of phosphorylated proteins for Erk2 was done as per manufacturer recommendations (BioRad). Data were calculated as ratio of phosphorylated Erk2 to total Erk2. The value of % control in the PRL group was shown.
BrdU and Ki-67 staining
Islets were cultured with or without 500μg/L of rhPRL at 37°C for 7 days in 5% CO2–humidified atmosphere. Culture media was exchanged every 2 days. To label newly synthesized DNA in dividing cells, 500 ng/mL of 5-bromo-2-deoxyuridine (BrdU; Roche, Laval, QC) was added to culture media 24 hrs before the assessment. Islet cells were dispersed by Accutase and fixed on glass slides with 2.5% paraformaldehyde (see above) (24). Epitopes were retrieved by heat induction with Antigen Decloaker 10X (Biocare Medical, Concord, CA) in a rice cooker for 10 minutes at 120°C. After blocking non-specific binding (Protein Block, 30 minutes, RT), cells were incubated for 2 hrs at RT with either mouse anti-BrdU (1:100, BD Biosciences, San Jose, CA) or mouse anti-Ki67 (clone MIB-1, 1:50; Dako), and anti-chicken insulin (1:500, Linco Research, St. Charles, MO). Then the cells were labeled for 1 hr at RT with AlexaFluor-488 goat anti-mouse IgG (1:200), AlexaFluor-647 goat anti-chicken IgG (1:200; all from Molecular Probes). The cell nuclei were stained twice for 10 minutes at RT with 4′, 6-diamidino-2-phenylindole (DAPI). The samples were analyzed using LSC/iCys (28, 29).
Delivery of pro-apoptotic stimuli to islet cells
Islet aliquots of 3,000IEQ were exposed for different periods of time to selected noxious stimuli to induce apoptosis 1 hr after pre-culture with or without 500μg/L rhPRL. S-nitroso-N-acetyl-dl-penicillamine (SNAP; 1.0 mM for 18 hrs; Baxter Healthcare Corporation, Deerfield, IL) was used as a nitric oxide (NO) donor. Hydrogen peroxide (H2O2; 50 μM for 18 hrs; Sigma) was used as source of oxidative stress. Islet Exposure for 24 hrs to a cytokine cocktail (50 U/ml of interleukin-1 β (IL-1β), 1,000 U/ml of tumor necrosis factor alpha (TNF-α) and 1,000 U/ml of interferon gamma (IFN-γ); all from R&D Systems, Minneapolis, MN) was used to mimic inflammation (30). After culture, islets were counted and processed for β-cell specific viability and for cellular composition assessment (24).
Measurement of inflammatory mediators
Islet aliquots (500 IEQ in 0.5 mL) were cultured with or without 500μg/L of rhPRL. After 24 hrs, supernatant from islet preparations were collected to determine the concentrations of pro-inflammatory mediators; namely, IL-1 β, interleukin-6 (IL-6), interleukin-8 (IL-8), IFN-γ, monocyte chemoattractant-1 (MCP-1), macrophage inflammatory protein 1 beta (MIP-1β), and Tissue necrosis factor (TNF-α), using Multi-Plex cytokine kits following the manufacturer’s protocol (Bio-Plex; Bio-Rad Laboratories) (26). Additionally, islets aliquots (500 IEQ) were homogenized, and tissue factor (TF) was measured by ELISA (Imubind Tissue Factor, American Diagnostica, Greenwich, CT, USA) (30). The amount of cytokines/chemokines and TF was normalized to total protein of the islet aliquot.
In vivo assessment of islet potency
Animal procedures approved by the IACUC were performed at the Diabetes Research Institute’s Preclinical Cell Processing and Translational Models Core. Athymic nu/nu (nude) mice (Harlan Laboratories, Indianapolis, IN) were housed at the Division of Veterinary Resources of the University of Miami School of Medicine in virus-antibody-free rooms using microisolated cages and with free access to autoclaved food and water. Animals were rendered diabetic via a single intravenous administration of 200 mg/kg of Streptozotocin (STZ; Sigma). Non-fasting blood glucose was assessed with a glucometer (OneTouch Ultra2, LifeScan, Milpitas, CA). Mice with sustained hyperglycemia (>300 mg/dL) were used as islet graft recipients. Human islet aliquots were cultured with or without rhPRL (500 μg/L) for 48 hrs and then 1,000 IEQ islets/ mouse were transplanted under the left kidney capsule of nu/nu mice. Non-fasting blood glucose values were assessed after transplant; reversal of diabetes was defined as stable non-fasting blood glucose <200 mg/dL. An intraperitoneal glucose tolerance test (IPGTT; 2 g/kg dextrose in saline given after overnight fasting) was performed in selected animals to assess graft performance over 60 minutes (31). Nephrectomy of the graft-bearing kidney was performed in animals achieving normoglycemia after transplantation to confirm return to hyperglycemia and exclude residual function of the native pancreas (31).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) and analyzed using Excel for Windows, SigmaPlot and GraphPad softwares for descriptive statistics and data plotting. Two samples were compared a using Wilcoxon sign rank test or Student’s t-test; statistical significance was considered for p-values <0.05.
Results
Prolactin improves human β-cell survival during culture
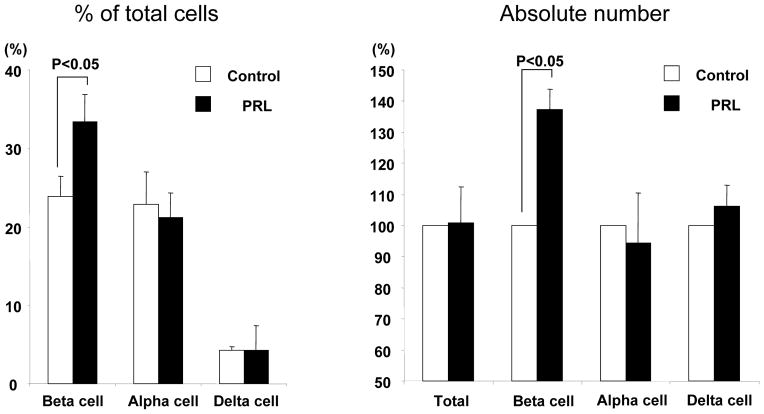
To investigate the effects of rhPRL on β-cell survival during culture, islet aliquots were cultured for 48 hr in conventional medium (MM1, control) with or without rhPRL. After culture, the recovery rate of islet in the PRL group was not significantly improved when compared to the control group (75.1±8.8% vs.70.2±6.1%, p= n.s.). Fractional β-cell viability assays showed no statistically significant difference between the experimental groups (PRL vs. Control, 59.1±10.4% vs.57.3±11.2%; p= n.s.). The cellular composition was also evaluated using immunocytofluorescence on dissociated cells by laser scanning cytometry (LSC/iCys) to estimate overall β-cell mass. No significant differences were observed between islets in PRL and control groups when assessing α-cell (21.1±3.1%, vs. 22.9±4.1%, respectively; p= n.s.) and δ-cell content (4.3±1.1% vs. 4.3±3.1%, respectively; p=n.s.). Conversely, the percentage of β-cell in the PRL group was significantly higher than in the control group (33.4±3.5% vs. 23.9±2.6%, respectively; p<0.05)(Figure 1A). The β-cell mass in the PRL group resulted significantly higher also when calculated based on the total protein of islet aliquots (Control vs. PRL=124.5 vs.171.0 μg, 137.3±6.6% of control; p<0.05)(Figure 1B).
Figure 1.
Cellular composition in islet preparation (A), Absolute endocrine cell mass (B). An aliquot of islet preparations was dissociated to obtain single cell suspensions. Fixed cells were stained with anti-C-peptide, glucagon, and somatostatin antibodies. The appropriate fluorochrome-conjugated secondary antibody was subsequently added. The stained samples were assessed by immunofluorescent assay using iCys/LSC. Each cellular composition was calculated using the formula [(β-, α- or δ- cell content %) / (β +α+ δ %)] × 100. (A). Absolute β-, α-and δ-cell mass were calculated with following formulas: absolute β-, α-or δ-cell mass = β-, α- or δ-cell (%) × protein content (μg)(B).
Effects of prolactin on human β-cell proliferation
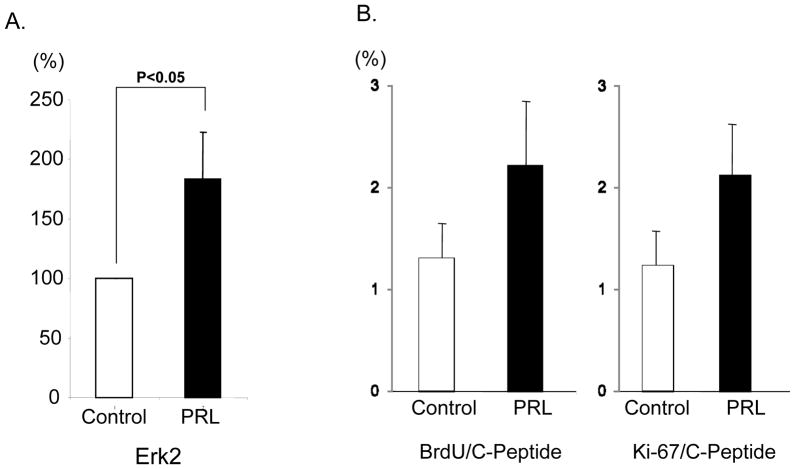
We investigated the effects of rhPRL on the human islet cell proliferation during pre-transplant culture. To this aim, Erk2 phosphorylation was assessed in islet aliquots cultured with or without rhPRL by the means of fluorescence-based quantitative measurement (BioPlex® system). Erk2 phosphorylation in the PRL group was significantly higher than control (183.2±39.7% of control, p<0.05)(Figure 2A). To further examine β-cell proliferation, human islets were cultured with MM1 in the presence or the absence of rhPRL for 7 days. C-peptide+ BrdU+ or C-peptide+Ki67+ double positive cells were quantitatively assessed using LSC/iCys. The percentage of C-peptide+BrdU+ or C-peptide+Ki67+ in the PRL group did not significantly increase when compared to the control group (BrdU; 2.22±0.69% vs. 1.31±0.37%; p=n.s, Ki67; 2.12±0.52% vs. 1.24±0.35%; p=n.s., respectively)(Figure 2B). These results suggest that the higher number of surviving β-cells in the PRL group during culture might not be caused by the β-cell proliferation with our protocol.
Figure 2. Analysis of proliferating β-cell cultured with or without rhPRL.
Erk2 phosphorylation was assessed by fluorescence-based quantitative measurement (A). Erk2 phosphorylation was significantly higher in islet aliquots cultured with rhPRL (PRL) than in those without rhPRL (control) (p<0.05). Islets were cultured with (PRL) or without (control) rhPRL for 7 days to examine β-cell proliferation (B). 5-bromo-2-deoxyuridine (BrdU) was added to culture media at 24 hours before the assessment to label newly synthesized DNA in dividing cells. Cell proliferation was evaluated by BrdU or Ki67 staining. There was no significant difference between the experimental groups.
Prolactin protects human β-cells from noxious stimuli
Prolactin is known for its cytoprotective properties. We investigated the cytoprotective effects of rhPRL on human β-cells against noxious stimuli acting on different pathways of stress-induced islet cell death. Before injury, islets were pre-cultured for 1 hr with or without rhPRL.
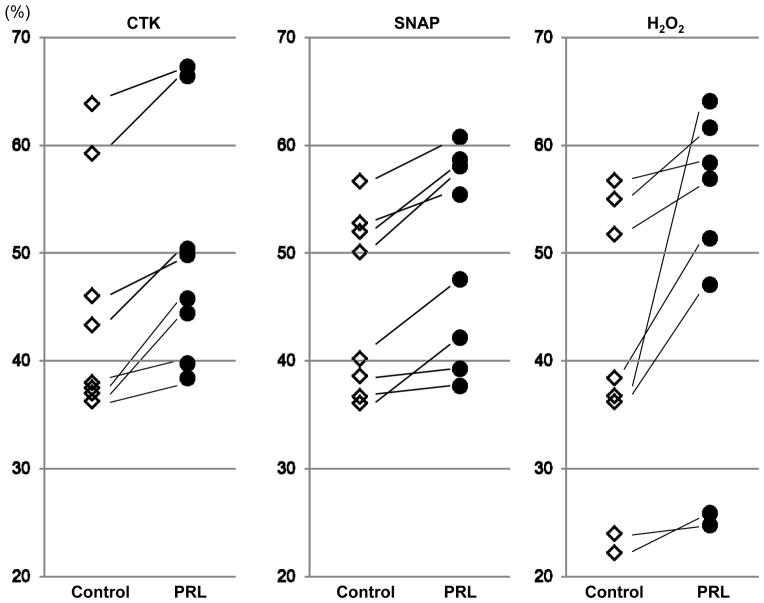
β-cell viability in islets treated with rhPRL was significantly improved in when compared to the control group after exposure to the cytokine cocktail (50.3±1.3% vs. 45.2 ±1.4%; p<0.05), NO donor (49.9±1.0% vs. 45.4±1.2%; p<0.05) and H2O2 (48.8±1.7% vs. 40.1±1.9%.; p<0.05, respectively)(Figure 3). These results suggest that the cytoprotective effects of rhPRL might mainly contribute to higher β-cell survival during culture.
Figure 3. Analysis of apoptosis in β-cell cultured with or without rhPRL after delivery of noxious stimuli.
Islet preparations cultured with or without rhPRL were incubated in the presence or absence of noxious stimuli, cytokine cocktail (IL1-β, TNF-α and IFN-γ), nitric oxide donor SNAP and H2O2. Apoptosis was analyzed by TMRE staining in the β-cell subsets. PRL treatment in any of the three conditions resulted in decreased apoptosis in β-cell subsets, suggesting β-cell specific anti-apoptotic effects of rhPRL.
Effects of prolactin on the production of inflammatory mediators from human islets in vitro
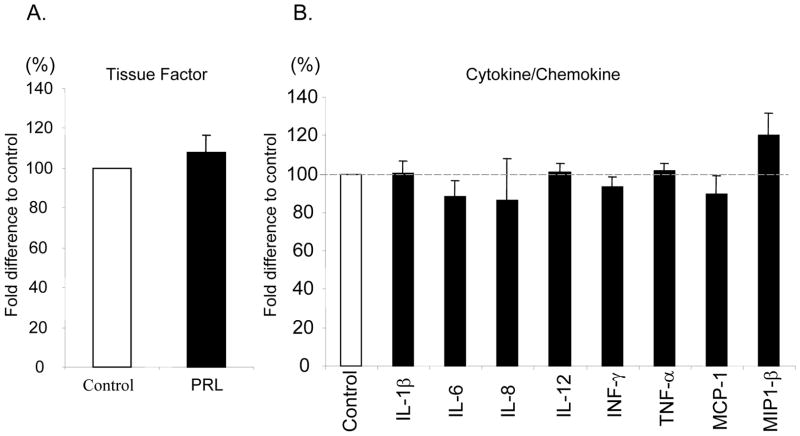
To examine the anti-inflammatory effects of rhPRL supplementation to the culture media of human islet preparations, cytokine/chemokine production in the supernatant was evaluated after 48 hrs of conventional culture. The production of IFN-γ, TNF-α, IL-1β, IL-6, IL-8, RANTES, MCP-1 and MIP-1β was comparable between the experimental groups (Figure 4). In addition, there was no significant difference in tissue factor levels in human islets (PRL vs. Control: 20.1±4.8 vs.17.7±3.6 pg/ml, respectively; p=n.s.). The data suggest that rhPRL supplementation to culture media can improve β-cell survival without affecting pro-inflammatory mediators and TF production.
Figure 4. Measurement of inflammatory mediators from islet preparations cultured with or without rhPRL.
To investigate the effects of rhPRL on cytokine/chemokine and tisssue factor production from islet preparations, islet aliquots were cultured with or without rhPRL for 48 hours. Tissue factor production (A) or Cytokine/chemokine production(B) were evaluated using ELIZA. There was no siginificant difference in sissue factor and cytokine/chemokine production between the experimental groups. Data is representative of four independent human islet preparations.
Prolactin treatment of human islets does not affect potency in vivo but improves long-term graft function
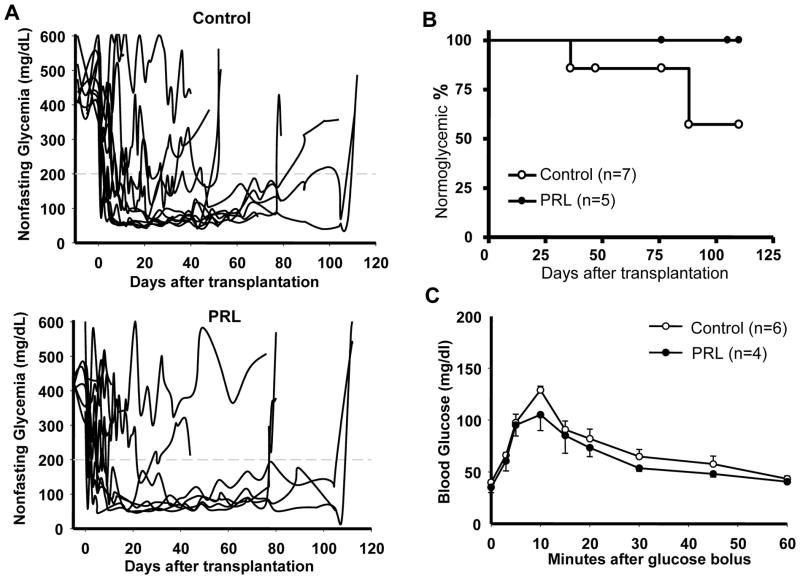
To evaluate islet quality after 48 hrs of culture with or without rhPRL, four independent human islet preparations were tested for in vivo islet potency test. After culture, islet aliquots of 1,000 IEQ were prepared from both experimental groups and transplanted into chemically-induced diabetic immunodeficient mice in (control group, n=10; PRL group, n=11). Seven out of 11 mice (63.6%) in the control group and 5 out of 10 mice (50.0%) in the PRL group reversed diabetes after transplantation (mean reversal time of 4.1±2.3 vs. 4.0±2.6 days, respectively; p=n.s.)(Figure 5A). Loss of graft function during the follow-up period was observed in 2/7 (28.5%) in the control group (on day 36 and 88, respectively), but in none (0/5, 0%) of the animals in the PRL group (p=n.s.) (Figure 5B). An intraperitoneal glucose tolerance test (IPGTT) performed in the mice achieving normoglycemia showed comparable glucose clearance between the two experimental groups (Figure 5C).
Figure 5. In vivo assessment of the effects of culturing human islets with PRL.
After 48 hr culture with or without PRL, human islet aliquots of 1,000 IEQ each were implanted under the kidney capsule of diabetic nude mice. A. Graft function was monitored by measuring nonfasting glycemia over the follow-up in recipients of control (n=11; Control, upper panel) or rhPRL-treated (n=11; Prolactin, lower panel) human islets. The star indicates animals displaying loss of graft function during follow-up. The arrowheads indicate nephrectomy of the graft-bearing kidney in selected animals. B. Loss of human islet graft function during the follow-up of animals that reverted diabetes after implant (Control, n=7; Prolactin, n=5). C. Intraperitoneal glucose tolerance test (IPGTT) was performed in animals with a functional human islet graft after two four weeks from islet transplantation (rhPRL, n=4; control, n=5).
Discussion
Numerous approaches have been proposed to improve culture conditions of islets (26, 30). Optimal cell culture conditions for islet transplantation should provide sufficient oxygen and nutrients in order to allow islet cells to recover from damages related to noxious insults induced by isolation, resulting in the reduction of islet cell loss. In this study, we investigated the possibility that rhPRL can be beneficial for pre-transplant culture of islet cells. Our data shows that rhPRL supplementation to the culture media significantly improved specifically β-cell survival. Overall, PRL appeared to improve β-cell survival without deteriorating islet quality and increasing immunogenicity (pro-inflammatory cytokine/chemokine and tissue factor production). The unique cytoprotective properties of PRL in targeting specifically β-cells may be of assistance in preserving viable β-cell mass during pre-transplant culture as well as in improving clinical outcome after islet transplantation.
A significant correlation has been recognized between β-cell mass and successful islet transplantation outcomes, which points to the fact that the estimation of transplanted β-cell mass may be important than that of the total islet quantity (namely, total IEQ)(24, 32). Many studies have reported that the cellular composition in human islet preparations significantly varies (24, 33, 34) possibly due to the different levels of vulnerability among islet cell subsets from stress and insults during the whole pre-islet transplantation process, including pancreas procurement, preservation, islet isolation and culture. The characteristics and function of each cell subset composing islet preparations are indeed different. In particular, β-cells may be particularly susceptible to oxidative stress due to the low anti-oxidant potential. Therefore, β-cell specific protection from damages during whole pre-islet transplantation processes should be considered in the optimization of culture condition for islet transplantation.
Lactogen hormones such as PRL, GH and placental lactogen (PL) are considered to have arisen from a common ancestral gene (12, 35). Lactogens increase during pregnancy leading to β-cell proliferation to adapt with increased fetal insulin demand (12). Only β-cells, amongst the cells comprised in islets, are known to have PRL receptors (36, 37). Prolactin can stimulate β-cell proliferation, glucose-induced insulin release, insulin gene expression and biosynthesis in fetal, newborn, and adult rat islets (10, 14, 15) and INS-1 cells (13). It has been, in fact, recognized that the β-cells undergo structural and functional modifications in pregnant rodents. Furthermore, β-cell specific proliferation can be induced in rodent islets by the means of extended culture in the presence of PRL (10). Moreover, the mitogenic effects of PRL have been observed in cultured human islets although this requires long incubation periods (11) or culture on coated dishes (17). In our study, we could not confirm the significant increase of β-cell proliferation with BrdU and Ki-67 staining by PRL supplementation, probably because islets were incubated with the culture protocol conventionally utilized in the clinical settings of islet transplantation consisting of floating condition (no tissue-treated flasks) with media supplemented with human serum albumin (without fetal bovine serum). However, we observed significant improvement in β-cell-specific survival, which may be caused by the cytoprotective effects of PRL resulting in prevention of apoptosis, rather than the stimulation of the β-cell proliferation in our experimental conditions. Indeed, lactogens have been recognized to not only cause the β-cell proliferation but also have anti-apoptotic effects on the β-cells. A role for PRL in the regulation of cell death and survival has been observed in lymphoid cells (38). Fujinaka et al. reported that lactogens, including PRL, directly protect rodent pancreatic β-cells against the cytotoxic effects of streptozotocin and dexamethasone (16). Many reagents that have cytoprotective effects on islet cells have been investigated and reported (39). Amongst them, Nicotinamide ameliorates cellular damage caused by noxious stimuli such as hydrogen peroxide and a combination of pro-inflammatory cytokines in vitro (40). The use of Nicotinamide during isolation and culture prior to transplantation has been shown to improve islet yields and islet quality by decreasing tissue factor and MCP-1 production in human islet preparations (30). Those pro-inflammatory mediators have been negatively associated with clinical islet transplant outcomes (41, 42). Moreover, activation of c-jun N terminal kinase (JNK) and nuclear factor-κB (NF-κB) are triggers for the production of pro-inflammatory cytokines/chemokines that can impair islet cell survival and function (43, 44). In addition, Emamaullee et al. recently reported that prevention of apoptosis by pan-caspase inhibitor in vitro and in vivo significantly improved human islet graft functions and longevity in a mouse model (45). Therefore, targeted inhibitors of these pro-inflammatory pathways could be useful to protect islet cells from stress during pre-islet transplantation processing (46, 47). Furthermore, numerous peptide hormones relating to islets, such as glucagon-like peptide-1 (GLP-1)(48), Lactogens (12), Hepatocyte growth factor (49), parathyroid hormone-related protein (PTHrP)(50) and insulin like growth factors (IGF), have been tested with, and promising results have been reported. However, many of these reagents and hormones improve viability of all the islet cell subsets in human islet preparations. Interestingly, in many cases, cytokine/chemokine production from islet preparations was also elevated, which may lead to β-cell damages through the direct toxic effect of cytokine/chemokine or via indirect effects by recruiting inflammatory cells to the transplant site. In our study, PRL did not increase pro-inflammatory cytokine/chemokine production from islet preparations. Pancreatic ductal cells are considered to be one of main sources in cytokine/chemokine production and do not have PRL receptors. In fact, we found no effects of rhPRL on viability and content in this study (data not shown).
To improve clinical outcomes in islet transplantation, quick revascularization of islet grafts after transplantation is a key. When compared to pancreas transplantation, implanted islets have less blood perfusion because of no vessels in the early post-transplant period. Johansson et al. have demonstrated that PRL supplementation to culture media improves revascularization, blood perfusion and oxygen tension of mouse and human islets implanted into immunodeficient mice (18). In addition, they have reported the beneficial effects of even systemic treatment of PRL using a rodent model. Although it is not realistic in a clinical setting when considering various influences of PRL throughout the organs, those results encourage us to consider the use of PRL during pre-transplant culture in a clinical setting. The proper combination of cytoprotective reagents and hormones may allow for further improvements in clinical islet transplantation.
In conclusion, PRL supplementation to pre-transplant culture media can specifically improve β-cell survival during culture through the prevention of apoptosis induced by insults from pre-transplant islet processing without increasing pro-inflammatory mediators in human islet preparations. Moreover, the function of the surviving β-cells remains intact as shown by in vivo potency into immunodeficient mice. Both in vitro and in vivo data in our study complement and extend recent reports of the cytoprotective properties of PRL for human islets (18).
β-cell specific cytoprotection by rhPRL supplementation to culture media may be of assistance in developing novel and efficient strategies to increase islet suitability for transplantation from a single donor pancreas with minimized risks or side effects.
Acknowledgments
This work was supported in part by NIH-NCRR, GCRC MO1RR16587, NIDDK RO1-DK55347-IU42RR016603, 5R01 DK25802, ICR 5U42RR016603, the Juvenile Diabetes Research Foundation International (4-2004-361) and the Diabetes Research Institute Foundation (diabetesresearch.org).
Abbreviations
- 7-AAD
7-aminoactinomycin D
- BrdU
5-bromo-2-deoxyuridine
- CIT
cold ischemic time
- FDA/PI
Fluorescein diacetate / propidium iodide
- GH
growth hormone
- IEQ
islet equivalent
- IFN-γ
interferon gamma
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- IL-8
interleukin-8
- LSC
Laser scaning cytometer
- MCP-1
monocyte chemoattractant-1
- MIP-1β
macrophage inflammatory protein 1 beta
- MM1A
Miami defined culture medium
- NG
Newport Green PDX acetoxymethylether
- NO
nitric oxide
- PFC
perfluorocarbon
- PL
placental lactogen
- PRL
prolactin
- RANTES
regulated upon activation, normal T cell expressed and secreted
- rhPRL
human recombinant prolactin
- SI
static incubation insulin release test
- SNAP
S-nitroso-N-acetyl-dl-penicillamine
- STZ
streptozotocin
- TF
Tissue factor
- TLM
Two layer method
- TMRE
tetramethylrhodamine ethyl ester
- TNF-α
tumor necrosis factor alpha
- UW
University of Wisconsin solution
References
- 1.Ricordi C, Strom TB. Clinical islet transplantation: Advances and immunological challenges. Nature Reviews Immunology. 2004;4 (4):258. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AMJ, Ricordi C, Hering BJ, et al. International trial of the edmonton protocol for islet transplantation. New England Journal of Medicine. 2006;355 (13):1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004;4 (3):390. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 4.Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5 (8):2037. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 5.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54 (7):2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 6.Goto M, Eich TM, Felldin M, et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78 (9):1367. doi: 10.1097/01.tp.0000140882.53773.dc. [DOI] [PubMed] [Google Scholar]
- 7.Ichii H, Sakuma Y, Pileggi A, et al. Shipment of human islets for transplantation. Am J Transplant. 2007;7 (4):1010. doi: 10.1111/j.1600-6143.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 8.Goss JA, Schock AP, Brunicardi FC, et al. Achievement of insulin independence in three consecutive type-1 diabetic patients via pancreatic islet transplantation using islets isolated at a remote islet isolation center. Transplantation. 2002;74 (12):1761. doi: 10.1097/00007890-200212270-00020. [DOI] [PubMed] [Google Scholar]
- 9.Pileggi A, Cobianchi L, Inverardi L, Ricordi C. Overcoming the challenges now limiting islet transplantation - A sequential, integrated approach. Immunology of Diabetes Iv: Progress in Our Understanding. 2006;1079:383. doi: 10.1196/annals.1375.059. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen JH. Effects of growth hormone, prolactin, and placental lactogen on insulin content and release, and deoxyribonucleic acid synthesis in cultured pancreatic islets. Endocrinology. 1982;110 (2):600. doi: 10.1210/endo-110-2-600. [DOI] [PubMed] [Google Scholar]
- 11.Brelje TC, Scharp DW, Lacy PE, et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132 (2):879. doi: 10.1210/endo.132.2.8425500. [DOI] [PubMed] [Google Scholar]
- 12.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29 (6):301. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 13.Petryk A, Fleenor D, Driscoll P, Freemark M. Prolactin induction of insulin gene expression: the roles of glucose and glucose transporter-2. J Endocrinol. 2000;164 (3):277. doi: 10.1677/joe.0.1640277. [DOI] [PubMed] [Google Scholar]
- 14.Tian Y, Laychock SG. Prolactin regulates adenylyl cyclase and insulin secretion in rat pancreatic islets. Mol Cell Endocrinol. 2003;204 (1–2):75. doi: 10.1016/s0303-7207(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 15.Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorenson RL. Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy. J Endocrinol. 2007;193 (3):367. doi: 10.1677/JOE-07-0043. [DOI] [PubMed] [Google Scholar]
- 16.Fujinaka Y, Takane K, Yamashita H, Vasavada RC. Lactogens promote beta cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem. 2007;282 (42):30707. doi: 10.1074/jbc.M702607200. [DOI] [PubMed] [Google Scholar]
- 17.Labriola L, Montor WR, Krogh K, et al. Beneficial effects of prolactin and laminin on human pancreatic islet-cell cultures. Mol Cell Endocrinol. 2007;263 (1–2):120. doi: 10.1016/j.mce.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Johansson M, Olerud J, Jansson L, Carlsson PO. Prolactin treatment improves engraftment and function of transplanted pancreatic islets. Endocrinology. 2009;150 (4):1646. doi: 10.1210/en.2008-1318. [DOI] [PubMed] [Google Scholar]
- 19.Jensen J, Galsgaard ED, Karlsen AE, Lee YC, Nielsen JH. STAT5 activation by human GH protects insulin-producing cells against interleukin-1beta, interferon-gamma and tumour necrosis factor-alpha-induced apoptosis independent of nitric oxide production. J Endocrinol. 2005;187 (1):25. doi: 10.1677/joe.1.06086. [DOI] [PubMed] [Google Scholar]
- 20.Holstad M, Sandler S. Prolactin protects against diabetes induced by multiple low doses of streptozotocin in mice. Journal of Endocrinology. 1999;163 (2):229. doi: 10.1677/joe.0.1630229. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura T, Kuroda Y, Suzuki Y, et al. 72-hour preservation of the canine pancreas by the 2-layer (Euro-collons solution Perfluorochemical) cold-storage method. Transplantation. 1989;47 (5):776. doi: 10.1097/00007890-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Ricordi C, Fraker C, Szust J, et al. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75 (9):1524. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 23.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37 (4):413. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 24.Ichii H, Inverardi L, Pileggi A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5 (7):1635. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 25.Ichii H, Miki A, Yamamoto T, et al. Characterization of pancreatic ductal cells in human islet preparations. Lab Invest. 2008;88 (11):1167. doi: 10.1038/labinvest.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mita A, Ricordi C, Miki A, et al. Anti-proinflammatory effects of sirolimus on human islet preparations. Transplantation. 2008;86 (1):46. doi: 10.1097/TP.0b013e31817c79c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornoni A, Cobianchi L, Sanabria NY, et al. The L-isoform but not D-isoforms of a JNK inhibitory peptide protects pancreatic beta-cells. Biochemical and Biophysical Research Communications. 2007;354 (1):227. doi: 10.1016/j.bbrc.2006.12.186. [DOI] [PubMed] [Google Scholar]
- 28.Zahr E, Molano RD, Pileggi A, et al. Rapamycin Impairs In Vivo Proliferation of Islet Beta-Cells. Transplantation. 2007;84 (12):1576. doi: 10.1097/01.tp.0000296035.48728.28. [DOI] [PubMed] [Google Scholar]
- 29.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50:2323. doi: 10.1007/s00125-007-0794-x. [DOI] [PubMed] [Google Scholar]
- 30.Ichii H, Wang X, Messinger S, et al. Improved human islet isolation using nicotinamide. Am J Transplant. 2006;6 (9):2060. doi: 10.1111/j.1600-6143.2006.01452.x. [DOI] [PubMed] [Google Scholar]
- 31.Ichii H, Pileggi A, Molano RD, et al. Rescue purification maximizes the use of human islet preparations for transplantation. Am J Transplant. 2005;5 (1):21. doi: 10.1111/j.1600-6143.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 32.Keymeulen B, Gillard P, Mathieu C, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proceedings of the National Academy of Sciences of the United States of America. 2006;103 (46):17444. doi: 10.1073/pnas.0608141103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Street CN, Lakey JRT, Shapiro AMJ, et al. Islet graft assessment in the Edmonton Protocol - Implications for predicting long-term clinical outcome. Diabetes. 2004;53 (12):3107. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 34.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. Journal of Histochemistry & Cytochemistry. 2005;53 (9):1087. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 35.Goffin V, Shiverick KT, Kelly PA, Martial JA. Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr Rev. 1996;17 (4):385. doi: 10.1210/edrv-17-4-385. [DOI] [PubMed] [Google Scholar]
- 36.Sorenson RL, Stout LE. Prolactin receptors and JAK2 in islets of Langerhans: an immunohistochemical analysis. Endocrinology. 1995;136 (9):4092. doi: 10.1210/endo.136.9.7649117. [DOI] [PubMed] [Google Scholar]
- 37.Galsgaard ED, Nielsen JH, Moldrup A. Regulation of prolactin receptor (PRLR) gene expression in insulin-producing cells. Prolactin and growth hormone activate one of the rat prlr gene promoters via STAT5a and STAT5b. J Biol Chem. 1999;274 (26):18686. doi: 10.1074/jbc.274.26.18686. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher-Chiappini SE, Compton MM, La Voie HA, Day EB, Witorsch RJ. Glucocorticoid-prolactin interactions in Nb2 lymphoma cells: antiproliferative versus anticytolytic effects. Proc Soc Exp Biol Med. 1993;202 (3):345. doi: 10.3181/00379727-202-43545. [DOI] [PubMed] [Google Scholar]
- 39.Pileggi A, Fenjves ES, Klein D, Ricordi C, Pastori RL. Protecting pancreatic beta-cells. Iubmb Life. 2004;56 (7):387. doi: 10.1080/15216540400006469. [DOI] [PubMed] [Google Scholar]
- 40.Hoorens A, Pipeleers D. Nicotinamide protects human beta cells against chemically-induced necrosis, but not against cytokine-induced apoptosis. Diabetologia. 1999;42 (1):55. doi: 10.1007/s001250051113. [DOI] [PubMed] [Google Scholar]
- 41.Piemonti L, Leone BE, Nano R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51 (1):55. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- 42.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360 (9350):2039. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 43.Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53 (11):2815. doi: 10.2337/diabetes.53.11.2815. [DOI] [PubMed] [Google Scholar]
- 44.Aikin R, Maysinger D, Rosenberg L. Cross-talk between phosphatidylinositol 3-kinase/AKT and c-Jun NH2-terminal kinase mediates survival of isolated human islets. Endocrinology. 2004;145 (10):4522. doi: 10.1210/en.2004-0488. [DOI] [PubMed] [Google Scholar]
- 45.Emamaullee JA, Davis J, Pawlick R, et al. The caspase selective inhibitor EP1013 augments human islet graft function arid longevity in marginal mass islet transplantation in mice. Diabetes. 2008;57 (6):1556. doi: 10.2337/db07-1452. [DOI] [PubMed] [Google Scholar]
- 46.Abdelli S, Abderrahmani A, Hering BJ, Beckmann JS, Bonny C. The c-Jun N-terminal kinase JNK participates in cytokineand isolation stress-induced rat pancreatic islet apoptosis. Diabetologia. 2007;50 (8):1660. doi: 10.1007/s00125-007-0704-2. [DOI] [PubMed] [Google Scholar]
- 47.Noguchi H, Nakai Y, Matsumoto S, et al. Cell permeable peptide of JNK inhibitor prevents islet apoptosis immediately after isolation and improves islet graft function. Am J Transplant. 2005;5 (8):1848. doi: 10.1111/j.1600-6143.2005.00985.x. [DOI] [PubMed] [Google Scholar]
- 48.Wideman RD, Yu ILY, Webber TD, et al. Improving function and survival of pancreatic islets by endogenous production of glucagon-like peptide 1 (GLP-1) Proceedings of the National Academy of Sciences of the United States of America. 2006;103 (36):13468. doi: 10.1073/pnas.0600655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Talavera JC, Garcia-Ocana A, Sipula I, Takane KK, Cozar-Castellano I, Stewart AF. Hepatocyte growth factor gene therapy for pancreatic islets in diabetes: reducing the minimal islet transplant mass required in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology. 2004;145 (2):467. doi: 10.1210/en.2003-1070. [DOI] [PubMed] [Google Scholar]
- 50.Cebrian A, Garcia-Ocana A, Takane KK, Sipula D, Stewart AF, Vasavada RC. Overexpression of parathyroid hormone-related protein inhibits pancreatic beta-cell death in vivo and in vitro. Diabetes. 2002;51 (10):3003. doi: 10.2337/diabetes.51.10.3003. [DOI] [PubMed] [Google Scholar]