Abstract
Objective
The critical window hypothesis of hormone therapy (HT) and cognitive function states that the effects of HT depend on the timing of initiation with respect to age and/or the menopausal transition and that optimal effects are evident with early initiation. This article reviews the clinical studies that bear on this hypothesis.
Methods
Recognizing that the typical pattern of HT use is early HT initiation, the review describes findings from observational studies of ever use of HT and observational studies that looked specifically at timing of HT on AD and cognitive test performance. Randomized trials of HT and verbal memory are discussed, and neuroimaging studies bearing on the hypothesis are reviewed.
Results
Observational data suggest that HT reduces the risk of Alzheimer’s disease (AD) generally. Three of three observational studies that specifically examined timing of initiation in relation to AD risk each provide support for the window, whereas three of five observational studies of HT timing and cognitive test performance do. Randomized clinical trials of estrogen therapy in younger women find support for the hypothesis. Conjugated equine estrogen/medroxyprogesterone acetate (CEE/MPA) increases risks regardless of timing. Little is known about the cognitive effects of other combination HT formulations.
Conclusion
A definitive trial to test the critical window hypothesis is not feasible. Evidence drawn from other sources provides initial support for the hypothesis. Although these findings are relevant to women who use HT to treat vasomotor symptoms, HT is currently not indicated for the treatment of cognitive complaints or dementia prevention.
Keywords: Estrogen, cognition, dementia, menopause, memory, neuroimaging
Alzheimer’s disease (AD) is now the sixth leading cause of death in the United States1, with approximately 5.4 million individuals with the disease2. Of the top 10 leading causes of death in the United States, AD shows the greatest sex difference, with more women than men dying from the disease3. A recent study of age-adjusted mortality rates from AD between 1999 and 2008 demonstrated that 20% more women than men died of the disease4. There is debate about whether this sex difference in the prevalence of AD is due to the greater longevity of women compared to men. Evidence against that explanation comes from an analysis of data pooled from four, population-based studies showing a greater incidence of AD in women compared to men; the cumulative risk for 65-year-old women to develop AD at the age of 95 years was 0.22 compared with 0.09 for men5. The prevalence of AD is expected to triple by 2050, with an estimated cost of 20 trillion dollars, including 11.4 trillion in costs to Medicare and 3.6 trillion in costs to Medicaid6. In light of the enormous costs associated with the disease, a medication that delayed the onset of the disease by just 5 years would be associated with significant cost savings. Conversely, a medication that hastened the onset of the disease would be associated with significant costs. No medication specifically prescribed for women has received greater attention in the field of AD research than hormone therapy (HT).
This review is focused on clinical studies bearing on the critical window hypothesis of HT and cognition. The critical window hypothesis, also known as the timing hypothesis or the critical period hypothesis, says that the effects of HT depend on the timing of initiation of treatment with respect to age and/or the onset of menopause, with benefits limited to early initiation 7, 8. This review first describes the epidemiological studies giving rise to the hypothesis that HT might impact the risk of AD. Then the history of studies giving rise to the timing hypothesis is reviewed and shows that the hypothesis preceded the later findings from the Women’s Health Initiative Study of Memory9, 10. Challenges in conducting a randomized controlled trial of early HT for AD prevention are then described to justify a focus on observational studies of AD and randomized trials of verbal memory. Three observational studies of AD are reviewed that specifically examined the timing hypothesis, each providing support for the hypothesis. Four observational studies are then described that examined the timing hypothesis on cognitive tests. Together, they show mixed results. Next randomized clinical trials of HT and verbal memory are summarized, based on evidence that verbal memory is a suitable proxy outcome for AD risk. Those data provide tentative support for benefits of early use of estrogen alone, and mixed findings with respect to estrogen plus progestin. Lastly, neuroimaging studies are reviewed that bear on the critical window hypothesis and that show initial evidence for enhanced function of the hippocampus and prefrontal cortex in early users. An evaluation of the body of evidence included in this review concludes that the literature generally provides support for the critical window hypothesis. Future directions and research priorities are then briefly discussed.
Observational Studies of HT and AD
The typical pattern of HT use in the U.S. was initiation of HT close in time to the final menstrual period and use of estrogen alone, most typically conjugated equine estrogen (CEE). For this reason, it is appropriate to review observational studies of the risk of HT and AD when evaluating the critical window hypothesis. To date there have been 18 observational studies examining the association between ever use of HT and AD. Fourteen of those studies were retrospective, case-control studies in which individuals with AD were matched to controls and exposure to HT was ascertained retrospectively11-19 or prospectively20-24. Four were prospective studies in which women were followed over time and the outcome measure was incident AD25-28. Meta-analyses of these studies demonstrated a significant reduction of 29 to 44%29-32 in the risk of AD among women who used HT compared to women who did not use HT. Notably, none of the studies demonstrated a significant increase in the risk of AD with HT. A significant limitation of those studies, however, was the “healthy user bias”; compared to women who did not use HT, women who used HT were healthier, particularly in terms of cardiovascular risk factors, and better educated. Since better cardiovascular health and higher educational status protect against AD, it was unclear whether it was truly the HT or alternatively the biases in characteristics of the women who went on HT that explained the findings.
Emergence of the Critical Window Hypothesis
In 2000, while the Women’s Health Initiative Memory Study (WHIMS) was in progress, findings from three randomized, placebo-controlled clinical trials of estrogen therapy for the treatment of women with a diagnosis of AD were published33-35. None of the trials showed efficacy. One trial examined 1.25 mg/d of CEE in 42 patients followed for 16 weeks33, another.625 or 1.25 mg/d of CEE in 120 patients followed for 15 months34, and a third 1.25 mg of CEE in 50 patients followed for 12 weeks 35. An editorial by Marder36 that accompanied the last of those trials33, noted that the discrepancy between the findings from treatment trials and observational studies and suggested that “Perhaps critical periods occur at menopause, when withdrawal of hormonal influence leads to increased brain susceptibility to pathologic processes” and that “perimenopause or immediately postmenopause might be the appropriate time for intervention.”7
The possibility that timing of initiation was a potential modifier of the effect of HT on cognitive function gained additional momentum with a 2002 publication from the Cache County Study27. That observational study was the first to stratify use of HT by current versus former use and within those strata, by duration of treatment. In agreement with previous observational studies, the overall finding was a reduced risk of AD among women who had used HT compared to those who had not. The new and striking finding was that compared to women who never used HT, former users of HT showed a reduced risk of AD but current users did not. Among current users, only those who had used for 10 or more years showed a reduced risk. Given that the average age of women in that study was 73, the implication was that HT might be ineffective in reducing the risk of dementia in the latent preclinical stage of the disease but effective if initiated earlier. An accompanying editorial by Resnick and Henderson8 noted “Because many women use HT for relatively brief durations around the menopause, the protective effect of ever-use therapy suggests the possibility of a critical period during the climacteric years, which are characterized by relatively rapid estrogen depletion.”
In 2003, the primary findings from the WHIMS were published10. The WHIMS remains the only randomized, placebo-controlled study of the effect of HT on the risk for dementia. The study had two arms, one for hysterectomized women where the active treatment was CEE alone, and the other for women with a uterus where the active treatment was CEE plus medroxyprogesterone acetate (CEE/MPA). The focus on those types of HT was clinically relevant because they were the most commonly used clinically at the time the WHI was launched. In order to study the hypothesis that HT was effective in the primary prevention of late-life AD, it was necessary to study women who were at risk of incident AD over the course of the study. Women enrolled in WHIMS therefore were aged 65 or older at the time of enrollment. From a public health perspective, it was thought that those women had the most to gain from the intervention since they were the most vulnerable to developing the disease, and no previous study had suggested any increase in risk of AD in women who were taking HT after age 65.
The findings from WHIMS were in striking contrast to findings from the observational studies, but consistent with the Cache County Study27 and with the lack of efficacy in AD patients33-35. In the CEE/MPA arm, with a sample size of 4532 women, there was a doubling of the risk of all-cause dementia with active treatment compared to placebo after an average follow up o 4 years10. The short time frame over which the increased risk for all-cause dementia emerged suggested that the increased risk of dementia did not reflect the primary initiation of neuropathology, but rather hastened existing neuropathology. Although AD was the initial primary outcome variable and although the majority of dementia cases in WHIMS participants were due to AD, there were too few cases of AD when study was prematurely halted due to other risks, so all-cause dementia became the primary outcome. Findings from the CEE alone arm, published in 2004, with a sample size of 2947 women followed over an average of 5 years, found no significant impact of CEE on dementia risk9. A direct comparison between the two arms of the study, however, revealed no statistical difference in dementia risk9. On that basis, WHIMS investigators did not distinguish between the effects of CEE and CEE/MPA.
One of the leading hypotheses to explain the increased risk of dementia with CEE/MPA was an increase ischemic burden that hastened the development of dementia9. Autopsy data from the Nun’s study had shown that given two clinical cases with equal burdens of AD neuropathology, the one with brain infarcts would exhibit poorer cognitive performance and be more likely to present with dementia than the one without infarcts37. In the WHI, CEE/MPA increased the risk of ischemic stroke by 30%38. It therefore seemed plausible that CEE/MPA might have increased subclinical cerebral vascular disease which in turn increased the risk of dementia. Findings from the WHIMS Magnetic Resonance Imaging (MRI) study, however, did not support that explanation; in a subsample of 1,403 WHIMS participants there was no difference in the volume of ischemic lesions between those who had been randomized to receive active treatment and those receiving placebo39. Despite a large sample size and use of strong imaging methods, WHIMS-MRI was not fully able to examine the impact of CEE/MPA on subclinical cerebrovascular disease because assessments of ischemic load were conducted on average 7 years after participants initiated treatment. Although the sample size in WHIMS-MRI was large, it is not possible to entirely rule out the possibility that groups differed in ischemic load at baseline or that CEE/MPA led to increases in ischemic volume early after treatment initiation. Notably, the key finding from WHIMS MRI was that women in the active treatment arm showed smaller brain volumes in the frontal lobe and hippocampus compared to women in the placebo arm40. Furthermore, the loss of hippocampal volume was most pronounced in those women who showed the poorest performance on a screening test for cognitive impairment prior to randomization to HT40. Later publications linked the loss of brain volume to the diagnosis of dementia in the WHIMS41. These results then suggested that HT was detrimental to older women who initiated HT when they were beginning to experience cognitive decline.
Challenges in Testing Critical Window Hypothesis for AD Prevention
In the decade since the publication of the WHIMS, there has been considerable debate about the external generalizability of WHIMS findings to perimenopausal and younger postmenopausal women and therefore about the clinical significance of WHIMS findings to more typical patterns of HT use. Prior to the WHI, 85% of women in the United States who initiated HT did so within one year of the final menstrual period. It was therefore possible that the discrepancy between the positive observational studies and the negative findings from WHIMS might be due to differences in the timing of treatment with respect to age and/or the final menstrual period. Testing this hypothesis using a randomized, placebo-controlled trial is not feasible because such a study would require: 1) randomization of large sample of perimenopausal/early postmenopausal women (WHIMS had over 7500 women); 2) longitudinal follow up for more than 20 years (until they were age 65 and older); 3) sufficient adherence; and 4) sufficient funding. Furthermore, the clinical relevance of such a study might be limited by changes in the doses and types of menopausal treatments used in the next decades, including use of new progestins, new selective estrogen modulators (SERMS) and tissue-specific estrogen complexes (TSEC).
The obstacles in conducting a clinical trial to test the timing hypothesis for prevention of AD necessitate that we examine evidence from other clinical research designs to evaluate the hypothesis. The next section reviews findings from such studies, including: observational studies that have examined the impact of timing of initiation of HT on AD risk and cognitive performance; randomized clinical trials of HT and verbal memory; and neuroimaging studies.
Observational Studies of HT Timing and Risk for AD
To date there have been three observational studies of the impact of timing of initiation of HT on the risk for dementia. All three find support for the hypothesis. The first was a case-control study (n = 426 AD patients, 545 relatives without dementia) from the Multi-Institutional Research in Alzheimer’s Genetic Epidemiology (MIRAGE) study, which examined risks of AD based on the age at which women initiated HT19. Only women in the youngest age tertile (ages 50-63) showed a reduced risk of AD. A second study was a prospective observational study from Kaiser Permanente (n = 5,504 women including 1,524 AD cases)42. Women who used HT only at midlife showed a 26% reduced risk of AD, those who used only late-life showed a 46% increased risk of dementia, and those who used during both periods showed neutral risk. The third study28 was a follow-up study from the Cache County Study27 where data were stratified by timing of initiation (n = 1,768 including 174 AD cases). There the results depended not only on timing of initiation but also on whether women used estrogen alone or estrogen in combination with a progestin. Women who initiated HT within five years of menopause onset showed a 30% reduced risk of AD, and the magnitude of risk was lowest for women who initiate early and continued to use HT for 10 years (37% reduced risk). Those who initiated later showed no protection, and those who initiated combination estrogen plus progestin later in life showed a nearly two-fold increase in risk, though that effect was a trend. Overall, then, observational studies of AD show reliable support for the critical window hypothesis. Also, the studies provide evidence that late life initiation of HT increases the risk of AD.
Verbal Memory as a Clinically Significant Outcome
Neuropsychological tests of verbal memory involve learning and recall of word lists, paragraphs, and stories. These tests are clinically useful because they have known normative standards and known clinical validity in predicting dementia. A meta-analysis of 32 clinical trials of HT demonstrated that the cognitive domain most sensitive to the effects of HT (positive or negative) is verbal memory43. The clinical relevance of this effect is high because deficits in verbal learning and memory have been shown to be a very strong predictor of who will develop dementia44-46. Notably, females show a life-long advantage in this cognitive domain compared to males, and unlike males, females begin to show declines with age only after age 5547, 48. Studies specifically examining verbal memory during the menopausal transition indicate that women show subtle deficits in verbal memory as they transition through the menopause49, 50.
Findings from an ancillary study to WHIMS, the WHI Study of Cognitive Aging (WHISCA), provide perhaps the strongest justification for focusing on verbal memory as a clinically relevant primary outcome. Whereas WHIMS focused on dementia outcomes, WHISCA focused on age-related changes in cognitive test performance51. CEE/MPA increased the risk for dementia in WHIMS10 and, as will be described in more detail later, CEE/MPA decreased verbal memory in WHISCA52. CEE alone had no impact on dementia in WHIMS9, and CEE alone had no impact on verbal memory in WHISCA53. This pattern of results suggests that verbal memory may be a good proxy for dementia risk when evaluating the critical window hypothesis. Findings from WHISCA underscore the importance of distinguishing not only between timing of initiation but also between estrogen alone and estrogen plus a progestogen.
Observational Studies of HT Timing and Cognitive Performance
Most women initiate HT within the “window,” therefore most observational studies reflect early initiation. A meta-analysis of observational studies concluded that HT positively impacts verbal memory function as measured by paragraph recall and word lists, but the level of heterogeneity across studies and inconsistencies across tests of verbal memory (even within the same study) are too great to draw any firm conclusions30.
The present investigation identified relevant articles for inclusion based on PubMed searches for the keywords “memory,” “dementia,” “cognition,” “Alzheimer’s disease” and “estrogen,” “estradiol,” “hormone therapy,” “hormone replacement therapy” and by searching reference sections from identified studies as well as review articles. For the review of articles addressing the timing hypothesis, the search terms “timing,” “critical window,” and “critical period” were used, and the reference sections of those articles were reviewed for additional articles. In the review of studies of verbal memory, only those studies using standardized neuropsychological measures of memory performance were included; studies using non-standardized, experimental measures of memory performance were not included. Additionally, for the review of randomized trials, only studies with a placebo-controlled arm were included, and the minimum treatment duration was two weeks.
Four prospective, observational studies have directly examined the impact of timing of initiation of HT on standardized neuropsychological tests, including verbal memory49, 54-56. Table 1 describes the study design and findings, which are mixed for all cognitive domains, including verbal memory. Two studies found no support for the critical window hypothesis, but found different results with respect to how HT impacts cognition generally. The Three Cities Study (3C) from France found no evidence to support the critical window hypothesis but found evidence for cognitive benefits regardless of timing55. Specifically, current use of HT was associated with better performance on tests of verbal fluency, working memory and psychomotor speed, but these associations did not depend on timing of initiation. Longer duration of treatment was associated with greater benefits. The Nurses’ Health Study (n = 16,514) also found no support for the critical window hypothesis and, in contrast to the 3C Study, actually found that HT was associated with slightly worse cognitive performance with estrogen alone and combination HT. The magnitude of this effect was small; a woman on HT performed as if she were 1 to 2 years older than a woman who was not on HT.
Table 1.
Observational cohort studies of timing of hormone therapy on cognitive outcomes
| Author (Year) Cohort |
N | Mean Age (Span) |
HT Use | Design | Tests | Results |
|---|---|---|---|---|---|---|
|
MacLennan (2006) REMEMBER |
428 | 70.7 (60+) |
Early: 72% E alone: 39% |
1 FTF Assessment |
|
|
|
Ryan (2009) Three Cities |
3130 | 74 (65+) |
Past: 16% Current: 15% Nonusers: 69% |
3 FTF assessments, 2 y apart |
|
|
|
Khoo (2010) LAW |
410 | (41-79) | Early: 38% Late: 10% Nonusers: 52% |
2 FTF assessments, 5 y apart |
|
|
|
Greendale (2009) SWAN |
2,362 | 46 (42-52) |
Current: 4% Nonusers: 27% Early: before FMP |
2 FTF assessments, baseline and 2 y apart |
|
|
| Kang & Grodstein NHS (2012) |
16,514 | 74 (70-81) |
Past: 35% E alone: 25% E+P: 9% Nonusers: 31% |
≤3 phone assessments, 2 y apart |
|
|
Notes: Abbreviations: E= estrogen; E+P = estrogen plus progestogen; FMP = final menstrual period; FTF = face-to-face; HT = hormone therapy; LAW= Longitudinal Assessment of Women study; NHS= Nurses’ Health Study; REMEMBER = Research into Memory, Brain function and Estrogen Replacement study; SWAN = Study of Women’s Health Across the Nation; trans = transdermal.
Three other observational studies, the Research into Memory, Brain function and Estrogen Replacement (REMEMBER) study57, Study of Women’s Health Across the Nation (SWAN)49 and the Longitudinal Assessment of Women (LAW)56, found partial support for the critical window hypothesis. Findings from 486 Australian women aged 70 and older in the REMEMBER study revealed that early use of HT was associated with enhanced global cognitive function and psychomotor speed57. A study of 2,362 women in SWAN found that prior use of HT (i.e., generally during the early postmenopause) conferred an advantage to performance on the immediate and delayed memory outcomes on the East Boston Memory Test (EBMT). Moreover, initiation before the final menstrual period conferred cognitive benefits whereas initiation after the final menstrual period conferred risks49. A study of 410 Australian women aged 41 to 79 enrolled in the LAW study found support for the critical window, but only in favor of estrogen alone. The LAW study examined memory performance in two face-to-face assessments 5 years apart56. Most women who took HT (80%) were early initiators, with 42% on unopposed estrogen. Early initiation of estrogen-alone was associated with a reduced risk of cognitive decline whereas early start of combination HT was associated with increase in risk for general memory.
In general, then, findings from observational studies provide mixed findings with respect to the critical window hypothesis. Notable limitations include the use of the East Boston Memory Test (EBMT), a test on which cognitively healthy women can easily reach maximum performance, in both the Nurses’ Health Study and SWAN. With repeated testing on that test, women are more likely to reach maximum performance. The LAW study used a memory test with a higher ceiling and level of difficulty that is more sensitive to changes in memory. The 3C study used transdermal estradiol, which may have different cognitive effects than oral estradiol which became more commonly used in the U.S. after the WHI.
Randomized, Placebo-Controlled Trials of HT and Verbal Memory
Tables 2 to 5 summarize findings from randomized, placebo-controlled trials that included neuropsychological tests of verbal memory as outcomes. These tables are updated from previous reviews58, 59. Trials using more experimental measures of verbal memory, such as those used in neuroimaging studies, were not included. In contrast to the observational studies, these trials provide some consistent findings with respect to timing and use of estrogen alone versus use of estrogen plus progestin.
TABLE 2.
Randomized, placebo-controlled trials of estrogen alone on verbal memory in younger peri- and postmenopausal women
| Author | N (All) Final ET/Pl |
Mean Age (SD or span) |
Prior HT Use (%) |
Menopausal Status/ Menopausal Symptoms/ Years since Menopausea |
Design | Duration | ET | Dose | Test | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Hackman & Galbraith (1976) | (?) 18 (9/9) |
(?) 29-68 |
? | 8 surgically menopausal patients/ 10 patients with symptoms (4 mild and 1 moderate in ET group)/ unknown y |
Parallel; 2 groups |
6 m | Cyclic oral piperazine E1 |
3.0 mg/d for 21 days (1.5 mg bid); 7 days off |
Immediate and Delayed Paragraph Recall & Paired Associates |
ET > PL on total memory score |
| Sherwin (1988) | (53) 50 |
43.6 (?) |
Likely none |
All surgical menopausal (BSO/TAH)/ Likely symptomatic / 0 y |
Crossover; 5 groups; 3 active treatments |
3 m | Monthly injections of E2 or TE or E2+TE |
E2 (10 mg) or TE (200 mg) or E2 dianthate (7.5 mg) + E2 benzoate (1.0 mg) + TE (150 mg) |
Immediate and Delayed Paragraph Recall |
Any ET > PL on all tests b |
| Phillips & Sherwin (1992) | (31) 19 10/9 |
48.2 (4.7) |
Likely none |
All surgical menopausal (BSO/TAH)/ Likely symptomatic / 0 y |
Parallel; 2 groups |
2 m | Monthly injections of E2 |
10 mg/m | Immediate and Delayed Paragraph Recall & Paired Associates |
ET > PL Both Immediate Measures & Delayed Paired Associates |
| Shaywitz (2003) | (60) 60 29/31 |
51.2 (32-64) |
27% | Postmenopausal/ 80% had menopausal symp- toms/ est. 3 y |
Crossover | 21 d | Continuous Oral CEE |
1.25 mg/d | Paragraph Recall, Paired Associates |
ET > PL |
| Dunkin (2005) | (26) 17 8/9 |
57.0 (6.9) |
6% | Postmenopausal/ No significant symptoms/ 7.9 y |
Parallel; 2 groups |
10 wk | Transdermal E2 patches |
0.1 mg/d | CVLT, Paragraph Recall, Verbal Paired Associates |
ET=PL |
| Joffe (2006) | (52) 50 26/24 |
51.0 (3.8) |
18% | Peri- (56%) and postmenopausal/ 62% had menopausal symptoms (32% mod- severe)/ < 5 y |
Parallel; 2 groups |
12 wk | Transdermal E2 patches |
0.05 mg/d | CVLT, WMS-R Verbal and Delayed |
ET>PL on perseverative errors |
| LeBlanc (2007) | (37) 32 14/18 |
52.3 (3.4) |
? | Peri- and Postmenopausal/ 46% high menopausal symptoms/ last menstrual period 3 and 36 months |
Cross sectional |
8 wk | Oral E2 | 2 mg/d | Paragraph Recall, Verbal Paired Associates |
ET=PL |
Note. If mean years since menopause was not provided in article, value was estimated as difference between current age and average age of menopause = 51.
53 subjects were originally recruited, 9 dropped out, and 6 additional women were recruited into study. Here all 3 active treatment groups are combined in results because they did not differ from one another. Their data are not reported in this table. Abbreviations: BSO= bilateral salpingo-oopherectomy; CEE = conjugated equine estrogen ; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; CVLT= California Verbal Learning Test; E1 = Estrone; E2 = Estradiol; ET= Estrogen therapy; HT = Hormone Therapy; mod = moderate; PL = Placebo; TE = testosterone enanthate.
TABLE 5.
Randomized, placebo-controlled trials of estrogen and progestogens on verbal memory in older postmenopausal women
| Author | N (All) Final HT/Pl |
Mean Age (SD or span) |
Prior HT Use (%) |
Menopausal Status/ Menopausal Symptoms/ Years since Menopausea |
Design | Duration | HT | Dose | Test | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Binder (2001) | (67) 52 34/18 |
81 (75-91) |
33% | Postmenopausal/ Likely minimal to none/ 34 y |
Parallel | 9 m | CEE alone in 59%; CEE/ trimonthly cyclic MPA in 41% |
0.625 mg/d; 5 mg/d for 13 at months 2, 5 and 8 |
Paired Associates |
HT = PL |
| Grady (2002) | (1328) 1063 517/ 546 |
66.8 (44-79) |
24% | Postmenopausal/15% Hot flashes, coronary disease in all/18 y |
Parallel | 4.2 y | CEE/MPA | 0.625 mg/d 2.5 mg/d |
Word List Learning and Recall |
HT < PLT1 Word List Learning |
| Resnick (2005) |
(2302) 1416 690/726 |
73.8 (3.7) |
22% | Postmenopausal/ 5% moderate-to-severe/ Unknown |
Parallel | 3.4 y | CEE/MPA | 0.625 mg/d 2.5 mg/d |
CVLT | HT < PL Learning & Long DelayT2 |
| Tierney (2009) | (142) 128 62/66 |
74.7 (6.9) |
27% | Postmenopausal/Likely minimal to none/ 26 y |
Parallel | 2 y | E2 + cyclic Norethindrone |
1 mg/d, 0.35 mg/ 3 d per week |
CVLT | HT = PL |
Notes: T1 = p-value was 0.06 for word list memory and .02 after controlling for age, education, years postmenopausal, statin use, Geriatric Depression Scale score, alcohol drinks per week, hot flushes, and trouble sleeping; T2 = p-value was 0.02 for learning and 0.01 for long delay. CEE = Conjugated equine estrogen; CVLT = California Verbal Learning Test; E2 = estradiol; HT= Hormone therapy; MPA = Medroxyprogesterone acetate; PL = Placebo.
Randomized Trials of Estrogen Alone on Verbal Memory in Younger Peri- and Postmenopausal Women
There have been seven clinical trials of estrogen alone in samples of younger postmenopausal women, defined here as trials where the mean age at randomization is 65 or younger60-66. (See Table 2.) The sample sizes in these studies range from 17 to 60, the treatment duration from 21 days to 6 months, and the mean ages of women in those samples ranged from 44 to 57. Five of the seven studies60, 63, 66-68 found beneficial effects of estrogen on verbal memory compared to placebo, and none found detrimental effects. Notably, one of those trials used a common measure of verbal memory, the California Verbal Learning Test (CVLT), and showed a significant impact on CVLT outcomes (e.g., proactive interference, perseverative errors) that are not typically reported in clinical trials and that are classified as measures of “executive function” rather than “verbal memory”66. Furthermore, although estradiol was found not to have an impact on CVLT measures of verbal learning in that trial, effects of estradiol on short- and long-delay free recall, two widely used outcome measures, were not reported and therefore cannot be ascertained66. Only one trial investigated cyclic estrogen and that trial found benefit60. Although these findings provide some support for the critical window hypothesis, it is clear that larger studies are needed. The need is particularly high for women who have their ovaries surgically removed at an early age, because observational data show that such women are at a 70% increased risk of AD unless they receive estrogen treatment69.
Randomized Trials of Estrogen Alone on Verbal Memory in Older Women
Table 3 shows the findings from the five randomized, placebo-controlled trials of estrogen alone in samples of women whose mean age was greater than 65 at randomization53, 70-73. The sample sizes in these five studies ranged from 38 to 886, the treatment duration ranged from 2 weeks to 5.7 years, and the mean ages ranged from 67 to 76. All five trials found neutral effects of estrogen alone treatment on verbal memory, including the two largest studies, one of ultra-low-dose transdermal estradiol in 417 women treated for two years71 and the other of CEE in 886 women treated for 5.7 years53. Overall, these data suggest no negative impact of estrogen alone on verbal memory in older postmenopausal women (see also74). Although some have argued that cyclic estrogen is the most biologically relevant regimen and therefore the most likely to be neuroprotective 75, there is surprisingly little research on cyclic estrogen treatment. Data from placebo-controlled trials in non-human provides very compelling evidence that only cyclic estrogen enhances cognition and the formation of synapses in the prefrontal cortex.76-78
TABLE 3.
Randomized, placebo-controlled trials of estrogen alone on verbal memory in older postmenopausal women
| Author | N (All) Final ET/PL |
Mean Age (SD or span) |
Prior HT Use (%) |
Menopausal Status/ Menopausal Symptoms/ Years since Menopausea |
Design | Duration | HT | Dose | Test | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Wolf (1999) | (40) 38 21/17 |
68.7 (5.8) |
? | Postmenopausal, 13 Hysterectomy (8 with BSO)/ Unknown / 17 y |
Parallel; 2 groups |
2 wk | Trans E2 |
0.1 mg/d | Word pair recall |
ET=PL |
| Yaffe (2006) | (417) 376 191/ 185 |
66.7 (5.0) |
? | Postmenopausal/16%/ ≥ 5 y |
Parallel; 2 groups |
24 m | Trans E2 |
0.014 mg/d |
Paragraph Recall, CERAD Word List |
ET=PL |
| Almeida (2006) | (115) 86 47/39 |
73.7 (3.8) |
38% | Postmenopausal/ 16%/28.7 y |
Parallel; 2 groups |
20 wk | Oral E2 |
2 mg/d | CVLT | ET=PL |
| Pefanco (2007) | (57) 45 (32)/(25)a |
75.5 (5.1) |
? | Postmenopausal, 32% had hysterectomy / Unknown |
Parallel; 2 groups |
36 m | Trans E2 |
0.25 mg/d |
Paired Associates Logical Memory |
ET=PL |
| Resnick (2009) | (886) 752 370/382 |
74.0 (3.8) |
48% | Postmenopausal with prior hysterectomy/ 9% /Unknown |
Parallel; 2 groups |
5.7 y a | CEE | 0.625 mg/d |
CVLT | ET=PL |
Notes: On average women were tested at the initial cognitive assessment 3 years after randomization in the WHI. The average follow-up after that initial assessment was 2.7 years. Abbreviations: BSO= bilateral salpingo-oopherectomy; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; CVLT= California Verbal Learning Test; E2 = Estradiol; ET= Estrogen therapy; HT = Hormone Therapy; PL = Placebo; Trans = transdermal.
Randomized Trials of Estrogen Plus Progestogen on Verbal Memory in Younger Postmenopausal Women
Table 4 shows results of five trials from combination estrogen plus progestogen in samples of women where the mean age was less than 6579-83, and includes two trials that also had an active estrogen alone arm79, 80. The sample sizes in these five studies ranged from 16 to 158, the treatment duration ranged from 2 to 12 months, and the mean ages ranged from 52 to 64 years. Two trials used continuous combined CEE/MPA (.625 mg/d + 2.5 mg/d) and both found trends (p< .06) for a decrease in verbal memory as measured by a modified version of the California Verbal Learning Test (CVLT). One was the Cognitive Complaints in Early Menopause Trial (COGENT), which involved 180 women with subjective memory complaints who were treated for four months81. (See Figure 1.) The other was a trial in 66 women who were treated for one year82. Two trials used estradiol valerate and a progestin, and each found significant improvements in verbal memory79, 83. Another trial used estradiol valerate plus norethisterone (0.7 mg/d) in 17 women followed for 6 months83. Two trials included both an estrogen alone arm (each estradiol valerate) and an estrogen plus progestin arm. One examined the effects of continuous combined estradiol valerate (2 mg/d) and dinogest (3 mg/d) in 49 women treated for three months and also included an active estradiol valerate alone arm; the combination arm showed improvements in verbal memory while the estradiol valerate alone arm showed improvement in numerical memory79. Another trial in 35 women examined the effects of estradiol valerate (2 mg/d) in combination with oral progesterone (100 mg/d), as well as estradiol valerate alone (2 mg/d), and found no effect in either arm80.
TABLE 4.
Randomized, placebo-controlled trials of estrogen and progestogen on verbal memory in younger postmenopausal women
| Author | N (All) Final HT/PL/G3/G4 |
Mean Age (SD or span) |
Prior HT (# m or %) |
Menopausal Status/ Menopausal Symptoms/ Years since Menopausea |
Design | Duration | HT | Dose | Test | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Linzmayer (2001) | (?) 49 16/16/17a |
57 (46-67) |
? | Postmenopausal/ Insomnia disorder & moderate-to-severe menopausal symptoms/est. 6 y |
Parallel 3 groups |
2 m | E2 Valerate + Dinogest; E2 Valerate |
2 mg/d + 3 mg/d; 2 mg/d |
Total Verbal Memory (Grunberger) |
HT>PL=ET associative memory; ET > PL numerical memory |
| Wolf (2005) | (51) 35 10/13/12b |
64 (58-75) |
13 y since use |
Postmenopausal, All hysterectomized/Unknown/ Hysterectomy at 44 y of age |
Parallel 3 groups |
6 m | E2 valerate + progesterone; E2 valerate |
2 mg/d + 100 mg/d; 2 mg/d |
Paragraph Recall, Paired Associates |
ET=HT= PL |
| Maki (2007) | (180) 158 78/80 |
52.2 (3.45) |
14 m | Postmenopausal/ All moderate to severe/ 43 m |
Parallel 2 groups |
4 m | CEE/MPA | 0.625 mg/d + 2.5 mg/d |
CVLT, Logical Memory |
HT < PLT1 CVLT Short & Long Delay |
| Maki (2009) | (70) 66 17/17/14/18c |
52.2 (3.45) |
43% | Postmenopausal/ ≥ 35 weekly hot flashes/ last menstrual period 6 m to 10 y prior |
Parallel 4 groups |
12 m | CEE/MPA; red clover; black cohosh |
0.625 mg/d + 2.5 mg/d; 120 mg/d; 128 mg/d |
CVLT, Logical Memory |
HT < PL; =BC=RCT2 CVLT Learning |
| Alhola (2010) | (?) 16 7/9d |
62.9 (3.0) |
69% 76 m |
Postmenopausal/Slight to Moderate/12 y |
Parallel 2 groupsb |
6 m | E2 valerate + norethisterone |
2 mg; 0.7 mg | RAVLT | HT>PL immediate recall |
Notes: Group 3 is the estradiol valerate alone arm. Study also included an open-label arm after the RCT, but results are presented for blinded arm only.
Group 3 is the estradiol alone arm.
Group 3 is the red clover arm, and Group 4 is the black cohosh arm.
Trial also included a premenopausal arm, but results are given for postmenopausal arm only. T1 = p-value was 0.054 for CVLT short delay and 0.06 for CVLT long delay; T2 = p-value was 0.056 for CVLT learning and 0.02 after controlling for vasomotor symptoms; BC = Black Cohosh; CEE = Conjugated equine estrogen; E2 = estradiol; G3 = Group 3; G4 = Group 4; HT= Hormone therapy; MPA = medroxyprogesterone acetate; PL = Placebo; RAVLT=Rey Auditory Verbal Learning Test; RC = red clover.
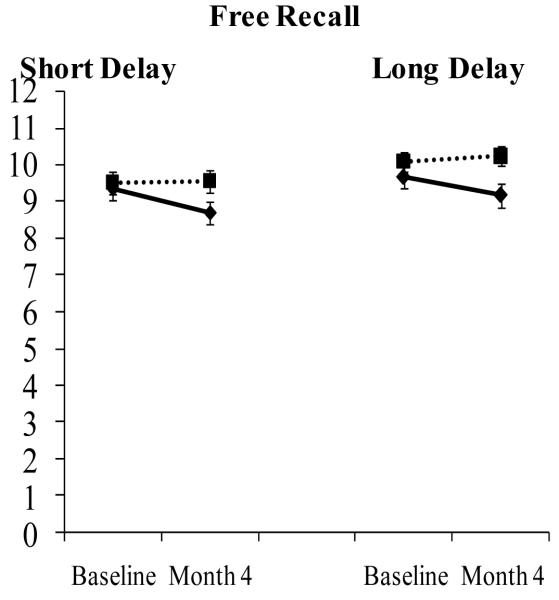
Figure 1.
Conjugated Equine Estrogen Plus Medroxyprogesterone Acetate Impairs Verbal Memory in Younger Postmenopausal Women.
Effect of combination CEE/MPA on verbal memory as measured by the California Verbal Learning Test in 158 postmenopausal women with a mean age of 52 years. p-value for the Group by Time interaction was 0.054 for short delay free recall and 0.06 for long delay free recall; women randomized to CEE/MPA declined significantly on short-delay free recall, p = .02. The HT-related decline in short- and long-delay free recall was 0.63 and 0.68 words. The CEE/MPA arm is shown in solid black lines and the placebo arm is shown in dotted lines. Adapted from Maki et al., 2007, Neurology.
Together these studies point out potential differences between types of progestins, and in agreement with WHIMS10 and WHISCA52 potential detrimental effects of CEE/MPA even to younger postmenopausal women. The latter finding argues against beneficial effects early in the critical window for that particular formulation of HT. Although sample sizes are limited, non-MPA-based progestins may provide some protection and perhaps benefit to verbal memory function if initiated early. There are no published studies on the effects of cyclic estrogen plus progestin on verbal memory in younger postmenopausal women, including cyclic CEE/MPA. Larger-n trials of estrogen alone versus estrogen plus progestin are also needed.
Randomized Trials of Estrogen Plus Progestogen on Verbal Memory in Older Postmenopausal Women
To date there have been four randomized, placebo-controlled trials of combination estrogen plus progestogen on neuropsychological measures of verbal memory in older postmenopausal women52, 84-86. (See Table 6.) The quality of these studies is high, with large sample sizes (range 52-1416) and long follow-ups (up to four years). Each of two trials of CEE/MPA, the Heart and Estrogen/progestin Replacement Study (HERS) involving 1063 women (mean age 66.8, range 44 to 79)85 and the WHISCA involving 1416 older postmenopausal women (mean age 74 years; all 65 years and older)52, found evidence of a negative impact of treatment on verbal memory. Although both were trends – HERS just missed statistical significance with a p-value of .06 and WHISCA had a p-value of .01 and .02 for two measures - the findings are consistent with evidence from WHIMS10 and from COGENT81.
Neuroimaging Studies and the Critical Window Hypothesis
Functional neuroimaging studies using functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) provide insights into the effects of HT on brain function as women are performing cognitive tasks or are at rest. Neuroimaging outcomes are typically more sensitive than neuropsychological test outcomes to drug interventions. Neuroimaging studies of memory typically involve an encoding condition and a retrieval condition and thereby help to determine what stage of memory HT might affect, encoding items into memory (learning) or retrieving those items from memory. In humans such studies provide biological plausibility for prevention of AD, particularly if HT is shown to positively alter activation in the hippocampus and other brain regions that are functionally impaired in preclinical and clinical AD. Again the focus here will be on neuroimaging studies during verbal memory tasks and on relevant structural neuroimaging studies. (See59 for a more comprehensive review of neuroimaging studies.) Initial insights into the neural targets of HT on brain function came from two PET studies from the Baltimore Longitudinal Study on Aging87, 88. The sample included 32 older women (mean age = 66) who were either on HT or never used HT. It is reasonable to assume that those who were on HT initiated therapy early since that was the typical pattern of HT use generally in the U.S. In both studies, participants completed neuroimaging assessments while resting and while performing a recognition test of words (verbal memory) and figures (visual memory) they had studied 30 minutes earlier. Results showed that on neuropsychological tests, users performed better than nonusers on tests of verbal memory and visual memory. On the imaging task, the groups showed differences in brain in brain areas that subserve memory, including the inferior frontal cortex and the parahippocampal gyrus. The second study89 followed that same group of women prospectively over a 2-year interval to look at the effect of HT on rate of brain aging. Over time, HT users showed increased blood flow to the right hippocampus, right entorhinal cortex, right posterior parahippocampal cortex, other temporal structures, and inferior frontal cortex compared with nonusers. On standardized neuropsychological tests, HT users again showed better memory performance. Importantly, decreased hippocampal activity is associated with an increased risk of dementia89, so the finding of increased hippocampal activity in HT users suggested protection.
A small fMRI study in perimenopausal and early postmenopausal women provided further evidence that HT enhanced the function of the prefrontal cortex during a word retrieval task.66 Five women randomized to receive transdermal estradiol (0.05 mg/day) and six randomized to receive placebo (mean age =51 years) completed the task before and after 12 weeks of therapy.66 Compared with placebo, estradiol enhanced activation in an area of prefrontal cortex that is involved in higher order executive functions such as planning and strategizing. Activation in prefrontal cortex during a spatial working memory task was also higher in the estradiol group (See also90, 91). These neuroimaging findings were in agreement with behavioral outcomes on a standardized verbal memory test administered outside the scanner; estradiol treatment did not enhance learning or delayed recall but instead decreased the number of executive errors associated with frontal dysfunction (e.g., impulsive errors and errors in updating of working memory). This study therefore suggests that transdermal estradiol enhances prefrontal function during memory tasks in women who were treated during the critical window.
Additional support for the critical window hypothesis came from an fMRI study that examined how early and continued use of HT use affects brain function and memory later in life92. The 34 participants (mean age = 60) came from the Melbourne Women’s Midlife Health Project. Exposure to HT was ascertained using prospectively collected menstrual diary data. All of the active users initiated HT before the final menstrual period. The a priori region of interest was the hippocampus, so the study did not examine prefrontal function. Data demonstrated that early and continued use of HT since the perimenopause was associated later in life with enhanced verbal recognition and enhanced function of the hippocampus and parahippocampal gyrus.
Together these neuroimaging findings are notable in light of data from older female monkeys treated with cyclic estradiol where treatment was associated with enhanced formation of spines in the prefrontal cortex78 and enhanced performance on tasks mediated by prefrontal cortex and medial temporal lobe76. Studies of pharmacological suppression of ovarian hormones with the gonadotropin-releasing hormone (GnRH) agonist analog, leuprolide acetate, provide evidence that loss of estrogen impairs memory and prefrontal function and that add-back estrogen reverses those effects93, 94. Additionally, some studies have found that the volume of the hippocampus is larger in women who use HT95-97 and varies depending on the timing of HT such that the shorter the delay in treatment between menopause and initiation of treatment, the greater the volume.96
Integrated Summary
The critical window hypothesis posits that the effects of HT on cognition depend on the timing of initiation of treatment with respect to age and/or the onset of menopause, and that benefits are limited to early initiation. It is important to recognize that the hypothesis preceded the WHIMS and is not simply an after-the-fact explanation of the findings from WHIMS. In light of the literature reviewed above, then, how does the evidence bear on the hypothesis? In evaluating the evidence it is important to recognize that the large majority of women in observational studies who used HT initiated treatment during the window. Thus, one can draw on the observational studies to draw conclusions.
As reviewed above, observational studies of ever use of HT when analyzed using meta-analytic techniques, reliably show a reduced risk of AD. Also as reviewed above, each of the three observational studies that specifically examined timing of initiation and/or age support the hypothesis in that early use was protective and later use was not or was detrimental19, 28, 42. Neuroimaging studies also provide support for the biological plausibility that early initiation of HT is associated with cognitive benefits, based on findings of enhanced hippocampal and prefrontal function and structure66, 87, 88, 92, 96, 97. Randomized clinical trials of HT and verbal memory provide preliminary evidence of a beneficial effect of estrogen alone on cognitive function in younger postmenopausal women60, 63, 66-68, but larger studies are clearly needed. Similarly, small randomized trials of estradiol valerate in combination with dinogest79 or norethindrone83 provide tentative support for cognitive benefits with those formulations. In contrast, continuous combined CEE/MPA does not appear to confer cognitive benefits and may lead to memory decline regardless of timing52, 81, 82, 85. Of the five observational studies that examined the timing of HT initiation on cognitive test performance, three provided support49, 56, 57, whereas one did not55 and one found a slight negative effect54. Overall then the literature suggests that early use of HT might be beneficial for cognitive function when the regimen is not continuous CEE/MPA.
An alternative view of the available evidence is that it supports the “healthy cell bias.” Whereas the critical window hypothesis posits that chronological and/or reproductive age is the critical determinant of HT effects, the healthy cell bias posits that it is the health of the women that is the critical determinant of HT effects98. Often good health is confounded with younger age or timing in relation to the menopausal transition so both theories predict cognitive benefits from HT in healthy younger postmenopausal women. However, only the healthy cell bias predicts that baseline cognitive function would predict the extent to which cognitive benefits or risks are observed with HT. Specifically, the healthy cell bias predicts that older women who are “healthy” - who perform at or above expected levels on cognitive tests – would benefit from HT even if they are outside the window. Similarly, the healthy cell bias predicts that women whose cognitive performance is poor, regardless of age, would experience the most adverse cognitive effects from HT.
Two findings from WHIMS support the healthy cell bias. First, the degree to which CEE adversely affected cognitive function was greatest among women with the lowest baseline scores on the Modified Mini-Mental State Exam (3MSE)99. Second, a structural neuroimaging study involving 1403 WHIMS participants showed HT-related volume loss in the frontal lobes and hippocampus that was most pronounced among women with low baseline 3MSE scores40. Additionally, a randomized trial of older postmenopausal women treated with cyclical estradiol and norethindrone found enhanced verbal memory in older women whose cognitive performance was at or above expected levels at baseline, but no benefit in women who were performing below expected levels86. Whether certain formulations of HT might benefit certain older women remains unclear, though caution is warranted since WHIMS10 and WHISCA52 highlight cognitive risks associated with CEE/MPA.
A top priority in this area of research is to identify a formulation of combination HT that effectively treats hot flashes but is safe for cognition. This need is underscored by findings from WHIMS10, WHISCA52, HERS85 and COGENT81 showing cognitive risks with continuous combined CEE/MPA. Initial findings from small-n randomized trials in younger postmenopausal women indicate that when combined with estradiol valerate, norethistrone83 and dinogest79 enhance verbal memory. Data from a larger sample of older postmenopausal women showed that when combined with estradiol, cyclic norethindrone had no negative impact on verbal memory overall and even might enhance verbal memory in older women whose cognitive performance was at or above expected levels86. It would be helpful to compare the cognitive effects of these progestins head-to-head. In the future, it is likely that women will have the option of tissue-selective estrogen complexes (TSEC), such as bazedoxifene and CEE, which combine estrogen with a selective estrogen receptor modulator (SERM) rather than a progestin. Other pressing research questions pertain to the effects of low-dose HT in light of the increase use of lower doses of oral preparations. Selective estrogen receptor modulators, particularly raloxifene, have been shown to have promise for reducing the risk for AD. A randomized, placebo-controlled clinical trial of raloxifene in 5,386 women followed for three years demonstrated that 120 mg of reduced the risk of mild cognitive impairment, the preclinical stage of AD, but 60 mg had no effect100.
The literature on the critical window hypothesis of HT and cognition has evolved in parallel with the literature on the critical window hypothesis of HT and cardiovascular disease (for a review see101). A meta-analysis of clinical trials showed that timing of HT significantly impacted the risk of coronary heart disease events; HT significantly reduced the risk by 32% in women who were fewer than 10 years from menopause, but had no impact in women more than 10 years102. A long-term follow-up study of the CEE arm of the WHI showed that after 10.7 years the impact of CEE on coronary heart disease events was more favorable in younger postmenopausal women than in older postmenopausal women103. CEE significantly reduced the risk of coronary heart disease by 41% among women aged 50 to 59 years at initiation of treatment103. Population-based studies with follow-ups of 20 years and longer show that cardiovascular disease risk factors at midlife, including hypertension, insulin resistance, and hypercholesterolemia, are associated with increased risk of Alzheimer’s disease later in life104-108. In this way, early use of HT might reduce risk of Alzheimer’s disease in part by lowering cardiovascular risk factors.
Findings from three randomized, placebo-controlled clinical trials that are in progress or recently completed will provide additional insights into the critical window hypothesis, particularly since their sample sizes in younger women exceed existing studies and the follow-up of each is long. The Kronos Early Estrogen Prevention Study (KEEPS) is a randomized trial of five years of treatment with cyclic micronized progesterone (200 mg for 12 days monthly) in combination with transdermal estradiol (50 microg weekly) or CEE (0.45 mg) on cognitive function and cardiovascular disease in 720 women aged 42 to 58 years within 36 months of their final menstrual period. KEEPS results are expected in 2013. ELITE (Early Versus Late Intervention Trial with Estradiol) is a randomized trial oral estradiol (1 mg/d) plus a vaginal progesterone gel for 10 days per month on cognitive function in 643 younger (< 6 years since menopause) and older postmenopausal women (10 or more years since menopause). ELITE will involve 643 postmenopausal women randomized based on years since menopause (i.e., less than 6 years or 10 years or more) to receive placebo or estradiol plus a vaginal progesterone gel for 10 days per month. The Women’s Health Initiative Memory Study of Younger Women (WHIMS-Y) is an ancillary study to the WHI HT trial. WHIMS-Y assesses the impact of CEE and CEE/MPA on global cognitive function in women who were aged 50-54 at enrollment in WHI. Results are expected in 2013.
Conclusions
Despite emerging evidence in favor of the critical window hypothesis, HT is not indicated for the treatment of cognitive complaints or the prevention of cognitive decline or dementia. The findings reviewed above are most relevant for women considering HT for the treatment of vasomotor symptoms. For hysterectomized women, the literature provides tentative support for beneficial cognitive effects with estrogen alone. Such treatment might be especially important for women who have their ovaries removed before the natural onset of the menopause69. The state of the science is not sufficient to inform women with a uterus of which HT formulation might be cognitively neutral or possibly beneficial. Results from KEEPS will provide important insights into transdermal estradiol and oral combination formulations for these women, and ELITE will provide insights into the cognitive effects of oral estradiol plus a vaginal progestin. Understanding these effects is a top priority in women’s health.
Acknowledgments
Funding/support: This study was supported in part by R01 MH083782-01. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure: P. Maki has received consultant fees from Depomed pharmaceuticals.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy SL, Xu J, Kochanek KD. Deaths: Preliminary Data for 2010. National Vital Statistics Reports. 2012. [PubMed]
- 2.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Archives of Neurology. 2003;60(8):1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.Kung H-S, Hoyert DL, Xu J, Murphy SL. Deaths: Final Data for 2005. CDC National Vital Statistics Report. 2008. [PubMed]
- 4.Moschetti K, Cummings PL, Sorvillo F, Kuo T. Burden of Alzheimer’s disease-related mortality in the United States, 1999-2008. J Am Geriatr Soc. 2012;60(8):1509–14. doi: 10.1111/j.1532-5415.2012.04056.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen K, Launer LJ, Dewey ME, et al. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53(9):1992–7. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 6.Alzheimer’sAssociation . Changing the Trajectory of Alzheimer’s Disease: A National Imperative. Chicago, IL: 2010. [Google Scholar]
- 7.Marder K, Sano M. Estrogen to treat Alzheimer’s disease: too little, too late? So what’s a woman to do? Neurology. 2000;54(11):2035–7. doi: 10.1212/wnl.54.11.2035. [DOI] [PubMed] [Google Scholar]
- 8.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288(2170-2) doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- 9.Shumaker S, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 10.Shumaker S, Legault C, Rapp S, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 11.Heyman A, Wilkinson WE, Stafford JA, Helms MJ, Sigmon AH, Weinberg T. Alzheimer’s disease: a study of epidemiological aspects. Ann Neurol. 1984;15(4):335–41. doi: 10.1002/ana.410150406. [DOI] [PubMed] [Google Scholar]
- 12.Amaducci LA, Fratiglioni L, Rocca WA, et al. Risk factors for clinically diagnosed Alzheimer’s disease: a case-control study of an Italian population. Neurology. 1986;36(7):922–31. doi: 10.1212/wnl.36.7.922. [DOI] [PubMed] [Google Scholar]
- 13.Broe GA, Henderson AS, Creasey H, et al. A case-control study of Alzheimer’s disease in Australia. Neurology. 1990;40(11):1698–707. doi: 10.1212/wnl.40.11.1698. [DOI] [PubMed] [Google Scholar]
- 14.Graves AB, White E, Koepsell TD, et al. A case-control study of Alzheimer’s disease. Annals of Neurology. 1990;28(6):766–74. doi: 10.1002/ana.410280607. [DOI] [PubMed] [Google Scholar]
- 15.Mortel KF, Meyer JS. Lack of postmenopausal estrogen replacement therapy and the risk of dementia. Journal of Neuropsychiatry. 1994;7:334–7. doi: 10.1176/jnp.7.3.334. [DOI] [PubMed] [Google Scholar]
- 16.Baldereschi M, Di Carlo A, Lepore V, et al. Estrogen-replacement therapy and Alzheimer’s disease in the Italian Longitudinal Study on Aging. Neurology. 1998;50(4):996–1002. doi: 10.1212/wnl.50.4.996. [DOI] [PubMed] [Google Scholar]
- 17.Harwood DG, Barker WW, Loewenstein DA, et al. A cross-ethnic analysis of risk factors for AD in white Hispanics and white non-Hispanics. Neurology. 1999;52(3):551–6. doi: 10.1212/wnl.52.3.551. [DOI] [PubMed] [Google Scholar]
- 18.Slooter AJ, Bronzova J, Witteman JC, Van Broeckhoven C, Hofman A, van Duijn CM. Estrogen use and early onset Alzheimer’s disease: a population-based study. J Neurol Neurosurg Psychiatry. 1999;67(6):779–81. doi: 10.1136/jnnp.67.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76(1):103–5. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner DE, Kukull WA, Stergachis A, et al. Postmenopausal estrogen replacement therapy and the risk of Alzheimer’s Disease: A population-based case-control study. American Journal of Epidemiology. 1994;140(3):262–7. doi: 10.1093/oxfordjournals.aje.a117245. [DOI] [PubMed] [Google Scholar]
- 21.Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women. Comparisons between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol. 1994;51(9):896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- 22.Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996;156(19):2213–7. [PubMed] [Google Scholar]
- 23.Waring SC, Rocca WA, Petersen RC, O’Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology. 1999;52(5):965–70. doi: 10.1212/wnl.52.5.965. [DOI] [PubMed] [Google Scholar]
- 24.Seshadri S, Zornberg GL, Derby LE, Myers MW, Jick H, Drachman DA. Postmenopausal estrogen replacement therapy and the risk of Alzheimer disease. Arch Neurol. 2001;58(3):435–40. doi: 10.1001/archneur.58.3.435. [DOI] [PubMed] [Google Scholar]
- 25.Tang MX, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348(9025):429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 26.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48(6):1517–21. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 27.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288(17):2123–9. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 28.Shao H, Breitner JC, Whitmer RA, et al. Hormone therapy and Alzheimer disease dementia: New findings from the Cache County Study. Neurology. 2012;79(18):1846–52. doi: 10.1212/WNL.0b013e318271f823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279(9):688–95. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 30.Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101(3):485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- 31.LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. Jama. 2001;285(11):1489–99. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- 32.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–81. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 33.Wang PN, Liao SQ, Liu RS, et al. Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology. 2000;54(11):2061–6. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- 34.Mulnard RA, Cotman CW, Kawas C, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA. 2000;283(8):1007–15. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 35.Henderson VW, Paganini-Hill A, Miller BL, et al. Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54(2):295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 36.Marder K, Tang MX, Alfaro B, et al. Postmenopausal estrogen use and Parkinson’s disease with and without dementia. Neurology. 1998;50(4):1141–3. doi: 10.1212/wnl.50.4.1141. [DOI] [PubMed] [Google Scholar]
- 37.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277(10):813–7. [PubMed] [Google Scholar]
- 38.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. Jama. 2003;289(20):2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 39.Coker LH, Hogan PE, Bryan NR, et al. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology. 2009;72(2):125–34. doi: 10.1212/01.wnl.0000339036.88842.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resnick SM, Espeland MA, Jaramillo SA, et al. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72(2):135–42. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espeland MA, Tindle HA, Bushnell CA, et al. Brain volumes, cognitive impairment, and conjugated equine estrogens. J Gerontol A Biol Sci Med Sci. 2009;64(12):1243–50. doi: 10.1093/gerona/glp128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69(1):163–9. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yesufu A, Bandelow S, Hogervorst E. Meta-analyses of the effect of hormone treatment on cognitive function in postmenopausal women. Womens Health (Lond Engl) 2007;3(2):173–94. doi: 10.2217/17455057.3.2.173. [DOI] [PubMed] [Google Scholar]
- 44.Linn RT, Wolf PA, Bachman DL, et al. The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52(5):485–90. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 45.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64(11):1853–9. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 46.Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64(6):862–71. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 47.Kramer J, Delis D, Daniel M. Sex differences in verbal learning. Journal of Clinical Psychology. 1988;44:907–15. [Google Scholar]
- 48.Kramer JH, Delis DC, Kaplan E, O’Donnell L, Prifitera A. Developmental sex differences in verbal learning. Neuropsychology. 1997;11(4):577–84. doi: 10.1037//0894-4105.11.4.577. [DOI] [PubMed] [Google Scholar]
- 49.Greendale GA, Huang MH, Wight RG, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72(21):1850–7. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage. Menopause. 2013 doi: 10.1097/GME.0b013e31827655e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women’s Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials. 2004;1(5):440–50. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 52.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91(5):1802–10. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 53.Resnick SM, Espeland MA, An Y, et al. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab. 2009;94(11):4152–61. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang JH, Weuve J, Grodstein F. Postmenopausal hormone therapy and risk of cognitive decline in community-dwelling aging women. Neurology. 2004;63(1):101–7. doi: 10.1212/01.wnl.0000132522.13574.67. [DOI] [PubMed] [Google Scholar]
- 55.Ryan J, Carriere I, Scali J, et al. Characteristics of hormone therapy, cognitive function, and dementia: the prospective 3C Study. Neurology. 2009;73(21):1729–37. doi: 10.1212/WNL.0b013e3181c34b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khoo SK, O’Neill S, Byrne G, King R, Travers C, Tripcony L. Postmenopausal hormone therapy and cognition: effects of timing and treatment type. Climacteric. 2010;13(3):259–64. doi: 10.3109/13697130903370316. [DOI] [PubMed] [Google Scholar]
- 57.MacLennan AH, Henderson VW, Paine BJ, et al. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13(1):28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- 58.Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann N Y Acad Sci. 2005;1052:182–97. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 59.Maki PM, Dumas J. Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med. 2009;27(3):250–9. doi: 10.1055/s-0029-1216278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hackman BW, Galbraith D. Replacement therapy and piperazine oestrone sulphate (‘Harmogen’) and its effect on memory. Curr Med Res Opin. 1976;4(4):303–6. doi: 10.1185/03007997609109322. [DOI] [PubMed] [Google Scholar]
- 61.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–57. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 62.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 63.Shaywitz SE, Naftolin F, Zelterman D, et al. Better oral reading and short-term memory in midlife, postmenopausal women taking estrogen. Menopause. 2003;10(5):420–6. doi: 10.1097/01.GME.0000060241.02837.29. [DOI] [PubMed] [Google Scholar]
- 64.Dunkin J, Rasgon N, Wagner-Steh K, David S, Altshuler L, Rapkin A. Reproductive events modify the effects of estrogen replacement therapy on cognition in healthy postmenopausal women. Psychoneuroendocrinology. 2005;30(3):284–96. doi: 10.1016/j.psyneuen.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 65.LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS. Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause. 2007;14(2):191–202. doi: 10.1097/01.gme.0000230347.28616.1c. [DOI] [PubMed] [Google Scholar]
- 66.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–22. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 67.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13(4):345–57. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 68.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17(5):485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 69.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 70.Wolf OT, Kudielka BM, Hellhammer DH, Torber S, McEwen BS, Kirschbaum C. Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology. 1999;24(7):727–41. doi: 10.1016/s0306-4530(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 71.Yaffe K, Vittinghoff E, Ensrud KE, et al. Effects of ultra-low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol. 2006;63(7):945–50. doi: 10.1001/archneur.63.7.945. [DOI] [PubMed] [Google Scholar]
- 72.Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition and quality of life. Neurobiol Aging. 2006;27(1):141–9. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Pefanco MA, Kenny AM, Kaplan RF, et al. The effect of 3-year treatment with 0.25 mg/day of micronized 17beta-estradiol on cognitive function in older postmenopausal women. J Am Geriatr Soc. 2007;55(3):426–31. doi: 10.1111/j.1532-5415.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 74.Schiff R, Bulpitt CJ, Wesnes KA, Rajkumar C. Short-term transdermal estradiol therapy, cognition and depressive symptoms in healthy older women. A randomised placebo controlled pilot cross-over study. Psychoneuroendocrinology. 2005;30(4):309–15. doi: 10.1016/j.psyneuen.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Toran-Allerand CD. Reply to ‘Hormone in the hot seat’. Nat Med. 2006;12(4):379–80. doi: 10.1038/nm0406-379b. [DOI] [PubMed] [Google Scholar]
- 76.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23(13):5708–14. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohm DT, Bloss EB, Janssen WG, et al. Clinically Relevant Hormone Treatments Fail to Induce Spinogenesis in Prefrontal Cortex of Aged Female Rhesus Monkeys. J Neurosci. 2012;32(34):11700–5. doi: 10.1523/JNEUROSCI.1881-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Y, Janssen WG, Hao J, et al. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14(2):215–23. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- 79.Linzmayer L, Semlitsch HV, Saletu B, et al. Double-blind, placebo-controlled psychometric studies on the effects of a combined estrogen-progestin regimen versus estrogen alone on performance, mood and personality of menopausal syndrome patients. Arzneimittelforschung. 2001;51(3):238–45. doi: 10.1055/s-0031-1300030. [DOI] [PubMed] [Google Scholar]
- 80.Wolf OT, Heinrich AB, Hanstein B, Kirschbaum C. Estradiol or estradiol/progesterone treatment in older women: no strong effects on cognition. Neurobiol Aging. 2005;26(7):1029–33. doi: 10.1016/j.neurobiolaging.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology. 2007;69(13):1322–30. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- 82.Maki PM, Rubin LH, Fornelli D, et al. Effects of botanicals and combined hormone therapy on cognition in postmenopausal women. Menopause. 2009 doi: 10.1097/gme.0b013e3181ace484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alhola P, Tuomisto H, Saarinen R, Portin R, Kalleinen N, Polo-Kantola P. Estrogen + progestin therapy and cognition: a randomized placebo-controlled double-blind study. J Obstet Gynaecol Res. 2010;36(4):796–802. doi: 10.1111/j.1447-0756.2010.01214.x. [DOI] [PubMed] [Google Scholar]
- 84.Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM. Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas. 2001;38(2):137–46. doi: 10.1016/s0378-5122(00)00214-0. [DOI] [PubMed] [Google Scholar]
- 85.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. American Journal of Medicine. 2002;113:543–8. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- 86.Tierney MC, Oh P, Moineddin R, et al. A randomized double-blind trial of the effects of hormone therapy on delayed verbal recall in older women. Psychoneuroendocrinology. 2009;34(7):1065–74. doi: 10.1016/j.psyneuen.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 87.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34(2):171–82. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- 88.Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21(2):373–83. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 89.Mosconi L, Tsui WH, De Santi S, et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology. 2005;64(11):1860–7. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- 90.Shaywitz SE, Shaywitz BA, Pugh KR, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281(13):1197–202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 91.Berent-Spillson A, Persad CC, Love T, et al. Early menopausal hormone use influences brain regions used for visual working memory. Menopause. 2010;17(4):692–9. doi: 10.1097/gme.0b013e3181cc49e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maki PM, Dennerstein L, Clark M, et al. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–43. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Craig MC, Fletcher PC, Daly EM, et al. Gonadotropin hormone releasing hormone agonists alter prefrontal function during verbal encoding in young women. Psychoneuroendocrinology. 2007;32(8-10):1116–27. doi: 10.1016/j.psyneuen.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 94.Craig MC, Fletcher PC, Daly EM, et al. Reversibility of the effects of acute ovarian hormone suppression on verbal memory and prefrontal function in pre-menopausal women. Psychoneuroendocrinology. 2008;33(10):1426–31. doi: 10.1016/j.psyneuen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 95.Eberling JL, Wu C, Haan MN, Mungas D, Buonocore M, Jagust WJ. Preliminary evidence that estrogen protects against age-related hippocampal atrophy. Neurobiol Aging. 2003;24(5):725–32. doi: 10.1016/s0197-4580(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 96.Erickson KI, Voss MW, Prakash RS, Chaddock L, Kramer AF. A cross-sectional study of hormone treatment and hippocampal volume in postmenopausal women: evidence for a limited window of opportunity. Neuropsychology. 2010;24(1):68–76. doi: 10.1037/a0017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol Aging. 2008;29(1):95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 98.Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- 99.Espeland M, Rapp S, Shumaker S, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 100.Yaffe K, Krueger K, Cummings SR, et al. Effect of raloxifene on prevention of dementia and cognitive impairment in older women: the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. Am J Psychiatry. 2005;162(4):683–90. doi: 10.1176/appi.ajp.162.4.683. [DOI] [PubMed] [Google Scholar]
- 101.Clarkson TB, Melendez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20(3):342–53. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- 102.Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med. 2006;21(4):363–6. doi: 10.1111/j.1525-1497.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137(3):149–55. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 105.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 106.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 107.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]