Abstract
Objective
In 2001 a randomized trial showed decreased mortality with early, goal-directed therapy in septic shock, a strategy later recommended by the Surviving Sepsis Campaign. Placement of a central venous catheter (CVC) is necessary to administer goal-directed therapy. We sought to evaluate nationwide trends in: 1) CVC utilization and 2) the association between early CVC insertion and mortality in patients with septic shock.
Design
We retrospectively analyzed the proportion of septic shock cases receiving an early (day of admission) CVC and the odds of hospital mortality associated with receiving early CVC from years 1998-2001 compared with 2002-2009.
Setting
Non-federal acute care hospitalizations from the Nationwide Inpatient Sample, 1998-2009.
Interventions
None
Patients
203,481 (population estimate: 999,545) cases admitted through an emergency department with principal diagnosis of septicemia and secondary diagnosis of shock.
Measurements and Main Results
From 1998-2009 population-adjusted rates of septic shock increased from 12.6 cases per 100,000 US adults to 78 cases per 100,000. During this time age-adjusted hospital mortality associated with septic shock declined from 40.4% to 31.4%. Early CVC insertion increased from 5.7% (95% CI 5.1-6.3%) to 19.2% (95% CI 18.7-19.5%) cases with septic shock, with an increased rate of early CVC placement identified after 2007. The rate of decline in age-adjusted hospital mortality was significantly greater for patients who received an early CVC (-4.2% per year, 95% CI -3.2, -4.2%) as compared with no CVC (-2.9% per year, 95% CI -2.3, -3.5%), p=0.016. Hospital mortality associated with early CVC insertion significantly decreased from a multivariable-adjusted odds ratio of 1.29 (95% CI 1.14-1.45) prior to 2001 to an adjusted odds ratio of 0.87 (95% CI 0.84-0.90) after 2001.
Conclusions
Placement of a CVC early in septic shock has increased 3-fold since 1998. The mortality associated with early CVC insertion decreased after publication of evidence-based instructions for CVC use.
Introduction
Population-based studies demonstrate that the case-fatality rate associated with severe sepsis has declined steadily during the past decade.(1-3) Multiple explanations for this change have been posited, including implementation of Surviving Sepsis Campaign guidelines,(4-7) reduction in nosocomial complications (e.g., venous thromboembolism, stress ulcer bleeding, ventilator-associated pneumonia),(8-11) or pseudo-improvements [e.g., changing International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding practices,(2,3) or earlier discharge of patients to long-term care hospitals].(3,12)
Based on the results of a randomized controlled trial by Rivers et al.,(13) the Surviving Sepsis Campaign Guidelines recommend early (i.e., within 6 hours) resuscitation in severe sepsis and septic shock.. Because measurement of CVP and central venous oxygen saturation requires insertion of a central venous catheter (CVC), guideline-recommended, early, goal-directed therapy cannot be implemented accurately without a CVC. Thus, study of the CVC in septic shock presents a unique opportunity to evaluate the evolution of utilization patterns and patient outcomes associated with a medical intervention before and after release of supporting evidence and guidelines.
Whereas Rivers et al. demonstrated lower mortality with use of early goal-directed therapy guided by measurements from a CVC, how widely CVCs are used and outcomes associated with CVC use in typical practice in the United States remains unknown. We evaluated nationwide trends in CVC placement, and compared mortality associated with early placement of a CVC for patients with septic shock in the 4 years before and 7 years after the publication of the Rivers trial. We hypothesized that early insertion of a CVC in septic shock has increased since publication of the Surviving Sepsis Campaign. Further, we hypothesized that in-hospital mortality associated with early placement of a CVC in septic shock has decreased after evidence-based therapeutic goals based on CVC measurements became widely available in 2001.(13)
Methods
Data Source
We examined hospitalizations from adults (age ≥18 years) using year 1998-2009 discharge data from the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.(14) The NIS is an approximate 20% stratified probability sample of all non-Federal acute care hospitals and contains de-identified clinical and resource use information from approximately 5-8 million hospital discharges yearly. NIS sampling strata are based on five hospital characteristics: ownership/control, teaching status, urban/rural location, US region and bed size. The 1998 NIS contained data from about 600 hospitals in 22 states and the 2009 NIS included data from approximately 1000 hospitals in 44 states. NIS elements include demographics, admission and discharge status, length of stay, up to 15 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes (increased to 25 diagnosis codes in 2009), and hospital characteristics. Study procedures were approved by the Boston University Medical Campus Institutional Review Board.
Septic Shock Definition
Inclusion criteria for the trial of early goal-directed therapy(13) upon which Surviving Sepsis Campaign Guidelines were based included patients admitted through the emergency department with suspected severe sepsis or septic shock characterized by the systemic inflammatory response syndrome,(15) and the presence of hypotension or an elevated blood lactate. Because blood lactate levels are unavailable in the NIS database, we included patients admitted through the emergency department with a principal diagnosis of sepsis or septicemia [previously validated ICD-9-CM codes septicemia (038.×), sepsis and severe sepsis (995.91 and 995.92, introduced 10/1/2002), septic shock (785.52, introduced 10/1/2003))(1-3,16-19) and a secondary diagnosis of shock or hypotension (ICD-9-CM codes 458.0, 458.8, 458.9, 785.5, 785.51, 785.52, 785.59, 796.3).(1-3) ICD-9-CM codes for sepsis, severe sepsis, and septic shock introduced in 2002 and 2003 require additional codes describing the infection, and in NIS data greater than 99% of cases with a code of 995.91, 995.92 or 785.52 also had a 038 code. Analysis of California State Inpatient data that include a “present on admission” modifier to diagnosis codes demonstrates that the sepsis diagnosis was coded as “present on admission” in 99% of patients with a principal diagnosis of sepsis or septicemia according to our algorithm.
Central venous catheter definition and ICD-9-CM validation
CVC placement was identified by ICD-9-CM procedure code 38.93. The resolution of procedure timing within the NIS is one day, thus “early” CVC placement was defined by placement of the CVC on the day of admission (Day 0). “Late” CVCs were defined as those placed after the day of admission. We validated the CVC ICD-9-CM code 38.93 code by retrospective chart review of 92 patients admitted to Boston Medical Center with septic shock ICD-9-CM codes in year 2009 and found 61% (95% CI 50-72%) sensitivity and 92% (95% CI 61-100%) specificity. Prior validation of ICD-9-CM 38.93 during 1996-1997 showed 40% sensitivity and 99% specificity.(20)
Covariates
Demographic data collected in the NIS included age, sex, race and ethnicity (coded as white, black, Hispanic or other). Hospital characteristics included United States Census regions, teaching status, urban or rural location, and bed size.(14) We selected comorbid conditions through enhanced Charlson and Elixhauser ICD-9-CM codes(21) for heart failure, myocardial infarction, obesity, diabetes mellitus, chronic obstructive pulmonary disease, dementia, human immunodeficiency virus infection, paralysis, chronic liver disease, chronic kidney disease, and metastatic or hematologic malignancy. Acute factors were assessed through ICD-9-CM codes for number and type of acute organ dysfunction diagnoses,(16, 22) electrolyte abnormalities, critical care procedures (arterial catheterization, pulmonary artery catheterization, dialysis, mechanical ventilation), infectious pathogen type (gram positive vs. gram negative bacteria vs. fungal vs. none reported), infection source and hospital strata characteristics. We estimated temporal trends in severity of illness through changes in the number of comorbid conditions, acute organ failures, and use of mechanical ventilation or dialysis. ICD-9-CM coding strategies are shown in Supplemental Digital Content Table 1; we restricted analyses to the first 15 ICD-9-CM diagnoses to decrease bias from the increasing number of available coding positions on the discharge record during the study period.
Statistical Analyses
The primary outcome measure was the proportion of septic shock discharges receiving an early CVC each year. We derived national estimates from the NIS using hospital weights with SAS version 9.1.3 (Cary, NC) surveyfreq, surveymeans and surveylogistic procedures. We performed multivariable logistic regression models with forward stepwise selection to determine factors associated with early CVC insertion during septic shock and used survey-weighted logistic regression to calculate effect estimates and standard errors for factors identified in conventional stepwise regression analysis. Annual percent change (APC) in placement of CVC, pulmonary artery, and peripheral arterial catheters was calculated with Joinpoint version 3.5.2 (Statistical Research and Applications Branch, National Cancer Institute, Bethesda, Maryland). To determine if the association between CVC status and mortality was modified by year, we included a CVC status by year interaction term. Because of immortal time bias(23) associated with late CVC insertion we excluded patients with late or unknown CVC timing from outcome analyses. We used the glimmix procedure to perform multivariable-adjusted logistic regression in a single model to calculate odds ratios (OR) each year for hospital mortality associated with early CVC as compared with no CVC. Primary outcome models were adjusted for patient demographics, comorbid conditions, acute organ failures, procedures, infection site, pathogen, and hospital characteristics (Supplemental Digital Content Table 2). Patients with missing data (0.5% of cases) were excluded from our analyses. Because acute organ failures may occur after CVC placement and lie on the causal pathway to mortality, we analyzed secondary models including only demographics, comorbid conditions and hospital characteristics. Finally, we compared early CVC-associated mortality prior to (1998-2001) and after (2002-2009) publication of the Rivers et al. study that demonstrated benefit to early, goal-directed therapy in severe sepsis and septic shock. A two-sided alpha level of 0.05 was selected for statistical significance.
Sensitivity analyses
Because patients receiving mechanical ventilation may receive a CVC for reasons other than resuscitation (e.g., administration of continuous sedative medications) and mechanical ventilation is a major prognostic determinant in severe sepsis,(3) we performed a sensitivity analysis stratifying by use of mechanical ventilation (ICD-9-CM 96.7). To eliminate confounding from the changing number of states contributing to NIS over time, we performed a sensitivity analysis including only the 20 states that contributed data each year from 1998-2009 (California, Colorado, Florida, Iowa, Illinois, Massachusetts, New Jersey, Washington, Wisconsin, Connecticut, Kansas, Maryland, New York, Oregon, South Carolina, Missouri, Tennessee, Georgia, Hawaii, and Utah). To examine whether trends in CVC insertion rates and associated outcomes were general trends associated with vascular access procedures in patients with septic shock, or are more specific to the CVC, we analyzed placement and outcome trends associated with the arterial catheter (ICD-9-CM 38.91) and pulmonary artery catheter (ICD-9-CM 89.63, 89.64, 89.66, 89.67, 89.68)(24) Finally, to address whether increasing early discharge of patients to other hospitals might confound the association of early CVC placement and hospital mortality, we performed an analysis that excluded patients discharged to another facility (e.g., long term or acute care hospitals).
Results
Septic shock epidemiology, 1998-2009
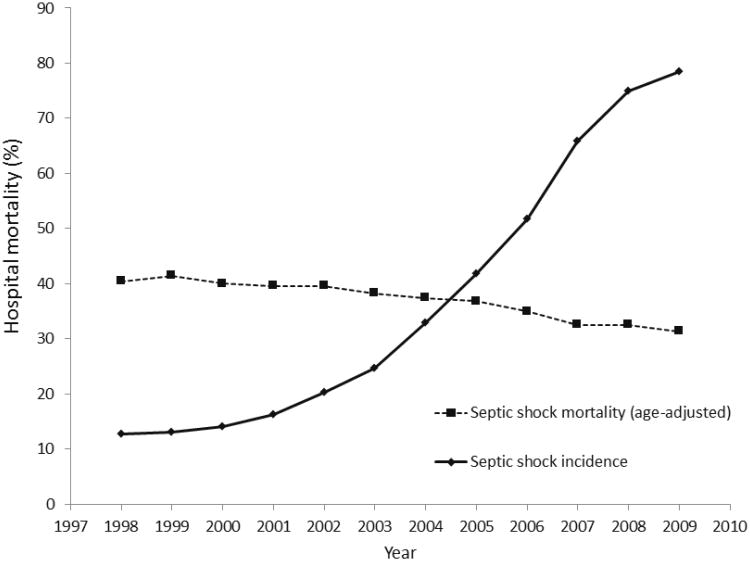
We identified 203,481 hospitalizations with septic shock representing approximately 1 million weighted discharges from years 1998-2009 (Supplemental Digital Content Figure). Patients with septic shock were an average 69 years of age [95% confidence interval (CI) 69.2-69.4]; 52% were women, with a racial/ethnic composition of 56% white, 11% black, 8% Hispanic and 25% other or unspecified race. Population-adjusted rates of septic shock increased from 12.6 cases per 100,000 US residents in 1998 to 78 cases per 100,000 in 2009. Despite evidence for increasing disease severity over time (Supplemental Digital Content Table 2), age-adjusted hospital mortality associated with septic shock hospitalization declined from 40.4% in 1998 to 31.4% in 2009 (Figure 1). Joinpoint regression models identified that septic shock-associated hospital mortality decreased more rapidly after 2004 [APC: -4% (95% CI -5 to -3%)] as compared with prior to 2004 [APC: -1.7% (95% CI -2.9 to -0.3%)], p=0.01.
Figure 1.
Trends in septic shock incidence and septic shock hospital mortality, 1998-2009.
Factors associated with early use of the central venous catheter
Characteristics of patients according to timing of CVC insertion are shown in Supplemental Digital Content Table 3. Multivariable-adjusted predictors of early CVC placement, as compared with no CVC, are shown in Table 1. Patients who received an early CVC were more likely to be younger, female, black race, with a greater number of acute organ failures, and to have Medicare as payer. Patients discharged from large, urban, or teaching hospitals were also more likely to receive an early CVC. Patients with metastatic malignancy, dementia, chronic kidney disease, chronic liver disease, and patients hospitalized in the Midwest region were less likely to receive an early CVC.
Table 1.
Factors associated with early placement of CVC (CVC) in patients with septic shock.
| Variable | Multivariable-adjusted predictors Early CVC vs. No CVC Odds Ratio (95% CI) |
|---|---|
| Age, per 1 year | 0.99 (0.99-0.99) |
| Sex, female | 1.12 (1.08-1.16) |
| Race/ethnicity | |
| White | Ref |
| Black | 1.08 (1.02-1.15) |
| Hispanic | 0.78 (0.73-0.83) |
| Other/Unspecified | 0.61 (0.58-0.64) |
| Per Comorbidity | 1.11 (1.08-1.14) |
| Paralysis | 1.36 (1.24-1.50) |
| Dementia | 0.79 (0.74-0.84) |
| Chronic liver disease | 0.90 (0.83-0.98) |
| Chronic kidney disease | 0.89 (0.84-0.94) |
| COPD | 0.95 (0.90-0.99) |
| Metastatic or hematologic malignanc | y 0.74 (0.70-0.79) |
| Per acute organ failure | 1.31 (1.28-1.34) |
| Hematologic failure | 0.94 (0.90-0.99) |
| Neurologic failure | 0.84 (0.80-0.90) |
| Hepatic failure | 0.86 (0.80-0.93) |
| Electrolyte abnormality | 1.10 (1.06-1.14) |
| Critical care interventions | |
| Arterial catheter | 3.45 (3.23-3.66) |
| Mechanical ventilation | 1.73 (1.66-1.81) |
| Dialysis | 0.76 (0.71-0.81) |
| Right heart catheterization | 0.69 (0.61-0.78) |
| Source of infection | |
| Respiratory | 1.08 (1.05-1.12) |
| Abdominal | 1.11 (1.05-1.17) |
| Skin or soft tissue | 1.19 (1.11-1.27) |
| Pathogen type | |
| Gram positive | REF |
| Gram negative | 0.87 (0.82-0.92) |
| None reported | 0.86 (0.82-0.90) |
| US Geographic Region | |
| Northeast | REF |
| Midwest | 0.68 (0.64-0.73) |
| South | 1.00 (0.95-1.05) |
| West | 1.17 (1.12-1.22) |
| Hospital Bed size | |
| Small | REF |
| Medium | 1.03 (0.97-1.09) |
| Large | 1.09 (1.03-1.16) |
| Hospital Location - Urban | 1.26 (1.18-1.35) |
| Teaching Hospital | 1.06 (1.02-1.10) |
| Payer | |
| Medicare | REF |
| Medicaid | 0.96 (0.90-1.02) |
| Private insurance | 0.90 (0.85-0.94) |
| Self-pay | 0.98 (0.88-1.09) |
| Other/No charge | 1.05 (0.93-1.18) |
| Per Year | 1.15 (1.14-1.15) |
‘Early’ CVC was placed on the day of admission, c-statistic 0.742
Trends in catheter placement
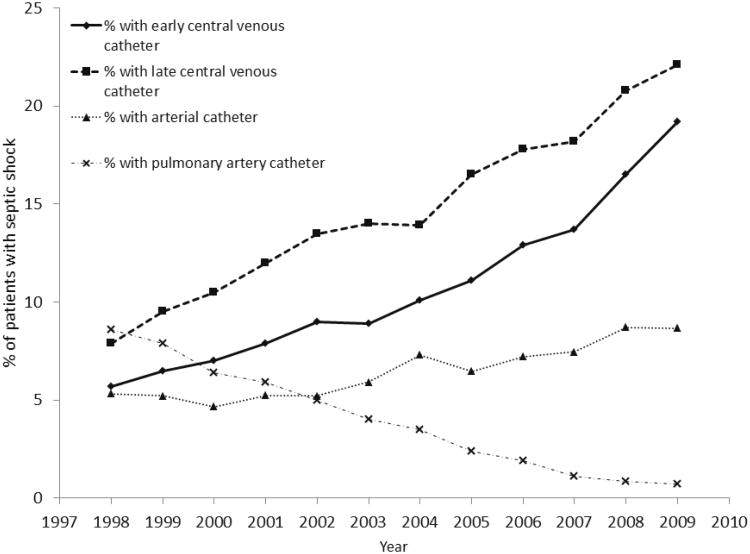
From 1998 to 2009, early CVC insertion increased from 5.7% (95% CI 5.1-6.3%) to 19.2% (95% CI 18.7-19.5%) of patients with septic shock (Figure 2). Jointpoint regression identified 2 distinct trends for early CVC placement: from 1998-2007 the APC in CVC use was 10.2% (95% CI 8.8-11.7%) and from 2007-2009 the APC increased to 17.8% (95% CI 12.4-23.4%). No change in trend was identified for late CVC use [APC 8.7% (95% CI 7.6-9.5%)]. The decline in placement of pulmonary artery catheters accelerated after 2004 [APC prior to 2004, -14.5% (95% CI -174 to -11.5); APC after 2004, -29% (-32.7 to -25.2)] Use of peripheral arterial catheters increased at a constant APC of 6% (95% CI 4.5-7.7%). Compared with patients who received an early CVC in 1998, patients who received an early CVC in 2009 were younger, more likely to be male, had more comorbidities and more acute organ failures (Supplemental Digital Content Table 4). Sensitivity analyses did not yield appreciably different results (Supplemental Digital Content Table 5).
Figure 2.
Trends in utilization of central venous catheter (CVC), pulmonary artery catheter, and peripheral arterial catheter, 1998-2009.
Trends in catheter-associated mortality
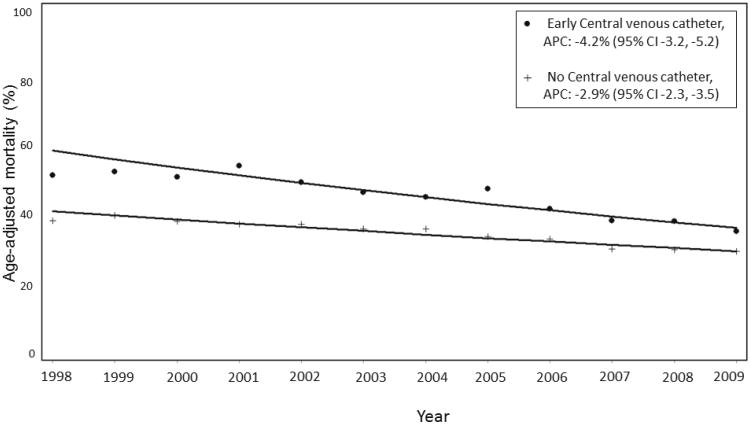
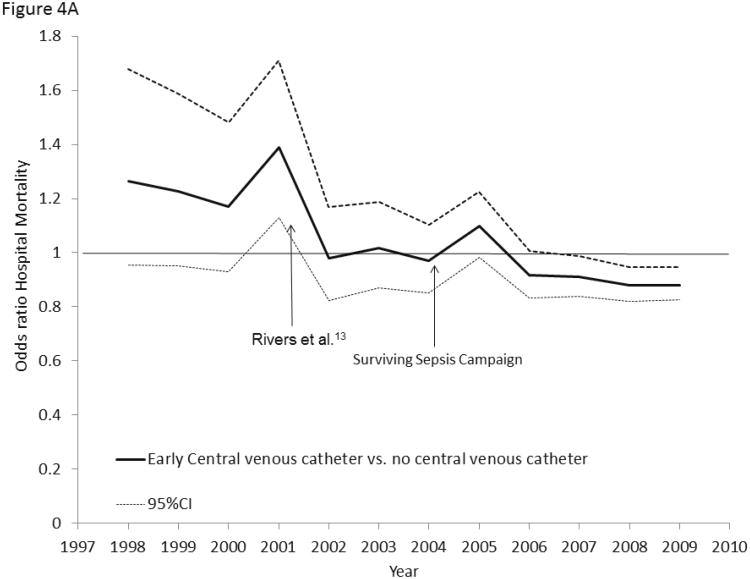
Receipt of early CVC was associated with a greater annual decline (APC -4.2%, 95% CI -3.2, -4.2%) in age-adjusted septic shock mortality than no CVC (APC -2.9, 95% CI -2.3, -3.5%), p=0.016 (Figure 3). Hospital mortality associated with early use of a CVC significantly decreased from a multivariable-adjusted odds ratio (OR) of 1.27 (95% CI 0.96-1.68) in 1998 to an OR of 0.88 (95% CI 0.83-0.95) in 2009 (P <0.001, Figure 4A). Hospital mortality associated with early CVC insertion was significantly lower after the 2001 publication of Rivers et al. [OR 0.87 (95% CI 0.84-0.90)] as compared with year 2001 or prior [OR 1.29 (95% CI 1.14-1.45)] with no significant trend in hospital mortality associated with receipt of an early CVC from 1998-2001 [APC 1.5% (-3.6, 6.9, p=0.34); OR for mortality in 1998 1.27 (0.95-1.68), OR for mortality in 2001: 1.39 (1.13.1.71), pinteraction =0.58].
Figure 3.
Comparison of age-adjusted hospital mortality trends in patients with septic shock who received early central venous catheter compared with no central venous catheter.
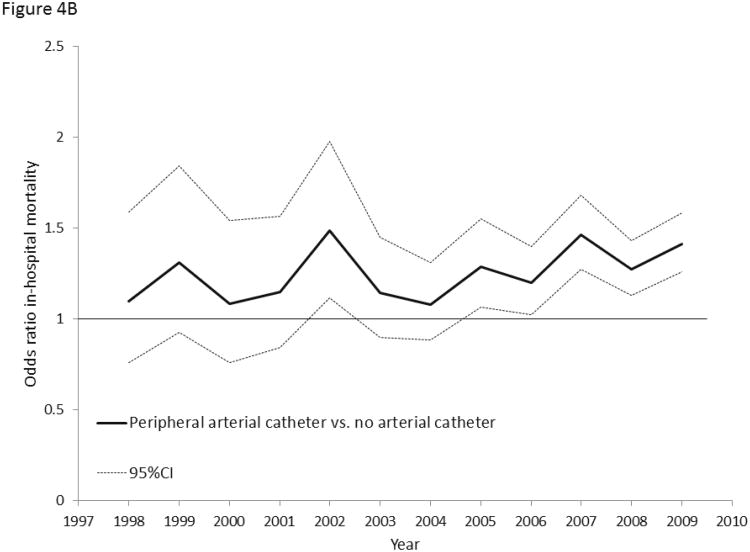
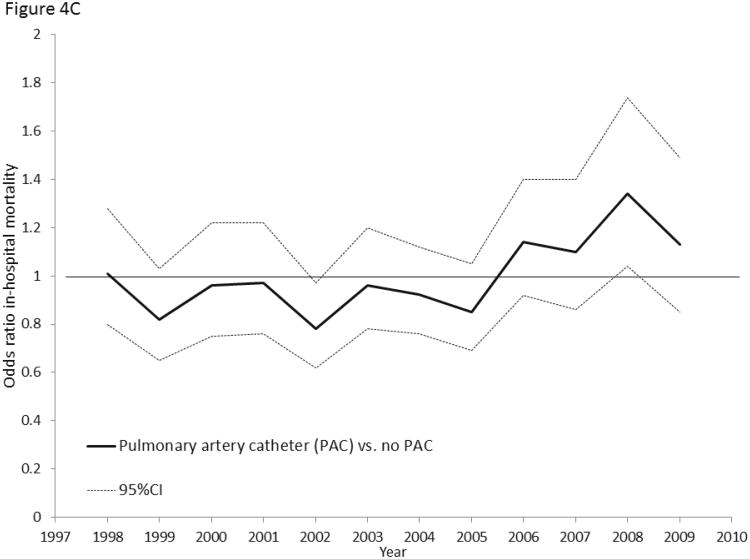
Figure 4.
4A: Multivariable-adjusted odds ratios for hospital mortality associated with early central venous catheter utilization (vs. no central venous catheter) in septic shock, 1998-2009. 4B: Multivariable-adjusted odds ratios for hospital mortality associated with early peripheral arterial catheter utilization (vs. no arterial catheter) in septic shock, 1998-2009. 4C: Multivariable-adjusted odds ratios for hospital mortality associated with pulmonary artery catheter utilization (vs. no pulmonary artery catheter) in septic shock, 1998-2009.
In contrast, hospital mortality associated with use of an arterial catheter (Pinteraction = 0.22, Figure 4B) or pulmonary artery catheter (Pinteraction=0.64, Figure 4C) did not change from 1998 to 2009. Sensitivity analyses showed similar trends in early CVC-associated mortality (Supplemental Digital Content Table 5).
Discussion
We performed a population-based assessment of CVC utilization trends and outcomes in patients with septic shock. A CVC facilitates delivery of medications and allows for measurement of CVP and central venous oxygen saturation. However, as shown by prior studies that evaluated the pulmonary artery catheter in the critically ill,(25-27) interventions that yield clinical data without evidence-based instructions for how to act upon the data may not improve outcomes. Similar to the lack of outcome benefit associated with use of the pulmonary artery catheter, we found that use of a CVC during early septic shock was not associated with improved adjusted mortality prior to publication of the Rivers et al. study (OR 1.29). However, after publication of the Rivers et al. study and release of the Surviving Sepsis Campaign guidelines, early CVC placement increased 3-fold and early CVC-associated adjusted mortality declined (OR 0.87).
We are not aware of other studies that have investigated population-based trends in septic shock CVC practice patterns. However, studies by Levy et al.(4) and Ferrer et al.(6) evaluated changing physician behavior in response to severe sepsis quality improvement interventions across multiple centers. Levy et al. found that 26% of patients reached CVP measurement goals prior to starting the Surviving Sepsis Campaign quality improvement initiative and 38% of patients reached CVP targets after the initiative. Ferrer et al. studied patients admitted to hospitals participating in Spain's Edusepsis improvement campaign from 2005-2007 and reported that only 21% of patients reached CVP goals at baseline and 26% reached CVP targets after the quality improvement intervention. Both studies showed that the quality improvement initiatives were associated with a reduction in mortality. Our finding that 19% of septic shock patients received an early CVC in 2009 is not directly comparable to the proportion of patients reaching CVP goals recorded in studies by Levy et al. and Ferrer et al. The NIS does not include data that would allow ascertainment of CVP resuscitation targets, and neither Levy et al. nor Ferrer et al. recorded the proportion of patients who received a CVC. Reade et al. surveyed emergency and critical care physician practice patterns during severe sepsis in 2007 and found that 71% of respondents stated they would place a CVC in a patient with septic shock and 44% would aim for CVP goal of 8-12mmHg.(28) However, survey responses variably correlate with actual physician practice.(29-31) These results highlight a limitation of our study - whereas early CVC placement is necessary to attempt early goal directed therapy, placement of a CVC is not sufficient to ensure that it is delivered effectively.
We identified several factors associated with early CVC placement in patients with septic shock. Patients receiving an early CVC were younger, and more likely to have a greater number of acute organ failures. However, certain comorbid conditions such as dementia, metastatic cancer and chronic liver, kidney or lung diseases were associated with a lower likelihood of receiving an early CVC. Many factors might explain these findings, including decisions to limit the invasiveness of care, or pre-existing central venous access (e.g. hemodialysis catheters or tunneled lines for chemotherapy) mitigating the need for a new CVC. Acute hematological or hepatic failures were associated with a decreased likelihood of receiving an early CVC; these conditions are often associated with coagulopathy that may complicate timely catheter insertion. Our findings of differences in CVC utilization patterns according to hospital size, teaching status, and region were similar to prior studies in heart failure (32) and myocardial infarction (33,34) demonstrating that larger, academic hospitals (35) may be more apt to adopt guidelines or quality improvement initiatives.(36)
Our study has limitations. Increasing utilization of early CVC in septic shock may potentially be explained by changing sensitivity of ICD-9-CM codes over time; however, ICD-9-CM validation in 2009 did not show substantially different sensitivity from prior validation of CVC claims in 1996-1997.(20) Furthermore, if the increase in CVC use over time merely reflected an increased tendency to code vascular access procedures, we would expect to observe similar increases in coding of pulmonary artery catheterization or peripheral arterial catheterization; no such trend was observed. Our data likely imperfectly estimate the true placement rates of early CVCs due to low sensitivity for the ICD-9-CM code, resolution of the procedure codes to the level of hospital day (making it impossible to ascertain whether CVC were placed within 6 hours of presentation), and the possibility that septic shock may have developed later during a hospitalization. We suspect that any misclassification of CVC status would not be associated with in-patient mortality over time, and thus is likely non-differential misclassification of CVC exposure that would bias our estimates towards the null. We could not ascertain the site of CVC placement (i.e., femoral, subclavian, internal jugular vein, peripherally inserted central catheter), which may affect the accuracy of CVP or SvO2 saturation measurements.
Moreover, associations between emerging clinical evidence and practice guidelines, increasing insertion of early CVCs and improved septic shock mortality cannot be assumed to be causal in an observational study using administrative data. We cannot exclude residual confounding from unmeasured covariates unavailable in NIS administrative claims data, such as information on vital signs, laboratory results, or timing of diagnoses. Additionally, we cannot exclude that rising rates of early CVC placement correlated with an increased quality of care in general, including concurrent implementation of other elements of the Surviving Sepsis bundle (e.g., early antibiotic administration) that could not be measured with NIS data. In addition, results may potentially be biased by temporal changes in coding practices,(37) as the validity of septic shock coding in particular has not been fully established However, if changes in coding practices were the explanation for our findings then we would expect to find that CVCs were placed in patients with decreasing illness severity over time. In contrast, we found that patients receiving early CVC had evidence of increasing illness severity over time (more comorbid conditions, mechanical ventilation, dialysis and greater number of acute organ failures) that would likely bias towards increasing odds of death associated with CVC placement. Further, similar outcome trends were not identified for other procedures for which evidence-based protocols do not currently exist (peripheral arterial catheters and pulmonary artery catheters). Notably, joinpoint regression analyses identified a significant change in septic shock mortality trends only after the 2004 publication of the Surviving Sepsis Campaign and the decline in septic shock mortality over time was greater in patients receiving early CVC compared with no CVC. Our results cannot be explained by the availability of new sepsis ICD-9-CM codes after 2002, as these codes only modify the existing 038 codes and do not increase sensitivity for ascertaining sepsis cases above that of 038 codes alone. Other studies have suggested that improvements in severe sepsis mortality may be the result of early patient discharge to other facilities,(3, 12) but our results were not altered by excluding patients discharged to other facilities. Thus, correlation between early CVC insertion and changing patient discharge practices are unlikely to explain our findings.
Conclusions
Severe sepsis-associated hospital mortality has declined during the past decade. We investigated trends in septic shock mortality associated with early CVC placement, a procedure necessary to implement early, goal-directed therapy recommended by the Surviving Sepsis Campaign. In patients likely to have septic shock, early CVC utilization increased and hospital mortality associated with early CVC placement improved during the time period after evidence-based guidelines for use of a CVC became widely available. We speculate that US healthcare providers have increasingly begun to implement evidenced-based recommendations regarding use of the CVC in the care of patients with septic shock, a behavior change associated with improved outcomes. In order to focus quality improvement efforts on strategies with high clinical impact, future studies should seek to investigate additional factors contributing to the decline in sepsis-associated mortality in chart-verifiable data sources.
Supplementary Material
Supplemental Table 1: International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) Codes.
Supplemental Table 2: Characteristics of patients with septic shock by year.
Supplemental Table 3: Characteristics of patients with septic shock stratified by timing of central venous catheter (CVC) insertion 1998-2009.
Supplemental Table 4: Clinical and demographic characteristics of patients who received an early central venous catheter (CVC) in 1998 and 2009.
Supplemental Table 5: Sensitivity analyses
Supplemental Figure: Flow chart of septic shock cohort case selection in Nationwide Inpatient Sample 1998-2009
Acknowledgments
Funding: K07 CA138772 (RSW) and the Department of Veterans Affairs (RSW).
No funding organization had a role in the design or conduct of the study.
Dr. Walkey had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Walkey, Wiener
Acquisition of data: Walkey, Wiener
Analysis and interpretation of data: Walkey, Wiener, Lindenauer.
Drafting of the manuscript: Walkey, Wiener, Lindenauer.
Critical revision of the manuscript for important intellectual content: Walkey, Wiener, Lindenauer.
Statistical analysis: Walkey, Wiener.
No author has conflicts of interest to declare.
Abbreviations
- APC
annual percent change
- CVC
central venous catheter
- CVP
central venous pressure
- CI
confidence interval
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- NIS
Nationwide Inpatient Sample
- OR
odds ratio
Footnotes
Work was performed at the Boston University School of Medicine.
Reprints will not be ordered
Contributor Information
Allan J. Walkey, The Pulmonary Center, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Renda Soylemez-Wiener, The Pulmonary Center, Division of Pulmonary and Critical Care Medicine, Boston University School of Medicine, Boston, MA USA. Center for Health Quality, Outcomes, & Economic Research, Edith Nourse Rogers Memorial VA Hospital, Bedford, MA, USA. The Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical School, Hanover, NH, USA.
Peter K. Lindenauer, Center for Quality of Care Research and Division of General Internal Medicine, Baystate Medical Center, Springfield MA, and Department of Medicine Tufts University School of Medicine, Boston MA USA.
References
- 1.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the united states: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 2.Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the united states 2003 to 2007. Crit Care Med. 2012 Mar;40:754–61. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 3.Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 4.Levy MM, Dellinger RP, Townsend SR, et al. The surviving sepsis campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in spain. JAMA. 2008;299:2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger RP, Carlet JM, Masur H, et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 8.Weiss CH, Moazed F, McEvoy CA, et al. Prompting physicians to address a daily checklist and process of care and clinical outcomes: A single-site study. Am J Respir Crit Care Med. 2011;184:680–686. doi: 10.1164/rccm.201101-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305:2175–2183. doi: 10.1001/jama.2011.697. [DOI] [PubMed] [Google Scholar]
- 10.Ho KM, Chavan S, Pilcher D. Omission of early thromboprophylaxis and mortality in critically ill patients: A multicenter registry study. Chest. 2011;140:1436–1446. doi: 10.1378/chest.11-1444. [DOI] [PubMed] [Google Scholar]
- 11.Bekaert M, Timsit JF, Vansteelandt S, et al. Attributable mortality of ventilator-associated pneumonia: A reappraisal using causal analysis. Am J Respir Crit Care Med. 2011;184:1133–1139. doi: 10.1164/rccm.201105-0867OC. [DOI] [PubMed] [Google Scholar]
- 12.Hall WB, Willis LE, Medvedev S, et al. The implications of long-term acute care hospital transfer practices for measures of in-hospital mortality and length of stay. Am J Respir Crit Care Med. 2012;185:53–57. doi: 10.1164/rccm.201106-1084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 14.Healthcare cost and utilization project (HCUP) agency for healthcare research and quality; rockville, MD: 1998-2009. HCUP Nationwide Inpatient Sample (NIS) www.hcup-us.ahrq.gov/nisoverview.jsp Edition. [PubMed] [Google Scholar]
- 15.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. the ACCP/SCCM consensus conference committee. american college of chest Physicians/Society of critical care medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 16.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the united states from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 17.Walkey AJ, Wiener RS, Ghobrial JM, et al. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulose JT, Cartin-Ceba R, Shoja A, et al. Comparison of international classification of Disease–Ninth revision (ICD–9) coding withretrospective case review for the diagnosis of septic shock. 2009 [Google Scholar]
- 19.Lagu T, Rothberg MB, Shieh MS, et al. What is the best method for estimating the burden of severe sepsis in the united states? J Crit Care. 2012 doi: 10.1016/j.jcrc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in international classification of diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 21.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 22.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the united states: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: Immortal time bias in observational studies. Am J Respir Crit Care Med. 2003;168:49–53. doi: 10.1164/rccm.200210-1231OC. [DOI] [PubMed] [Google Scholar]
- 24.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the united states, 1993-2004. JAMA. 2007;298:423–429. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 25.Connors AF, Jr, Speroff T, Dawson NV, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT investigators. JAMA. 1996;276:889–897. doi: 10.1001/jama.276.11.889. [DOI] [PubMed] [Google Scholar]
- 26.Harvey S, Harrison DA, Singer M, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-man): A randomised controlled trial. Lancet. 2005;366:472–477. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–24. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 28.Reade MC, Huang DT, Bell D, et al. Variability in management of early severe sepsis. Emerg Med J. 2010;27:110–115. doi: 10.1136/emj.2008.070912. [DOI] [PubMed] [Google Scholar]
- 29.Krishnagopalan S, Johnson EW, Low LL, et al. Body positioning of intensive care patients: Clinical practice versus standards. Crit Care Med. 2002;30:2588–2592. doi: 10.1097/00003246-200211000-00031. [DOI] [PubMed] [Google Scholar]
- 30.Peabody JW, Luck J, Glassman P, et al. Comparison of vignettes, standardized patients, and chart abstraction: A prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 31.Rethans JJ, Sturmans F, Drop R, et al. Does competence of general practitioners predict their performance? comparison between examination setting and actual practice BMJ. 1991;303:1377–1380. doi: 10.1136/bmj.303.6814.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heidenreich PA, Zhao X, Hernandez AF, et al. Patient and hospital characteristics associated with traditional measures of inpatient quality of care for patients with heart failure. Am Heart J. 2012;163:239–245.e3. doi: 10.1016/j.ahj.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Krumholz HM, Chen J, Rathore SS, et al. Regional variation in the treatment and outcomes of myocardial infarction: Investigating new England's advantage. Am Heart J. 2003;146:242–249. doi: 10.1016/S0002-8703(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 34.Allison JJ, Kiefe CI, Weissman NW, et al. Relationship of hospital teaching status with quality of care and mortality for medicare patients with acute MI. JAMA. 2000;284:1256–1262. doi: 10.1001/jama.284.10.1256. [DOI] [PubMed] [Google Scholar]
- 35.Ayanian JZ, Weissman JS. Teaching hospitals and quality of care: A review of the literature. Milbank Q. 2002;80:569–93,v. doi: 10.1111/1468-0009.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nau DP, Garber MC, Lipowski EE, et al. Association between hospital size and quality improvement for pharmaceutical services. Am J Health Syst Pharm. 2004;61:184–189. doi: 10.1093/ajhp/61.2.184. [DOI] [PubMed] [Google Scholar]
- 37.Lindenauer PK, Lagu T, Shieh M, et al. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA: The Journal of the American Medical Association. 2012;307:1405–1413. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) Codes.
Supplemental Table 2: Characteristics of patients with septic shock by year.
Supplemental Table 3: Characteristics of patients with septic shock stratified by timing of central venous catheter (CVC) insertion 1998-2009.
Supplemental Table 4: Clinical and demographic characteristics of patients who received an early central venous catheter (CVC) in 1998 and 2009.
Supplemental Table 5: Sensitivity analyses
Supplemental Figure: Flow chart of septic shock cohort case selection in Nationwide Inpatient Sample 1998-2009