Abstract
The growing field of epigenetics and human behavior affords an unprecedented opportunity to discover molecular underpinnings of mental health disorders and pave the way for the development of preventive intervention programs. Maternal depression during pregnancy is a serious public health issue and leads to a fourfold increase in the likelihood that the child will develop depression. We describe how mood disorders, particularly depression, may be shaped by early life stress, programming, and epigenetic processes and pathways showing how these processes could lead to depression in childhood. Implications of this approach to the study of mental health disorders for preventive interventions are discussed.
Keywords: maternal depression, childhood depression, programming, epigenetics, stress, developmental origins
Introduction
The study of epigenetics and human behavior is just beginning. Merging these two disciplines is an unprecedented opportunity to discover the molecular basis of human behavior, including the development of mental health disorders and preventive intervention programs1. Approximately 1 in 5 children develop a mental illness during their lifetime, and many of these children are reared in environments characterized by early life stress2, including exposure to maternal depression. In this article, rather than provide a “generic” model for the development of mental disorders, we focus on depression, a mood disorder that is highly co-morbid with anxiety. We highlight the development of depression because depression may in part be shaped by prenatal and postnatal pathways of early life stress, programming, and epigenetic mechanisms. We also focus on depression because it is a significant public health problem.
The prevalence of clinical depression in pregnant women is approximately 22%, with 25% of these mothers also suffering from postpartum depression3. A child whose mother is depressed is 4 times more likely to develop a psychiatric disorder4. This increased risk may be due, in part, to physiologic and behavioral sequelae of early life stress produced by maternal depression, and the potential developmental programming effects of depression on child mental health5. The concept of programming is based on epidemiological studies suggesting that an adverse fetal environment, originally indicated by low birth weight, is associated with the development many decades later of adult cardiovascular and metabolic disorders6. These findings lead to the developmental origins of health and disease (DOHaD) concept that fetal adjustments to cues from the intrauterine environment can affect disease susceptibility in adulthood7.
Stress is the organism’s response to a challenge that affects the internal milieu (homeostasis) of the organism and involves activation of neurobiological systems that preserve viability such as the HPA axis and related physiological systems. These neurobiological systems are altered by environmental factors because developmental plasticity enables neural pathways to be shaped by experience. In response to prenatal environmental cues such as stressors, structure and function can be reprogrammed in the fetus. The idea that environmental factors or experiences can alter physiological systems is known as programming. Developmental plasticity enables environmental factors to re-program or alter the set points of physiological systems, in preparation for the postnatal environment. If the re-programming is not compatible with the postnatal environment, maladaptive physiology can lead to later disease.
The identification of factors in the intrauterine environment related to the development of mental disorders and of the mechanisms responsible for linking these factors to infant outcomes may enable us to understand causal mechanisms that are involved in these disorders. Moreover, adversity in the postnatal environment brings additional early life stress and requires continued adaptation. Developmental plasticity extends beyond the prenatal period and can modulate or reverse the effects of prenatal programming through postnatal programming. However, not all children with early life stress develop mental health disorders because early life stress has different effects at different time points. In fact, programming reflects the action of factors during sensitive developmental periods making specific physiological pathways vulnerable at different times. Thus, the effects on development depend both on the nature and timing of environmental challenges.
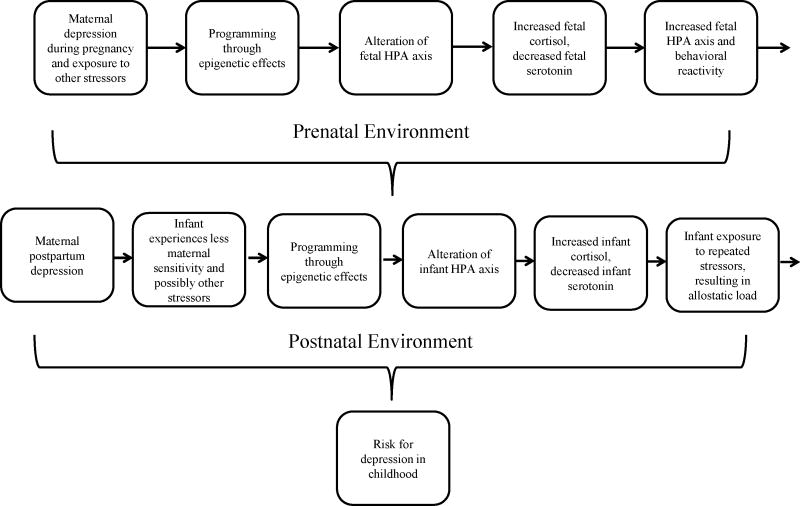
Epigenetic changes that alter gene expression are proposed as a central mechanism that can explain adaptation to the prenatal and postnatal environment. Early experience can affect the development of mental health disorders by the biological embedding of insults during sensitive developmental periods and by accumulating damage over time due to chronic stress8. Below we describe how exposure to prenatal and postnatal maternal depression may lead to increased risk for development of depression in the offspring via epigenetic mechanisms (Fig 1).
Figure 1.
Epigenetic transmission of maternal depression from parent to offspring: Hypothesized prenatal and postnatal pathways leading to risk for childhood depression.
Epigenetic Transmission of Maternal Depression to Offspring: Prenatal Pathways
Exposure to maternal mood disorders prenatally may lead to long-term emotional, behavioral, and social problems in offspring9. Adults with depression often hypersecrete cortisol and exhibit prolonged elevations in cortisol10. Furthermore, infants whose mothers are depressed during pregnancy have higher cortisol levels11 compared to infants whose mothers are not depressed. There is also altered fetal neurobehavior among depressed mothers12. In addition, infants whose mothers were depressed in pregnancy and in infancy had higher baseline cortisol levels than children never exposed to maternal depression or exposed to maternal depression only in infancy13. These studies provide increasing evidence that the fetal environment has a strong influence on these adverse behavioral outcomes by altering programming of the developing brain including the infant neuroendocrine system14. During early development, neural networks are formed and behavioral pathways are programmed making them vulnerable to environmental influences15 although the molecular mechanisms underlying these relationships are unclear16.
The fetus is exposed to physiological sequelae of the mother’s depression in the intrauterine environment via placental transfer of altered hormones and neurotransmitters as well as restricted uterine blood flow17. These alterations may be due to direct changes in physiology related to maternal depression during pregnancy; specifically, elevated circulating cortisol, elevated norepinephrine, and decreased serotonin11,18. Eighty to ninety percent of maternal cortisol is metabolized while it passes through the placenta, though higher levels of maternal cortisol lead to higher levels of fetal exposure to cortisol which have harmful effects on the fetus19. Alterations in maternal neurotransmitters are reflected in the fetus. Neonatal levels of cortisol, norepinephrine, and serotonin reflect maternal biochemistry11. Urinary cortisol is elevated and the 5-HIAA serotonin metabolite is decreased in mothers with depression and also in neonates of these mothers11. Glucocorticoid exposure can affect synaptogenesis, neurotransmitter function, and glucocorticoid receptor expression in the offspring’s developing brain and thus, also can impact the development of the HPA axis, as well as the autonomic nervous system (ANS).
Fetal exposure to maternal cortisol disrupts the programming of fetal biological stress response systems. The norepinephrine transporter (NET), 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) and the glucocorticoid receptor (NR3C1) are placental genes that have been implicated in perturbations of the hypothalamic pituitary adrenocortical (HPA) axis. Placental NET and 11β-HSD-2 program the intrauterine neuroendocrine environment during development. They protect the fetus from excess catecholamines and glucocorticoids, which have harmful effects on the fetus. 11β-HSD-2 in particular converts maternal cortisol to inert cortisone protecting the fetus from exposure to maternal cortisol. Rat pups born to mothers who did not express 11β-HSD-2 were exposed to higher levels of maternal glucocorticoids and had lower birth weights, and exhibited more anxiety compared to rat pups born to parents who did express 11β-HSD-220. Prenatal stress reduces 11β-HSD-2 activity in rodents, which is related to increased exposure to glucocorticoids21. This increased exposure to glucocorticoids, or inhibition of 11β-HSD-2, results in decreased birth weight, increases in hyperglycemia, hypertension, and increased HPA axis reactivity and increased anxiety in rodent models21. We found increased placental DNA methylation leading to decreased expression of 11β-HSD-2 in healthy term infants with the lowest birth weights, and that this increased methylation was associated with poorer scores in quality of movement in those newborns22.
The NR3C1 gene encodes the glucocorticoid receptor, is activated by cortisol and also regulates 11β-HSD-2. Maternal depression during the third trimester of pregnancy is associated with increased methylation of NR3C123 at a location analogous to the site found to be hypermethylated in rat pups exposed to less maternal nurturing care24. In an animal model of depression, male rodents exposed to chronic variable stress early in gestation spent more time immobile during a tail suspension test and a forced swim test, suggestive of learned helplessness, and they ingested significantly more of a sugar solution25. They also exhibited increased NR3C1 methylation at the same site that had previously been linked to decreased expression of NR3C124, and increased HPA reactivity. We found increased methylation of NR3C1 in the placenta related to decrements in the newborn infant’s ability to track and follow animate and inanimate stimuli26. At this same region, increased methylation of NR3C1 in infant blood samples after birth was also associated with increased cortisol response in infants at 3 months postpartum23. Taken together, these human studies suggest complementary epigenetic processes among humans and rat models.
The HPA axis and serotenergic system are intricately linked and exert bidirectional effects. Serotonin stimulates the HPA axis such that increases in 5-HT can stimulate the release of glucocorticoids27. Corticotropin releasing hormones may also affect the serotonergic system. Elevated cortisol levels lower brain serotonin function which may predispose the mother to mood disorders. The serotonin transport gene, SLC6A4 mediates placental uptake of serotonin. Selective serotonin reuptake inhibitors (SSRIs) bind to SLC6A4 and block serotonin reuptake, thereby increasing synaptic serotonin levels. Mothers both with untreated mood disorders (depression and/or anxiety) and mothers who took SSRIs had increased SLC6A4 gene expression compared to women without a history of a mood disorder and no current mood disorder28. Maternal depression during pregnancy is associated with decreased maternal and infant DNA methylation of the SLC6A4 gene which would also increase gene expression resulting in increased 5-HT reuptake, lowering serotonin and increasing risk for depression. Thus, abnormalities in cortisol secretion and 5-HT function may be part of a pathway in which cortisol is a biological mediator that lowers brain 5-HT function, increasing susceptibility to depression. In sum, these studies suggest a pathway to later depression informed by a developmental origins approach. In this pathway, prenatal depression is transmitted to the fetus via reprogramming of placental genes through epigenetic mechanisms that impact serotonin levels and the HPA axis and related physiological systems.
Epigenetic Transmission of Maternal Depression to Offspring: Postnatal Pathways
In addition to depression, other postnatal stressors confer risk for mood disorders in offspring. Adults raised in low socioeconomic status (SES) environments during their first 5 years of life show up-regulation of genes in leukocytes which are responsive to adrenergic signals and down-regulation of genes containing response elements for NR3C1, potentially reflecting maladaptation to increased cortisol exposures during this early developmental period29.
One mechanism for the transmission of psychopathology involves parenting behavior, which may itself be a form of postnatal stress. Poorer maternal care is associated with greater physiological reactivity in animals24 and humans30. Maternal care in rodents, defined as licking and grooming and arched-back nursing (LG-ABN), alters offspring behavioral and physiological response to stress via methylation of GR24. High licking and grooming initiates a cascade of events in the hippocampus. Increases in serotonin lead to greater NGF1-A expression, activating the GR exon 17 promoter. Offspring of high LG-ABN mothers exhibit demethylation of this promoter, increasing gene expression. Higher levels of GR gene expression leads to enhanced feedback sensitivity of the HPA system and lower cortisol levels in response to stress. Disruption of the GR gene results in negative feedback impairments and increased HPA activity that is maintained into adulthood24. Adult offspring of high LG-ABN mothers show reduced corticosterone responses to acute stress and increased hippocampal glucocorticoid receptor mRNA expression compared to low LG-ABN adult offspring24. These physiological differences mirrored behavior responses to stress. Behaviorally, these adults showed decreased startle responses, increased open-field exploration and shorter latencies to eat food when in a novel environment. In cross-fostering studies the differences in behavioral responses to stress were associated with the rearing mother, and not the biological mother24. Maternal sensitivity, the ability to detect the infant’s cues or signals and vary behavior promptly and appropriately may be the human analogue of LG-ABN. It not only results in more physical contact and tactile stimulation, but also provides stimulation that is appropriate for the infant. Some mothers with mood disorders are less sensitive when interacting with their infants5, which may be another mechanism by which infants of mothers with mood disorders show alterations in the HPA axis. For instance, infants of insensitive mothers with depression and anxiety had higher baseline cortisol30.
One particularly pernicious form of early life stress is trauma. In humans, exposure to trauma early in life is associated with an earlier onset of depression and increased number of depressive episodes31. Trauma exposure is related to epigenetic alterations in genes associated with HPA axis regulation. Rodents exposed to daily, 3 hour separations from their mother for the first 10 days of life show greater depression-like behaviors (e.g., reduced coping and memory deficits)32 and hypomethylation of the AVP gene involved in cortisol secretion resulting in upregulation of AVP, mRNA expression and sustained activity of the HPA axis. Compared to suicide completers without a history of childhood abuse, those with a history of abuse had decreased GR mRNA and increased methylation of an NR3C1 promoter33. Pups of female rodents exposed to maltreatment (e.g., who were stomped on, dropped during transport, dragged, rejected, roughly handled, and neglected) had greater methylation of BDNF, a gene involved in regulating neural plasticity in the prefrontal cortex and hippocampus. There were also deficits in BDNF gene expression that persisted into adulthood and the following generation34. There was greater methylation of BDNF among offspring of maltreated rodents, even among offspring who were cross-fostered and raised by mothers who had never been maltreated.
Adult survivors of childhood trauma with the risk allele FKBP5, another gene that regulates glucocorticoid activity, are more likely to exhibit demethylation in this risk allele35. Demethylation only happens in childhood, and does not occur if the individual was abused as an adult. Demethylation enhances expression of FKBP5 and leads to increased glucocorticoid receptor resistance35. Glucocorticoid receptor resistance is triggered by prolonged exposure to chronic stress and leads to increased inflammatory response and the likelihood of chronic inflammatory diseases such as heart disease and type II diabetes36. Childhood maltreatment or adversity is associated with increased NR3C1 promoter methylation and related to attenuated cortisol responses to the Dex/CRH test37. Adults with PTSD have more unmethylated genes associated with immune system function suggesting PTSD effects on epigenetic processes that affect immune system activation38. Adults exposed to the World Trade Center attacks and who had PTSD, compared to those exposed to the attacks without PTSD, had less whole blood gene expression of FKBP539. The serotonergic system is also implicated in studies of adult survivors of childhood abuse. Adults who were abused as children had greater methylation of SLC6A4, which could lower serotonin activation and lead to depression in this population40. Adults with the ss variant of 5HTTLPR, a variable nucleotide repeat upstream of the transcription start site of the serotonin transporter gene, had more unresolved loss or trauma, but only if the 5HTTLPR was less methylated41. The ll variant carriers who had higher methylation were also more likely to have unresolved loss or trauma41,42. Higher methylation of the ss variant was associated with less unresolved loss or trauma. These studies, although primarily retrospective, suggest that the experience of abuse may lead to epigenetic alterations in a range of genes involved in the HPA response and serotonin function.
Risk for Depression in Children Based on Early Life Stress, Programming and Epigenetics
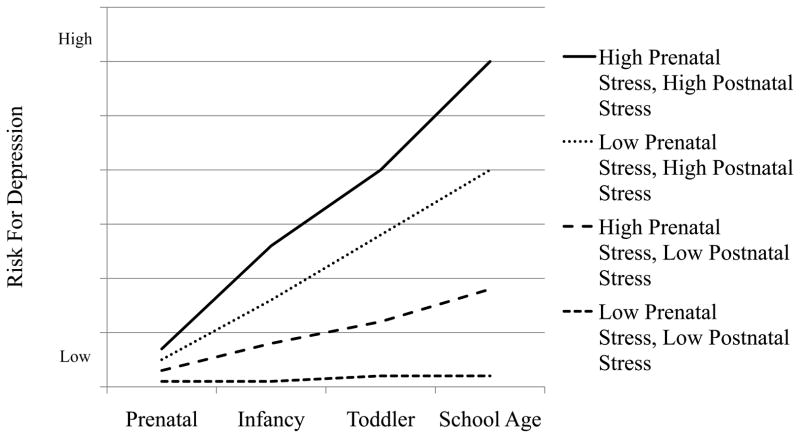
The development of depression in children (Fig. 2) can be modeled from a developmental origins perspective involving programming and epigenetic processes. Four trajectories are shown with increasing risk for depression based on prenatal and postnatal programming driving epigenetic processes to adjust to environmental challenges. Infants at the highest risk for depression would experience maternal depression during pregnancy and maternal postpartum depression, both of which could be exacerbated by additional environmental adversities. In this scenario, fetal exposure to maternal depression during pregnancy leads to elevated circulating cortisol, elevated norepinephrine, and decreased serotonin. Placental genes that mediate uptake of serotonin, SLC6A4, and regulate the neuroendocrine environment, NET, 11β-HSD-2 and NR3C1, reprogram the HPA axis to alter set points for the postnatal activation of the HPA axis and related physiological systems. This reprogramming results in increased cortisol secretion and decreased serotonin levels. The infant’s threshold of HPA reactivity to the postnatal environment is lowered due to reprogramming in anticipation of an adverse postnatal environment. In the short term, this increased physiological reactivity, which would result in increased behavioral reactivity as well, would be protective by buffering the child from postnatal environmental adversity. The well described “fight or flight” stress response is adaptive in a hostile environment. However, it would also make the child more susceptible to the effects of maternal postpartum depression and long term chronic stress. Mother’s with postpartum depression are less sensitive which can be further exacerbated by a highly reactive infant. Decreased sensitivity may in turn impact the infant’s response to stress reactivity. The long term effect on the physiological response to stress can result in hypocortisolism, or a blunted cortisol response which eventually becomes an inadequate stress response. This process has been referred to as “allostatic load” or the wear and tear of the body produced by the repeated activation of the HPA axis and related biological stress systems43. Prolonged activation of the neuroendocrine stress axis is related to physical disease and mental health disorders in children including mood disorders44. Thus, while the fetus was programmed for the postnatal environment, the “double whammy” of both prenatal and postpartum maternal depression during the sensitive developmental periods of prenatal development and infancy would overcome the child’s ability to reprogram and re-adapt. Furthermore, epigenetic processes would not be sufficiently altered and the inability of the serotonin system to recover would predispose the child to depression. However, plasticity during early development may not be an ongoing process. Consistent with the theory of allostatic load, with repeated exposure to stressors in postnatal life, the HPA system may become less plastic and less likely to respond flexibly to stress. Evidence that young children exposed to significant, repeated stress early in life exhibit a less flexible HPA response to stress in the form of blunted cortisol supports this idea.
Figure 2.
Risk for depression in children based on early life stress, programming and epigenetic mechanisms.
The second highest risk for depression may occur when the prenatal environment is benign (the fetus does not experience poor maternal mood) but postnatally, the infant is exposed to postpartum depression and/or high levels of stress. Although it might seem counterintuitive at first to suggest that a benign prenatal environment could lead to serious risk for depression, in this case, the fetus is prepared for a comparably benign postnatal environment but is then confronted with poor parenting and high stress. The stress response system has not been altered to protect the infant from adversity and is highly vulnerable to dysregulation due to DNA methylation of genes, mentioned above, implicated in perturbations of the HPA axis and serotonin levels. In addition, maternal postpartum depression independent of maternal depression during pregnancy is related to depression in the child. Allostatic processes may also be involved. Postnatal reprogramming may enable some of these children to adapt to this unexpected environment and develop coping strategies and avoid depression. Although this argument ignores a purely genetic explanation, epigenetic processes are gene-environment interactions, thus genotype by epigenetic interactions that would undoubtedly be involved.
The infant at third highest risk for depression may experience maternal depression during pregnancy and other stressors but a benign postnatal environment. In this case the fetus is programmed to prepare for adversity and may show HPA over-reactivity and behavioral dysregulation in early infancy. Developmental plasticity will enable the infant, with the support of appropriate maternal sensitivity in a low stress environment, to reprogram and “right itself.” And, for obvious reasons, the lowest risk for depression is when there is no prenatal or postnatal depression and low environmental stress.
We recognize that this is an overly simplistic model and that many factors have not been included. In addition to genotype, we would have to add to this model not only the amount of stress, but the nature and timing of the stress. We focused on maternal sensitivity because that is especially germane for depression but some conditions such as trauma or maltreatment, especially when suffered early in life during sensitive periods, could have a stronger impact on mental health outcome than other adversities in the environment. We also recognize that it is naïve to think that only a few genes would be involved in these complex scenarios or that DNA methylation is the only epigenetic process involved. We chose to focus on depression because of its public health importance and because it gave us the opportunity to “drill down” and focus on one mental health disorder. Clearly, changes in the model could be used to explore mood or other mental health disorders as well as mental health disorders in general. Our goal here was to describe an approach (one of many possible) to identify potential pathways that could help us understand molecular mechanisms that are involved in the later development of depression and mood disorders in general.
Future Directions
The long term implications of this discussion are potentially “game-changing” both in terms of our understanding of molecular mechanisms involved in the development of mental health disorders and for treatment. As described above, there is a growing animal and human literature suggesting that epigenetic processes are involved in the development of depression and other mental health disorders especially through effects on the HPA axis and related physiological systems. However, for obvious reasons, the kinds of experimental manipulations that are conducted with animals (e.g. cross fostering) cannot be conducted on humans. As a result human studies are associational and have been unable to establish causality. However, behavioral intervention paradigms represent an experimental manipulation often used in human research to provide more mechanistic implications. Given the evidence that parenting behavior in the rodent has epigenetic effects that alter HPA axis and behavioral reactivity in offspring, a human study could be designed to determine if an intervention to improve parenting (e.g. increase maternal sensitivity) would also result in decreased DNA methylation of GR, and less cortisol and behavioral reactivity in the infant. Showing that these changes can be induced through a behavioral intervention would not only support causal mechanisms at the molecular level that could be involved in the development of mental disorders but also have far reaching implications for intervention on several levels.
First, the intervention itself, improving maternal sensitivity, could be applied to populations at risk for poor parenting such as maternal depression to determine if depression (and presumably other mental health disorders) in the children can be prevented. Second, interventions could target precise aspects of parenting aimed at inducing specific epigenetic changes related to psychopathology. Third, interventions could be developed that target physiologic pathways implicated in mental health outcomes, identify epigenetic mechanisms that affect these pathways and identify environmental cues (e.g. behavior) that trigger the identified epigenetic mechanisms. Fourth, to the extent that these epigenetic changes are transmitted to the next generation, the development of mental disorders could be attenuated in the future. In sum, these are exciting times as the intersection of epigenetics and human behavior ushers in a new era in our understanding and approach to the treatment of mental health disorders including benefits for future personalized diagnostics and therapies for psychiatric disorders.
Acknowledgments
Source of Funding: National Institute of Health: R01MH094609 (to CM) and F32DA032175 (to EC).
Footnotes
Conflicts of Interest
For the remaining authors none were declared. None of the authors have conflicts of interest.
Contributor Information
Barry M. Lester, Center for the Study of Children at Risk, Warren Alpert Medical School of Brown University, and Women and Infants Hospital of Rhode Island.
Elisabeth Conradt, Center for the Study of Children at Risk, Warren Alpert Medical School of Brown University, and Women and Infants Hospital of Rhode Island.
Carmen J. Marsit, Department of Pharmacology and Toxicology and of Community and Family Medicine, Geisel School of Medicine at Dartmouth, Hanover, NH.
References
- 1.Lester BM, Marsit CJ, Conradt E, Bromer C, Padbury JF. Behavioral epigenetics and the developmental origins of child mental health disorders. Journal of Developmental Origins of Health and Disease. 2012;3:395–408. doi: 10.1017/S2040174412000426. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. Bmj. 2001;323:257–60. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hay DF, Pawlby S, Waters CS, Perra O, Sharp D. Mothers’ antenatal depression and their children’s antisocial outcomes. Child development. 2010;81:149–65. doi: 10.1111/j.1467-8624.2009.01386.x. [DOI] [PubMed] [Google Scholar]
- 5.Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Dev Psychopathol. 2012;24:1361–76. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker D. Mothers, Babies and Health in Later Life. Edinburgh and New York: Churchill Livingstone; 1998. [Google Scholar]
- 7.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–50. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA: the journal of the American Medical Association. 2009;301:2252–9. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 9.Salisbury AL, Wisner KL, Pearlstein T, Battle CL, Stroud L, Lester BM. Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depress Anxiety. 2011;28:1008–19. doi: 10.1002/da.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–6. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 11.Field T, Diego M, Dieter J, et al. Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development. 2004;27:216–29. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Monk C, Sloan RP, Myers MM, et al. Fetal heart rate reactivity differs by women’s psychiatric status: an early marker for developmental risk? Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:283–90. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–84. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 14.Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. International journal of peptides. 2011;2011:837596. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millan MJ. An epigenetic framework for neurodevelopmental disorders: From pathogenesis to potential therapy. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Sandman CA, Davis EP. Neurobehavioral risk is associated with gestational exposure to stress hormones. Expert review of endocrinology & metabolism. 2012;7:445–59. doi: 10.1586/eem.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–9. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- 18.Lundy BL, Jones NA, Field T, et al. Prenatal depression effects on neonates. Infant Behavior and Development. 1999;22:119–29. [Google Scholar]
- 19.Meyer JS. Biochemical effects of corticosteroids on neural tissues. Physiol Rev. 1985;65:946–1020. doi: 10.1152/physrev.1985.65.4.946. [DOI] [PubMed] [Google Scholar]
- 20.Holmes MC, Abrahamsen CT, French KL, Paterson JM, Mullins JJ, Seckl JR. The mother or the fetus? 11beta-hydroxysteroid dehydrogenase type 2 null mice provide evidence for direct fetal programming of behavior by endogenous glucocorticoids. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:3840–4. doi: 10.1523/JNEUROSCI.4464-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–89. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PloS one. 2012;7:e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 24.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 25.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bromer C, Marsit CJ, Armstrong DA, Padbury JF, Lester B. Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Dev Psychobiol. 2012 doi: 10.1002/dev.21061. n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanley SNR, Van de Kar LD. Serotonin and the Neuroendocrine Regulation of the Hypothalamic–Pituitary–Adrenal Axis in Health and Disease. Vitamins and hormones. 2003;66:189–255. doi: 10.1016/s0083-6729(03)01006-9. [DOI] [PubMed] [Google Scholar]
- 28.Ponder KL, Salisbury A, McGonnigal B, Laliberte A, Lester B, Padbury JF. Maternal depression and anxiety are associated with altered gene expression in the human placenta without modification by antidepressant use: implications for fetal programming. Dev Psychobiol. 2011;53:711–23. doi: 10.1002/dev.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller GE, Chen E, Fok AK, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan LA, Evans L, Monk C. Effects of mothers’ prenatal psychiatric status and postnatal caregiving on infant biobehavioral regulation: can prenatal programming be modified? Early Hum Dev. 2008;84:249–56. doi: 10.1016/j.earlhumdev.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernet CZ, Stein MB. Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depress Anxiety. 1999;9:169–74. [PubMed] [Google Scholar]
- 32.Murgatroyd C, Patchev AV, Wu Y, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 33.McGowan PO, Sasaki A, D’Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth TL. Lasting Epigenetic Influence of Early-Life Adversity on the BDNF Gene. Biological psychiatry (1969) 2009;65:760. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klengel T, Mehta D, Anacker C, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen S, Janicki-Deverts D, Doyle WJ, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5995–9. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PloS one. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin M, Aiello AE, Wildman DE, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9470–5. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yehuda R, Cai G, Golier JA, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–11. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 40.Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at SLC6A4 is linked to family history of child abuse: an examination of the Iowa Adoptee sample. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2010;153B:710–3. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Ijzendoorn MH, Caspers K, Bakermans-Kranenburg MJ, Beach SR, Philibert R. Methylation matters: interaction between methylation density and serotonin transporter genotype predicts unresolved loss or trauma. Biol Psychiatry. 2010;68:405–7. doi: 10.1016/j.biopsych.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van IMH, Caspers K, Bakermans-Kranenburg MJ, Beach SR, Philibert R. Methylation matters: interaction between methylation density and serotonin transporter genotype predicts unresolved loss or trauma. Biol Psychiatry. 2010;68:405–7. doi: 10.1016/j.biopsych.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- 44.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]