Abstract
Background and Purpose
Neighborhood characteristics may influence the risk of stroke and contribute to socioeconomic disparities in stroke incidence. The objectives of this study were to examine the relationship between neighborhood socioeconomic status (NSES) and incident ischemic stroke and examine potential mediators of these associations.
Methods
We analyzed data from 3834 whites and 785 African Americans enrolled in the Cardiovascular Health Study, a multicenter, population-based, longitudinal study of adults ages ≥65 years from four U.S. counties. The primary outcome was adjudicated incident ischemic stroke. NSES was measured using a composite of six census tract variables. Race-stratified multilevel Cox proportional hazard models were constructed, adjusted for sociodemographic, behavioral, and biologic risk factors.
Results
Among whites, in models adjusted for sociodemographic characteristics, stroke hazard was significantly higher among residents of neighborhoods in the lowest compared to the highest NSES quartile (Hazard Ratio [HR] =1.32; 95% CI 1.01-1.72), with greater attenuation of the HR after adjustment for biologic risk factors (HR=1.16; 0.88-1.52) than for behavioral risk factors (HR=1.30; 0.99-1.70). Among African Americans, we found no significant associations between NSES and ischemic stroke.
Conclusions
Higher risk of incident ischemic stroke was observed in the most disadvantaged neighborhoods among whites, but not among African Americans. The relationship between NSES and stroke among whites appears to be mediated more strongly by biologic than behavioral risk factors.
Characteristics of the neighborhood in which a person lives may influence the risk of stroke and may help to explain why stroke disproportionately affects disadvantaged persons in the United States, including those with low income, less education, and minority status.1-5 There is a strong, independent association between residence in a socioeconomically disadvantaged neighborhood and incident coronary heart disease, even after adjustment for individual socioeconomic status (SES).6-9 Research from outside the US suggests a relationship between low area SES and stroke.10-13 Nonetheless, only a few investigators in the U.S. have addressed the question in stroke,10, 14, 15 and they have generally conducted cross-sectional analyses that do not adjust for individual SES and, with few exceptions,10, 12 do not examine the role of traditional stroke risk factors as mediators of observed associations between neighborhood SES and stroke. We aimed to improve upon these prior studies by examining the relationship between neighborhood SES and incident stroke and exploring the mediators of these associations in the Cardiovascular Health Study (CHS), a large population-based, longitudinal study of coronary heart disease and stroke in adults 65 years of age and older. The CHS data are well suited to address these gaps in the nascent literature on socioeconomically disadvantaged neighborhoods in the U.S. and incident stroke because participants’ addresses are geocoded; their sociodemographic, behavioral, and biologic stroke risk factors are well characterized; and their follow up for stroke endpoints is over 10 years.
Methods
The CHS is a longitudinal, population-based study of cardiovascular disease, as detailed previously.16, 17 Briefly, participants were randomly sampled from Medicare eligibility lists in four U.S. communities: Forsyth County, North Carolina; Washington County, Maryland; Sacramento County, California; and Pittsburgh (Allegheny County), Pennsylvania. Eligible participants were ages 65 years or older, not institutionalized, and not requiring a proxy respondent at the time of recruitment.
Ischemic Stroke
The primary outcome was first ischemic stroke adjudicated by a cerebrovascular disease end-point committee that also classified stroke subtype (ischemic, hemorrhagic, and unknown type) and determined whether death was caused by stroke.16, 18, 19
Neighborhood Socioeconomic Status
Participants’ baseline home addresses were geocoded to identify the residential census tract defined in the 1990 U.S. decennial Census. Census tracts were used as a proxy for neighborhood, given their relatively small spatial and population size. Although neighborhood definitions vary widely and are not perfectly captured by such administrative units, census tract characteristics have been shown to be robust predictors of health.20
The neighborhood socioeconomic status (NSES) index used in this study has been previously described in studies of the CHS population.21, 22 It was constructed by summing the Z-scores of six census-derived SES indicators that represent the area’s physical and social resources: 1) median household income; 2) median value of housing units; 3) % households with interest, dividend, or rental income; 4) % of residents ≥25 with a high school degree; 5) % of residents ≥25 with a college degree; and 6) % of residents in executive, managerial, or professional specialty occupations. Quartile 1 represented the highest residential NSES, and quartile 4, the lowest. Neighborhood SES differed between African Americans and whites (Figure). Fewer than 25% of African Americans in the highest race-specific quartile overlapped with whites in the cohort, with even less overlap between the race-specific 2nd and 3rd quartiles of neighborhood SES for whites and African Americans. Thus, we constructed separate race-specific NSES index quartiles for whites and African Americans and conducted race-stratified analyses to examine neighborhood associations with stroke.
Figure 1.
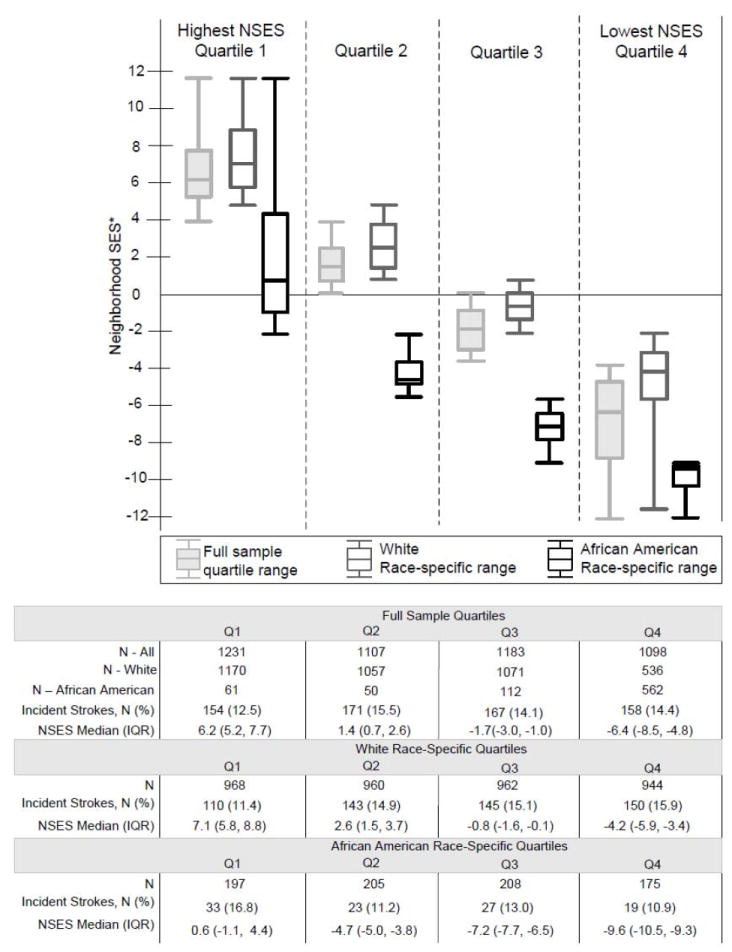
Neighborhood Socioeconomic Status (NSES): Overall vs. Race-specific quartiles ranges*
*The neighborhood socioeconomic status (NSES) index is the sum of the Z-scores of six variables from the 1990 U.S. Census, measured at the census tract level:1) median household income; 2) median value of housing units; 3) % households with interest, dividend, or rental income; 4) % of residents >25 with a high school degree; 5) % of residents >25 with a college degree; and 6) % of residents in executive, managerial, or professional specialty occupations. A higher NSES score is associated with more neighborhood socioeconomic resources.
Covariates
Sociodemographic characteristics, including age, sex, race, median household income, and education were all reported in the baseline survey. Health behaviors reported in the interview included smoking history, physical activity, alcohol use, and diet. Dietary intake was available for the original cohort only. Biologic characteristics included in the models were subclinical cardiovascular disease, atrial fibrillation, hypertension, diabetes and total cholesterol to HDL cholesterol ratio. Subclinical cardiovascular disease was defined as evidence of any of the following: Ankle-arm index <0.9; Carotid stenosis >25%; internal carotid thickness >80th percentile; common carotid thickness >80th percentile; major EKG abnormalities; abnormal EF or wall motion on echocardiogram; and claudication or angina on Rose Questionnaire.22
Study Sample
Baseline data were available for 5888 participants (Supplemental Figure S1). We excluded from the analyses 39 adults who were neither white nor African American. Of the 4925 whites, we excluded 875 participants whose addresses were not geocoded or whose addresses matched to block groups with fewer than 100 persons, fewer than 30 housing units per block, or with more than 33% persons in group quarters, e.g. military bases; 149 with prevalent stroke, and 67 with a prior TIA, leaving an analytic sample of 3834, with 652 incident strokes (548 adjudicated as ischemic). Among the 924 African Americans, 68 could not be geocoded, 58 had a prevalent stroke, and 15 reported a prior TIA, resulting in an analytic sample of 785, 129 of whom had an incident strokes (102 ischemic).
Analyses
Means and percentage distributions of participant characteristics for those with and without incident ischemic stroke were generated by race. To examine whether NSES was associated with incident ischemic stroke, we constructed multilevel Cox proportional hazard models that adjusted for demographic, behavioral, and/or biologic characteristics. Multilevel regression models provide a mechanism for decomposing the variation in outcome variables of interest into separate components due to individual-level and neighborhood-level effects23 and enable us to estimate and test for neighborhood effects on the outcome variables while allowing for unobserved heterogeneity at the individual and neighborhood levels. By accounting for possible correlation of the error terms for individuals within the same census tract, they yield more accurate estimates of regression coefficients and standard errors. Participants who had no ischemic stroke events prior to the study end date were censored at their first non-ischemic stroke or at the time of death, if death was unrelated to stroke; otherwise, they were censored at June 30, 2006. The race-specific multivariable models included the same covariates with one exception: there were too few African Americans with a diagnosis of atrial fibrillation to include this variable in the model.
Mediation Analysis
To determine whether observed associations of NSES and ischemic stroke were mediated by behavioral or biologic risk factors, we used methods described by Baron et al.24 This approach allows us to decompose the association indirect (through the mediating risk factor) and direct effects. For the mediation analysis, we first examined the direct association between NSES and incident ischemic stroke, then tested whether NSES was associated with either the biologic or the behavioral risk factors (as dependent variables) and whether these risk factors, in turn, were significantly associated with incident ischemic stroke. The indirect effects were estimated by calculating the proportion change in the hazard ratio for models with and without the risk factors. We used bootstrapping with 1000 repetitions to estimate standard errors of the indirect effects.25 In the mediation analyses, all regression equations were adjusted for individual sociodemographic characteristics.
Sensitivity analyses
First, to determine whether prior TIA influenced the relationship between NSES and incident ischemic stroke, we examined the NSES effect by including participants with a history of TIA in the model. We conducted a second sensitivity analysis to examine the role of dietary factors. Because nutrition assessments were only available for participants in the original cohort, we included measures of fat and salt intake in a series of models restricted to white participants. As a third set of sensitivity analysis, we took three approaches to incorporating biologic risk factors that were measured longitudinally, such as SBP and DBP. In separate models, we first incorporated the baseline value of these covariates, then the last available measurement prior to incident ischemic stroke, death, or study end date; and finally, we included them as time-varying covariates.23
All participants gave written informed consent and all study protocols were approved by the Institutional Review Boards (IRBs) of participating institutions. These analyses were reviewed and approved by the UCLA IRB.
Results
Descriptive Results
Compared to those included in the analyses, excluded white participants had higher rates of several demographic, behavioral, and biologic risk factors for stroke; excluded African Americans generally had lower rates of the biologic risk factors than those included in the analyses (Supplemental Table S3). Over the mean 11.5 years of surveillance (12.2 years for whites and 10.8 years for African Americans), 548 (14.3%) of the whites and 102 (13%) of the African Americans were diagnosed with incident ischemic stroke (Table 1). Whites resided in 317 of the 363 census tracts represented (median 8 participants per tract; interquartile range (IQR) 3-15), and African Americans in 162 tracts (median 2 participants per tract; IQR 1-6)
Table 1.
Characteristics of study population at baseline
| Whites (n=3834) N (%) OR mean (SD) | African Americans (n=785) N (%) OR mean (SD) | |
|---|---|---|
| Incident Ischemic Stroke | 548 (14.3) | 102 (13.0) |
| Demographics Characteristics | ||
| Age, mean (SD) | 72.7 (5.6) | 72.7 (5.5) |
| Female | 2223 (58.0) | 503 (64.1) |
| Education | ||
| Less than high school | 993 (26.0) | 343 (44.0) |
| Income | ||
| Less than $25,000 | 2106 (54.9) | 582 (74.1) |
| Behaviors | ||
| Smoking status | ||
| Never smoked | 1796 (46.9) | 384 (49.1) |
| Former smoker | 1600 (41.7) | 272 (34.8) |
| Current smoker | 437 (11.4) | 126 (16.1) |
| Alcohol use | ||
| 0 drinks per week | 1777 (46.5) | 515 (66.2) |
| 1–7 drinks per week | 1527 (40.0) | 210 (27.0) |
| >7 drinks per week | 515 (13.5) | 53 (6.8) |
| Physical activity (kcals past two weeks), mean (SD) | 1884 (2104) | 1069 (1436) |
| Biologic Risk Factors | ||
| Atrial fibrillation | 96 (2.5) | 8 (1.0) |
| Subclinical cardiovascular disease* | 2494 (65.1) | 552 (70.3) |
| Hypertension† | 1528 (39.9) | 472 (60.3) |
| Diabetes‡ | 530 (13.9) | 192 (25.4) |
| Total/HDL ratio, mean (SD) | 4.2 (1.3) | 3.8 (1.0) |
Ankle-arm index≤0.9, Carotid stenosis>25%, internal carotid thickness>80th percentile, major EKG abnormalities, abnormal EF or wall motion on echocardiogram, or claudication or angina on Rose Questionnaire.
Hypertension categories: SBP≥160 mmHg, DBP≥95 mmHg.
Diabetes: Fasting blood glucose above 126 mm/dL or diabetes diagnosis and diabetes medication.
NSES and Incident Ischemic Stroke (Table 2)
Table 2.
Incident Ischemic stroke, hazard ratio
| Unadjusted | Model 1 (adjusted for age, sex, income and education) | Model 2 (Model 1 + behavioral risk factors*) | Model 3 (Model 1 + biological risk factors†) | Model 4 (Model 1 + behavioral* + biological risk factors†) | |
|---|---|---|---|---|---|
| Writes (n=3834) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
| Neighborhood SES | |||||
| Q1 (Highest), ref., N=968 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2, N=960 | 1.33 (1.04–1.71)§ | 1.27 (0.98–1.63) | 1.27 (0.98–1.64) | 1.20 (0.93–1.56) | 1.21 (0.93–1.56) |
| Q3, N=962 | 1.42 (1.11–1.83)§ | 1.27 (0.92–1.65) | 1.26 (0.97–1.64) | 1.17 (0.90–1.52) | 1.17 (0.90–1.52) |
| Q4 (Lowest), N=944 | 1.56 (1.22–2.00)§ | 1.32 (1.01–1.72)§ | 1.30 (0.99–1.70) | 1.16 (0.88–1.52) | 1.15 (0.88–1.51) |
| Test of trend | P=0.0004 | P=0.069 | P=0.09 | P=0.42 | P=0.44 |
| African Americans (n=785)‡ | |||||
| Neighborhood SES | |||||
| Q1 (Highest) ref., N=197 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2, N=205 | 0.74 (0.43–1.25) | 0.68 (0.39–1.16) | 0.66 (0.39–1.14) | 0.70 (0.40–1.23) | 0.68 (0.39–1.20) |
| Q3, N=208 | 0.84 (0.51–1.40) | 0.70 (0.41–1.17) | 0.63 (0.37–1.07) | 0.72 (0.42–1.24) | 0.64 (0.37–1.12) |
| Q4 (Lowest), N=175 | 0.71 (0.41–1.25) | 0.60 (0.33–1.07) | 0.59 (0.33–1.08) | 0.67 (0.37–1.22) | 0.67 (0.36–1.23) |
| Test of trend∥ | P=0.30 | P=0.08 | P=0.05 | P=0.17 | P=0.13 |
Behavioral Risk Factors: smoking, alcohol use, and physical activity.
Biologic Risk Factors: EKG abnormalities, subclinical cardiovascular disease, hypertension, diabetes, Total/HD-cholesterol.
Atrial fibrillation was not included as a biologic risk factor in the African American.
P<0.05 in comparison to Q1.
Test of model fit for nested models compared to full model (Model 4)
Among whites, in the unadjusted models, ischemic stroke hazard was higher in all quartiles of neighborhood SES compared with the highest neighborhood SES (Quartile 1); the hazard ratio (HR) ranged from 1.33 (95% CI: 1.04-1.71) for Quartile 2 to 1.56 (95% CI: 1.22-2.00) for Quartile 4 (the lowest quartile of NSES) (Unadjusted Model). After adjusting for age, sex, income, and education (Model 1), only the lowest NSES quartile was associated with a significantly higher stroke hazard, HR=1.32 (95% CI: 1.01-1.72). With additional adjustment for behavioral risk factors resulted (Model 2), the HR did not change appreciably: HR 1.30 (95% CI: 0.99-1.70). Adjustment for biologic risk factors (Model 3) resulted in the most substantial reduction in the NSES HR in Quartile 4, with a HR=1.16 (95% CI: 0.88-1.52). This estimate changed little in the full model (Model 4), adjusted for demographic, behavioral, and biologic risk factors, HR 1.15 (95% CI: 0.88-1.51). In the full model, other covariates associated with stroke hazard were older age, lower educational attainment, subclinical cardiovascular disease, a diagnosis of hypertension, borderline hypertension, and diabetes (Supplemental Table S4).
Among African Americans, in contrast to whites, the stroke hazard was lower in all quartiles of NSES compared to Quartile 1, but never reached statistical significance in either the unadjusted or adjusted models. In the fully adjusted model, the HR ranged from 0.64 (95% CI: 0.37-1.12) to 0.68 (95% CI: 0.39-1.20). The only covariate that remained significant in the full model was alcohol use. Compared to participants who did not drink alcohol, those who reported 1-7 drinks per week had a lower ischemic stroke hazard; reporting more than 7 drinks per week was not associated with incident stroke.
Among whites, biologic risk factors accounted for approximately 13.0% (95% CI: 4.3%-21.0%) of the association between NSES and incident ischemic stroke. In contrast, behavioral risk factors, which were not found to be significant mediators, accounted for only 1.2% (95% CI: -0.2%-9.2%) of the association. (Supplemental Table 3)
Our findings remained robust in the sensitivity analyses. Including participants with a history of TIA did not appreciably alter the associations in any of the models. Incorporating dietary cereal fiber or salt intake into models restricted to whites did not change the main findings in the models. Use of baseline biologic measurements, last observed biologic measurements, and time-varying covariates all produced comparable results, so we presented only results for the baseline measurements.
Discussion
In this longitudinal study, we found higher incidence of ischemic stroke among whites residing in the most disadvantaged neighborhoods. Our analyses suggest that this relationship is mediated primarily through higher levels of biologic risk, such as hypertension, diabetes, hyperlipidemia, and atherosclerotic disease, in lower SES neighborhoods. In contrast, among African Americans, residents of neighborhoods with lower NSES had lower incidence of ischemic stroke than those in higher SES neighborhoods, though this difference did not reach statistical significance.
Our finding that NSES is mediated by biologic risk factors does not mean that neighborhood characteristics do not influence stroke risk, nor does it mean that the behavioral risk factors are not important. Instead these results underscore the importance of understanding the relationship between neighborhood characteristics and both behavioral and biologic stroke risk factors over the life course. The cardiometabolic dysregulation that contributes to conditions such as hypertension, diabetes, hyperlipidemia and their complications appears to be influenced by both individual and community level disadvantage. Recent research suggests that neighborhood disadvantage is independently associated with such measures of biologic risk26, and that the incidence of diabetes in a community is associated with specific features of low SES communities, among them few resources for physical activity (e.g. parks and recreation areas) and poor access to healthy food.27 The neighborhood socioeconomic disparities in stroke risk evident in our study seem to be mediated by the prevalence of stroke risk factors. Thus, public health efforts to reduce the morbidity and mortality associated with stroke may require more coordinated intervention that incorporates both individual and community components.
We interpret the finding of a positive association between NSES and incident ischemic stroke among African Americans with caution. First, the relatively small African Americans sample in these analyses may not be representative of black residents of the four communities. The differences by race in the strength and direction of the association between NSES and incident ischemic stroke may have several possible explanations. There may have been unmeasured confounders of the relationship, among them life course exposures (e.g. maternal malnutrition) that differed between whites and African-Americans.28 Further, because the vast majority of African Americans lived in neighborhoods whose NSES was in the lowest overall quartile for both races, variation among African Americans may have been too limited to observe a significant effect of NSES. Finally, although in the general population stroke incidence rates are higher for African Americans than whites at every age, the greatest disparity is seen in young and middle-aged blacks, peaking among adults ages 35-44 years.29, 30 If African Americans in the four study communities suffered strokes before becoming eligible for Medicare, they would have been less likely to have been enrolled in the CHS cohort (because of age restrictions, earlier death, or functional limitations that deterred study participation) and, even if enrolled, would have been excluded from these analyses, which were limited to those without a prior stroke. The remaining African Americans in the analytic cohort may thus represent “healthy survivors,” an effect that would be particularly pronounced if stroke at a younger age were associated with NSES. There is clearly a need for additional investigation into the association between neighborhood SES and stroke risk in a large, population-based cohort of African Americans.
This study has several strengths. We have a large population-based cohort of older adults who were followed longitudinally for many years, and thus we were able to assess a substantial number of incident ischemic strokes. We also had extensive individual and neighborhood socioeconomic data in addition to detailed data on participants’ health behaviors and clinical characteristics.
The study also has potential limitations. Although CHS participants were derived from a representative sample of Medicare enrollees, only four geographical areas in the US were represented. Further, the enrolled participants may not have reflected the racial/ethnic diversity of these areas: some racial/ethnic groups, particularly Latinos, may be under-represented among Medicare enrollees, and participants who did not self-identify as white or African American were excluded. As the aging population becomes more diverse, the influence of place on the risk of stroke will need to be studied in cohorts of Latinos, Asians, Pacific/Islanders, particularly those who reside in racially and ethically varied communities. We used baseline residential addresses and did not incorporate information on whether participants moved over the study period. However, residential mobility appears to be relatively low by age 35 and declines rapidly thereafter, thus using the characteristics of the neighborhood of residence at baseline for those who move is unlikely to bias the estimates that would be obtained if the characteristics of destination neighborhoods were available.31-33 Finally, management of stroke and treatment targets for the biologic risk factors has changed appreciably since the study’s inception. Nonetheless, disparities resulting from individual and neighborhood disadvantage likely have persisted and may be more pronounced, as diffusion of new therapies is often delayed to vulnerable individuals and communities.
The findings of this study suggest a need to incorporate public health and community-level interventions into the individual efforts to reduce stroke risk in diverse, low income, and underserved communities. Additional research is needed to understand more fully which features of disadvantaged neighborhoods have the strongest influence on stroke and its risk factors, how specific neighborhood characteristics, such as stressful social environments or inadequate social support, a lack of safe places to exercise or to obtain healthy foods, or an overabundance of fast food outlets may influence the health behaviors and biologic factors that contribute to stroke, and whether these associations differ by race/ethnicity.34
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the American Heart Association PRT-Spina Outcomes Research Center #0875135N and by N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chsnhlbi.org/pi.htm.
Footnotes
Disclosures
none
References
- 1.Avendano M, Kawachi I, Van Lenthe F, Boshuizen HC, Mackenbach JP, Van den Bos GA, et al. Socioeconomic status and stroke incidence in the US elderly: the role of risk factors in the EPESE study. Stroke. 2006;37:1368–1373. doi: 10.1161/01.STR.0000221702.75002.66. [DOI] [PubMed] [Google Scholar]
- 2.Gillum RF, Mussolino ME. Education, poverty, and stroke incidence in whites and blacks: the NHANES I Epidemiologic Follow-up Study. J Clin Epidemiol. 2003 Feb;56:188–195. doi: 10.1016/s0895-4356(02)00535-8. [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Howard VJ, Katholi C, Oli MK, Huston S. Decline in US stroke mortality: an analysis of temporal patterns by sex, race, and geographic region. Stroke. 2001;32:2213–2220. doi: 10.1161/hs1001.096047. [DOI] [PubMed] [Google Scholar]
- 4.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36:374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 5.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;29117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 6.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 7.Sundquist K, Malmstrom M, Johansson SE. Neighbourhood deprivation and incidence of coronary heart disease: a multilevel study of 2.6 million women and men in Sweden. J Epidemiol Community Health. 2004;58:71–77. doi: 10.1136/jech.58.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkleby M, Sundquist K, Cubbin C. Inequities in CHD incidence and case fatality by neighborhood deprivation. Am J Prev Med. 2007;32:97–106. doi: 10.1016/j.amepre.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimaud O, Bejot Y, Heritage Z, Vallee J, Cadot E, Giroud M, et al. Incidence of stroke and socioeconomic neighborhood characteristics: an ecological analysis of dijon stroke registry. Stroke. 2011;42:1201–1206. doi: 10.1161/STROKEAHA.110.596429. [DOI] [PubMed] [Google Scholar]
- 10.Aslanyan S, Weir CJ, Lees KR, Reid JL, McInnes GT. Effect of area-based deprivation on the severity, subtype, and outcome of ischemic stroke. Stroke. 2003;34:2623–2628. doi: 10.1161/01.STR.0000097610.12803.D7. [DOI] [PubMed] [Google Scholar]
- 11.Brown P, Guy M, Broad J. Individual socio-economic status, community socio-economic status and stroke in New Zealand: a case control study. Soc Sci Med. 2005;61:1174–1188. doi: 10.1016/j.socscimed.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Engstrom G, Jerntorp I, Pessah-Rasmussen H, Hedblad B, Berglund G, Janzon L. Geographic distribution of stroke incidence within an urban population: relations to socioeconomic circumstances and prevalence of cardiovascular risk factors. Stroke. 2001;32:1098–1103. doi: 10.1161/01.str.32.5.1098. [DOI] [PubMed] [Google Scholar]
- 13.Thrift AG, Dewey HM, Sturm JW, Paul SL, Gilligan AK, Srikanth VK, et al. Greater incidence of both fatal and nonfatal strokes in disadvantaged areas: the Northeast Melbourne Stroke Incidence Study. Stroke. 2006;37:877–882. doi: 10.1161/01.STR.0000202588.95876.a7. [DOI] [PubMed] [Google Scholar]
- 14.Lisabeth LD, Diez Roux AV, Escobar JD, Smith MA, Morgenstern LB. Neighborhood environment and risk of ischemic stroke: the brain attack surveillance in Corpus Christi (BASIC) Project. Am J Epidemiol. 2007;165:279–287. doi: 10.1093/aje/kwk005. [DOI] [PubMed] [Google Scholar]
- 15.Morgenstern LB, Escobar JD, Sanchez BN, Hughes R, Zuniga BG, Garcia N, et al. Fast food and neighborhood stroke risk. Ann Neurol. 2009;66:165–170. doi: 10.1002/ana.21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 17.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 18.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley P, et al. Surveillance and ascertainment of cardiovascular events : The Cardiovascular Health Study. Annals of Epidemiology. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 19.Longstreth WT, Jr, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56:368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 20.Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health. 2000;21:171–192. doi: 10.1146/annurev.publhealth.21.1.171. [DOI] [PubMed] [Google Scholar]
- 21.Diez-Roux AV, Kiefe CI, Jacobs DR, Jr, Haan M, Jackson SA, Neito FJ, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001;11:395–405. doi: 10.1016/s1047-2797(01)00221-6. [DOI] [PubMed] [Google Scholar]
- 22.Nordstrom CK, Diez Roux AV, Jackson SA, Gardin JM. The association of personal and neighborhood socioeconomic indicators with subclinical cardiovascular disease in an elderly cohort. The cardiovascular health study. Soc Sci Med. 2004;59:2139–2147. doi: 10.1016/j.socscimed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2. New Jersey, NJ: Wiley; 2002. [Google Scholar]
- 24.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 25.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 26.Bird CE, Seeman T, Escarce JJ, Basurto-Davila R, Finch BK, Dubowitz T, et al. Neighbourhood socioeconomic status and biological ‘wear and tear’ in a nationally representative sample of US adults. J Epidemiol Community Health. 2010;64:860–865. doi: 10.1136/jech.2008.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auchincloss AH, Diez Roux AV, Mujahid MS, Shen M, Bertoni AG, Carnethon MR. Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: the Multi-Ethnic study of Atherosclerosis. Arch Intern Med. 2009;169:1698–1704. doi: 10.1001/archinternmed.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker DJ. Intrauterine programming of coronary heart disease and stroke. Acta Paediatr Suppl. 1997;423:178–182. doi: 10.1111/j.1651-2227.1997.tb18408.x. [DOI] [PubMed] [Google Scholar]
- 29.Chong JY, Sacco RL. Epidemiology of stroke in young adults: race/ethnic differences. J Thromb Thrombolysis. 2005;20:77–83. doi: 10.1007/s11239-005-3201-9. [DOI] [PubMed] [Google Scholar]
- 30.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 31.Jackson M, Mare RD. Understanding the Effects of Neighborhoods on Children: Cross-Sectional and Longitudinal Measures of the Neighborhood Experience. Proceedings of the Population Association of America Annual Meeting, 2000; Los Angeles, CA. 2004. [Google Scholar]
- 32.Kunz J, Page ME, Solon G. Are Point-In-Time Measures of Neighborhood Characteristics Useful Proxies for Children’s Long-Run Neighborhood Environment? Economics Letters. 2003;79:231–239. [Google Scholar]
- 33.South SJ, Crowder KD. Residential mobility between cities and suburbs: race, suburbanization, and back-to-the-city moves. Demography. 1997;34:525–538. [PubMed] [Google Scholar]
- 34.Clark CJ, Guo H, Lunos S, Aggarwal NT, Beck T, Evans DA, et al. Neighborhood cohesion is associated with reduced risk of stroke mortality. Stroke. 2011;42:1212–1217. doi: 10.1161/STROKEAHA.110.609164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


