Abstract
Understanding the mode of action for lipoxygenase (LOX) inhibitors is critical to determining their efficacy in the cell. The pseudoperoxidase assay is an important tool for establishing if a LOX inhibitor is reductive in nature, however, there have been difficulties identifying the proper conditions for each of the many human LOX isozymes. In the current paper, both the 234 nm decomposition (UV) and iron-xylenol orange (XO) assays are shown to be effective methods of detecting pseudoperoxidase activity for 5-LOX, 12-LOX, 15-LOX-1 and 15-LOX-2, but only if 13-(S)-HPODE is used as the hydroperoxide substrate. The AA products, 12-(S)-HPETE and 15-(S)-HPETE, are not consistent hydroperoxide substrates since they undergo a competing transformation to the di-HETE products. Utilizing the above conditions, the selective 12-LOX and 15-LOX-1 inhibitors, probes for diabetes, stroke and asthma, are characterized for their inhibitory nature. Interestingly, ascorbic acid also supports the pseudoperoxidase assay, suggesting that it may have a role in maintaining the inactive ferrous form of LOX in the cell. In addition, it is observed that nordihydroguaiaretic acid (NDGA), a known reductive LOX inhibitor, appears to generate radical species during the pseudoperoxidase assay, which are potent inhibitors against the human LOX isozymes, producing a negative pseudoperoxidase result. Therefore, inhibitors that do not support the pseudoperoxidase assay with the human LOX isozymes, should also be investigated for rapid inactivation, to clarify the negative pseudoperoxidase result.
Keywords: lipoxygenase, pseudoperoxidase, hydroperoxide, inhibitor
1.1 Introduction
The inflammatory response in humans is regulated by fatty acid signaling cascades, which are initiated by the oxidation of polyunsaturated fatty acids. Three classes of enzymes catalyze this oxidation: cyclooxygenase (COX) [1]; cytochrome P450 [2]; and lipoxygenase (LOX) [3], the latter of which is the focus of this study. Lipoxygenases (LOX) are a family of iron containing metalloenzymes that utilize a non-heme iron center to incorporate molecular oxygen into a variety of fatty acids. There are three main LOXs of pharmacological importance, 5-LOX, 12-LOX and 15-LOX. They are named according to their oxygenation position on arachidonic acid (AA)[4], generating the hydroperoxyeicosatetraenoic acid (HPETE) product [5]. HPETEs are responsible for maintaining the homeostasis of the inflammatory response [6], and have also been implicated in many human diseases, such as asthma [7], psoriasis [8], atherosclerosis [9], cancer [10], heart disease [11,12] and diabetes [13], to name a few.
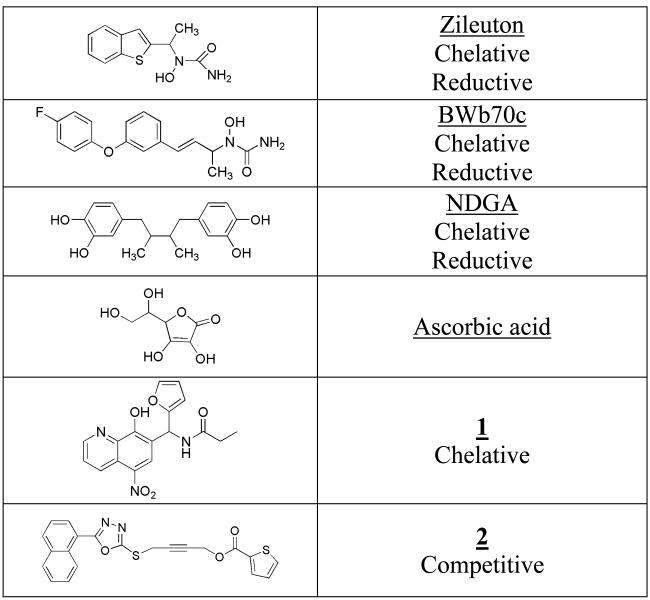
Due to the important role LOX plays in human disease, numerous inhibitors for LOX have been reported [14-28], which can be generally classified into three categories. There are reductive inhibitors (such as Zileuton [21,22], BWb70c [19,20,26], NDGA [27,28], chelative (such as compound 1 [29]) and competitive/mixed inhibitors (such as compound 2 [30])), shown in Figure 1. Nevertheless, only one compound has been approved as a drug, Zileuton [21,22], a potent and selective 5-LOX inhibitor [20,23]. It contains an N-hydroxyurea moiety, which chelates to the active ferric ion and reduces it to the inactive ferrous ion [23-25]. Many other reductive inhibitors of LOX have been found, such as N-hydroxyureas, hydroxybenzofurans, hydroxamic acids, hydroxylamines, and catechols [18-20,26], indicating the ease of which LOX isozymes can be inhibited in this manner. However, it is challenging to determine whether a particular inhibitor of LOX is reductive because it is difficult to concentrate human LOX isozymes and therefore the direct visualization of the active site iron by electron paramagnetic resonance (EPR) is not possible. Interestingly, Zileuton and other hydroxamic acids were initially designed to chelate the iron center of LOX [21,25], but it was later determined, using the UV pseudoperoxidase assay, that Zileuton also reduced the active site iron of 5-LOX [18]. Nordihydroguaiaretic acid (NDGA), found in the Larrea tridentata plant, is another example of a non-specific LOX inhibitor, which possesses a dual mode of inhibition [27,31,32]. NDGA contains a catechol moiety, which binds to the active site ferric ion, but it also reduces it to the ferrous ion, with the concomitant oxidation of the catechol moiety to the semiquinone. This reactivity is also seen with the non-heme iron enzyme, catechol dioxygenase, whose catechol substrate is activated to the semiquinone by the active site ferric ion for oxidation by molecular oxygen [32-34].
Figure 1.
Classifications of general LOX inhibitors.
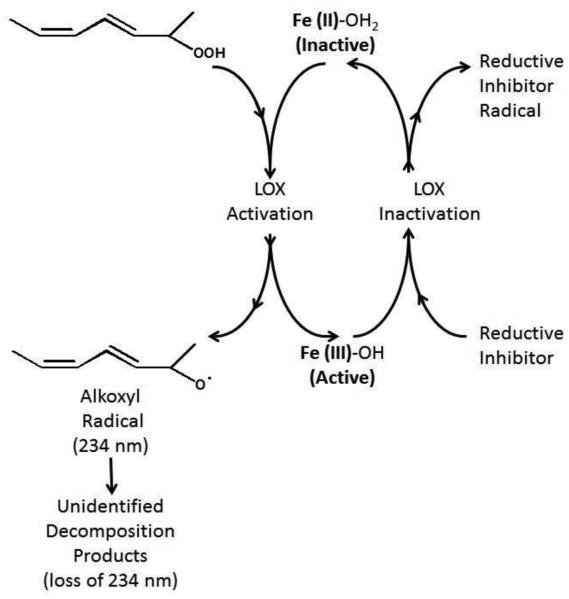
Considering that direct detection of the reduced active site iron by EPR is not practical for many human LOX isozymes, the typical method for determining whether an inhibitor is reductive in nature is the pseudoperoxidase reaction. This reaction follows the reduction of the fatty acid hydroperoxide product by the ferrous ion to the alkoxyl radical, generating the active ferric form of LOX (Figure 2). However, for this process to be catalytic, a reducing inhibitor is required to reduce the ferric ion back to its ferrous form. This cycling results in the degradation of both the hydroperoxide product and the reducing inhibitor to their corresponding radicals. The reaction is typically witnessed by the reduced absorbance at 234 nm [35-37], due to the decomposition of the resulting alkoxyl radical, triggering a loss in the conjugation of the hydroperoxide product. However, this assay is not without its difficulties when Riendeau and coworkers observed that NDGA and 13-(S)-HPODE did not support the pseudoperoxidase assay with 5-LOX [37]. An alternative method for researchers to investigate reductive inhibitors is to monitor their ability to quench the free radical of 1,1-diphenyl-2-picrylhydrazyl (DPPH), but this method is not reliable for predicting the reductive activity of LOX inhibitors. DPPH is considered a general indicator of the cellular reduction potential [38,39], which is distinct from the reduction potential of the various LOX isozymes. Alternatively, several methods can be employed to detect the loss of the hydroperoxide product directly, such as iodine oxidation [40], radiolabeling [41], thiobarbituric acid (TBA) [42], enzymatic oxidation of dyes [43], and coupled oxidation of NADH [44]. Unfortunately, these assays are tedious and subject to various confounding factors. Simplified methods have been developed, such as the fluorescent indicator, diphenyl-1-pyrenylphosphine (DPPP) [45], and the visible indicator, iron-xylenol orange (XO) [46,47], both of which are oxidized by the hydroperoxy-lipids, changing their spectroscopic properties. These methods are robust and have been successfully utilized for high-throughput inhibitor screening of LOX inhibitors [29,30,45,48].
Figure 2.
The LOX pseudoperoxidase reaction scheme.
Given the need for a robust pseudoperoxidase procedure to determine the inhibitory mechanism of reducing inhibitors, we have utilized both the conjugated diene decomposition (UV) and iron-xylenol orange (XO) pseudoperoxidase assays to determine the best hydroperoxides for the pseudoperoxidase reaction against the human isozymes, 5-LOX, 12-LOX, 15-LOX-1 and 15-LOX-2. In addition, the reductive nature of a variety of disease related LOX inhibitors were determined utilizing the aforementioned UV and XO assays.
1.2 Materials and Methods
1.2.1 Materials
All commercial fatty acids (Sigma-Aldrich Chemical Company, and NuCheck) were stored at −80 °C for a maximum of 6 months. LOX products were generated by reacting substrate with the appropriate LOX isozyme (13-(S)-HPODE from soybean LOX-1 and LA, 15-(S)-HPETE from 15-LOX-2 and AA, 12-(S)-HPETE from 12-LOX and AA). Product generation was performed as follows. A 2 L solution of 50-100 μM substrate in the appropriate buffer (50 mM Borate pH 9.2 for soybean LOX-1, 25 mM HEPES pH 7.5 for 15-LOX-2 and 25 mM HEPES pH 8 for 12-LOX) was run to completion, quenched with 10 mL acetic acid, extracted three times with dichloromethane, evaporated to dryness, and reconstituted in MeOH for HPLC purification. The products were HPLC purified using an isocratic elution of 55% Acetonitrile: 45% H2O: 0.1% acetic acid. All products were tested with enzyme to show that no residual substrate was present and subjected to analytical HPLC to test for purity. Zileuton, BWb70c and NDGA were purchased from Sigma/Aldrich Chemicals. The inhibitors, compounds 1, and 2 were previously characterized and kindly provided by the NIH Chemical Genomics Center (NCGC). All other chemicals were reagent grade or better and were used without further purification.
1.2.2 Overexpression and Purification of 5-Human Lipoxygenase, 12-Human Lipoxygenase, and the 15-Human Lipoxygenases
Human reticulocyte 15-lipoxygenase-1 (15-LOX-1) [49] and human platelet 12-lipoxygenase (12-LOX) [49] and human prostate epithelial 15-lipoxygenase-2 (15-LOX-2) [50] were expressed as N-terminally, His6-tagged proteins and purified to greater than 90% purity. Human leukocyte 5-lipoxygenase was expressed as a non-tagged protein and used as a crude ammonium sulfate protein fraction, as published previously [51].
1.2.3 Evaluation of Hydroperoxides as Pseudoperoxidase Substrates
The ability of various HPETEs to serve as substrates to the pseudoperoxidase activity was investigated with 20 μM BWb70c and the LOX isozymes. Pseudoperoxidase activity measurements were conducted on a Perkin-Elmer Lambda 40 UV/Vis spectrometer using a universal assay buffer for all human LOXs screened (50 mM Sodium Phosphate (pH 7.4), 0.3 mM CaCl2, 0.1 mM EDTA, and 0.01% Triton X100). The hydroperoxide concentration was 20 μM of 13-(S)-HPODE for all LOX isozymes investigated. However, in the case of 12-HPETE and 15-HPETE, 20 μM was used for 5-LOX and 15-LOX-1, whereas 40 μM was used in the case of 12-LOX and 15-LOX-2. This higher concentration of hydroperoxide was due to competing reactions for these two LOX isozymes (vide infra). The following concentrations of each isozyme were used (400 nM of 12-LOX, 300 nM of 15-LOX-1, 2 μM of 15-LOX-2) in 2 mLs of buffer, on an oscillating shaker (22 °C). The reaction was initiated by addition of 20 μM BWb70c, a known reductive inhibitor. The reaction was incubated for 30 minutes and quenched with two parts of the iron-xylenol orange solution (25 mM H2SO4, 100 mM xylenol orange, and 250 mM ferrous sulfate, solubilized in 90:10 methanol:water) [46,47]. In order to determine if any competing reactions degraded the hydroperoxide, the hydroperoxide stability (i.e. maximal absorption at 590 nm) was determined through the use of two controls. The first control measured an inhibitor/hydroperoxide solution with iron-xylenol orange added, to account for any degradation of the hydroperoxide by the inhibitor. The second control measured an enzyme/hydroperoxide solution with iron-xylenol orange added, to account for any breakdown of the hydroperoxide by the LOX isozyme, without the inhibitor present. These two controls allowed for the determination of the background percentage, which was subsequently subtracted from the measured percentage. In all cases, the inhibitors did not degrade a significant amount of hydroperoxide product, however, some LOX isozymes did degrade 12-(S)-HPETE and 15-(S)-HPETE by increasing their absorbance at 280 nm, presumably through converting the HPETEs to di-HETEs. Due to this slow consumption of hydroperoxide by 12-LOX and 15-LOX-2, higher concentrations of hydroperoxides (40 uM) were required to ensure a sufficient rate of the pseudoperoxidase reaction, relative to that of the aforementioned background reactions. In all assays, the primary absorption peaks for the hydroperoxide lipids and their oxidized metabolites were confined to the UV region, resulting in no overlap between their absorption bands and that of the 590 nm oxidized iron-xylenol orange product. Controls to establish the endpoint of the pseudoperoxidase reaction (i.e. 100% degradation of the hydroperoxide) were conducted by measuring an iron-xylenol orange solution with enzyme and inhibitor, but no hydroperoxide added. Given the large error for these results, 20% or less, a positive result for pseudoperoxidase activity, after the subtraction of control rates, was considered a loss of greater than 45% absorption at 590 nm in 30 minutes. The amount of enzyme varied for each LOX isozyme, so this minimal level of detection corresponds to approximately 1.3 mol/min/mol for 12-LOX, 1.5 mol/min/mol for 15-LOX-1 and 0.25 mol/min/mol for 15-LOX-2.
1.2.4 Iron-xylenol Orange Pseudoperoxidase Inhibitor Assay
The reductive properties of the inhibitors were determined by monitoring the pseudoperoxidase activity of lipoxygenase in the presence of 40 μM inhibitor and 20 μM 13-(S)-HPODE. Increased concentrations of inhibitors were used in this assay to drive the reaction to completion. Pseudoperoxidase activity measurements were conducted as described above. All inhibitors were ran alone with 13-(S)-HPODE to account for direct breakdown of 13-(S)-HPODE, and to account for absorbance changes from individual inhibitors. The control inhibitors for this assay were Zileuton and BWb70c, known reductive inhibitors.
1.2.5 UV Pseudoperoxidase Activity Assay
The pseudoperoxidase activity rates were determined with BWb70c as the reducing inhibitor, 13-(S)-HPODE as the oxidizing product and the following isozymes; 12-LOX, 15-LOX-1 and 15-LOX-2. Activity for all isozymes was determined by monitoring the decrease at 234 nm (product degradation) in buffer (50 mM Sodium Phosphate (pH 7.4), 0.3 mM CaCl2, 0.1 mM EDTA, 0.01% Triton X100, and 20 μM 13-(S)-HPODE), with a Perkin-Elmer Lambda 40 UV/Vis spectrometer. The following concentrations of each isozyme were used (400 nM of 12-LOX, 300 nM of 15-LOX-1, 2 μM of 15-LOX-2), in 2 mLs of buffer and constantly stirred with a rotating stir bar (22 °C). The reaction was initiated by addition of 20 μM inhibitor (at a 1:1 ratio to product), and the initial rate recorded. The percent consumption of 13-(S)-HPODE was recorded for each of the isozymes, with a loss of product less than 35% not being considered as significant activity. Individual controls were conducted with inhibitor alone with product and enzyme alone with product. These negative controls established the baseline for the assay, reflecting non-pseudoperoxidase dependent hydroperoxy product decomposition.
1.2.6 Residual Oxygenase activity after the Pseudoperoxidase Reaction
To evaluate whether inactivation was occurring as a result of pseudoperoxidase cycling, the LOX residual activity was measured after a set amount of pseudoperoxidase turnover was completed. Activity is characterized by direct measurement of the product formation with the increase of absorbance at 234 nm using a Perkin-Elmer Lambda 40 UV/Vis spectrometer. All human isozymes utilized the same buffer (50 mM Sodium Phosphate (pH 7.4), 0.3 mM CaCl2, 0.1 mM EDTA, and 0.01% Triton X100) and were constantly stirred with a rotating stir bar in 2 mLs of buffer (22 °C). Pseudoperoxidase reactions were initiated as described above, except in the case of 15-LOX-1, where compound 2 (Figure 1) was screened at lower inhibitor concentration (10 μM), due to the high potency of this inhibitor (compound 5 in our previous publication [30], IC50 = 19 nM)). Oxygenase activity was evaluated 2 min post initiation of the pseudoperoxidase assay by the addition of 20 μM AA to the reaction mixture. This time interval was determined to be sufficient to inactivate the isozymes with NDGA. Residual activity was determined by comparing the initial rates with inhibitor and 13-(S)-HPODE versus inhibitor alone, since the inhibitor itself lowers the rate of the oxygenation.
1.3 Results and Discussion
1.3.1 Evaluation of Hydroperoxides as Pseudoperoxidase Substrates
To further understand the pseudoperoxidase activity of the various LOXs, the primary products from AA and LA, 13-(S)-HPODE, 12-(S)-HPETE and 15-(S)-HPETE, were screened to determine if they were substrates for the XO pseudoperoxidase assay (Table 1). It was observed that 13-(S)-HPODE was the most effective substrate for all the LOX isozymes tested, as seen by the large consumption of the hydroperoxide product. Interestingly, 12-(S)-HPETE and 15-(S)-HPETE were not effective pseudoperoxidase substrates, especially with 12-LOX and 15-LOX-2. We attribute this to the fact that these LOX products can also be oxygenation substrates, resulting in doubly oxygenated products (i.e. di-HETEs). For both 12-LOX and 15-LOX-2, the oxygenation rates were comparable to the pseudoperoxidase rates, making measurements difficult. For these two isozymes, the HPETE concentration was increased to 40 μM to obtain more reliable data. In the case of 15-LOX-2, this increase in concentration allowed for a measurable pseudoperoxidase rate, above that of the oxygenation rate. However, these conditions did not allow for measurable pseudoperoxidase rates for 12-LOX. Therefore, 13-(S)-HPODE is the most reliable substrate for all of the LOX isozymes with the XO pseudoperoxidase assay.
Table 1.
Product decomposition percentages with the XO pseudoperoxidase assay.a
| 13-(S)-HPODE | 12-(S)-HPETE | 15-(S)-HPETE | |
|---|---|---|---|
| 5-LOX | 75%b | 80% | 100% |
| 12-LOX | 50% | <20%c | <20%c |
| 15-LOX-1 | 100% | 100% | 75% |
| 15-LOX-2 | 100% | 50%c | 50%c |
The assay was conducted over a 30 minute turnover period, using 20 μM hydroperoxide and 20 μM BWb70c within 50 mM Sodium Phosphate (pH 7.4), 0.3 mM CaCl2, 0.1 mM EDTA, and 0.01% Triton X100.
All values had an error of 20% or less.
Due to the oxygenation reaction, 40 μM hydroperoxide was used in these reactions.
1.3.2 Iron-xylenol Orange Pseudoperoxidase Inhibitor Assay
LOX inhibitors were screened against 5-LOX, 12-LOX, 15-LOX-1 and 15-LOX-2 to evaluate their ability to reduce the active ferric form of the isozyme. Initially the iron-xylenol orange (XO) pseudoperoxidase assay with 13-(S)-HPODE was utilized with the well-characterized reductive inhibitors, Zileuton and BWB70c (Figure 1). These two inhibitors were active against all isozymes screened, indicating that each isozyme is capable of oxidizing these two inhibitors and reducing 13-(S)-HPODE to complete the pseudoperoxidase cycle (Figure 2 and Table 2). 5-LOX and 15-LOX-1 displayed the most consistent pseudoperoxidase activities, displaying the greatest total consumption of 13-(S)-HPODE. Compound 1, does not support the assay, indicating that it is not a reductive inhibitor [29]. Compound 1 is a potent and selective 12-LOX inhibitor that is currently being investigated for its activity against diabetes and heart disease [29]. It has been proposed that compound 1 chelates the active site iron, similarly to Zileuton, however, it was not known at the time whether it was reductive in nature, since EPR spectroscopy was not possible. The current data indicates that compound 1 is distinct from Zileuton in that it does not reduce the ferric ion to the inactive ferrous state. The 15-LOX-1 inhibitor, compound 2, also does not display reductive LOX isozyme activity. Compound 2 is a potential therapeutic for stroke and these results are consistent with its inability to reduce the standard free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH) [30]. Interestingly, ascorbic acid (Figure 1), a known reductant with high concentrations in the cell, also supported the XO pseudoperoxidase assay. This is consistent with its chelative structure and reductive nature and suggests that ascorbic acid may facilitate the conversion of LOX isozymes to their inactive ferrous form in the cell. NDGA, however, did not support the XO pseudoperoxidase assay with any of the LOX isozymes. These results are inconsistent with our previous work, which demonstrated that NDGA and a variety of its derivatives displayed reductive activity against soybean LOX-1 [27]. These conflicting data suggested that the human LOX isozymes may interact differently with NDGA than soybean LOX-1, as previously seen with 5-LOX (vide infra) [37].
Table 2.
XO assay percent conversion results of LOX isozymes with various inhibitors.a
| Compound | 5-LOX | 12-LOX | 15-LOX-1 | 15-LOX-2 |
|---|---|---|---|---|
| Zileuton | 100% b | 50% | 100% | 100% |
| BWb70C | 100% | 50% | 100% | 100% |
| NDGA | 0% | 0% | 0% | 0% |
| Ascorbic acid | 50% | 50% | 85% | 75% |
| 1 | 0% | 0% | 0% | 0% |
| 2 | 0% | 0% | 0% | 0% |
The assay was conducted over a 30 minute turnover period, using 20 μM hydroperoxide and 40 μM inhibitor within 50 mM Sodium Phosphate (pH 7.4), 0.3 mM CaCl2, 0.1 mM EDTA, and 0.01% Triton X100.
All values had an error of 20% or less.
1.3.3 UV Pseudoperoxidase Activity Assay
As mentioned above, an alternative method to the XO pseudoperoxidase assay is to measure the decomposition of the hydroperoxide by monitoring the decrease at 234 nm. Unlike the XO pseudoperoxidase assay, in which the signal is produced by the hydroperoxide reacting directly with the ferrous xylenol orange complex, the UV pseudoperoxidase assay follows the decrease in absorbance (234 nm), which is a secondary decomposition of the alkoxyl radical after the hydroperoxide oxidation of the ferrous center [35,37]. Even though the UV pseudoperoxidase assay does not directly measure the decomposition of the hydroperoxide, it does have the advantage of being a continuous assay, allowing for the rate determination of pseudoperoxidase activities amongst the various human isozymes. Utilizing this method, BWb70c was screened against the lipoxygenase isozymes and observed that 15-LOX-l had the greatest Vmax at 20 uM 13-(S)-HPODE, with a rate of 3.3 +/− 0.06 mole/sec/mole, ~20 times the velocity of 12-LOX, and ~250 times that of 15-LOX-2 (Table 3). NDGA, however, did not display UV pseudoperoxidase activity with any of these three LOX isozymes, consistent with the results of the XO pseudoperoxidase assay. It should be noted that the low percent decomposition of 13-(S)-HPODE with the UV assay is due to the fact that this assay is detecting the secondary decomposition of the alkoxyl radical, not the primary decomposition of the hydroperoxide, as seen in the XO assay.
Table 3.
Rate comparison of LOX isozymes with the UV pseudoperoxidase activity.a
| 12-LOX | 15-LOX-1 | 15-LOX-2 | |
|---|---|---|---|
| Vmax
(mole/sec/mole) |
0.14 +/− 0.01 |
3.3 +/− 0.06 |
0.013 +/− 0.001 |
| Conversion (%) | 40% | 50% | 40% |
The assays were conducted using 20 μM hydroperoxide and 20 μM BWb70C within 50 mM Sodium Phosphate (pH 7.4), 0.3 mM CaCl2, 0.1 mM EDTA, and 0.01% Triton X100. The percent conversion was evaluated over a 30 minute time period.
1.3.4 Residual Oxygenase activity after the Pseudoperoxidase Reaction
Previous to this publication, NDGA was shown to be a reductive inhibitor against soybean LOX-1 [27], which was consistent with its catechol structure, but it was inactive in the 5-LOX pseudoperoxidase assay [37]. In this investigation, NDGA also did not support the pseudoperoxidase assay with 5-LOX, nor with 12-LOX, 15-LOX-1 and 15-LOX-2. A possible explanation for this lack of pseudoperoxidase activity by NDGA could be due to its radical chemistry and the auto-inactivation of the human LOX isozymes. It is known that human LOX isozymes auto-inactivate [52], presumably through oxidation of active site residues by radical intermediates generated in the catalytic process, but it has not been proven conclusively [53]. Considering that the pseudoperoxidase assay generates both hydroperoxide and inhibitor radicals (the one electron reduced hydroperoxide and the one electron oxidized inhibitor, Figure 2) it is possible that either radical could inactivate LOX. Therefore, the activity of the LOX isozymes were determined by adding AA, after a significant amount of 13-(S)-HPODE and reducing inhibitor were consumed via the pseudoperoxidase activity, and measuring the residual LOX activity (Table 4). By this method, LOX treated with a BWB70c/13-(S)-HPODE mixture showed significant activity after the pseudoperoxidase assay, relative to BWb70c alone. Note that the addition of BWB70c does inhibit the enzyme, but enough residual activity is observed to establish the relative activity of the LOX isozyme with and without product present. The competitive inhibitors, compounds 1 and 2, also retained residual activity, which would be expected from non-reducing inhibitors. In contrast, it was observed that 12-LOX, 15-LOX-1 and 15-LOX-2 did not demonstrate any oxygenase activity after the pseudoperoxidase turnover, when 13-(S)-HPODE and NDGA were added, relative to NDGA alone. As mentioned above, since the inactivity of LOX observed with 13-(S)-HPODE and NDGA is relative to inhibitor alone, this lack of activity is not due to the inherent inhibitory activity of NDGA, but rather is due to the cycling of the pseudoperoxidase activity with both 13-(S)-HPODE and NDGA being present. This data is consistent with the inactivation of human LOX isozymes being due to the presence of NDGA radicals generated through the pseudoperoxidase activity. This data also indicates that NDGA is a reducing inhibitor, but that a secondary radical is possibly the potent inhibitor to the human LOX isozymes. This is consistent with the work of Riendeau and coworkers, where they observed that NDGA and 13-(S)-HPODE did not support the pseudoperoxidase assay with 5-LOX [37]. We are currently investigating the nature of this radical and how it inactivates LOX, possibly thru a suicide inhibitor mechanism. It should be noted that it is unlikely that the 13-(S)-HPODE radicals, generated during the pseudoperoxidase activity, inactivate the LOX isozymes. The reaction of various reductive inhibitors (e.g. BWb70c, Zileuton and ascorbic acid) and 13-(S)-HPODE do not affect the oxygenase activity of LOX, even though 13-(S)-HPODE radicals are generated in all of these reactions.
Table 4.
Residual activity after completion of the pseudoperoxidase assay.a
| Enzyme | 13-(S)- HPODE/ NDGA |
13-(S)- HPODE/ BWb70c |
13-(S)- HPODE/ Compound #1 |
13-(S)- HPODE/ Compound #2 |
|---|---|---|---|---|
| 12-LOX | 0% b | 80% | 100% | 100% |
| 15-LOX-1 | 0% | 50% | 100% | 100% |
| 15-LOX-2 | 10% | 50% | 100% | 100% |
Enzyme was incubated for 2 min in 20 μM hydroperoxide and 20 μM inhibitor within 50 mM Sodium Phosphate (pH 7.4), 0.3 mM CaCl2, 0.1 mM EDTA, and 0.01% Triton X100. Residual activity was measured by addition of 20 μM AA, following the increase in absorbance at 234 nm.
All values had an error of 20% or less.
1.4 Conclusion
These results demonstrate that both the UV and XO assays are effective methods of detecting pseudoperoxidase activity for 5-LOX, 12-LOX, 15-LOX-l and 15-LOX-2, if 13-(S)-HPODE is used as the hydroperoxide substrate. The AA products, 12-(S)-HPETE 15-(S)-HPETE, are not consistent hydroperoxide substrates since they undergo a competing tranformation to the di-HETE products. These two assays are also effective methods for determining whether a particular inhibitor is reductive in nature with 5-LOX, 12-LOX, 15-LOX-l and 15-LOX-2 but there is a caveat. Reductive inhibitors generate radicals during the pseudoperoxidase assay, which in the case of NDGA can inactivate human LOX isozymes. Therefore inhibitors, which do not support the pseudoperoxidase assay, should also be investigated for rapid inactivation of the LOX isozyme in order to clarify the negative pseudoperoxidase result. In comparing the two pseudoperoxidase assays, both do not measure hydroperoxide levels directly, with the UV assay measuring a side reaction that records only partial degradation of the hydroperoxide. However, both assays do possess particular advantages, with the XO assay allowing for a high-throughput approach, as previously reported [46,47]. In contrast, the UV assay requires less set-up time, provides pseudoperoxidase rates and allows for the determination of enzyme inactivation. Given the advantages and disadvantages of these two assays, careful thought should be given when utilizing either method. Finally, the fact that ascorbic acid supports both the UV and XO pseudoperoxidase assays may imply wider consequences of the biological reactive state of LOX since ascorbic acid could help maintain the inactive ferrous form of LOX isozymes in the cell.
Acknowledgements
The authors would like to acknowledge Drs. David Maloney and Ganesha Rai for inhibitors.
Abbreviations
- LOX
lipoxygenase
- 15-LOX-2
human epithelial 15-lipoxygenase-2, 15-LOX-l, human reticulocyte 15-lipoxygenase-1
- 12-LOX
human platelet 12-lipoxygenase
- soybean LOX-1
soybean lipoxygenase 1
- 5-LOX
human 5-lipoxygenase
- COX
cyclooxygenase
- AA
arachiclonic acid
- LA
linoleic acid
- 12-HPETE
12-(S)-hydroperoxyeicosatetraenoic acid
- 15-HPETE
15-(S)-hydroperoxyeicosatetraenoic acid
- LA
linoleic acid
- 13-HPODE
13-(S)-hydroperoxyoctadecadienoic acid
- NDGA
nordihydroguaiaretic acid
- DPPH
l,l-diphenyl-2-picrylhydrazyl
- TBA
thiob arbituric acid
- XO
iron-xylenol orange
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by the National Institutes of Health (GM56062) and the Juve nile Diabetes Research Foundation (40-2009-711).
References
- 1.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Capdevila JH, Falck JR, Estabrook RW. Cytochrome P450 and the arachidonate cascade. FASEB J. 1992;6(2):731–736. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- 3.Haeggstrom JZ, Wetterholm A. Enzymes and receptors in the leukotriene cascade. Cell Mol Life Sci. 2002;59(5):742–753. doi: 10.1007/s00018-002-8463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim. Biophys. Acta. 1992;1128(2-3):117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 5.Solomon EI, Zhou J, Neese F, Pavel EG. New Insights from Spectroscopy into the structure/function relationships of lipoxygenases. Chem. Biol. 1997;4(11):795–808. doi: 10.1016/s1074-5521(97)90113-7. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim Biophys Acta. 1994;1212(1):1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 7.Nakano H, Inoue T, Kawasaki N, Miyataka H, Matsumoto H, Taguchi T, Inagaki N, Nagai H, Satoh T. Synthesis and biological activities of novel antiallergic agents with 5-lipoxygenase inhibiting action. Bioorg. Med. Chem. 2000;8(2):373–380. doi: 10.1016/s0968-0896(99)00291-6. [DOI] [PubMed] [Google Scholar]
- 8.Fogh K, Kragballe K. Eicosanoids in inflammatory skin diseases. Prostaglandins & other lipid mediators. 2000;63(1-2):43. doi: 10.1016/s0090-6980(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 9.Capra V, Bäck M, Barbieri SS, Camera M, Tremoli E, Rovati GE. Eicosanoids and Their Drugs in Cardiovascular Diseases: Focus on Atherosclerosis and Stroke. Medicinal research reviews. 2012 doi: 10.1002/med.21251. [DOI] [PubMed] [Google Scholar]
- 10.Rao CV, Janakiram NB, Mohammed A. Lipoxygenase and Cyclooxygenase Pathways and Colorectal Cancer Prevention. Current colorectal cancer reports. 2012:1–9. doi: 10.1007/s11888-012-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeung J, Apopa PL, Vesci J, Kenyon V, Rai G, Jadhav A, Simeonov A, Holman TR, Maloney DJ, Boutaud O. Protein kinase C regulation of 12-lipoxygenase-mediated human platelet activation. Molecular pharmacology. 2012;81(3):420–430. doi: 10.1124/mol.111.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR, Holinstat M. Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. Journal of Lipid Research. 2012 doi: 10.1194/jlr.M026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver JR, Holman TR, Imai Y, Jadhav A, Kenyon V, Maloney DJ, Nadler JL, Rai G, Simeonov A, Taylor-Fishwick DA. Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Molecular and Cellular Endocrinology. 2012 doi: 10.1016/j.mce.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Koeberle A, Zettl H, Greiner C, Wurglics M, Schubert-Zsilavecz M, Werz O. Pirinixic Acid Derivatives as Novel Dual Inhibitors of Microsomal Prostaglandin E2 Synthase-1 and 5-Lipoxygenase. Journal of Medicinal Chemistry. 2008;51(24):8068–8076. doi: 10.1021/jm801085s. [DOI] [PubMed] [Google Scholar]
- 15.McMillan RM, Walker ER. Designing therapeutically effective 5-lipoxygenase inhibitors. Trends Pharmacol Sci. 1992;13(8):323–30. doi: 10.1016/0165-6147(92)90100-k. [DOI] [PubMed] [Google Scholar]
- 16.Deschamps JD, Kenyon VA, Holman TR. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg Med Chem. 2006;14(12):4295–301. doi: 10.1016/j.bmc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 17.Segraves EN, Shah RR, Segraves NL, Johnson TA, Whitman S, Sui JK, Kenyon VA, Cichewicz RH, Crews P, Holman TR. Probing the activity differences of simple and complex brominated aryl compounds against 15-soybean, 15-human, and 12-human lipoxygenase. J Med Chem. 2004;47(16):4060–5. doi: 10.1021/jm049872s. [DOI] [PubMed] [Google Scholar]
- 18.Falgueyret JP, Hutchinson JH, Riendeau D. Criteria for the identification of non-redox inhibitors of 5-lipoxygenase. Biochemical Pharmacology. 1993;45(4):978–81. doi: 10.1016/0006-2952(93)90185-y. [DOI] [PubMed] [Google Scholar]
- 19.Pergola C, Werz O. 5-Lipoxygenase inhibitors: a review of recent developments and patents. Expert Opinion on Therapeutic Patents. 2010;20(3):355–375. doi: 10.1517/13543771003602012. [DOI] [PubMed] [Google Scholar]
- 20.Robert N Y. Inhibitors of 5-lipoxygenase: a therapeutic potential yet to be fully realized? European Journal of Medicinal Chemistry. 1999;34(9):671–685. [Google Scholar]
- 21.Carter GW, Young PR, Albert DH, Bouska J, Dyer R, Bell RL, Summers JB, Brooks DW. 5-lipoxygenase inhibitory activity of zileuton. J Pharmacol Exp Ther. 1991;256(3):929–37. [PubMed] [Google Scholar]
- 22.McGill KA, Busse WW. Zileuton. The Lancet. 1996;348(9026):519–524. doi: 10.1016/S0140-6736(95)12297-4. [DOI] [PubMed] [Google Scholar]
- 23.Bell RL, Young PR, Albert D, Lanni C, Summers JB, Brooks DW, Rubin P, Carter GW. The discovery and development of zileuton: an orally active 5-lipoxygenase inhibitor. Int J Immunopharmacol. 1992;14(3):505–10. doi: 10.1016/0192-0561(92)90182-k. [DOI] [PubMed] [Google Scholar]
- 24.Musser JH, Kreft AF. 5-Lipoxygenase: properties, pharmacology, and the quinolinyl(bridged)aryl class of inhibitors. Journal of Medicinal Chemistry. 1992;(14):35, 2501–2524. doi: 10.1021/jm00092a001. [DOI] [PubMed] [Google Scholar]
- 25.Lewis AJ, Glaser KB, Sturm RJ, Molnar-Kimber KL, Bansbach CC. Strategies for the development of new antiarthritic agents. International journal of immunopharmacology. 1992;14(3):497–504. doi: 10.1016/0192-0561(92)90181-j. [DOI] [PubMed] [Google Scholar]
- 26.Yeadon M, Dougan F, Petrovic A, Beesley J, Payne A. Effect of BW B70C, a novel inhibitor of arachidonic acid 5-lipoxygenase, on allergen-induced bronchoconstriction and late-phase lung eosinophil accumulation in sensitised guinea-pigs. Inflammation Research. 1993;38:8–18. doi: 10.1007/BF02027207. [DOI] [PubMed] [Google Scholar]
- 27.Whitman S, Gezginci M, Timmermann BN, Holman TR. Structure-activity relationship studies of nordihydroguaiaretic acid inhibitors toward soybean, 12-human, and 15-human lipoxygenase. J. Med. Chem. 2002;45(12):2659–2661. doi: 10.1021/jm0201262. [DOI] [PubMed] [Google Scholar]
- 28.Reddanna P, Krishna Rao M, Channa Reddy C. Inhibition of 5-lipoxygenase by vitamin E. FEBS letters. 1985;193(1):39–43. doi: 10.1016/0014-5793(85)80075-2. [DOI] [PubMed] [Google Scholar]
- 29.Kenyon V, Rai G, Jadhav A, Schultz L, Armstrong M, Jameson JB, Perry S, Joshi N, Bougie JM, Leister W, Taylor-Fishwick DA, Nadler JL, Holinstat M, Simeonov A, Maloney DJ, Holman TR. Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. J Med Chem. (2nd) 2011;54(15):5485–97. doi: 10.1021/jm2005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rai G, Kenyon V, Jadhav A, Schultz L, Armstrong M, Jameson JB, Hoobler E, Leister W, Simeonov A, Holman TR, Maloney DJ. Discovery of potent and selective inhibitors of human reticulocyte 15-lipoxygenase-1. J Med Chem. 2010;53(20):7392–7404. doi: 10.1021/jm1008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walenga RW, Showell HJ, Feinstein MB, Becker EL. Parallel inhibition of neutrophil arachidonic acid metabolism and lysosomal enzyme secretion by nordihydroguaiaretic acid. Life Sciences. 1980;27(12):1047–1053. doi: 10.1016/0024-3205(80)90028-4. [DOI] [PubMed] [Google Scholar]
- 32.Kemal C, Louis-Flamberg P, Krupinski-Olsen R, Shorter A. Reductive Inactivation of Soybean Lipoxygenase-1 by Catechols. Biochemistry. 1987;26:7064–7072. doi: 10.1021/bi00396a031. [DOI] [PubMed] [Google Scholar]
- 33.Nelson MJ, Cowling RA, Seitz SP. Structural Characterization of Alkyl and Peroxyl Radicals in Solutions of Purple Lipoxygenase. Biochemistry. 1994;33(16):4966–4973. doi: 10.1021/bi00182a027. [DOI] [PubMed] [Google Scholar]
- 34.Pham C, Jankun J, Skrzypczak-Jankun E, Flowers RA, Funk MO. Structure of the Soybean Lipoxygenase-NitroCatechol Complex. Biochemistry. 1998;37:17952–17957. doi: 10.1021/bi981989t. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, Brash AR. On the Role of Molecular Oxygen in Lipoxygenase Activation. Journal of Biological Chemistry. 2010;285(51):39876–39887. doi: 10.1074/jbc.M110.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamulitrat W, Hughes MF, Eling TE, Mason RP. Superoxide and peroxyl radical generation from the reduction of polyunsaturated fatty acid hydroperoxides by soybean lipoxygenase. Archives of biochemistry and biophysics. 1991;290(1):153–159. doi: 10.1016/0003-9861(91)90601-e. [DOI] [PubMed] [Google Scholar]
- 37.Riendeau D, Falgueyret JP, Guay J, Ueda N, Yamamoto S. Pseudoperoxidase Activity of 5-Lipoxygenase Stimulated by Potent Benzofuranol and N-Hydroxyurea Inhibitors of the Lipoxygenase Reaction. Biochem. J. 1991;274:287–292. doi: 10.1042/bj2740287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Li J, Follett PL, Zhang Y, Cotanche DA, Jensen FE, Volpe JJ, Rosenberg PA. 12-Lipoxygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes. European Journal of Neuroscience. 2004;20(8):2049–58. doi: 10.1111/j.1460-9568.2004.03650.x. [DOI] [PubMed] [Google Scholar]
- 39.van Leyen K, Arai K, Jin G, Kenyon V, Gerstner B, Rosenberg PA, Holman TR, Lo EH. Novel lipoxygenase inhibitors as neuroprotective reagents. Journal of Neuroscience Research. 2008;86(4):904–9. doi: 10.1002/jnr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mair R, Graupner AJ. Determination of organic peroxides by iodine liberation procedures. Analytical Chemistry. 1964;36(1):194–204. [Google Scholar]
- 41.Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. Journal of Photochemistry and Photobiology B: Biology. 2001;63(1):103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 42.Jiang ZY, Woollard ACS, Wolff SP. Lipid hydroperoxide measurement by oxidation of Fe 2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991;26(10):853–856. doi: 10.1007/BF02536169. [DOI] [PubMed] [Google Scholar]
- 43.Ferrer AS, Santema JS, Hilhorst R, Visser AJWG. Fluorescence detection of enzymatically formed hydrogen peroxide in aqueous solution and in reversed micelles. Analytical biochemistry. 1990;187(1):129–132. doi: 10.1016/0003-2697(90)90429-d. [DOI] [PubMed] [Google Scholar]
- 44.Frew JE, Jones P, Scholes G. Spectrophotometric determination of hydrogen peroxide and organic hydropheroxides at low concentrations in aqueous solution. Analytica Chimica Acta. 1983;155:139–150. [Google Scholar]
- 45.Dahlström M, Forsström D, Johannesson M, Huque-Andersson Y, Björk M, Silfverplatz E, Sanin A, Schaal W, Pelcman B, Forsell PKA. Development of a fluorescent intensity assay amenable for high-throughput screening for determining 15-lipoxygenase activity. Journal of biomolecular screening. 2010;15(6):671–679. doi: 10.1177/1087057110373383. [DOI] [PubMed] [Google Scholar]
- 46.Waslidge NB, Hayes DJ. A colorimetric method for the determination of lipoxygenase activity suitable for use in a high throughput assay format. Anal Biochem. 1995;231(2):354–8. doi: 10.1006/abio.1995.0063. [DOI] [PubMed] [Google Scholar]
- 47.Gay C, Collins J, Gebicki JM. Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem. 1999;273(2):149–55. doi: 10.1006/abio.1999.4208. [DOI] [PubMed] [Google Scholar]
- 48.Deschamps JD, Gautschi JT, Whitman S, Johnson TA, Gassner NC, Crews P, Holman TR. Discovery of platelet-type 12-human lipoxygenase selective inhibitors by high-throughput screening of structurally diverse libraries. Bioorganic & Medicinal Chemistry. 2007;15(22):6900–8. doi: 10.1016/j.bmc.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amagata T, Whitman S, Johnson TA, Stessman CC, Loo CP, Lobkovsky E, Clardy J, Crews P, Holman TR. Exploring sponge-derived terpenoids for their potency and selectivity against 12-human, 15-human, and 15-soybean lipoxygenases. J Nat Prod. 2003;66(2):230–5. doi: 10.1021/np020462l. [DOI] [PubMed] [Google Scholar]
- 50.Vasquez-Martinez Y, Ohri RV, Kenyon V, Holman TR, Sepulveda-Boza S. Structure-activity relationship studies of flavonoids as potent inhibitors of human platelet 12-hLO, reticulocyte 15-hLO-1, and prostate epithelial 15-hLO-2. Bioorganic & Medicinal Chemistry. 2007;15(23):7408–25. doi: 10.1016/j.bmc.2007.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson SJ, Hoobler EK, Riener M, Loveridge ST, Tenney K, Valeriote FA, Holman TR, Crews P. Using enzyme assays to evaluate the structure and bioactivity of sponge-derived meroterpenes. Journal of Natural Products. 2009;72(10):1857–63. doi: 10.1021/np900465e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds CH. Inactivation of soybean lipoxygenase by lipoxygenase inhibitors in the presence of 15-hydroperoxyeicosatetraenoic acid. Biochemical Pharmacology. 1988;37(23):4531–7. doi: 10.1016/0006-2952(88)90669-7. [DOI] [PubMed] [Google Scholar]
- 53.Gan QF, Witkop GL, Sloane DL, Straub KM, Sigal E. Identification of a Specific Methionine in Mammalian 15-Lipoxygenase which is Oxygenated by the Enzyme Product 13-HPODE - Dissociation of Sulfoxide Formation from Self-Inactivation. Biochemistry. 1995;34(21):7069–7079. doi: 10.1021/bi00021a019. [DOI] [PubMed] [Google Scholar]