Abstract
Neural progenitor cells have been proposed as a therapy for central nervous system disorders, including neurodegenerative diseases and trauma injuries, however their accessibility is a major limitation. We recently isolated Tuj1+ cells from skeletal muscle culture of Nestin-GFP transgenic mice however whether they form functional neurons in the brain is not yet known. Additionally, their isolation from nontransgenic species and identification of their ancestors is unknown. This gap of knowledge precludes us from studying their role as a valuable alternative to neural progenitors. Here, we identified two pericyte subtypes, type-1 and type-2, using a double transgenic Nestin-GFP/NG2-DsRed mouse and demonstrated that Nestin-GFP+/Tuj1+ cells derive from type-2 Nestin-GFP+/NG2-DsRed+/CD146+ pericytes located in the skeletal muscle interstitium. These cells are bipotential as they generate either Tuj1+ cells when cultured with muscle cells or become “classical” α-SMA+ pericytes when cultured alone. In contrast, type-1 Nestin-GFP-/NG2-DsRed+/CD146+ pericytes generate α-SMA+ pericytes but not Tuj1+ cells. Interestingly, type-2 pericyte derived Tuj1+ cells retain some pericytic markers (CD146+/PDGFRβ+/NG2+). Given the potential application of Nestin-GFP+/NG2-DsRed+/Tuj1+ cells for cell therapy, we found a surface marker, the nerve growth factor receptor, which is expressed exclusively in these cells and can be used to identify and isolate them from mixed cell populations in nontransgenic species for clinical purposes.
Keywords: skeletal muscle, neural cells, pericytes
INTRODUCTION
Neural progenitor-based therapies are rapidly emerging as a potential strategy for central nervous system (CNS) regeneration in patients with neurodegenerative diseases or injury. The aim of this therapy is to replace, repair, or enhance the biological functions of damaged tissues. However, neural progenitors availability, immune reaction and ethical concerns are limitations for its medical application. The skeletal muscle, as one of the largest organs in the body, represents an alternative source of stem cells, which can be easily obtained in large quantities through a biopsy procedure performed in an outpatient clinic (Wu et al., 2010). Thus, the skeletal muscle may be a convenient and enriched source of neural cells that may circumvent these limitations.
Previously, we isolated Nestin-GFP+ cells in cultured adult skeletal muscle from Nestin-GFP transgenic mice, which exhibit characteristics of neural progenitors such as morphology, specific neural markers profile, replicative capacity, ability to form neurospheres, and functional response to neurotransmitter (Birbrair et al., 2011). Our laboratory is working to determine whether these cells form functional neurons in the brain.
However, as their origin remains unclear, here, we aimed to determine their ancestor(s) in the skeletal muscle. The skeletal muscle is highly vascularized and contains a variety of mononucleated cells, including blood, endothelial, fibroblasts, myofiber satellite, immune, and pericytes. Pericytes can be recognized by their position in the microvasculature more than by a precise phenotype (Feng et al., 2011; Sa-Pereira et al., 2012). Their role as stem cells contributing to formation of tissues other than blood vessels has been reported in numerous publications (Alliot-Licht et al., 2001; Caplan, 2007; Crisan et al., 2008; Dellavalle et al., 2007; Dore-Duffy et al., 2011; Feng et al., 2011; Lin et al., 2008; Maier et al., 2010; Nehls and Drenckhahn, 1993; Shi and Gronthos, 2003). Although pericytes may have inherent potential to differentiate into multiple lineages if exposed to appropriate epigenetic signals, this capacity may differ between tissues (Bianco et al., 2008; Sacchetti et al., 2007; Shi and Gronthos, 2003). Here, by using the Nestin-GFP/NG2-DsRed transgenic mouse, we found that skeletal muscle derived Tuj1+ cells differ from classic pericytes but derive from a pericyte subtype that expresses a specific combination of markers, Nestin-GFP+/NG2-DsRed+/CD146+. Interestingly, we also determined that another pericyte subtype, Nestin-GFP-/NG2-DsRed+/CD146+, found in the skeletal muscle, does not have the ability to differentiate into Tuj1+ cells under the same conditions. Moreover, we found that pericyte derived neural Tuj1+ cells are the only cells to express NGF receptor (NGFR, p75) in skeletal muscle cultures, which can be used as a surface marker to distinguish and isolate them from all other cell types obtained from nontransgenic species.
This work demonstrates the heterogeneity of the pericyte population in the skeletal muscle and its distinct differentiation potential. Based on this analysis, we envision the possibility of using pericyte derived Nestin-GFP+/NGF receptor+/Tuj1+ cells to treat diverse pathologies, including neurodegenerative diseases, neoplasias, and CNS trauma lesions.
MATERIALS AND METHODS
Animals
Nestin-GFP transgenic mice were maintained homozygous for the transgene on the C57BL/6 genetic background (Mignone et al., 2004). C57BL/6 wild-type mice were used as controls. NG2-DsRed transgenic mice expressing DsRed-T1 under the control of the NG2 promoter (Zhu et al., 2008) were purchased from the Jackson Laboratory. Nestin-GFP mice were crossbred with NG2-DsRed mice to generate Nestin-GFP/NG2-DsRed double-transgenic mice. All mouse colonies were housed at Wake Forest School of Medicine (WFSM) in a pathogen-free facility of the Animal Research Program under 12:12-h light/dark cycle and fed ad libitum. Both male and female homozygous mice were used, and their ages ranged from 3 to 5 months. Animal handling and procedures were approved by the WFSM Animal Care and Use Committee.
Flexor digitorum brevis (FDB) muscle culture preparation
FDB muscle from Nestin-GFP transgenic, NG2-DsRed transgenic, Nestin-GFP/NG2-DsRed transgenic, and C57BL/6 wild-type mice were used for most experiments in this work. FDB muscle was preferred over more traditional muscles for most experiments because it is small and flat, allowing more complete dissociation by trituration in a single step, shortening the experiment significantly (Zhang et al., 2011). Methods for FDB culture preparation have been described (Birbrair et al., 2011). Briefly, muscles were carefully dissected away from the surrounding connective tissue and minced, then digested by gentle agitation in 0.2% (w/v) Worthington’s type-2 collagenase in Krebs solution at 37°C for 2 hours. They were resuspended in growth medium and dissociated by gentle trituration. The growth medium used to plate cell cultures consisted of DMEM-high glucose (Invitrogen, Carlsbad, CA, USA), supplemented with 2% L-glutamine, 50 U/ml penicillin and 50 mg/ml streptomycin, 10% (v/v) horse serum (Invitrogen) and 0.5% (v/v) CEE (Gemini Bio-products, West Sacramento, CA, USA). It supported both proliferation and differentiation of myogenic cells (Zammit et al., 2004).
Immunocytochemistry
Cultured cells were fixed with 4% PFA for 30 minutes, then permeabilized in 0.5% Triton X-100 (Sigma, St. Louis, MO, USA), and blocked to saturate nonspecific antigen sites using 5% (v/v) goat serum/PBS (Jackson Immunoresearch Labs, West Grove, PA, USA) overnight at 4°C. The next day, the cells were incubated with primary antibodies at room temperature for 4 h and visualized using appropriate species-specific secondary antibodies conjugated with Alexa Fluor 488, 568, 647 or 680 at 1:1000 dilution (Invitrogen). They were counterstained with Hoechst 33342 reagent at 1:2000 dilution (Invitrogen) to label the DNA and mounted on slides for fluorescent microscopy with Fluorescent Mounting Medium (DakoCytomation, Carpinteria, CA, USA).
Primary antibodies
Table 1 shows antibodies, their dilution, and source.
Table 1.
Antibodies, concentration, and source
| Antibody | Dilution | Source | Location |
|---|---|---|---|
| Rabbit monoclonal anti-Tuj1 | 1:800 | Covance | Princeton, NJ |
| Mouse monoclonal anti-α-smooth muscle actin | 1:1000 | Sigma | St. Louis, MO |
| Rabbit polyclonal anti-NG2 Chondroitin Sulfate | 1:100 | Chemicon-Millipore | Temecula, CA |
| Rabbit anti-PDGFRβ | 1:250 | Dr. W. Stallcup | Sanford-Burnham Medical Research Institute, CA |
| Rabbit monoclonal anti-NGF receptor (p75) | 1:200 | Epitomics | Burlingame, CA |
| Rat monoclonal anti-CD146 | 1:100 | Miltenyi biotec | Auburn, CA |
| CD146(LSEC)-FITC conjugated | 1:500 | Miltenyi biotec | Auburn, CA |
| Mouse monoclonal anti-connexin 43 (Cx43IF1) | 1:100 | Fred Hutchinson Cancer Research Center | Seattle, WA |
| Rat anti-CD31 (PECAM-1) | 1:100 | BD Biosciences | San Jose, CA |
| Rabbit anti-NGFR | 1:100 | Advanced Targeting Systems | San Diego, CA |
| PerCP/Cy5.5 anti-mouse CD146 | 1:500 | BioLegend | San Diego, CA |
Skeletal muscle processing
To detect DsRed and GFP fluorescence, nondissociated extensor digitorum longus (EDL) muscles from 3-month-old Nestin-GFP/NG2-DsRed mice were dissected, fixed in 4% paraformaldehyde overnight, immersed in 10%, 20%, and 30% sucrose solutions for 60, 45, and 30 minutes, respectively, embedded in OCT, and rapidly frozen in liquid nitrogen to prepare 10-μm thick cryosections. Muscle sections were fixed with 4% PFA for 30 minutes, then permeabilized in 0.5% Triton X-100 (Sigma), and blocked to saturate nonspecific antigen sites using 5% (v/v) goat serum/PBS (Jackson Immunoresearch Labs) overnight at 4°C. The next day, the sections were incubated with primary antibodies at room temperature for 4 h and visualized using appropriate species-specific secondary antibodies conjugated with Alexa Fluor 488, 568, 647 or 680 at 1:1000 dilution (Invitrogen). Muscle sections were counterstained with Hoechst 33342, mounted on slides using Fluorescent Mounting Medium (DakoCytomation), and examined with fluorescence microscopy.
Microscopy, Cell Imaging, and Counting
An inverted motorized fluorescent microscope (Olympus, IX81, Tokyo, Japan) with an Orca-R2 Hamamatsu CCD camera (Hamamatsu, Japan) was used for image acquisition. Camera drive and acquisition were controlled by a MetaMorph Imaging System (Olympus, Center Valley, PA, USA). Ten arbitrary microscopic fields were counted in each immunostained plate, and values pooled from parallel duplicates per time point and individual experiment.
Fluorescence-activated cell sorting (FACS)
FACS was carried out on a BD FACS (Aria Sorter, San Jose, CA, USA) at 4°C and a pressure of 20 psi, using a laser at the 488-nm line, a 530/30 band pass filter, a 100-μm sorting tip, and a 34.2 kHz drive frequency, sterilized with 10% bleach. This instrument allowed us to characterize cells by size as well as fluorescence. Low flow rate improved sorting purity. Data acquisition and analyses were performed using BD FACS Diva 5.0.3 software, gated for a high level of GFP, DsRed, PerCP/Cy5.5 and/or APC expression. For instance, the clear separation of GFP+ from GFP- cells (Birbrair et al., 2011), and DsRed+ from DsRed- cells explains the ease of sorting. Sorted cells were re-analyzed to confirm their fluorescence profile.
Isolation of NGFR+/Tuj1+ cells from skeletal muscle cultures of nontransgenic mice by FACS
FDB cultures from young-adult (3-5-month) C57BL/6 wild-type mice were prepared as described above. After 7 days, the cells were scraped from the dishes, dissociated by trituration and resuspended in 5 mM EDTA in PBS for 15 min at 4°C. After centrifuging at 1500 rpm for 5 min, the supernatant was removed, and the pellet resuspended in growth medium. Aggregates were removed by passing them through a 40-μm cell strainer prior to sorting. After counting, the cells were centrifuged at 1500 rpm for 5 min and resuspended in 100-μl 1% FBS in PBS /106 cells. An aliquot was used as an unlabeled control, while the remaining cells were labeled with 5 μg/mL of rabbit anti-NGFR antibody (Advanced Targeting Systems) for 1 hour. This antibody has been used to isolate NGFR+ cells from the brain (Schnitzler et al., 2008). After one washing, the cells were labeled with APC anti-rabbit antibody (Invitrogen) for 45 minutes and, after a second washing, resuspended in 1% FBS in PBS and analyzed for forward scatter and NGFR-APC to sort out NGFR+ cells. After sorting, 2-3×104 cells/cm2 were plated on laminin (Invitrogen) precoated dishes (Fisher Scientific, Pittsburgh, PA). Purity, neural morphology, and Tuj1 expression were analyzed after 4 days in culture.
Cell isolation from Nestin-GFP/NG2-DsRed mice skeletal muscle by FACS
A pool of hindlimb muscles, excluding those from the foot, was used for experiments that required a large number of cells as indicated. Cells were sorted immediately after skeletal muscle dissociation (time 0). Hindlimb muscles from young-adult (3-5 month) Nestin-GFP/NG2-DsRed transgenic mice were prepared as described (Birbrair et al., 2011). Briefly, muscles were carefully dissected away from the surrounding connective tissue and minced, then digested by gentle agitation in 0.2% (w/v) type-2 collagenase in Krebs solution at 37°C for 2 hours, and dissociated by trituration and resuspension in 0.25% trypsin/0.05% EDTA in PBS for 15 minutes at 37°C. After centrifuging at 1500 rpm for 5 minutes, the supernatant was removed, and the pellet resuspended in growth medium. Aggregates were removed by passing them through a 40-μm cell strainer prior to sorting. Cells were centrifuged at 1500 rpm for 5 minutes. The supernatant was removed, and the pellet resuspended in 1% FBS in PBS and analyzed for GFP and DsRed fluorescence to sort the different cell populations based on these two markers. Isolated Nestin-GFP+/NG2-DsRed-, Nestin-GFP-/NG2-DsRed+, Nestin-GFP+/NG2-DsRed+, and Nestin-GFP-/NG2-DsRed- cells were used for single cell RT-PCR or cultured either separately or together with FDB culture from wild-type mice. Neural morphology, and Nestin-GFP, Tuj1, and α-SMA expression were analyzed after 8 days in culture.
Single cell RT-PCR
After sorting, Nestin-GFP+/NG2-DsRed-, Nestin-GFP-/NG2-DsRed+, Nestin-GFP+/NG2-DsRed+, or Nestin-GFP-/NG2-DsRed- cells were transferred to a separate tube containing 1 ml of growth medium, homogenized and counted. The cells were diluted to obtain a concentration of 1 cell / 10 μl. The resulting cell suspension was dispensed into laminin pre-coated wells of a 24-well plate, putting only 10 μl in each well containing 200 μl of growth medium. Each well was expected to yield none or one cell per well, based on our experience with this technique previously. Cells were identified visually using an inverted motorized fluorescent microscope (Olympus). For cell harvest, fine tip transfer pipets (Fisher Scientific) coupled to a standard patch-clamp pipettes pulled from borosilicate glass (Boralex, WPI, Sarasota, FL, USA) using a Flaming Brown micropipette puller (P97, Sutter Instrument Co., Novato, CA, USA) were used. A pipette was moved into the bath solution under positive pressure. Under visual control, the tip of the pipette was gently attached to the selected cell and suction was applied, until the cell entered the tip of the pipette. The pipette was removed quickly from the bath. Under a dissecting microscope, the content of the pipette was ejected into a PCR tube, centrifuged at 1500 rpm for 5 minutes and all the solution removed. The cell was resuspended in the RNA protecting solution: (4 μl of H2O with 15 U RNAse-inhibitor (Promega, Fitchburg, WI, USA), 25 mM DTT). Then, we added 2 μl of nucleotide/detergent solution (10 mM Tris–HCl, pH 8.0, 25 μM random hexamers (Promega), 2.5 mM of each dNTP (Promega), 0.2% Nonidet P40 (Roche, Indianapolis, IN, USA), denatured at 70 °C for 5 minutes and placed it on ice for 5 minutes. After this, 2 μl of 5X First-strand buffer (Invitrogen), 0.5 μl of 200 mM DTT, 20 U RNAse-inhibitor (Promega) and 100 U superscript III reverse transcriptase (Invitrogen) were added. The RT reaction (final volume: 10 μl) was incubated at 37 °C for 1 h and stopped at 95 °C for 5 minutes. As negative controls, the RT reactions were performed in the absence of either cell (only growth medium tested) or reverse transcriptase.
cDNA purification
For cDNA purification, the QIAEX II gel extraction Kit (Qiagen, Valencia, CA, USA) was used. The complete RT reaction (10 μl) was mixed with 80 μl QX1 binding buffer and 1.5 μl DNA-binding matrix per RT reaction at 25 °C for 15 minutes mixing every 2 minutes to keep QIAEX in suspension. The binding matrix was pelleted (13,000 rpm, 2 minutes) and washed twice in 90 μl ice-cold, ethanol-based PE-buffer (Qiagen). The DNA-binding matrix was dried at 37 °C for 10 minutes to remove the ethanol completely. Finally, the cDNA was eluted with an appropriate volume (>5 μl) of 1 mM Tris-HCl, pH 8.5 at 50 °C for 3 minutes. The binding matrix was pelleted (13,000 rpm, 2 minutes) and the supernatant containing cDNA material was stored at - 80 °C until use for PCR.
Polymerase Chain Reaction (PCR)
The cDNA was amplified by PCR using the primers included in Table 2. PCR Master Mix was purchased from Promega. Each PCR reaction contained 1× Promega PCR Master Mix, 1 μM of each primer, and the cDNA of the cell used in each case (Nestin-GFP+/NG2-DsRed-, Nestin-GFP-/NG2-DsRed+, Nestin-GFP+/NG2-DsRed+, or Nestin-GFP-/NG2-DsRed- cell). The volume of each reaction was brought up to 50 μl with water. DNA amplification was carried out as follows: denaturation at 94°C for 2 minutes, followed by 35 cycles of 94°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes. After 35 cycles, the reactions were incubated at 72°C for 7 minutes to increase the yield of amplification. PCR products were verified with DNA 2% agarose gel electrophoresis.
Table 2.
Genes, GenBank Accession numbers, Coding regions, and Primers
| Gene | GenBank Accession numbers | Coding regions | Forward primer and positions | Reverse primer and positions |
|---|---|---|---|---|
| Nestin | NM_016701.3 | CDS: 111-5705 | AGGACCAGGTGCTTGAGAGA (2248- 2267) | TCCTCTGCGTCTTCAAACCT (2383- 2364) |
| NG2 | NM_139001.2 | CDS: 87-7070 | GCACGATGACTCTGAGACCA (3020-3039) | AGCATCGCTGAAGGCTACAT (3242-3223) |
| CD146 | NM_023061.2 | CDS: 33-1797 | AAGAGGAGAGCACCGATGAA (922-941) | TTACTTTCTGCCTCGCAGGT (1147-1128) |
| PDGFRβ | NM_001146268.1 | CDS: 430-3729 | CCGGAACAAACACACCTTCT (2511-2530) | TATCCATGTAGCCACCGTCA (2656-2637) |
| GAPDH | NM_008084.2 | CDS: 51-1052 | GTGGCAAAGTGGAGATTGTTGC C (118-140) | GATGATGACCCTTTTGGCTCC (407-387) |
Cell isolation using CD146-PerCP-Cy5.5 antibody from Nestin-GFP mouse skeletal muscle by FACS
Hindlimb muscle cells were isolated from young-adult (3-5-month) C57BL/6 wild-type mice as described above. After counting, cells were centrifuged at 1500 rpm for 5 min and resuspended in 100-μl 1% FBS in PBS /106 cells. An aliquot was collected for use as unlabeled control, while the remaining cells were labeled with PerCP/Cy5.5 anti-mouse CD146 antibody for 1 hour. After washing, the cells were resuspended in 1% FBS in PBS and sorted using GFP and PerCP/Cy5.5 fluorescence. Isolated Nestin-GFP+/CD146-PerCP-Cy5.5-, Nestin-GFP-/CD146-PerCP-Cy5.5+, Nestin-GFP+/CD146-PerCP-Cy5.5+, and Nestin-GFP-/CD146-PerCP-Cy5.5- cells were cultured either separately or with medium from 7-day-old FDB culture. Neural morphology and Tuj1 expression were analyzed after 8 days in culture.
Isolation of CD146+/CD31- cells from skeletal muscle by FACS
Cells isolation from hindlimb muscles from young-adult (3-5-month) C57BL/6 wild-type mice was done as described above (Birbrair et al., 2011). To exclude endothelial cells (CD31+), magnetic separation was performed using a MACS system (Miltenyi Biotec Inc., Auburn, CA). Briefly, dissociated cells were resuspended in MACS buffer (Miltenyi) (90-μl/107 cells) and labeled with endothelial cell marker anti-CD31 beads (Miltenyi) by adding 10-μl beads/107 cells and incubating for 15 min at 4°C. After washing, the cells were resuspended in 500-μl buffer/108 cells. Magnetic separation of the cells was performed on a MACS column (Miltenyi), where CD31+ endothelial cells were retained and eluted only upon removal from the magnetic field, according to the manufacturer’s instructions. CD31- cells, obtained from MACS sorting, were centrifuged at 1500 rpm for 5 min. The supernatant was removed, and the pellet resuspended in 1% FBS in PBS. An aliquot of these cells was collected for use as unlabeled control, while the remaining cells were labeled with CD146-FITC-conjugated antibody (Miltenyi) for 1 hour. After washing, the cells were resuspended in 1% FBS in PBS and analyzed for forward scatter and CD146-FITC to sort the CD146+ and CD146- cells that were cultured either separately, together, or with medium from 7 day-old FDB culture. Neural morphology and Tuj1 expression were analyzed after 8 days in culture.
Statistical Analysis
Results are expressed as the mean ± s.e.m. Statistical significance was assessed using analysis of variance (ANOVA) followed by t-test using Prism GraphPad. P < 0.05 was considered significant.
RESULTS
Nestin-GFP+/Tuj1+ cells share some markers with pericytes
Nestin-GFP+/Tuj1+ cells are obtained from a pool of hindlimb skeletal muscle interstitial cells. As their properties are poorly understood (Birbrair et al., 2011), we sought to define their relationship with mesenchymal cells and lineage, by examining their marker-expression profile. All Nestin-GFP+ cells have neural morphology and express Tuj1 (class III β tubulin), a neural progenitor marker (Erceg et al., 2008), after 7 days in culture (Birbrair et al., 2011). At this culture time, Nestin-GFP+ cells did not exhibit classical markers of pericytes, connexin 43 (Cx43) and α-SMA (Figs. 1A, B), and their morphological properties, small cytoplasm and thin, multipolar extensions, differed from classic fibroblastoid pericytes (Farrington-Rock et al., 2004). Cx43, which has been reported in fibroblasts (Zhang et al., 2008) and pericytes (Mogensen et al., 2011), was found in the pool of Nestin-GFP- cells, representing 10 ± 2.0 % of all cells in culture. The α-SMA marker, which has been found in vascular smooth muscle cells (Bockmeyer et al., 2012) and pericytes (Mogensen et al., 2011), is also present in Nestin-GFP-cells, accounting for 29 ± 5.8 % of all cells (Figs. 1A, B). All Nestin-GFP+/Tuj1+ cells exhibit some markers of pericytes, CD146+ /PDGFRβ+/NG2+ (Fig. 1A, B), while some Nestin-GFP-cells also present them in cultures in a small percent (4.8 ± 1.4 % CD146+, 5.2 ± 1.1 % PDGFRβ+, and 3.8 ± 0.6 % NG2+ cells) (Fig. 1A, B). Very few Nestin-GFP+ cells negative for these three markers were detected (CD146-: 0.2 ± 0.2 %, PDGFRβ-: 0.3 ± 0.3 %, and NG2-cells: 0.1 ± 0.1 %). Previous studies show that neural progenitors derive from the CNS microvascular pericytes and retain some of its markers (Bonkowski et al., 2011; Dore-Duffy et al., 2006). Taken together, these results suggest that Nestin-GFP+/Tuj1+ cells derive from pericytes or have a common ancestor.
Figure 1. FDB-derived Nestin-GFP+/Tuj1+ cells, cultured for 7 days, express some pericytic markers.
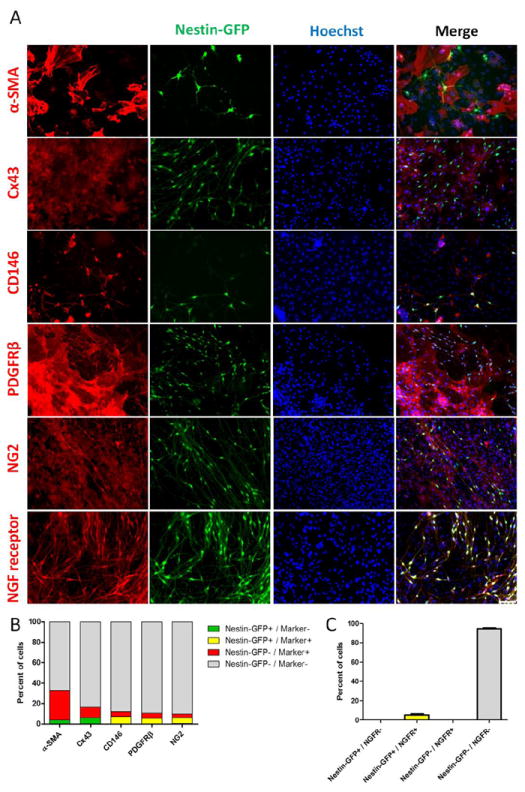
A. Antibodies against α-SMA and Connexin-43 (Cx43) recognized a population of pericytes but did not overlap with nestin-GFP+ neural cells, while other pericytic markers (CD146, PDGFRβ, and NG2) were found in both pericytes and nestin-GFP+/Tuj1+ cells. Nuclei were stained with Hoechst. B. Percent of cells expressing various cell markers in Nestin-GFP+ or Nestin-GFP- cells. Cells expressing α-SMA, Cx43, CD146, PDGFRβ, and NG2 in Nestin-GFP+ (yellow) or Nestin-GFP- (red) populations. Nestin-GFP+ cells that did not express the marker are in green. Markers are described in the x-axis. Note that Nestin-GFP+ neural cells lack some pericytic markers (α-SMA and Cx43), while expressing others (CD146, PDGFRβ, and NG2). NGFR (p75) identifies neural progenitors in 7 day-old FDB-derived cultures. NGFR was detected exclusively in nestin-GFP+/Tuj1+ cells. Representative immunocytochemistry of Nestin-GFP and NGFR fluorescence overlap is shown in A. NGFR overlapped with GFP which is expressed in the germline under the nestin control element in the Nestin-GFP transgenic mouse. C. Percent of cells sharing nestin-GFP and NGFR (p75) expression demonstrating that all NGFR overlapped with Nestin-GFP+ neural cells. No Nestin-GFP- cells expressed NGFR (p75) in culture. Data are mean ± s.e.m. (n=3). Scale bar = 100 μm.
After a broad screening for surface markers, we found that NGFR (p75) is expressed exclusively in all Nestin-GFP+/Tuj1+ cells in FDB cultures, accounting for 5.4 ± 2.4 % of the total cell number (Fig. 1A, C). NGFR expression appears in culture but not in the skeletal muscle (data not shown), consistently with previous reports (Fanburg-Smith and Miettinen, 2001; Sakaguchi et al., 2005). NGFR is a surface marker that could be used to isolate Tuj1+ cells from non-transgenic animal species. We did not detect NGFR in α-SMA+ pericytes in culture (data not shown). As a result, we purified Tuj1+ neural cells from wild-type mouse skeletal muscle cultures by FACS using NGFR as a surface marker (Suppl. Fig. 1A, B).
The NG2-DsRed transgene allows identifying Tuj1+/NGFR+ potential neural progenitors and α-SMA+ pericytes
The nerve/glia antigen-2 (NG2) proteoglycan is expressed in CNS neural progenitors (Aguirre et al., 2004; Kucharova and Stallcup, 2010; Nishiyama et al., 2002) and pericytes throughout the body (Ozerdem et al., 2001). To determine more clearly whether NG2+ muscle-derived Tuj1+ cells differ from pericytes, we analyzed NG2-DsRed+ cells derived from FDB muscle from NG2-DsRed transgenic mice at days 3 and 10 in culture. Our analysis was done on day 3 in cultures because DsRed fluorescence disappears later on, despite the persistent NG2 protein expression (Fig. 2A, B). At this time point, more than half of the NG2+ cells exhibit DsRed fluorescence (66 ± 11 % DsRed+ cells /NG2+ cells) (Fig. 2A, B). We identified NG2-DsRed+ cells with two distinct morphologies (Table 3): one with a big soma, no processes, and a fibroblast-like aspect that stained positive for α-SMA, negative for Tuj1 and NGFR (Fig. 2D and Suppl. Fig. 2A), which probably corresponds to pericytes described in the literature (Mogensen et al., 2011)(Table 3); and another with a small soma, long, thin processes that stained negative for α-SMA but positive for Tuj1 and NGFR (Fig. 2D and Suppl. Fig. 2A), which probably corresponds to Nestin-GFP+/Tuj1+ cells (Table 3). The number of Tuj1+ and NGFR+ cells was very similar (34 ± 7.2 % and 35 ± 14 %, respectively) (Fig. 2C), suggesting that they correspond to the same population, possibly potential neural progenitors. Co-immunostaining analysis showed that Tuj1+ cells were NGFR+ as expected (Suppl. Fig. 2B). A significant fraction of NG2-DsRed+ cells is α-SMA+ (44 ± 10%), which matches up a bigger cell population (Fig. 2C, D and Suppl. Fig. 2A), possibly classic pericytes (Table 3). The remaining fraction (~20%) corresponds to non-typified cells.
Figure 2. FDB-derived DsRed+ cells from NG2-DsRed mice lose NG2-DsRed fluorescence with time in culture despite persistent NG2 protein expression.
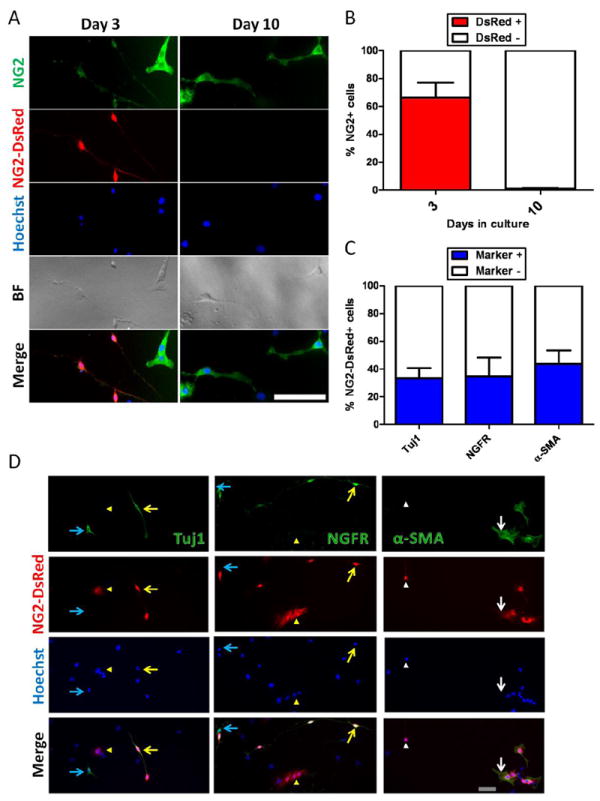
A. Cells were grown for 10 days and fixed at days 3 and 10. More than half kept the intense DsRed fluorescence at day 3, but it was drastically reduced by day 10. B. Percent of DsRed+ and DsRed- cells at days 3 and 10 in the NG2 protein + population. Note the complete disappearance of DsRed fluorescence at day 10 in culture. Data are mean ± s.e.m. (n=3). FDB-derived NG2-DsRed cells isolated from NG2 transgenic mice show two cell populations in culture: Tuj1+/NGF receptor+ neural cells and α-SMA+ pericytes. C. Relative proportions of Tuj1, NGF receptor, and α-SMA in the NG2-DsRed+ population. Data are mean ± s.e.m. (n=3). Cells were grown for 3 days, fixed and stained for Tuj1 (class III β tubulin), NGF receptor (NGFR, p75), and α-SMA (D). Their corresponding NG2-DsRed fluorescence, Hoechst staining, and merged images are displayed. Note that Tuj1 and NGF receptor staining was positive in cells with neural morphology (neural cells) (yellow arrow), but negative in cells with fibroblast-like morphology of pericytes (yellow arrowhead); while α-SMA stained pericytes (white arrow), but did not stain cells with neural morphology (white arrowhead). Light blue arrows indicate Tuj1 and NGFR positive cells in which the DsRed fluorescence disappeared (A, B). (A) Scale bar = 100 μm, (D) Scale bar = 20 μm.
Table 3.
Properties of skeletal muscle-derived Nestin-GFP+ neural cells and pericytes
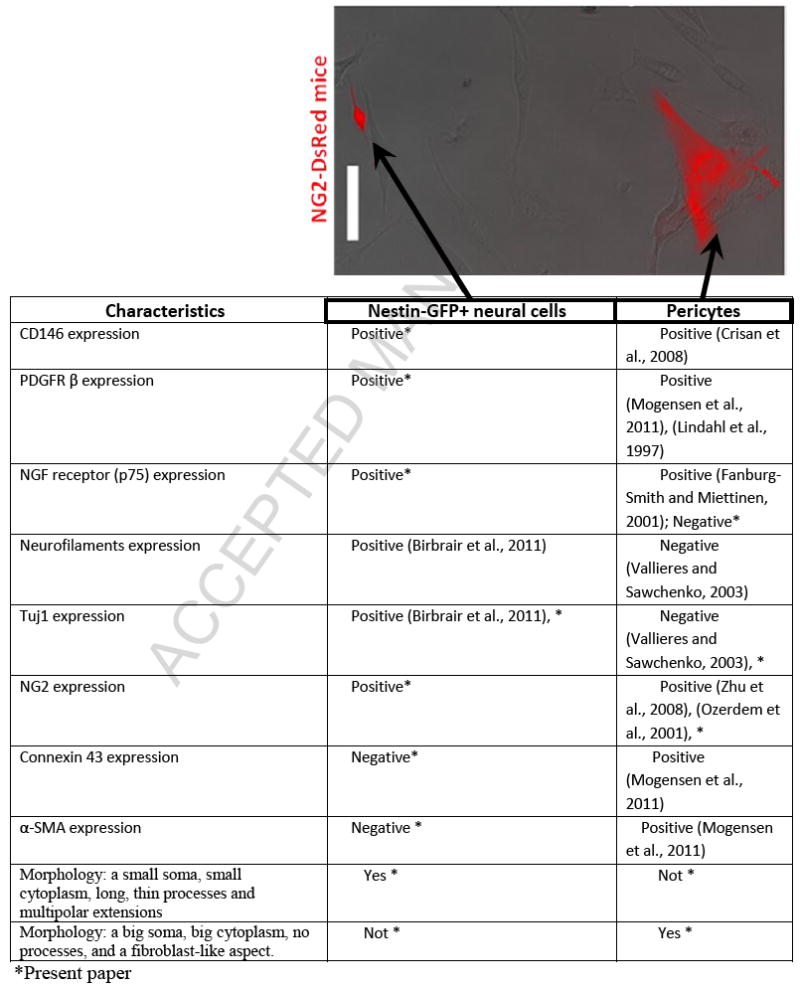
|
Skeletal muscle-derived Nestin-GFP+/NG2-DsRed+ cells correspond to Nestin-GFP+/Tuj1+ cells
To determine whether Nestin-GFP+ and NG2-DsRed+ cells exhibiting small soma and long, thin processes, correspond to the same population, we generated a double Nestin-GFP/NG2-DsRed transgenic mouse and examined FDB-derived cells at day 3 in culture (Fig. 3). We found that 7.3 ± 1.0 % of the cells were Nestin-GFP+/NG2-DsRed+ potential neural progenitors, all exhibiting small cytoplasm and multiple extensions (Fig. 3A) and expressing Tuj1 (Fig. 3C), but not α-SMA (Fig. 3D); while 9.8 ± 1.4% were Nestin-GFP+/NG2-DsRed-. These cells express fainter GFP fluorescence than Nestin-GFP+/NG2-DsRed+/Tuj1+ cells, which indicates that they possibly correspond to activated satellite cells losing Nestin-GFP fluorescence with culture time (Birbrair et al., 2011; Day et al., 2007), or unidentified cells (Fig. 3A, B). A smaller fraction, accounting for 5.6 ± 1.2 % of the cells, was Nestin-GFP-/NG2-DsRed+ with a fibroblastic morphology, expressing α-SMA (Fig. 3D), but not Tuj1 (Fig. 3C), corresponding to classic pericytes (Fig. 3A, B, C, and D). The large percent of remaining cells (~77%) corresponds, possibly, to macrophages, Schwann cells, fibroblasts, myoblasts, and endothelial cells, among others.
Figure 3. Skeletal muscle-derived NG2-DsRed+ and Nestin-GFP+ neural cells overlap in double transgenic Nestin-GFP/NG2-DsRed mice.
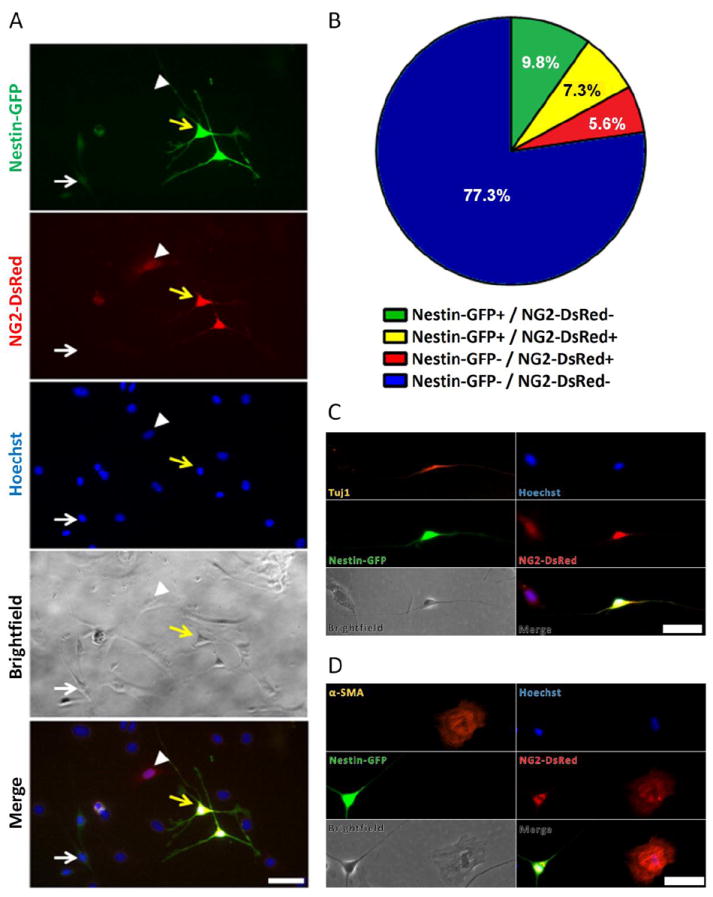
FDB muscle-derived cells from Nestin-GFP/NG2-DsRed mice were grown for 3 days and fixed. A. Nestin-GFP and NG2-DsRed cells and their corresponding, Hoechst 33342, brightfield, and merge images are illustrated. The white arrow indicates Nestin-GFP+/ NG2-DsRed- cells; the yellow arrow, Nestin-GFP+/ NG2-DsRed+ cells; and arrowheads indicate Nestin-GFP-/ NG2-DsRed+ cells. GFP fluorescence is brighter in Nestin-GFP+/NG2-DsRed+ cells than /Nestin-GFP+/NG2-DsRed- cells, probably because, in the latter, it is disappearing with time in culture. Also, Nestin-GFP-/NG2-DsRed+ cells have fibroblast-like morphology, while the Nestin-GFP+/NG2-DsRed+ cells have more neural morphology with long, thin processes. B. Pie chart representing the fraction of Nestin-GFP+/NG2-DsRed- (green), Nestin-GFP+/NG2-DsRed+ (yellow), Nestin-GFP-/NG2-DsRed+ (red), and Nestin-GFP-/NG2-DsRed- (blue) cells. Circle area represents the total number of cells. Values are expressed as the mean percent (n = 5). C. Tuj1 (orange) is expressed exclusively in Nestin-GFP+/NG2-DsRed+ cells. D. α-SMA (orange) is expressed only in Nestin-GFP-/NG2-DsRed+ cells while it is absent in Nestin-GFP+/NG2-DsRed+ cells. Scale bars = 50 μm.
Nestin-GFP+ cells co-express NG2-DsRed in vivo
As Nestin-GFP+/Tuj1+ cells are NG2-DsRed+ in culture and derive from GFP+ cells in vivo (Birbrair et al., 2011), we explored whether they overlap in vivo as well. We found 113 ± 19 Nestin-GFP-/NG2-DsRed+ cells/mm2 (Fig. 4) in extensor digitorum longus (EDL) muscle cross-sections, likely corresponding to classic pericytes; 44 ± 3.3 Nestin-GFP+/NG2-DsRed-cells/mm2 (Fig. 4), credibly corresponding to satellite cells that do not generate Tuj1+ cells in culture (Birbrair et al., 2011; Day et al., 2007); and 125 ± 11 Nestin-GFP+/NG2-DsRed+ cells/mm2 (Fig. 4), most probably corresponding to cells that give rise to Tuj1+ cells. These results suggest that neural cells derive from Nestin-GFP+/NG2-DsRed+ cells.
Figure 4. NG2-DsRed and Nestin-GFP expression by various cell populations in EDL muscle from Nestin-GFP/NG2-DsRed mice in vivo.
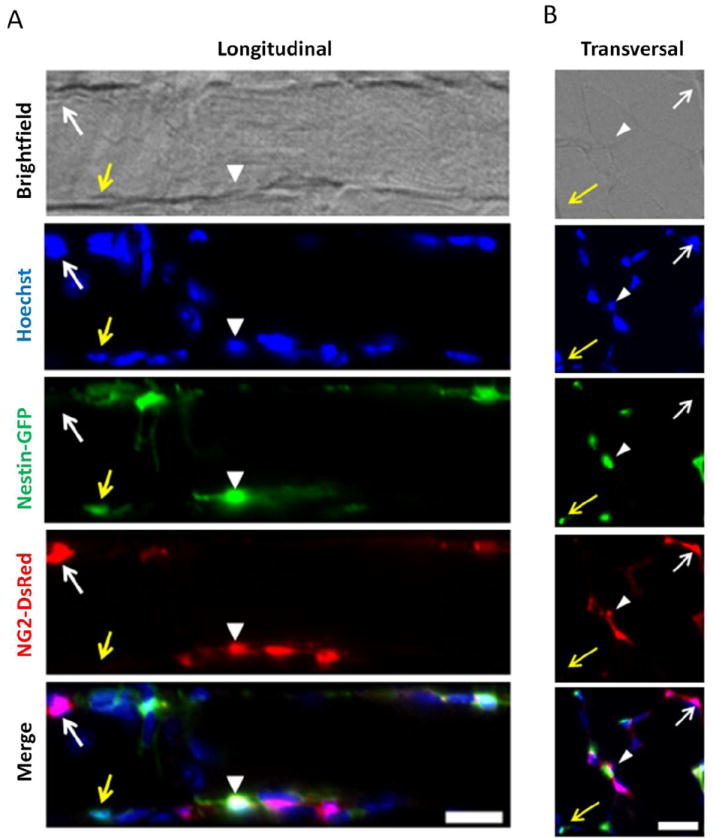
A. Longitudinal section of EDL muscle from the double transgenic Nestin-GFP/NG2-DsRed/ mice. B. EDL muscle transverse sections. Yellow arrows indicate Nestin-GFP+/NG2-DsRed- cells; white arrows, Nestin-GFP-/NG2-DsRed+ cells; and arrowheads, cells positive for both markers. Scale bars = 20 μm.
Staining skeletal muscle sections for PDGFRβ, CD146, and the blood vessel CD31 marker shows pericytes in proximity to endothelial cells (Suppl. Fig. 3). We found that both Nestin-GFP-/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed+ cells expressed the pericytic markers PDGFRβ and CD146 (Suppl. Fig. 3A) and lined (Suppl. Fig. 3B, C) or wrapped around (Suppl. Fig. 3D, E, F, and G) microvessels in skeletal muscle cross-sections.
Pericyte heterogeneity has been described in the spinal cord scar tissue (Goritz et al., 2011). Here, we hypothesize that, in skeletal muscle, Nestin-GFP+/NG2-DsRed+ cells are pericytes that differ from the classic ones, Nestin-GFP-/NG2-DsRed+, and possibly give rise to Nestin-GFP+/NG2-DsRed+/Tuj1+ cells in culture. However, the origin of Nestin-GFP+ potential neural progenitors remains unclear.
Nestin-GFP+/Tuj1+ cells derive from Nestin-GFP+/NG2-DsRed+ pericytes
Previous studies have demonstrated that Nestin+/NG2+ CNS microvascular pericytes and PDGFRβ pericytes can differentiate into neural progenitors (Bonkowski et al., 2011; Dore-Duffy et al., 2006; Jung et al., 2011; Nakagomi et al., 2011). Dore-Duffy et al. showed that neural progenitors retain the pericyte markers as Nestin and NG2 and express differentiation markers (Dore-Duffy et al., 2006). These data, combined with our observation that skeletal muscle-derived Nestin-GFP+/Tuj1+ cells share markers with CNS neural progenitors and pericytes, suggest that the Nestin-GFP+ neural cells may form, at least in part, from perivascular cells/microvascular pericytes, widely distributed in the skeletal muscle.
To test this hypothesis, we sorted different cell populations from the skeletal muscle of Nestin-GFP/NG2-DsRed mice (Nestin-GFP+/NG2-DsRed-; Nestin-GFP-/NG2-DsRed+; Nestin-GFP+/NG2-DsRed+; and Nestin-GFP-/NG2-DsRed- cells) (Suppl. Fig. 4A, B and Suppl. Fig. 5A). Then, we analyzed these cells by single cell RT-PCR to identify which correspond to pericytes (Suppl. Fig. 5B). Cell variety is certainly far greater than presumed using standard methods. Because of the heterogeneous cell population within tissue homogenates or cultures, various protocols have been developed to quantify specific transcripts from single cells (Chow et al., 1998; Klein et al., 2002; Liss, 2002) (Morris et al., 2011; Tsuzuki et al., 2001). RT-PCR in single cells rather than a cell sorted fraction allowed us to investigate more in depth molecular differences between and within cell subpopulations. The four cells tested from each group showed similar results.
As CD146 is a marker of pericytes associated with pluripotent activity (Dore-Duffy et al., 2011; Maier et al., 2010), we examined its expression (Suppl. Fig. 5B), in addition to PDGFRβ, another pericytic marker (Winkler et al., 2010) in the skeletal muscle. Both CD146 and PDGFRβ were present in dissociated Nestin-GFP-/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed+ cells but not in cells that did not express NG2 proteoglycan (n=4) (Suppl. Fig. 5B). These results show that the two sorted cell populations correspond to two pericyte subtypes, Nestin-GFP-/NG2-DsRed+/CD146+/ PDGFRβ+ and Nestin-GFP+/NG2-DsRed+/CD146+/ PDGFRβ+ cells.
The progeny of cells sorted immediately after isolation from the Nestin-GFP/NG2-DsRed mice skeletal muscle was analyzed (Fig. 5A,B). Nestin-GFP+/NG2-DsRed-, Nestin-GFP-/NG2-DsRed+, Nestin-GFP+/NG2-DsRed+, or Nestin-GFP-/NG2-DsRed- cells cultured alone did not give rise to Nestin-GFP+/Tuj1+ cells with their typical neural phenotype at day 8 (Fig. 5A). To mimic the FDB culture microenvironment that favors the appearance of Tuj1+ cells with neural morphology, cells were cultured with dissociated FDB muscle from wild-type mice and analyzed 8 days later. Only Nestin-GFP+/NG2-DsRed+ cells formed 15 ± 2.1 typical Nestin-GFP+ with neural morphology / mm2 (Fig. 5A, B), which were Tuj1+ (Fig. 5B), while Nestin-GFP+/NG2-DsRed-, Nestin-GFP-/NG2-DsRed+, and Nestin-GFP-/NG2-DsRed- cells did not give rise to any Nestin-GFP+/Tuj1+ cells in culture (Fig. 5A), supporting our hypothesis that Nestin-GFP+/Tuj1+ cells arise exclusively from Nestin-GFP+/NG2-DsRed+/CD146+ pericytes in the skeletal muscle.
Figure 5. Cells expressing Nestin, NG2 and CD146 generate Tuj1+ cells in culture.
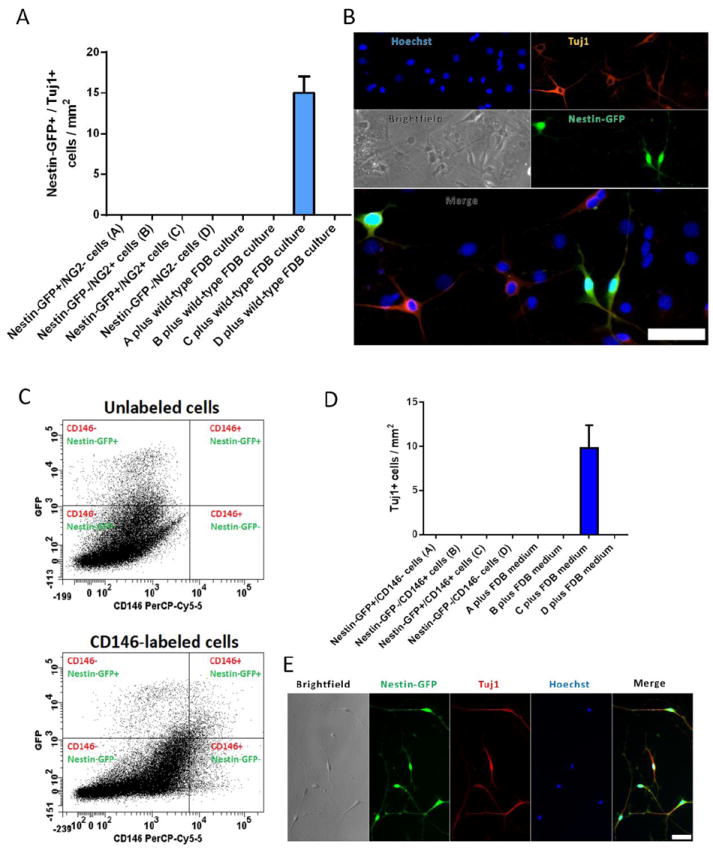
Mononucleated cells, isolated from hindlimb muscles from young-adult Nestin-GFP/NG2-DsRed mice, were immediately sorted after enzymatic dissociation. (A) Number of neural cells (Nestin-GFP+/Tuj1+, with neural morphology) after 8 days in various culture conditions: Nestin+/NG2-, Nestin-/NG2+, Nestin+/NG2+, or Nestin-/NG2- cells cultured alone; and Nestin+/NG2-, Nestin-/NG2+, Nestin+/NG2+, or Nestin-/NG2- cells co-cultured with wild-type mouse FDB culture. The number of plated cells right after sorting was approximately 5,000 per dish in both conditions. Only Nestin-GFP+/Tuj1+ cells with 2 or more thin processes were counted. Data are mean ± s.e.m. (n=5). B. Representative image of Nestin-GFP+ neural cells derived from Nestin-GFP+/NG2-DsRed+ cells cultured with cells derived from wild-type FDB muscle co-stained with Tuj1 antibody. Notice that Nestin-GFP-/Tuj1+ cells shown in this image represent neural cells derived from wild-type cells. C and D. Mononuclear cells, isolated from hindlimb muscles from young-adult Nestin-GFP mice, were labeled with CD146-PerCP-Cy5.5-conjugated antibody and sorted as different cell populations. C. Representative dot plots showing CD146+ cells versus GFP fluorescence with gate set using unlabeled cells. D. Number of Tuj1+ cells after 8 days in various culture conditions: Nestin+/CD146-, Nestin-/CD146+, Nestin+/CD146+, or Nestin-/CD146- cells cultured alone; and Nestin+/CD146-, Nestin-/CD146+, Nestin+/CD146+, or Nestin-/CD146- cells co-cultured with medium from WT mouse FDB culture. The number of plated cells right after sorting was approximately 5,000 per dish in both conditions. Data are mean ± s.e.m. (n=5). E. Representative image of Nestin-GFP+ neural cells derived from Nestin-GFP+/CD146+ cells cultured with medium from WT mouse FDB culture co-stained with Tuj1 antibody. Scale bars = 50 μm.
To confirm our results based on CD146 expression, we sorted cells from the skeletal muscle of Nestin-GFP mice immediately after isolation and analyzed their progeny (Fig. 5 C, D, and E). Nestin-GFP+/CD146-PerCP-Cy5.5-, Nestin-GFP-/CD146-PerCP-Cy5.5+, Nestin-GFP+/CD146-PerCP-Cy5.5+, and Nestin-GFP-/CD146-PerCP-Cy5.5- cells cultured alone did not give rise to Nestin-GFP+/Tuj1+ cells with a neural phenotype after 8 days in culture (Fig. 5D). To mimic the FDB culture microenvironment that favors the appearance of Tuj1+ cells with neural characteristics (Birbrair et al., 2011), cells were cultured with medium from a 7-day-old FDB culture. While Nestin-GFP+/ CD146-PerCP-Cy5.5-, Nestin-GFP-/ CD146-PerCP-Cy5.5+, and Nestin-GFP-/ CD146-PerCP-Cy5.5- cells produced no cells with neural morphology, the Nestin-GFP+/ CD146-PerCP-Cy5.5+ cells produced approximately 10 Tuj1+ cells / mm2 (Fig. 5D, E), supporting the concept that Tuj1+ cells arise from Nestin-GFP+/CD146+ pericytes in the skeletal muscle. Based on these results, together with our previous work (Birbrair et al., 2011), which shows that only Nestin-GFP+ cells from the skeletal muscle interstitium give rise to Tuj1+ cells in culture, we suggest that potential neural progenitors derive from Nestin-GFP+/NG2-DsRed+/CD146+ pericytes in the skeletal muscle interstitium.
To confirm that our results were not an artifact of a transgenic mouse culture, we used wild-type mice and, based on CD146 expression, sorted cells negative for endothelial cell marker CD31 immediately after isolation from the skeletal muscle and analyzed their progeny (Suppl. Fig. 6A, B). CD146- cells did not give rise to Tuj1+ cells with a neural phenotype after 8 days in culture, while CD146+ cells formed less than 1 Tuj1+ cell / mm2 (Suppl. Fig. 6B). However, when CD146+ cells and CD146- cells were co-cultured, approximately 23 Tuj1+ cells / mm2 appeared, with 2 or more processes (Suppl. Fig. 6B). To determine the source of these neural cells, either CD146+ or CD146- cells were co-cultured with medium from a 7-day-old FDB culture. While the negative cells produced no cells with neural morphology, the positive cells produced approximately 15 Tuj1+ cells / mm2 (Suppl. Fig. 6B), supporting the concept that Tuj1+ cells arise from CD146+ pericytes located in the interstitium of wild-type mice skeletal muscle.
Nestin-GFP+/NG2-DsRed+ and Nestin-GFP-/NG2-DsRed+ cells form α-SMA+ pericytes but not neural cells
We used various markers to track the fate of cells dissociated from the skeletal muscle of Nestin-GFP/NG2-DsRed mice that do not become Tuj1+ cells when cultured by themselves. As αSMA has a serum response element in its promoter (Bushel et al., 1995), αSMA-cells do not necessarily become pericytes. However, less than 30% of freshly isolated pericytes are αSMA+, but 100% become αSMA+ as they differentiate in culture (Katyshev and Dore-Duffy, 2012).
α-SMA+ pericytes were found to derive from Nestin-GFP+/NG2-DsRed+ (3.3 ± 1.0 α-SMA+ cells / mm2) and Nestin-GFP-/NG2-DsRed+ (4.4 ± 1.4 α-SMA+ cells / mm2), but not from Nestin-GFP+/NG2-DsRed- (Fig. 6). Nestin-GFP+/NG2-DsRed- cells probably correspond to satellite cells (Birbrair et al., 2011; Day et al., 2007) and give rise to myoblasts in culture, which are α-SMA- (Fig. 6).
Figure 6. Nestin-GFP+/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed- cultures form α-SMA+ pericytes but not neural cells.
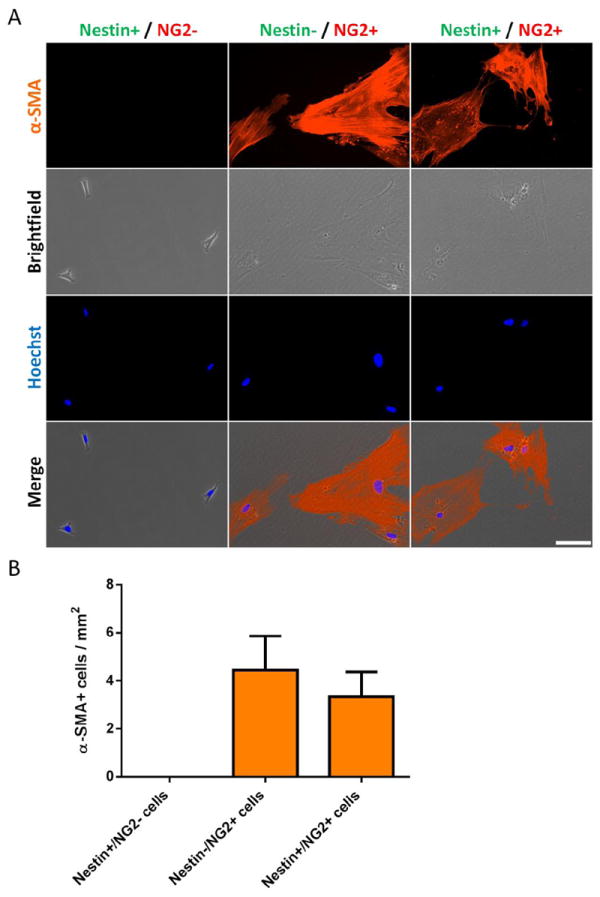
Mononucleated cells, isolated from hindlimb muscles from young-adult Nestin-GFP/NG2-DsRed mice, were immediately sorted after enzymatic dissociation (Suppl. Fig. 4A, B). A. Representative images of staining for α-SMA marker (orange) staining of Nestin+/NG2-, Nestin-/NG2+, or Nestin+/NG2+ cells cultured after 8 days. Hoechst 33342, brightfield, and merge images are also shown. Scale bar = 100 μm. B. Number of pericytes (α-SMA+, with fibroblastic morphology) in Nestin+/NG2-, Nestin-/NG2+, or Nestin+/NG2+ cell population cultured for 8 days. The number of plated cells right after sorting was approximately 5,000 per dish. Data are mean ± s.e.m. (n=5).
DISCUSSION
Nestin-GFP+/Tuj1+cells share pericyte markers
We found that NG2 proteoglycan, a known pericyte marker (Ozerdem et al., 2001), is expressed in Nestin-GFP+/Tuj1+ cells. It has also been identified in resident glial progenitors (Stallcup and Beasley, 1987), with neural progenitor activity after brain injury (Yokoyama et al., 2006; Zawadzka et al., 2010). Using NG2-DsRed transgenes we distinguished Tuj1+/NGF receptor+/NG2-DsRed+ cells (Nestin-GFP+/Tuj1+ cells) from α-SMA+/NG2-DsRed+ pericytes (Table 3). Progenitors that express NG2 may play a role as multipotent neural cells differentiating into neurons, astrocytes and oligodendrocytes in the brain (Richardson et al., 2011). As Nestin-GFP+/Tuj1+ cells express various pericyte markers (CD146, PDGFRβ and NG2), and neural cells derived from CNS pericytes retain pericytic markers (Dore-Duffy et al., 2006), we hypothesize that skeletal muscle perivascular cells expressing NG2 exhibit properties similar to those in the CNS. Pericytes with a potential to form neural cells have not been reported previously in the skeletal muscle.
Nestin-GFP+/Tuj1+ cells derive from Nestin-GFP+/NG2-DsRed+/CD146+ pericytes
As vasculature has a role in tissue induction during embryogenesis (Lammert et al., 2001; Matsumoto et al., 2001), possibly stem cells located in the adult vasculature are actively involved in tissue repair. The presence of mesenchymal stem cells (MSCs) in multiple adult tissues suggests that they have a common origin. Recently, attention has turned to pericytes as candidates to be MSCs due to their broad organ distribution (Alliot-Licht et al., 2001; Crisan et al., 2008; da Silva Meirelles et al., 2008; Lin et al., 2008; Nehls and Drenckhahn, 1993; Satokata et al., 2000; Shi and Gronthos, 2003). However, whether all MSCs are pericytes is not clear. Pericytes can be recognized more by their position in the microvasculature than a precise phenotype. Perivascular cells originate from mesenchymal cells that condense on the abluminal side of the endothelial tube (Clark, 1925) (Drake et al., 1998; Hungerford and Little, 1999). That MSCs are pericytes, has been proposed based on shared markers in vivo and in vitro (Caplan, 2008). However, although cells throughout the vasculature have a similar morphology and marker expression profile, they have several different developmental origins, making a single blood vessel appear very complex developmentally (Majesky, 2007; Majesky et al., 2011). Isolation of perivascular cells followed by long-term culture provides compelling evidence that pericytes act as stem cells (Caplan, 2007; Dellavalle et al., 2011; Dore-Duffy et al., 2011; Maier et al., 2010; Sacchetti et al., 2007). Their potential to contribute to tissue formation in addition to vessels has been established by numerous studies in various tissues (Alliot-Licht et al., 2001; Crisan et al., 2008; Dellavalle et al., 2011; Dellavalle et al., 2007; Feng et al., 2011; Lin et al., 2008; Nehls and Drenckhahn, 1993; Shi and Gronthos, 2003). Interestingly, pericytes from different tissues have different differentiation capacities as MSCs from distinct tissues (Bianco et al., 2008; Shi and Gronthos, 2003). However, it is not yet clear if pericytes are pluripotent due to the artifactual nature of many in vitro studies.
Our results provide evidence that although Nestin-GFP+/Tuj1+ cells express some common markers, they differ from classic pericytes (Table 3). Blood vessel walls in the SNC harbor a reserve of pericytes with neurogenic capacity, as reported recently (Dore-Duffy, 2008; Dore-Duffy et al., 2006; Dore-Duffy et al., 2011; Jung et al., 2011; Nakagomi et al., 2011). However, whether peripheral pericytes differentiate into cells with neural lineage characteristics is unknown. Our results support this possibility; we show that pericytes, derived from the skeletal muscle, are the ancestors of neural cells seen in skeletal muscle cultures. We also demonstrate that only a specific subtype of pericytes has the potential to differentiate into Tuj1+ cells. It is well known that pericytes are heterogeneous based on the expression of various markers (Bondjers et al., 2006; Goritz et al., 2011), but, to our knowledge, the concept that different types of pericytes are present in the skeletal muscle interstitium, is novel. We show that their potential to form Tuj1+ cells is restricted exclusively to type-2 (Nestin-GFP+/NG2-DsRed+/CD146+), but not type 1 (Nestin-GFP-/NG2-DsRed+/CD146+) pericytes under the same conditions (Fig. 7). Nestin-GFP+ neural cells derived from type-2 pericytes (Nestin-GFP+/NG2-DsRed+/CD146+ pericytes) and isolated from skeletal muscle continue to express pericyte markers, in agreement with a previous work showing that some pericyte markers are retained in neural cells derived from CNS pericytes (Dore-Duffy et al., 2006).
Figure 7. A schematic representation of the two pericyte subtypes found in the skeletal muscle.
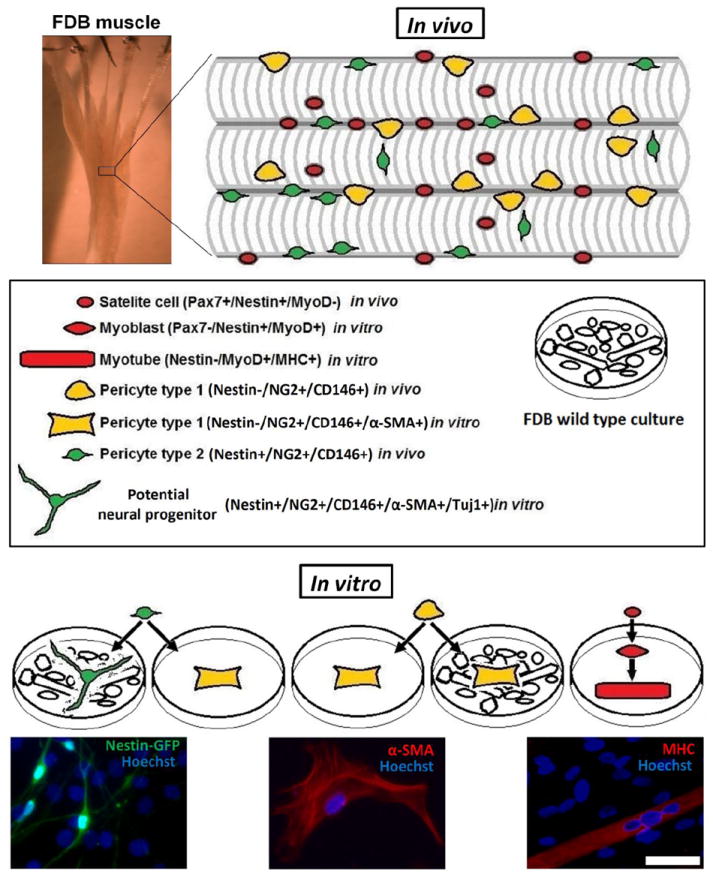
Skeletal muscle is composed of bundles of multinucleated myofibers (in vivo). Each fiber carries a population of satellite cells (Pax7+/Nestin+/MyoD-) (red), which reside between the myofiber plasma membrane and the surrounding basal lamina. Two types of pericytes are present in the skeletal muscle interstitium: type 1 (Nestin-/NG2+/CD146+) (yellow) and type 2 (Nestin+/NG2+/CD146+) (green). (in vitro) After muscle dissociation into single cells and cultured for 7 days, these cells exhibit distinct differentiation potential: myoblasts (Pax7-/Nestin+/MyoD+) (red) and multinucleated myotubes (Nestin-/MyoD+/MHC+) (red) are derived from satellite cells (red); type 1 pericytes (yellow) remain as pericytes (Nestin-/NG2+/D146+/α-SMA+) (yellow, fibroblast-like aspect) when cultured alone or together with wild-type FDB culture; type 2 pericytes (green), similarly to type 1 pericytes, generate pericytes (yellow, fibroblast-like aspect) if cultured alone, but, in contrast to them, form potential neural progenitors (Nestin+/NG2+/CD146+/α-SMA-/Tuj1+) (green, thin processes and multipolar extensions) if co-cultured with wild-type FDB culture. Scale bar of image with FDB muscle = 500 μm. Scale bar of microscopic fluorescent images = 50 μm.
The relationship between pericyte subtypes is unknown. They may represent developmental stages of the same cell. Future studies will use a Cre/loxp system such as NG2-CreER or/and Nestin-CreER mice to clarify the cell lineage and relationship between the two subtypes reported here.
We found cells co-expressing markers of immature neurons (Tuj1, Neurofilaments) (Birbrair et al., 2011) and oligodendrocyte progenitors, such as NG2 and NGF receptor (p75)(Chang et al., 2000), which is consistent with previous studies showing that cells derived from CNS pericytes express markers characteristic of pericytes, neurons, and glia, simultaneously (Dore-Duffy et al., 2006) (Bonkowski et al., 2011).
Although the presence of potential neural progenitors in skeletal muscle derived cultures has been reported previously (Alessandri et al., 2004; Arsic et al., 2008; Birbrair et al., 2011; Schultz and Lucas, 2006), their role has been difficult to establish. It has been reported that pericytes can migrate away from their location (capillary surface) in response to various stimuli and possibly assume different roles (Bonkowski et al., 2011). Secreted factors may change the culture microenvironment of skeletal muscle derived pericytes and promote their differentiation into neural cells as shown here. Blocking the Wnt/beta-catenin signaling pathway (Liebner and Plate, 2010; Stenman et al., 2008) inhibited pericyte differentiation into chondrocyte. This pathway is active in NG2+ cells differentiating into NG2 glia cells (White et al., 2010). Possibly inhibitors of this pathway, are present in the skeletal muscle in vivo, but become nonfunctional in the culture system.
Two pericyte subpopulations are present in the skeletal muscle with defined differentiation potential
Cultured alone both types of skeletal muscle pericytes (Types 1 and 2) (Fig. 7) form α-SMA+ pericytes instead of neural cells. Differentiation into the neural lineage only happens if type 2 pericytes are cultured with dissociated wild-type FDB containing various cell types able to secrete neurogenic factors (Birbrair et al., 2011). Interestingly, type-1 pericytes do not form neural cells, even in these same conditions. In this work, we show that type-1 pericytes are unipotential (restricted to form α-SMA+ pericytes), while type-2 pericytes are bipotential (may form α-SMA+ pericytes or Tuj1+ cells). This intriguing finding merits further research, as pericytes have been associated with growth and regeneration of various postnatal tissues (Crisan et al., 2008; Dellavalle et al., 2011; Dore-Duffy, 2008; Dore-Duffy et al., 2006; Feng et al., 2011). Identifying specific pericyte subtypes can help to select the most appropriate cells for therapy of various diseases.
Use of NGFR (p75) as a marker to isolate Tuj1+ cells from nontransgenic animals
Skeletal muscle derived potential neural progenitors have been characterized in Nestin-GFP transgenic mice by several markers, including Tuj1 (class III β tubulin) expression (Birbrair et al., 2011). Unfortunately, these markers could not be used for sorting purposes, as they all are not surface proteins. Here, we found that these cells express exclusively another neural progenitor marker on their surface, NGFR (p75) (Chang et al., 2000), which can be used to sort them out in a mixed cell population from nontransgenic animals. NFGR has been shown in brain neural progenitors (Bernabeu and Longo, 2010). Although, this marker appears in pericytes (Fanburg-Smith and Miettinen, 2001), it is absent in the adult skeletal muscle (Fanburg-Smith and Miettinen, 2001). Probably skeletal muscle pericytes do not express this marker as we did not detect it in any cells other than cells with neural morphology in culture.
Conclusion
Previous data from our laboratory showed that Tuj1+ neural cells can be isolated from skeletal muscle cultures (Birbrair et al., 2011). These cells express neural markers as Tuj1, Neu-H, Neu-L, and Nestin-GFP, respond to glutamate, form neurospheres, and clearly differ from endothelial (CD31-) and Schwann cells (S100β-). Here, we describe, for the first time, two pericyte subtypes based on their markers and differentiation potential. Also, we found that Nestin-GFP+/Tuj1+ cells derive from type-2 pericytes, which retain some pericyte markers (Fig. 7), while type-1 pericytes do not have the potential to differentiate into the neural lineage under the same conditions (Fig. 7). Additionally, potential neural progenitors can be efficiently isolated by sorting skeletal muscle cultures based on the surface receptor NGFR, expressed exclusively in these cells.
Supplementary Material
Highlights.
Two pericyte subpopulations are present in the skeletal muscle
Skeletal muscle pericyte subtypes differ in their differentiation potential
NGFR may be a useful marker to isolate neural cells from skeletal muscle culture
Acknowledgments
The present study was supported by a PUSH grant from the Wake Forest Comprehensive Cancer Center to Drs. Akiva Mintz and Osvaldo Delbono, grants from the National Institutes of Health/National Institute on Aging (AG13934 and AG15820) to Dr. Osvaldo Delbono, the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332), and the National Institute of Aging (R01AG040209), National Institute of Mental Health (R01MH092928) and NYSTEM to Grigori N. Enikolopov. We thank Dr. James Wood for his expert support on Flow Cytometry of the Comprehensive Cancer Center of Wake Forest School of Medicine (WFSM), Dr. Xin Feng from the Department of Otolaryngology of WFSM, for providing the monkey muscle samples and Dr. W. Stallcup from the Sanford-Burnham Medical Research Institute, CA, USA for sharing the rabbit anti-PDGFRβ antibody with us.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri G, Pagano S, Bez A, Benetti A, Pozzi S, Iannolo G, Baronio M, Invernici G, Caruso A, Muneretto C, Bisleri G, Parati E. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet. 2004;364:1872–1883. doi: 10.1016/S0140-6736(04)17443-6. [DOI] [PubMed] [Google Scholar]
- Alliot-Licht B, Hurtrel D, Gregoire M. Characterization of alpha-smooth muscle actin positive cells in mineralized human dental pulp cultures. Arch Oral Biol. 2001;46:221–228. doi: 10.1016/s0003-9969(00)00115-1. [DOI] [PubMed] [Google Scholar]
- Arsic N, Mamaeva D, Lamb NJ, Fernandez A. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res. 2008;314:1266–1280. doi: 10.1016/j.yexcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Bernabeu RO, Longo FM. The p75 neurotrophin receptor is expressed by adult mouse dentate progenitor cells and regulates neuronal and non-neuronal cell genesis. BMC Neurosci. 2010;11:136. doi: 10.1186/1471-2202-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One. 2011;6:e16816. doi: 10.1371/journal.pone.0016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmeyer CL, Kern DS, Forstmeier V, Lovric S, Modde F, Agustian PA, Steffens S, Birschmann I, Traeder J, Dammrich ME, Schwarz A, Kreipe HH, Brocker V, Becker JU. Arteriolar vascular smooth muscle cell differentiation in benign nephrosclerosis. Nephrol Dial Transplant. 2012 doi: 10.1093/ndt/gfr811. [DOI] [PubMed] [Google Scholar]
- Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellstrom M, Lindahl P, Betsholtz C. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J. 2006;20:1703–1705. doi: 10.1096/fj.05-4944fje. [DOI] [PubMed] [Google Scholar]
- Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS. 2011;8:8. doi: 10.1186/2045-8118-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow N, Cox C, Callahan LM, Weimer JM, Guo L, Coleman PD. Expression profiles of multiple genes in single neurons of Alzheimer’s disease. Proc Natl Acad Sci U S A. 1998;95:9620–9625. doi: 10.1073/pnas.95.16.9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ER, C EL. The development of adventitial (Rouget) cells on the blood capillaries of amphibian larvae. American Journal of Anatomy. 1925;35:239–264. [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14:1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Mehedi A, Wang X, Bradley M, Trotter R, Gow A. Immortalized CNS pericytes are quiescent smooth muscle actin-negative and pluripotent. Microvasc Res. 2011;82:18–27. doi: 10.1016/j.mvr.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ, Hungerford JE, Little CD. Morphogenesis of the first blood vessels. Ann N Y Acad Sci. 1998;857:155–179. doi: 10.1111/j.1749-6632.1998.tb10115.x. [DOI] [PubMed] [Google Scholar]
- Erceg S, Lainez S, Ronaghi M, Stojkovic P, Perez-Arago MA, Moreno-Manzano V, Moreno-Palanques R, Planells-Cases R, Stojkovic M. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS One. 2008;3:e2122. doi: 10.1371/journal.pone.0002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanburg-Smith JC, Miettinen M. Low-affinity nerve growth factor receptor (p75) in dermatofibrosarcoma protuberans and other nonneural tumors: a study of 1,150 tumors and fetal and adult normal tissues. Hum Pathol. 2001;32:976–983. doi: 10.1053/hupa.2001.27602. [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A. 2011;108:6503–6508. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999;36:2–27. doi: 10.1159/000025622. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Bahn JJ, Jeon D, Kim JH, Kim S, Won CH, Kim M, Lee SK, Roh JK. Multipotent PDGFRbeta-expressing cells in the circulation of stroke patients. Neurobiol Dis. 2011;41:489–497. doi: 10.1016/j.nbd.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Katyshev V, Dore-Duffy P. Pericyte coculture models to study astrocyte, pericyte, and endothelial cell interactions. Methods Mol Biol. 2012;814:467–481. doi: 10.1007/978-1-61779-452-0_31. [DOI] [PubMed] [Google Scholar]
- Klein CA, Seidl S, Petat-Dutter K, Offner S, Geigl JB, Schmidt-Kittler O, Wendler N, Passlick B, Huber RM, Schlimok G, Baeuerle PA, Riethmuller G. Combined transcriptome and genome analysis of single micrometastatic cells. Nat Biotechnol. 2002;20:387–392. doi: 10.1038/nbt0402-387. [DOI] [PubMed] [Google Scholar]
- Kucharova K, Stallcup WB. The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience. 2010;166:185–194. doi: 10.1016/j.neuroscience.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Liebner S, Plate KH. Differentiation of the brain vasculature: the answer came blowing by the Wnt. J Angiogenes Res. 2010;2:1. doi: 10.1186/2040-2384-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Liss B. Improved quantitative real-time RT-PCR for expression profiling of individual cells. Nucleic Acids Res. 2002;30:e89. doi: 10.1093/nar/gnf088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CL, Shepherd BR, Yi T, Pober JS. Explant outgrowth, propagation and characterization of human pericytes. Microcirculation. 2010;17:367–380. doi: 10.1111/j.1549-8719.2010.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108:365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Mogensen C, Bergner B, Wallner S, Ritter A, d’Avis S, Ninichuk V, Kameritsch P, Gloe T, Nagel W, Pohl U. Isolation and functional characterization of pericytes derived from hamster skeletal muscle. Acta Physiol (Oxf) 2011;201:413–426. doi: 10.1111/j.1748-1716.2010.02206.x. [DOI] [PubMed] [Google Scholar]
- Morris J, Singh JM, Eberwine JH. Transcriptome analysis of single cells. J Vis Exp. 2011 doi: 10.3791/2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi T, Molnar Z, Nakano-Doi A, Taguchi A, Saino O, Kubo S, Clausen M, Yoshikawa H, Nakagomi N, Matsuyama T. Ischemia-Induced Neural Stem/Progenitor Cells in the Pia Mater Following Cortical Infarction. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0279. [DOI] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry. 1993;99:1–12. doi: 10.1007/BF00268014. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70:661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa-Pereira I, Brites D, Brito MA. Neurovascular Unit: a Focus on Pericytes. Mol Neurobiol. 2012 doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Schnitzler AC, Lopez-Coviella I, Blusztajn JK. Purification and culture of nerve growth factor receptor (p75)-expressing basal forebrain cholinergic neurons. Nat Protoc. 2008;3:34–40. doi: 10.1038/nprot.2007.477. [DOI] [PubMed] [Google Scholar]
- Schultz SS, Lucas PA. Human stem cells isolated from adult skeletal muscle differentiate into neural phenotypes. J Neurosci Methods. 2006;152:144–155. doi: 10.1016/j.jneumeth.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K, Lambolez B, Rossier J, Ozawa S. Absolute quantification of AMPA receptor subunit mRNAs in single hippocampal neurons. J Neurochem. 2001;77:1650–1659. doi: 10.1046/j.1471-4159.2001.00388.x. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BD, Nathe RJ, Maris DO, Nguyen NK, Goodson JM, Moon RT, Horner PJ. Beta-catenin signaling increases in proliferating NG2+ progenitors and astrocytes during post-traumatic gliogenesis in the adult brain. Stem Cells. 2010;28:297–307. doi: 10.1002/stem.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wang S, Chen B, An X. Muscle-derived stem cells: isolation, characterization, differentiation, and application in cell and gene therapy. Cell Tissue Res. 2010;340:549–567. doi: 10.1007/s00441-010-0978-4. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Sakamoto A, Kameda K, Imai Y, Tanaka J. NG2 proteoglycan-expressing microglia as multipotent neural progenitors in normal and pathologic brains. Glia. 2006;53:754–768. doi: 10.1002/glia.20332. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Carvajal JJ, Golding JP, Morgan JE, Summerbell D, Zolnerciks J, Partridge TA, Rigby PW, Beauchamp JR. Myf5 expression in satellite cells and spindles in adult muscle is controlled by separate genetic elements. Dev Biol. 2004;273:454–465. doi: 10.1016/j.ydbio.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Birbrair A, Wang ZM, Taylor J, Messi ML, Delbono O. Troponin T nuclear localization and its role in aging skeletal muscle. Age (Dordr) 2011 doi: 10.1007/s11357-011-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kanter EM, Laing JG, Aprhys C, Johns DC, Kardami E, Yamada KA. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun Adhes. 2008;15:289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


