Abstract
Sialic acid binding Ig-like lectins or Siglecs vary in their specificity for sialic acid containing ligands and are mainly expressed by cells of the immune system. Many siglecs are inhibitory receptors expressed in innate immune cells that regulate inflammation mediated by DAMPs and PAMPs. This family also includes molecules involved in adhesion and phagocytosis and receptors that can associate with the ITAM containing DAP12 adaptor. Siglecs contribute to the inhibition of immune cells both by binding to cis-ligands (expressed in the same cells) as well as by responding to pathogen derived sialoglycoconjugates. They can help maintain tolerance in B lymphocytes, modulate the activation of conventional and plasmacytoid dendritic cells, and contribute to the regulation of T cell function both directly and indirectly. Siglecs modulate immune responses influencing almost every cell in the immune system, and are of relevance both in health and disease.
Keywords: inhibitory receptors, tolerance, autoimmunity, inflammation, clonal ignorance
Introduction
Recognition events mediated by receptors of immense diversity lie at the heart of the immune system. Beyond the recognition of pathogens by receptors of innate and adaptive immunity, many distinct lock-and-key events drive adhesion, cell-cell interaction, cell migration, co-stimulatory signaling and inhibitory signaling events in the immune system. The importance of carbohydrates as recognition elements in biology was brought home by a calculation made many decades ago by John Clamp and popularized by Nathan Sharon (1). This calculation has since been refined by Roger Laine (2). It makes the point that while any three amino acids can be combined to yield a maximum of only 33 or 27 tripeptides, when one takes into account the multiple reactive hydroxyl groups, isomeric forms, possible branch-points and other key features of carbohydrate structure, three typical monosaccharides can be joined together to potentially yield an astonishing 27, 648 different trisaccharides. Carbohydrates have the potential to contribute to tremendous structural diversity in nature. It is perhaps not surprising that carbohydrate-binding proteins play significant roles in pathogen recognition, cell adhesion, migration, activation and inhibition events in the immune system.
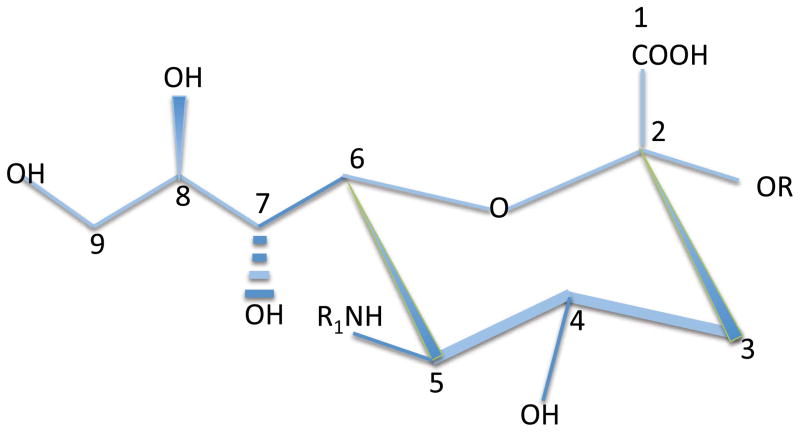
Sialic acids (Sias) are a family of nine-carbon sugars primarily expressed by vertebrates as well as by some microbial pathogens that have evolved the ability to evade the immune system in their vertebrate hosts (3). These negatively charged sugars decorate the termini of a number of glycoconjugates including N-linked glycan chains on proteins, O-linked mucin chains, and glycosphingolipids. The first numbered carbon atom on sialic acids is the carboxylic carbon. The remaining carbons make up a ring (including C-2 through C-6) and an exocyclic side chain that includes C-7, C-8, and C-9 (Fig. 1). Neuraminic acids are sialic acids that are of the most relevance from the perspective of this review and these members of this carbohydrate family are characterized by an amino group at the C-5 position. This amino group is N-acetylated to form N-acetyl neuraminic acid (Neu5Ac) and in most vertebrates the acetyl group can be converted by a hydroxylase, CMP-N-acetyl hydroxylase (CMAH) to an N-glycolyl group to yield N- glycolyl neuraminic acid or Neu5Gc. In humans CMAH is non-functional (reviewed in 3) and Neu5Gc is not synthesized. Available hydroxyl groups at the C-4, -7, -8 and -9 positions can be esterified in many different ways, including acetylation, sulfation, methylation, and phosphorylation to name but a few post-synthetic changes. One modification that will be considered in some depth in this review is the O-acetylation of sialic acid that occurs in the 9-OH position.
Figure 1.
A schematic view of the structure of sialic acid
Sialic acids are usually in the terminal positions of sugar chains and the isomeric form in which the carboxylic group is in the axial orientation (facing “upwards”) is by convention referred to as the α-form; when the hydroxyl group at C-2 is axially oriented it is called the (β form. The C-2 α-hydroxyl of the terminal sialic acid residue in a sugar chain is frequently covalently linked to a hydroxyl group on the C-3 or C-6 position of a pre-terminal galactose or N-acetylgalactosamine forming α2–3 or α2–6 linkages. A multitude of sialyltransferases exist that can generate these linkages depending on the underlying core structure on the relevant glycolipids, O-linked mucins, or N-glycans. A subsequent sialic acid may be linked to a previous α2–3 or α2–6 linked sialic acid moiety by an α2–8 linkage where the terminal sialic acid is linked via its C-2 hydroxyl group to the hydroxyl group on C-8 (in the exocyclic side chain) of the previous sialic acid. A distinct set of sialyltransferases can generate this α2–8 linkage in some gangliosides, in some glycoproteins and also in polysialic acid chains found in some glycoconjugates, mainly in the nervous system. The plethora of enzymes that generate, remove, and modify these terminal sialic moieties underlies the remarkable versatility of the sialoglycome. This diversity and plasticity at glycan termini has been exploited by the immune system over evolutionary time to provide regulatory mechanisms in a range of immune cells, and it is these mechanisms which will be the main subject of this review.
Sialic acids caught the attention of virologists and immunologists as host recognition elements that bind the hemagglutinins or spike proteins of type A and B influenza viruses. These hemagglutinins represent viral sialic acid binding lectins. Neutralizing antibodies against these viruses sometimes block the sialic acid binding site of these hemagglutinins and thwart infection. Complement factor H was the first vertebrate sialic acid binding lectin to be discovered. It binds to the exocyclic side chain of sialic acids on host cells and prevents the interaction of complement factor B with C3b, thus impeding the formation of the alternative pathway C3 convertase. It also recruits a specific protease, Factor I, that mediates cleavage and destruction of C3b. Many microbial pathogens have evolved the ability to synthesize sialic acids in order to evade vertebrate innate immunity either by recruiting factor H or, as discussed later in this review, by triggering sialic acid binding receptors on host innate immune cells to prevent their robust activation. A major role for sialic acids in the immune system emerged with the discovery of sialic acid containing ligands for Selectins, C-type or calcium-dependent lectins that regulate the rolling of leukocytes as part of the homing process.
The molecular cloning of a cDNA encoding CD22, a protein expressed primarily on B lymphocytes, revealed that this protein possessed multiple Ig like domains (4), and in 1991 it was reported that CD22 could bind to two sialated glycoproteins, suggesting that this Ig superfamily member was a sialic acid binding lectin (5). That same year the sheep erythrocyte receptor, a prominent protein on macrophages was purified and characterized and re-named sialoadhesin based on its ability to bind sialoglycoconjugates (6). Sialoadhesin and the myelin associated glycoprotein were also subsequently recognized as being sialic acid binding lectins that were type I integral membrane proteins with extracellular immunoglobulin-like domains (7, 8). These lectins, along with CD22 were recognized to be part of a family initially called the sialoadhesin family (8) or the I-type (for Ig like) lectin family (9) to distinguish these lectins from the large family of C-type or calcium-dependent lectins. In 1998 Crocker and Varki established a consensus nomenclature with others in the field, and since then the members of this growing family of sialic acid binding Ig domain containing lectins have been called Siglecs (10). It should be pointed out that some Siglecs, in spite of their name, probably do not bind sialic acid ligands while others, despite their ability to bind sialosides in vitro, could well be involved in the in vivo recognition of some other anionic biological structures. A number of distinct sialic acid containing oligosaccharide sequences have been shown, at least in vitro, to be highly specific for certain Siglecs, as depicted in Fig. 2. Knowledge regarding the specificity of different Siglecs is currently incomplete but is rapidly evolving (11 – 14).
Figure 2.
Glycan binding specificities of selected human and murine Siglecs. *While human CD22 binds to both Neu5Ac and Neu5Gc, murine CD22 is specific for Neu5Gc.
All Siglecs contain an N-terminal Ig like domain that has nine beta strands and close sequence homology to Ig V regions (a V-set domain). A critical arginine residue in this domain (found in most but not all members of the family) makes a salt bridge with the ionized carboxylic group of sialic acid in Siglec ligands; two aromatic residues in this domain are also conserved and contribute to ligand recognition (15 – 21). A relatively conserved loop between two of the beta strands also contributes significantly to carbohydrate recognition and can undergo a conformational change upon sialic acid binding as suggested by co-crystallization studies of Siglecs and sialoside ligands. This Ig V-type domain is followed by one to sixteen Ig C-2 domains that also possess sequence homology to Ig V domains but (like typical Ig C domains) have only seven beta strands each. Some contribution to sialic acid binding may also be attributed to the most N-terminal C-2 type Ig domain. The V-set and first C-2 domain contain additional cysteine residues that contribute to an inter-domain disulfide bridge as well as intra β-strand disulfide bonds. These additional disulfide bridges are thought to contribute to sialic acid recognition by Siglecs.
Many, but not all, Siglecs contain inhibitory signaling motifs in their cytoplasmic tails. These motifs will be discussed below. A large fraction of Siglecs are therefore an important source of inhibitory signals that can attenuate immune responses and dampen inflammation, perhaps in certain circumstances inducing lymphocyte exhaustion or even cell death. Other Siglecs, however, lack these inhibitory signaling motifs and mediate other diverse functions dependent on sialic acid recognition as will be discussed below.
This review will primarily examine how Siglecs regulate immune function. The reader is referred to a number of excellent reviews on Siglecs that have addressed the role of these lectins in immune function, but which also make available a stronger evolutionary perspective than will be provided here (14, 22 – 25).
Siglec families
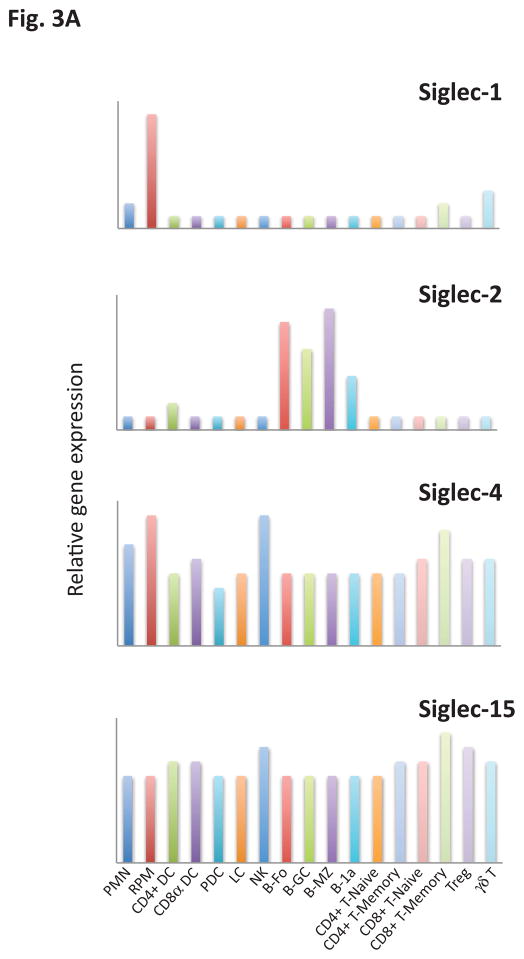
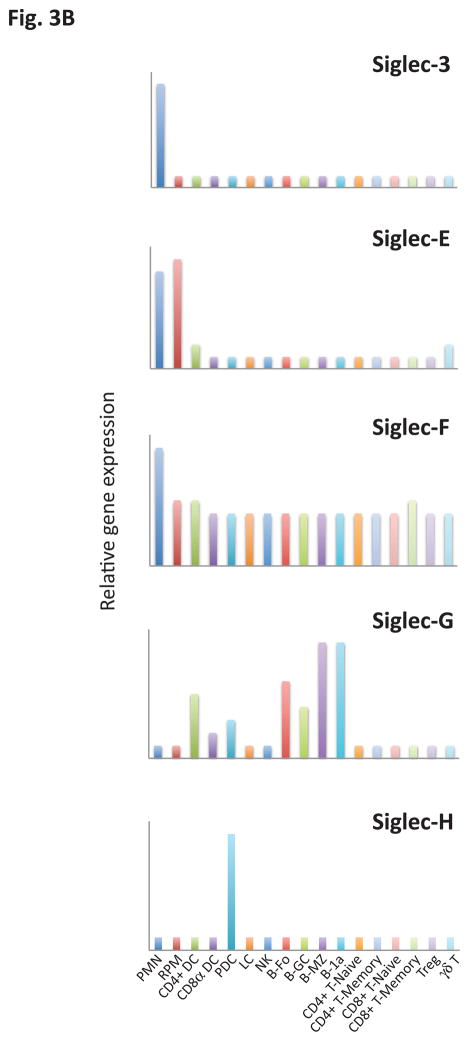
Siglecs are primarily expressed by cells of the immune system. They are conventionally divided into two broad groups that are both functionally diverse, even in terms of signaling mechanisms. In this conventional classification one category of Siglecs represents those that are conserved structurally between rodents, humans and other vertebrates and share about 25–30% identity. These include Siglecs 1, 2, 4 and 15 (Table I). Another separate group of Siglecs includes Siglec 3/CD33 and CD33-related Siglecs which have high homology to CD33 in their extracellular domains (50–85% identity), are still evolving by gene duplication events, vary considerably in number between species and, as is the case in humans, can even vary between individuals of the same species. CD33 related Siglecs in humans are numbered (e.g. Siglecs -3, -5, -6, -7, -8, -9, -10, -11, -12, -14 and -16), while CD33 related murine Siglecs (other than Siglec-3) are identified by a distinct alphabetical nomenclature. Some of these murine Siglecs (Siglecs -E, -F, -G and -H), have functional paralogs in humans that appear to have been generated by convergent evolution. CD33-related Siglecs are also fairly diverse in terms of their signaling motifs and downstream pathways of activation. There is no evidence so far for a significant degree of functional redundancy either in the Siglecs conserved across species or in the CD33-related group of Siglecs. Although knowledge regarding both gene expression and protein expression patterns is evolving, it is already known that many Siglecs that are of relevance from an immune function standpoint are expressed in a very cell type specific manner. For instance, among the murine CD33 related Siglecs, CD33 is expressed mainly in granulocytes (26), Siglec E is expressed primarily in neutrophils, monocytes and conventional dendritic cells (27), Siglec F is primarily expressed in eosinophils and mast cells (13, 28, 29), Siglec-G is primarily expressed in B cells and some dendritic cells (30, 31), and Siglec H is primarily expressed in plasmacytoid dendritic cells (32 – 34). Examination of publicly available gene-expression data from the Immgene database reveals a very diverse pattern of gene expression profiles for murine Siglecs (Fig. 3).
Table I.
Classification of Murine and Human Siglecs based on sequence conservation (Presumed functional paralogs are placed in the same row for the CD33 family)
| CONSERVED HUMAN AND MOUSE | HUMAN CD33 FAMILY | MURINE CD33 FAMILY |
|---|---|---|
| Siglec 1 (Sialoadhesin) | ||
| Siglec 2 (CD22) | ||
| Siglec 4 (MAG) | ||
| Siglec 15 | Siglec 3 (CD33) | Siglec 3 (CD33) |
| Siglec 5 | ||
| Siglec 6 | ||
| Siglec 7 | ||
| Siglec 8 | Siglec F | |
| Siglec 9 | Siglec E | |
| Siglec 10 | Siglec G | |
| Siglec 11 | ||
| Siglec 12 | ||
| Siglec 14 | ||
| Siglec 16 | ||
| Siglec H |
Figure 3.
Relative gene expression of (a) conserved and (b) CD33-related (non-conserved) Siglecs across key innate and adaptive immune cells. Values in each panel are normalized to the maximum expression of the respective Siglec across the represented cell types and presented in a linear scale. PMN, Polymorphonuclear leukocyte; RPM, Red pulp macrophage; CD4+ DC, CD4+ dendritic cell; CD8α+ DC, CD8α+ dendritic cell; PDC, Plasmacytoid dendritic cell; LC, Langerhans cell; NK, NK cell; B.Fo, Follicular B cell, B.GC, Germinal center B cell; B.MZ, Marginal Zone B cell; B-1a, B-1a peritoneal B cell; CD4+ T.naive, CD4+ naïve T cell; CD4+ T. memory, CD4+ memory T cell; CD8+ T. naive, CD8+ naive T cell; CD8+ T.memory, CD8+ memory T cell; Treg, Regulatory T cell; γδ T, gamma-delta T cell.
This work benefited from data assembled by the ImmGen Consortium
Heng, T et al. Immunological Genome Project Consortium. Nat Immunol. 10:1091–1094 (2008).
Human Siglec 12 is a pseudogene in many, but not all, human subjects (35). The in-frame version of Siglec 12 expressed in some individuals contains two N-terminal Ig-like V-set domains which lacks the arginine residue required for sialic acid binding. The natural ligand/s for this siglec remain to be identified (35). The apparently ongoing evolution of CD33 family Siglecs has led to suggestions that sialic acid expressing pathogens in the microbial environment are perhaps constantly driving this poorly understood process (24, 36, 37).
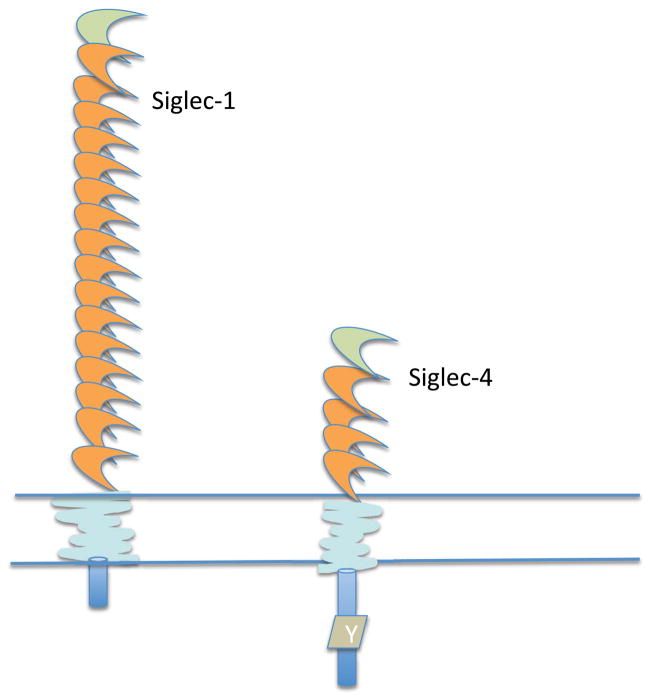
Siglecs may also be categorized into three groups based on features of the transmembrane and cytoplasmic tails of individual Siglecs that likely reflect the major mechanisms by which they mediate their biological functions. The first group is made up of Siglec-1 (sialoadhesin) and Siglec-4 (myelin associated glycoprotein or MAG), lectins that lack inhibitory signaling cytosolic motifs and possess neutral transmembrane domains (Fig. 4). Siglec-4 does contain an ITIM-like tyrosine in its cytoplasmic tail, but no ITIM motif itself. These Siglecs may primarily mediate adhesion events. The biological role of Siglec-1 will be explored later in this review in the context of innate immunity and also when discussing T cell activation; Siglec-4, which is expressed at high levels in oligodendrocytes and Schwann cells, and at much lower levels in almost all immune cells, will not be discussed further in this review.
Figure 4.
Siglecs that lack defined cytoplasmic tail signaling motifs or a charged transmembrane residue. The cytoplasmic tail of Siglec-4 has a tyrosine residue (Y) that does not fit into any known consensus for signaling. It has been referred to in the literature as a Fyn phosphorylation site.
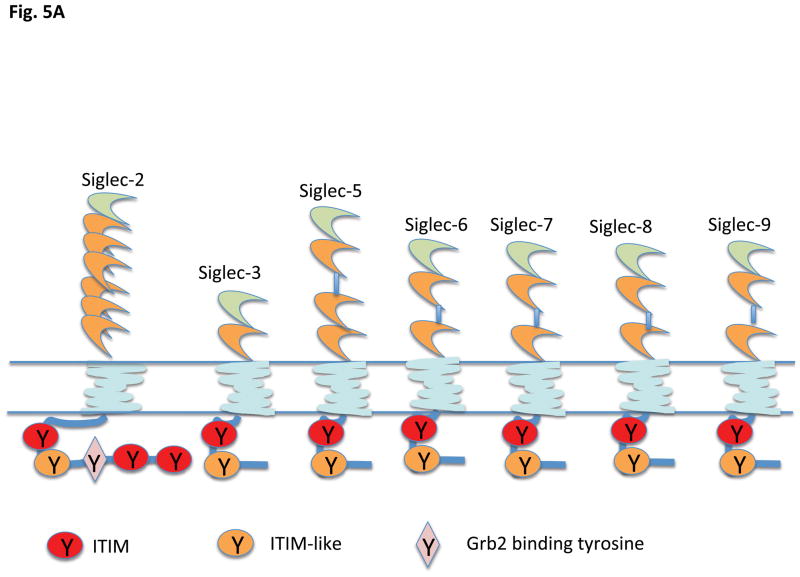
The second group of Siglecs in this type of functional classification is the largest, and includes members in which the major biological function may be to set an inhibitory tone mediated by cytosolic ITIMs (immunoreceptor tyrosine based inhibitory motifs) with the general consensus I/V/L/SxYxxL/V (a hydrophobic residue at position −2, and a leucine or a valine at the +3 position) (Figures 5A and B). Some of these Siglecs also contain an ITIM-like motif with the consensus sequence D/E xYxEV/IK/R which does not fully conform to an immunoreceptor tyrosine based switch motif or ITSM (consensus sequence TxYxxV/I) found in the cytoplasmic tails of SLAM family proteins. The signature Siglec of this inhibitory category is CD22, which may be capable of orchestrating both inhibitory and activating signals, as discussed later in this review. In this family of Siglecs, ligand recognition results in an induction of accessibility of the cytosolic ITIM tyrosine and the ITIM like tyrosine to Src family kinases such as Lyn, which can phosphorylate cytosolic ITIM tyrosines, which then in turn recruit tyrosine phosphatases such as SHP-1 or SHP-2 that can attenuate signal transduction (38, 39). Mutagenesis studies have revealed that the ITIM tyrosines play a more pronounced inhibitory role than the ITIM-like tyrosine residues (39 – 42). In mice this group of inhibitory lectins includes CD22/Siglec-2, Siglec-E, Siglec-F and Siglec-G. Human siglecs that contain ITIM motifs include Siglecs -2, -3, -5, -6, -7, -8, -9, -10, -11 and -12 (Fig. 5) (5, 35, 36, 43 – 50) Phosphorylation of ITIM tyrosines may not only dampen signaling downstream of activating receptors (as a consequence of SHP-1 and SHP-2 recruitment), but the ITIM phosphotyrosines can also contribute to internalization and turnover as discussed later. A general view of ITIM containing Siglecs is that they probably maintain a constitutive “inhibitory tone” as they are bound to cognate sialoglycoconjugates in the same cell in which they are expressed. Exposure of the host to a pathogen, even if the pathogen lacks surface sialoglycans, may either alter Siglec expression or alter cellular levels of Siglec ligands. Pathogens that do express surface sialic acid might engage cognate Siglecs to attenuate inflammation as part of immune evasion, but innate immune cell activation may also be modulated in order for the host to generate specific protective responses. Pathogens might also exploit inhibitory siglecs as conduits for cellular entry.
Figure 5.
Siglecs that contain ITIM motifs in their cytoplasmic tails A) Human and B) Murine. Siglec 12 is refrred to as Siglec XII in human by convention since the in-frame human form lacks the crucial arginine for sialic acid recognition (but the arginine is present in other primates).
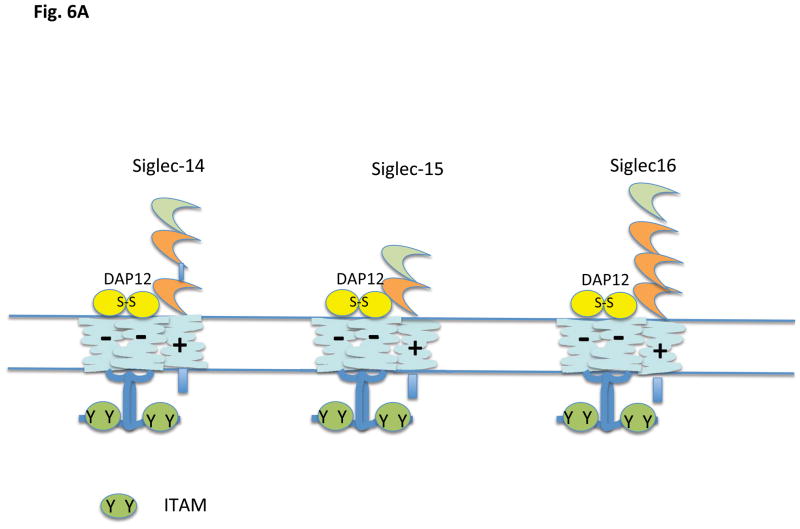
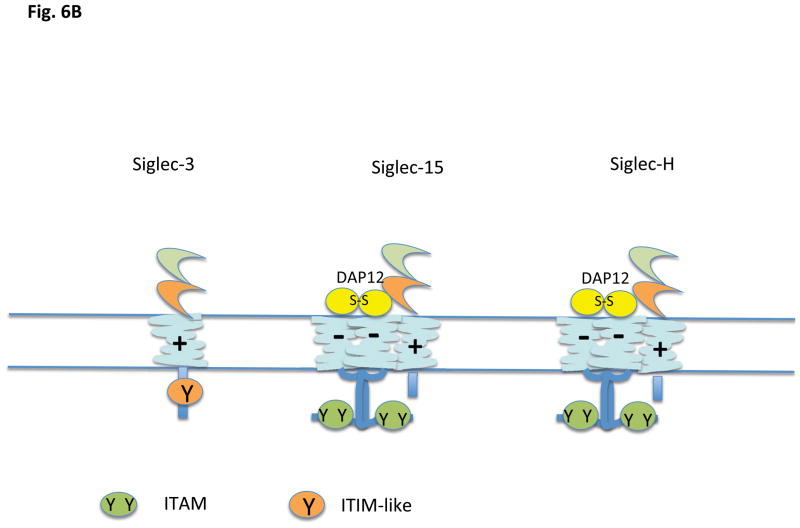
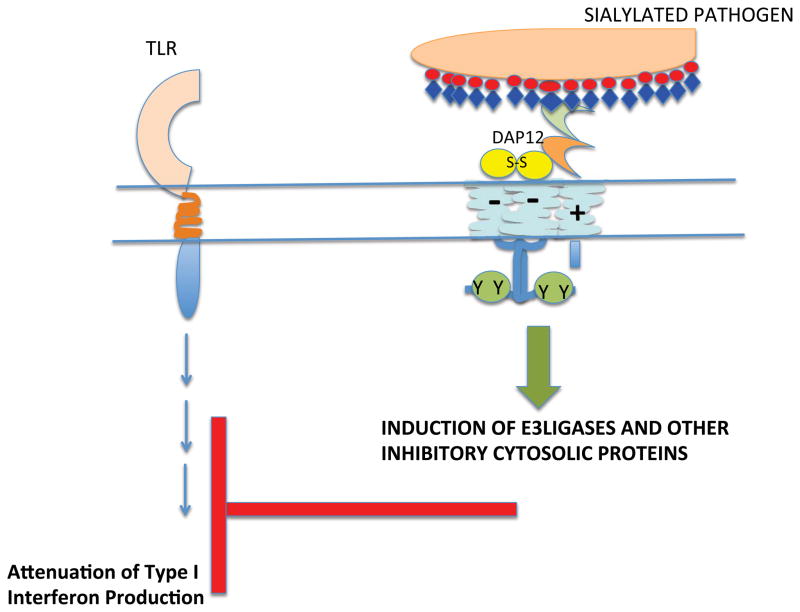
There is a third category of Siglecs, members of which contain a positively charged residue in the transmembrane anchor region (Fig. 6 A and B). These Siglecs can in general associate with a disulfide-linked homodimer of DAP12 (DNAX associated protein of 12 kilodaltons) which contains an aspartate transmembrane residue and a cytosolic ITAM motif (YxxL/I [x]6-7YxxL/I); in the case of Siglec-15, the inhibitory receptor may also associate with DAP 10, another signaling chain with a negatively charged transmembrane residue but a cytosolic YxxM motif. Based on data from other DAP12 containing receptors whose ligands are known, ligation of these ITAM linked Siglecs would be predicted to lead to a conformational change resulting in enhanced accessibility of the cytosolic tyrosine containing motifs of either DAP12 or DAP10 to non-receptor tyrosine kinases, resulting in tyrosine phosphorylation of the ITAM of DAP12 to facilitate Syk family tyrosine kinase recruitment and activation, or phosphorylation of the tyrosine in the YxxM motif of DAP10 resulting in PI-3 kinase recruitment and activation. The murine Siglecs that fall into this category are Siglec-3, Siglec-H and Siglec-15. Murine Siglec-3/CD33 contains an ITIM-like motif in the cytoplasmic tail as well as a positively charged residue in the transmembrane region and association with DAP12 has not been formally demonstrated for this murine siglec. In humans, Siglecs with positively charged residues in the transmembrane region include Siglecs-14, -15, and -16 (14, 51 – 53) but not Siglec-3 (43). Siglec-13 is a related gene that also contains a positively charged transmembrane residue. It is expressed in most primates, but the gene has been deleted in humans (http://carta.anthropogeny.org/moca/topics/siglec-13). As will be discussed later primarily in the context of the biology of plasmacytoid dendritic cells, DAP12 ITAMs can potentially contribute to the attenuation of TLR signaling. A similar phenomenon has been noted downstream of integrin mediated DAP 12 activation.
Figure 6.
Siglecs that contain a transmembrane lysine (+). A) Human, and B) Murine. Siglec 15 is shown associated with DAP12; it can also associate with DAP10. Murine CD3 has not yet been shown to associate with DAP12 experimentally.
Cytosolic DAP12 tyrosines may in the appropriate context be phosphorylated by Src-family kinases, leading to the recruitment and activation of Syk. Activation may contribute to the transcriptional induction of E3 ligases that facilitate the turnover of TRAF6 downstream of TLRs (54). In this way ITAM containing Siglecs may attenuate pathogen induced TLR activation. While ITAMs that shut off signaling pathways have sometimes been referred to as inhibitory ITAMs (55, 56), the concept of ITAMs mediating unique inhibitory signals has long been studied in the immune context. B cell anergy and T cell anergy both involve antigen receptor linked ITAM activation, but the biochemical pathways induced inhibit lymphocyte activation. It is not clear if ITAM-associated Siglecs typically bind to cis-ligands or are activated primarily in trans. Potential ways in which they function will be considered in the section on Siglec-H and plasmacytoid dendritic cells.
It has been suggested that Siglec-5 and Siglec-14 are paired receptors, as are also Siglecs-11 and -16 (50, 52, 53). One member of each pair has a cytosolic ITIM motif and the other member associates with DAP12 and has the potential to be an activating receptor. The first two Ig domains of both the inhibitory and potentially activating member of each pair are virtually identical, and it has been therefore implied that these Siglec pairs function in a Yin-Yang fashion and have evolved in order to provide a balance of signaling in response to pathogen encounter. While such a notion is interesting, the evidence that these pairs of receptors are tightly expressed together in vivo remains scanty and is not supported by publicly available gene expression data. Because antibodies to Siglecs-5 and -14 cross-react, co-expression at the protein level has been hard to establish. The same issue complicates the study of Siglecs-11 and -16. Detailed gene expression profiling and Western blots on different cell types may eventually provide more definitive evidence as to whether these Siglecs are actually expressed in a coordinate manner in different cell types.
Siglecs and Innate Immunity
The majority of Siglecs are expressed primarily in innate immune cells. Some information on the in vivo function of selected murine Siglecs has been obtained from the study of specific gene-targeted mice but many postulated aspects of Siglec function in innate immune cells remain to be confirmed by in vivo approaches. Potential functions of Siglecs in innate immune cells include a role in the internalization of sialic acid expressing pathogens by phagocytosis, the attenuation of inflammation in response to both sialic acid expressing and non-sialated pathogens by inhibitory signaling, the attenuation of DAMP (damage associated molecular pattern) mediated inflammation by Siglec inhibitory signaling, and the inhibition of Natural Killer Cell activation by terminal sialic acid moieties on mucins. The potential role of Siglecs in influencing the lifespans of myeloid cells in general will also be considered. While dendritic cells may be considered in the context of innate immunity we will consider the potential roles for Siglecs in plasmacytoid and conventional dendritic cells separately.
Pathogen internalization by innate immune cells: phagocytosis and endocytosis by Siglecs
Some Siglecs are capable of phagocytosing pathogens that express cell surface sialic acid moieties. Such interactions represent the recognition of sialic acid containing ligands in-“trans” (as opposed to “cis”-interactions in which the sialic acid moiety and Siglec are in the same cell). Sialoadhesin/Siglec-1 has an extended extracellular region with 17 Ig-like domains, is expressed on a number of macrophage populations (that stain with the MOMA-1 antibody in mice) and has been implicated in the interactions of macrophages with lymphocytes. It is also expressed on monocyte derived dendritic cells and its role in T cell driven inflammatory responses will be discussed in the context of lymphocyte activation and T cell polarization later in the chapter. Sialoadhesin was long thought to be primarily involved in adhesion events, and a number of earlier studies demonstrated the absence of the induction of phagocytosis following the binding of this Siglec to its ligands (57); more recent studies indicate that sialoadhesin may participate in the phagocytosis of pathogens that synthesize or acquire terminal Neu5Acα2-3Gal moieties on glycoconjugates (58). Sialoadhesin recognizes such sialic acid containing moieties on the lipopolysaccharide of the Gram negative bacterium Neisseria meningitides and can mediate phagocytosis of these bacteria (59). Comparison of wild type macrophages and those from a sialoadhesin knockout animal provide the strongest evidence for an in vivo role for a Siglec in the phagocytic process. However given the absence in sialoadhesin of cytosolic motifs that are known to facilitate the internalization process, it is assumed that some other receptor that drives endocytosis might be co-ligated by the microbial ligand. Sialoadhesin has also been shown to recognize similar sialic acid moieties on the O-linked carbohydrate termini of mucins of T. cruzi, the trypanosome that causes Chagas’ disease. This interaction facilitates macrophage–T.cruzi interactions and also contributes to the internalization of the trypanosome (60). Sialoadhesin on porcine alveolar macrophages has been shown to bind Neu5Acα2-3Gal moieties on N-glycans on proteins of the porcine respiratory and reproductive syndrome virus and mediate viral internalization (61).
Siglec-5, a human Siglec which contains a cytosolic ITIM motif as well as an ITIM-like motif, can also recognize the Neu5Acα2-3Gal moiety on the lipopolysaccharide of N. meningitides and in vitro studies employing transfected COS cells has shown it to be capable of phagocytosis of this Gram negative species (59). Siglec-5 has also been implicated in the clearance of apoptotic bodies by macrophages (62), but there is little evidence to indicate that apoptotic cells are more avidly decorated with Neu5Acα2-3Gal moieties (63, 64). Like most Siglecs with cytosolic ITIM motifs, Siglec-5 is normally bound to sialic acid containing ligands in cis. Endocytic activity of Siglecs has been also demonstrated in vitro for Siglecs -7 and -9, Siglec-F and CD22 (65). It is unclear whether any of these inhibitory Siglecs actually participate in pathogen phagocytosis in macrophages or dendritic cells in vivo. It is possible that some inhibitory Siglecs may be utilized by pathogens for cellular entry. However it is unclear whether Siglecs other than Siglec-1 function in vivo as phagocytic receptors that capture their sialogylcoconjugate cargo for pathogen destruction and/or for antigen presentation. Endocytosis may in some circumstances contribute to signal transduction itself or may be a part of the process by which Siglecs are turned over either as part of normal constitutive degradation or in an accelerated manner following signal transduction. The recruitment to tyrosine phosphorylated Siglec ITIMs of E3 ligases such as the SOCS complex or Cbl (66 – 69) may reflect the need for poly-ubiquitination and proteasomal degradation (in the case of SOCS-3) or mono-ubiquitination and lysosomal targeting of activated Siglec complexes (mediated by Cbl), as a part of a turnover process that terminates inhibitory signaling.
While CD22 is expressed at the highest level on B cells and is generally viewed as a “B-cell” Siglec, some CD22 is expressed on dendritic cells; since endocytosis by CD22 has been studied in some detail it is useful to consider this Siglec in the context of endocytosis in this section. CD22 ITIM motifs were the first Siglec structural determinants linked to endocytosis. ITIM tyrosines in general fit the YxxΦ consensus (where Φ is a hydrophobic residue) and these motifs contribute to the association of integral membrane proteins with the AP2 complex and their subsequent internalization into clathrin coated vesicles (70). Site-directed mutagenesis of CD22 cytosolic ITIM tyrosines has established the role for these motifs in the association of CD22 with the AP50 protein of the AP2 complex and CD22 endocytosis has been shown to be an AP2 and clathrin dependent event. There is, however, some disagreement about the fate of endocytic vesicles containing CD22. While earlier studies suggested that endocytosed CD22 is targeted for lysosomal degradation (71) more recent studies suggest that CD22 recycles much like the transferrin receptor (70, 72). There are significant teleological reasons to believe that in-vivo sialoside ligands should not be delivered to lysosomes in B cells by CD22, although such a function would be theoretically acceptable in dendritic cells. Antigen specificity in T-B interactions depends on lysosomal delivery of peptides for MHC class II mediated antigen presentation by the B cell antigen receptor alone and not by other endocytic receptors. It is possible that CD22 can contribute to endocytosis when associated with membrane-IgM or IgD (via both sialic acid dependent and independent mechanism perhaps), while the specificity of the cargo internalized is completely dependent on the antigen-specificity of the B cell receptor. It remains to be determined whether or not sialic acid containing antigens can be internalized via CD22 and processed in B cells or in any other cell types.
Studies on Siglec-F, a Siglec which mediates a potential inhibitory function in eosinophils (discussed later in the review), have shown that endocytosis by this Siglec is also dependent on ITIM and ITIM-like tyrosines, but vesicles are internalized by a clathrin-independent, but ARF6-dependent, mechanism (65). Siglec-F does not recycle, but can deliver internalized cargo to lysosomes. Most Siglecs with ITIM tyrosines presumably have the ability to use their cytosolic tails for cargo internalization. Different Siglecs with ITIM tyrosines may possibly recognize distinct sialic acid containing ligands on different set of pathogens and mediate their internalization into innate immune cells for destruction or antigen presentation, dependent on the cell type. The study in the years to come of Siglec-E and Siglec-F knockout dendritic cells and macrophages should help resolve whether inhibitory Siglecs are important for pathogen phagocytosis in vivo.
As will be discussed for Siglec-H later in this review, even Siglecs that have positively charged transmembrane residues and no cytosolic tyrosine residues could potentially participate in cargo internalization for antigen presentation. The associated tyrosine residues in DAP12 might be capable of contributing to endocytosis; however, it is unclear that the capture of cargo is a physiologically relevant function of ITAM associated Siglecs.
Siglecs and the attenuation of inflammatory responses
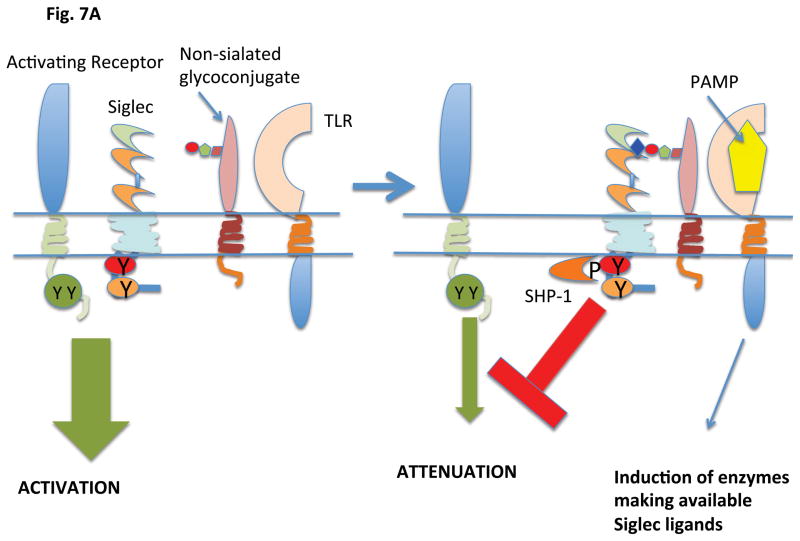
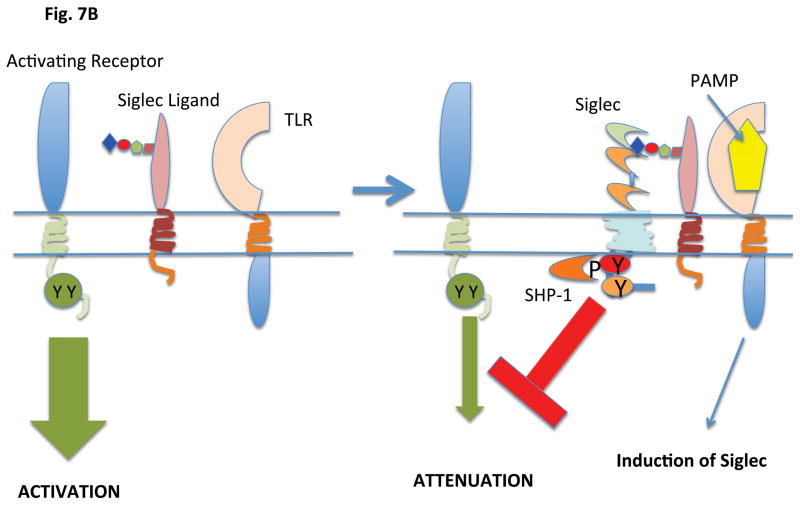
Most Siglecs appear to have evolved to attenuate innate immune responses and to control inflammation in responses to a variety of pathogens most of which are non-sialated microbes. Siglecs presumably maintain an inhibitory state by binding to self-ligands in cis. Alterations in the expression of siglecs and/or of siglec ligands may represent means by which inhibitory signaling is upregulated in the context of infection and inflammation (Figure 7). Siglec–E is expressed on neutrophils in mice and its expression can be induced on macrophages by ligands for TLR2, 4, 7 and 9 in a Myd88 dependent manner (73). In cells activated by LPS, the induced Siglec-E is tyrosine phosphorylated and recruits the tyrosine phosphatases SHP-1 and SHP-2. Siglec-E attenuates NF-κB activation and the consequent synthesis and secretion of TNF and IL-1. Recruited SHP-2 can inhibit the activation of the TBK1 kinase that functions downstream of TLRs to induce IFN-β.
Figure 7.
Induction of ligand or Siglec expression by pathogens can regulate inhibitory signaling
A. Activation of a TLR by a PAMP results in the generation of a Siglec ligand by regulating an enzyme or enzymes that regulate ligand availability. The generation of a Siglec ligand results in inhibitory signaling being brought into play
B. Activation of a TLR by a PAMP results in the induction of Siglec expression. This results in inhibitory signaling being brought into play.
The activation of neutrophils by LPS (via TLR4) results in the transcriptional induction of Siglec-9 and the enhanced recruitment of SHP-2 to the phosphorylated ITIM tyrosine of Siglec-9, similar to the Siglec-E phenomenon in mice (74). SHP-2 antagonizes TRIF mediated signaling downstream of endosomal TLRs and attenuates the secretion of Type I interferons. Siglec-9 perhaps contributes to the attenuation of Type I interferon release during gram negative bacterial infections when Type I interferons may only be tangentially helpful, and in these circumstances, the α2–3 Sia ligands that facilitate this process could well be self glycoprotein derived N-glycans. Siglec-9 can also be triggered by N-glycans on viral glycoproteins presumably to attenuate inflammation driven by viruses. Studies on the modulation of expression of different siglecs induced by different inflammatory stimuli as well as an analysis of the alterations in the glycome landscape in the context of these stimuli will help provide a better picture of how siglecs contribute to the attenuation of inflammation.
In general, in cells exposed to pathogens, Siglecs may be induced and also bind to the appropriate self-sialoglycoconjugates that ligate these inhibitory receptors in order to prevent uncontrolled inflammation. A fine balance obviously has to be achieved: sufficient inflammation must be generated in order to eliminate pathogens, but not too much in order to avoid tissue damage. Pathogens sometimes seek to disrupt this balance. It is possible that higher density trans-ligands on certain pathogens may provide more potent inhibitory signals than the constitutive or induced signals delivered by self-ligands, primarily in cis, for Siglecs.
Immune Evasion by Pathogens via Siglec Ligation
A number of pathogens either synthesize sialic acids or acquire them from the host. While a number of bacteria including Group B Streptococci, Neisseria species, and Campylobacter jejuni synthesize sialic acids, some pathogens scavenge host sialic acids. Although the acquisition of sialic acid by pathogens can contribute to the evasion of host complement, appropriately sialated glycoconjugates may also trigger inhibitory Siglecs on host innate immune cells and thus attenuate host protective responses. While this type of triggering event might reflect immune evasion by pathogens it may also represent a protective response that serves to prevent excessive inflammation in the host.
The most interesting case of sialic acid scavenging is that mediated by the unique trans-sialidase (TS) enzyme of the protozoan parasite Trypanosoma cruzi, the causative agent of Chagas’ disease. This enzyme cleaves sialic acid moieties from host glycoconjugates and transfers them in α2-3 linked form to T. cruzi mucin O-glycan termini. More heavily sialylated T. cruzi strains elicit greater parasitemia, an effect attributed both to the ability of sialic acid moieties to mask sub-terminal carbohydrate epitopes and protect the parasite from the lytic activity of anti-galactosyl antibodies, as well as to the ability of glycoproteins decorated with terminal sialic to inhibit complement activation (75, 76). Immune evasion by heavily sialylated T cruzi also involves Siglec ligation and will be considered later in this review in the context of regulatory effects in dendritic cells.
The capsular polysaccharide of Group B Streptococci displays a repeated Neu5Acα2-3Galβ1-4GlcNAc structure that resembles that seen at the termini of many vertebrate glycoproteins. Siglec-9 on neutrophils binds to host α2-3 linked Sias in cis presumably to generate an “inhibitory tone” by tyrosine phosphorylation of ITIM motifs and recruitment of SHP-1 or SHP-2. The presence of group B streptococcal polysaccharides of serotype III in trans however induces inhibitory signaling presumably because the high-valency of theα2-3Sia motif on the polysaccharide displaces cis-acting self-glycans (Fig. 8). Ligation of Siglec-9 attenuates the oxidative burst in neutrophils, results in a reduction in the formation of neutrophil extracellular traps, and favors survival of group B Streptococci (77, 78).
Figure 8.
Siglecs may be activated in cis by self-sialoglycoconjugates or in trans by sialylated pathogens
A. Tonic inhibition refers to inhibitory signaling based on ongoing ligation in cis of a Siglec by its ligand
B. A sialylated pathogen may displace a cis-ligand and more potently engage an inhibitory Siglec.
Group B streptococci of serotype Ia express a cell wall protein (the β protein) that can bind to Siglec-5 on host white blood cells in a sialic acid independent manner and induces the recruitment of SHP-2, This protein-protein interaction facilitates microbial-leukocyte attachment but attenuates neutrophil activation, inhibits receptor mediated phagocytosis, and reduces streptococcal clearance. Siglec-5 is thus subverted into contributing to a bacterial immune evasion mechanism (79).
One way that vertebrates appear to counter the pathogenicity of sialylated pathogens is to use sialidases to strip the sialic acid from microbial intruders. O-acetylation of sialic acids on group B streptococci prevent sialidase mediated removal of sialic acid from capsular polysaccharides and does not significantly compromise the evasion of complement. However O-acetylated sialic acid on the capsule is incapable of ligating Siglec-9 and the microbe appears to have achieved a critical balance of partial O-acetylation in order to successfully evade the complement system and also induce Siglec mediated inhibition of leukocyte activation (80, 81). The subversion of Siglecs for immune evasion is perhaps not restricted to pathogens. Many tumors secrete mucins that engage Siglec-9 and attenuate IL-12 production (82) as part of an evasion strategy similar to one described in more detail later in this review in a pathogen context.
If sialylated glycans on pathogens displace cis-acting sialoglycoconjugates, what then are the differences in signaling between cis-ligands and high density trans ligands? Is the frequency and/or duration of ITIM tyrosine phosphorylation enhanced by higher avidity trans-ligands? Is SHP-1 recruitment measurable in the context of cis-interactions and can this be distinguished experimentally from presumably enhanced recruitment that occurs with pathogen sialogycan engagement? Do pathogens better engage both ITIM and ITIM-like tyrosines while cis-ligands perhaps only engage ITAMs? Numerous issues that relate to the biology of the immune evasion process still require to be addressed.
Attenuation of DAMP mediated inflammation by Siglecs
Ongoing cell damage or cell death results in the release of damage associated molecular patterns (DAMPs) that can activate NOD like receptors and the inflammasome, and also activate other pattern recognition receptors such as TLRs. Among the DAMPs that are induced on damaged and dying cells, are HMGB1 (high mobility group protein B1), HSP70 and HSP90. Some DAMPs can associate with a sialoglycoprotein, CD24, that exists in many different glycoforms that vary in their N-glycan termini – subset of these glycoforms have the appropriate forms of Siglec ligands as side chains. CD24 associates particularly well with HMGB1, a prototypic DAMP. CD24 also has the ability to interact with human Siglec-10 but not with Siglecs -7, -9 and -11 (83, 84). CD24 bound to HMGB1 can associate with Siglec 10 in human innate immune cells, forming a ternary complex. The association of CD24 with Siglec -10 depends on the critical arginine residue in the V-set domain of Siglec-10 that is involved in sialic acid recognition. HMGB1 and a subset of CD24 glycoforms form a similar ternary complex with Siglec-G in murine splenic cells. Siglec G is expressed not only on B cells but also on some myeloid cells including dendritic cells. The ligation of Siglecs by DAMP-CD24 complexes helps reduce NFκB activation and prevents uncontrolled inflammation in the context of tissue damage (83). In CD24 null mice, the absence of this inhibitory axis results in a marked enhancement of the inflammatory response mediated by DAMPs but not by TLR ligands. Some enhancement of inflammation by DAMPs is also seen in Siglec-G null mice confirming the relevance of this axis in vivo.
It has been shown that in microbial sepsis, microbial sialidases can remove sialic acid residues from CD24, abrogate CD24-SiglecG or CD24-Siglec 10 interactions and thus enhance the inflammatory process (85). Sialidase inhibitors protect mice from bacterial sepsis in a CD24 and Siglec G dependent fashion. Studies on sialidase-deficient bacterial mutants confirm the importance of this enzyme as a virulence factor in sepsis and highlight the importance of Siglecs in controlling damage mediated inflammatory responses (85).
Siglecs therefore participate in the attenuation of both pathogen and non-pathogen mediated inflammatory responses. In models of allergic inflammation the absence of Siglec-F exacerbates eosinophilic responses (86) and in the absence of Siglec-G the expansion of B-1 a B cells can be linked to uncontrolled activation of NFκB (31). Siglec-1 in human microglial cells and Siglec F in murine microglia can contribute to the attenuation of inflammation in the brain induced by extracellular aggregates and apoptotic neural fragments (87 – 89).
The regulation of the life span of myeloid cells in the context of inflammation by Siglec-8, Siglec- 9 and Siglec-F
One mechanism by which the half-lives of myeloid cells such as neutrophils and eosinophils might be regulated may involve apoptotic elimination of these cells by siglec-ligation following activation. Siglec-8 in humans and Siglec-F in mice are functional convergent paralogs that are primarily expressed in eosinophils and mast cells (13, 86, 90, 91). Siglec-9 is expressed in neutrophils and other innate immune cells in humans (48). The sulfated sialic acid containing glycolipid, 6′-sulfo-sialyl Lewisx (6-sulfo-sLex or Neu5Acα2-3Galβ1-4(Fucα1-3)(6-O-sulfo)GlcNAc) has been established in vitro to be a high affinity ligand for Siglec-8 as well as for Siglec F, and the related sulfated glycolipid 6-sulfo-sialyl Lewisx is the best known ligand for Siglec-9. It has been established that eosinophils and neutrophils readily undergo apoptosis when Siglec-8 and Siglec-9 are respectively crosslinked with specific antibodies (92 – 94). This apoptotic process occurs most readily when these granulocytes are in a pro-inflammatory cytokine milieu, suggesting that these Siglecs might help regulate the turnover of activated granulocytes in the context of inflammation. Mitochondrial apoptosis pathways are induced when Siglec ligation results in the generation of reactive oxygen species (ROS); neutrophils from subjects with chronic granulomatous disease are not eliminated by apoptosis following Siglec-9 crosslinking since ROS generation is defective. Since both Siglec-8 and Siglec-9 contain cytosolic ITIM tyrosines, crosslinking presumably results in tyrosine phosphorylation of the ITIM and recruitment of SHP-1 or SHP-2. How these events result in ROS generation is unclear.
The administration of an antibody against Siglec-F in mice causes a specific depletion of eosinophils (95). It is possible that this depletion reflects the activation-linked apoptotic death of eosinophils which would be consistent with the view that Siglecs-8, Siglec-9 and Siglec F are normally triggered to induce their apoptotic removal. However these data could merely signify antibody-based depletion similar to the therapeutic depletion of B cells by anti-CD20 antibodies for instance. Siglec F knockout mice have proved useful in better understanding the role of inhibitory Siglecs in regulating the life spans of myeloid cells in vivo. While these mice are phenotypically normal, when placed in an allergen sensitization based asthma model, these mice exhibit eosinophilia in the blood, bone-marrow and tissues because of decreased apoptotic clearance (96). Overall these studies suggest a potential role for inhibitory Siglecs in the regulation of the lifespan of eosinophils and neutrophils.
What signaling differences result in inhibition of inflammation versus the induction of apoptosis is unclear. It is also unclear how exactly triggering of these presumptively turnover inducing Siglecs occurs in vivo. The possibility that some of these studies on apoptosis reflect the artificial triggering of Siglecs by antibodies and not an in vivo phenomenon remains an issue of some concern.
Polymorphisms in Siglec-8 have been linked to asthma (91) although no difference in ligand binding was noted between proteins encoded by the risk allele and the wild type allele.
Regulation of NK cell function by Siglec-7
Siglec-7 is primarily expressed on human NK cells and recognizes Neu5Acα2-8Neu5Acα2-6Gal moieties present on certain b series gangliosides such as GD3, GT1b and GQ1b, but these moieties are also present on some glycoproteins. NK cells differ from other lymphocytes in that they express ST8SiaVI which generates α2-8 linked disialic acid moieties on O-linked glycans of NK cells (46, 97, 98). NK cells express these endogenous ligands for Siglec-7 that can presumably ligate Siglec-7 and possibly maintain these cells in an inhibited state. It has been suggested that certain tumors may be refractory to NK cell killing because these tumors express GD3 and other NK cell ligands that might send inhibitory signals via Siglec-7. There are a number of unexplained issues. Normally Siglec-7 on NK cells is constitutively bound to its natural sialoside ligands apparently to initiate inhibitory signaling. This kind of phenomenon has been referred to in the field as “masking” since in the absence of sialidase treatment the siglec being studied cannot bind to an exogenous, often synthetic, sialoside ligand. This nomenclature is potentially confusing since normal biology is visualized as the passive “masking” of a structure being interrogated by an artificial investigator-initiated intrusion, while in fact the masked state likely reflects an active physiologically important ligand-receptor interaction in cis that generates a constitutive inhibitory tone. It is possible that Siglec-7 contributes to inhibitory signaling in NK cells that can be further enhanced as part of an immune evasion phenomenon by tumors such as melanomas and renal cell carcinomas. This begs the question. What happens to Siglec-7 mediated inhibitory signaling when an NK cell confronts a virally infected target cell that fails to express a ligand for a classical Ig family KIR or C-type lectin inhibitory receptor? Is NK cell activation accompanied by reduced ST8SiaVI activity or enhanced neuraminidase activity? Is Siglec-7 always “ON” or is its activity regulated? The identification of an inhibitory Siglec in murine NK cells and perhaps an eventual conditional deletion study might or might not provide answers to some of the fundamental questions on the function of Siglecs in NK cells that remain to be answered.
During HIV infection, immune evasion involves the induction of dysfunctional NK cell subsets which significantly downregulate Siglec-7 expression (99). The biologic relevance of this Siglec alteration is not understood.
Siglecs, Dendritic Cells and T cell activation
Dendritic cells (DCs) integrate signals from antigens and microbial products in order to provide instructions to T cells during an immune response generally resulting in a specific activation phenotype or sometimes the induction of regulatory T cells. A large number of lectins, including a variety of siglecs, are expressed on dendritic cells. Many of these lectins, especially C-type lectins, contribute to antigen capture but they typically also induce intracellular signals. Glycans on or secreted by microbes may activate a number of C-type lectin receptors but signaling may also be modulated by Siglecs – ligated either in cis by sialoglyconjugates in the DC itself or by pathogen derived sialoglycans. How exactly various glycan and non-glycan derived signals are integrated in order for a DC to secrete IL-12 or some other specific cytokine instead is still unclear, and understanding how siglecs contribute to this process represents a major challenge for the field.
In this section, a potential role for Siglec activation in regulating TLR signaling in plasmacytoid dendritic cells will be initially discussed. The participation of siglecs in conventional dendritic cells, as well as in T cells themselves, in regulating T cell activation and polarization will be considered subsequently.
Siglecs and the regulation of plasmacytoid dendritic cell function
Plasmacytoid dendritic cells (pDCs) are key orchestrators of innate immunity, most notably through the generation of an anti-viral state through the secretion of type I interferons. pDCs may also contribute to the induction of tolerance by promoting the generation of antigen-specific T regulatory cells. Siglec-H is a specific surface marker for murine pDCs (32, 34). Intracellular Siglec-H expression has also been described in splenic marginal zone ER-TR9-positive macrophages and lymph node medullary cord sialoadhesin-positive macrophages (34). Perhaps Siglec-H is inefficiently transported to the plasma membrane (possibly because of the absence of DAP12) or is constitutively endocytosed in these specialized macrophage subsets. A Siglec-H-GFP knockin reporter mouse shows that the Siglec-H promoter is active in pDCs and to some extent in cDCs as well (100). The homozygous form of this reporter mouse will undoubtedly help provide important information regarding the function of Siglec-H.
Siglec-H possesses all of the conserved structural features that are thought to contribute to sialic acid recognition. These include an essential arginine at position 125 (β strand F) that is predicted to form a salt bridge with the carboxyl group of sialic acid; the aromatic amino acids, phenylalanine, immediately before strand A, and tryptophan within strand G, that are expected to make hydrophobic contacts with sialic acid; and the unusual pattern of cysteines in domains 1 & 2 that form intra-β sheet and interdomain disulfide bonds. Nonetheless, in vitro solid-phase assays to evaluate binding of Siglec-H-Fc recombinant protein to red blood cells and attempts to detect Siglec-H binding to sialylated polyacrylamide conjugates (that bind avidly to other siglecs) have failed to demonstrate recognition of sialic acid by this siglec (34). A plausible molecular explanation for this is that Siglec-H contains 2 unpaired cysteines in domain 1 (distinct from those described above), one immediately upstream of strand A, and the other immediately downstream of strand G. These could participate in an intersheet disulfide bond that may reduce the distance between the two β sheets, thereby preventing interaction with sialic acid. These unpaired cysteines are conserved in rat Siglec-H, which, in addition lacks the critical arginine involved in sialic acid binding (34). Thus, although it is possible that murine Siglec-H has evolved to engage a very specific type of carbohydrate ligand-potentially on a pathogen, it is likely that Siglec-H binds to a ligand in vivo that does not contain sialic acid.
Siglec-H contains a transmembrane lysine residue that facilitates its interaction with a negatively charged aspartate in the transmembrane region of DAP12. DAP12-deficient pDCs do not stain with anti-Siglec-H antibodies and Siglec-H appears to require DAP12 in order to be transported to the cell membrane, just like other DAP 12 associated receptors (101).
Murine pDCs have been divided recently into two functionally distinct subsets. One subset expresses the CD9 tetraspannin and low (but clearly discernible) levels of Siglec-H (102). This subset secretes large amounts of type I interferons following stimulation with TLR-7, -8, & -9 agonists, induces cytotoxic T lymphocyte (CTL) activation, and generates protective anti-tumor immunity. A second murine pDC subset does not express CD9, expresses high levels of Siglec-H, and secretes negligible levels of type I interferons. This subset can contribute to the generation of antigen-specific Foxp3 positive Tregs, and is inefficient at inducing anti-tumor immunity. Interestingly, the majority of newly formed pDCs mobilized from the bone marrow are Siglec-H low expressors that are capable of producing IFN-α, while the majority of pDCs in peripheral tissues are Siglec-H high expressors that have lost this ability, but instead promote immune tolerance. Cells of both pDC subsets express IRF-7, the main IFN-α transcription factor, similar levels of TLR9, and the pDC-defining transcription factor, E2-2. Given the potential inhibitory role of Siglec H in attenuating Type IIFN synthesis and secretion discussed below, it appears that Siglec-H levels may determine the functional phenotype of pDC subsets: low Siglec H levels are presumably permissive for Type I interferon synthesis and secretion, while high Siglec H levels presumably inhibit TLR activation and constrain Type I interferon production.
Ligation of Siglec-H using antibodies has revealed an inhibitory role for this Siglec, discussed briefly in a previous section (Fig. 9). DAP12 homodimers contain ITAM motifs that are typically phosphorylated by Src-family tyrosine kinases and recruit Syk, triggering signaling cascades linked in general to cellular activation. Antibody crosslinking of Siglec-H on murine pDCs, however, attenuates TLR9 induced Type I interferon synthesis and secretion (32). In addition, DAP12-deficient pDCs lack surface Siglec-H expression and secrete more IFN-α upon stimulation with CpG oligodeoxydinucleotides (ODNs) than do wild type pDCs (101). For many ITAM linked immune receptors low avidity interactions result in inhibitory consequences while high avidity interactions result in cell activation. For some ITAM containing receptors in PDCs, including ILT7, BDCA-2, NKp44 and Siglec-H high avidity interactions nevertheless result in the attenuation of TLR induced Type IIFN production. It appears that in myeloid cells a DAP12-Syk-calcium-Pyk2 pathway coordinately induces inhibitory cytokines such as IL-10 and molecules that compromise TLR signaling and thus contribute to the attenuation of TLR linked Type I IFN secretion. Some of the proteins induced include SOCS3 – which inhibits JAKs, A20- which inhibits TRAF6, ABIN-3 which inhibits NFκB, and the transcriptional regulators, Hes1 and STAT3, that suppress the synthesis of inflammatory cytokines (54).
Figure 9.
Engagement of a DAP12 associated Siglec may result in the induction and/or activation of proteins that can compromise TLR signaling and reduce the secretion of type I interferons in plasmacytoid dendritic cells.
It is not clear whether trans ligands actually interact with Siglec-H in vivo. Since the suppression of TLR ligand induced Type I interferon production can be shown to be DAP12 dependent in vitro using isolated pDCs, it appears likely that Siglec-H is ligated by a cis-ligand, albeit a non-sialic acid containing one. Antibody crosslinking of Siglec-H results in efficient delivery into a lysosomal compartment, suggesting that Siglec-H might be capable of pathogen capture and delivery into endosomes (33).
While Siglec-H appears to be important in murine pDCs, it is unclear which member of the Siglec family, if any, mediates similar functions in human pDCs. Given the strong possibility that Siglec-H might not actually recognize sialic acid containing ligands, it is quite possible that some non-Siglec receptor on human pDCs recognizes similar ligands to Siglec-H and provides a similar function. Indeed NKp44, a DAP12 associated receptor on human pDCs, can be triggered by specific antibodies to inhibit type I interferon secretion. Given that all the available data on Siglec-H function has so far been generated using antibody ligation, more direct approaches to identify specific in vivo ligands for Siglec-H and for other pDC receptors in mice and humans will greatly help is obtain a better understanding of the function of this lectin and consequently of pDCs as well.
Siglecs in conventional dendritic cells and the regulation of T cell activation and polarization
When dendritic cells encounter microbial products in tissue sites they are triggered typically through pattern recognition receptors, alter their gene expression profiles, acquire the ability to efficiently present antigens, migrate to lymph nodes and activate specific T cells. This process, often called “maturation” is accompanied by the altered expression of a range of glycosyltransferases as well as documented changes in glycosylation (103). Genes involved in the synthesis of glycan core structures, sugar donors, and transporters are expressed at high levels in immature DCs and their levels remain unchanged upon TLR-induced maturation. However genes involved in the terminal modifications of glycan structures are markedly upregulated during DC maturation. Gene expression analyses of sialyltransferases were corroborated by mass spectrometry and lectin binding assays and indicated that maturation was associated with an increase in α2-3Neu5Ac containing glycans that are capable of binding to sialoadhesin and α2-8 disialic acid moieties that have the ability to bind to Siglec-7, and a decrease in α2-6 linked sialic acid containing glycoconjugates. Sialoadhesin is not believed to be involved in cis-interactions and why mature DCs express higher levels of α2-3Neu5Ac is unclear. It is possible that the higher levels of α2-3Neu5Ac facilitate interactions between DCs or facilitate macrophage-DC interactions.
Immature DCs express higher levels of α2-6 sialic acid containing glycans that are considered to be tolerogenic and can favor Treg generation. The mechanism by which α2-6 sialic acid containing glycans in immature DCs might contribute to Treg generation is not known. Siglec-1 and PD-L1, a ligand for the PD-1 inhibitory receptor are both induced in human monocyte-derived DCs that have been exposed to rhinoviruses, and such DCs are weak stimulators of T cells in mixed lymphocyte reactions, potently inducing an anergic state (104, 105). Interestingly, monoclonal antibody blockade of PD-L1 alone was not sufficient to revert the deep anergic state in T cells triggered by rhinovirus treated DCs, however, the combined blockade of Siglec-1 and PD-L1 restored the in vitro stimulatory capacity of DCs. T cells express the adhesion molecule CD43, a known ligand of Siglec-1 that is normally removed during APC-T cell interaction from the immunological synapse to the distal pole of the T cell; thus, it is possible that Siglec-1 on DCs may engage CD43 in trans to interfere with such CD43 relocalization and synapse formation. Siglec-7, on the other hand, is present on immature DCs, but its expression diminishes following LPS activation (106). Increases in α2-8 sialylation and ligation of Siglec-7 during DC maturation may potentially contribute to endocytosis/recycling and thus decreased expression of this receptor, as has been shown for other CD33-related Siglecs; alternatively, the extent of α2-8 linked sialic acid containing glycoconjugates in DCs may be coordinately regulated with Siglec-7 expression during maturation to maintain Siglec-7 activity within a fine range. While the changes in the binding in vitro of Fc fusion proteins of Siglec-1 and Siglec-7 to mature versus immature DCs that have been demonstrated (103) are consistent with the noted alterations of the DC glycome, the in vitro binding of Siglec-2 (which is also expressed on DCs and may be therefore potentially capable of cis interactions) also increased with maturation (106). This was a surprising result since Siglec-2 binds best to α2-6 linked sialic acid containing ligands that were predicted by gene expression analysis to be reduced on mature DCs. As pointed out by the authors, these discrepant findings could be explained by maturation-triggered mobilization of molecules (including α2,6-linked sialoconjugates) stored in DC granules. An alternative explanation might be provided by the results of a study that showed that immature DCs can inhibit BCR-induced proliferation in a contact-dependent manner that requires B cell expression of Siglec-2, but not the presence of α2,6-linked sialic acid-containing Siglec-2 ligands on either DCs or B cells (107). Instead, the group reported that DCs express a second, novel Siglec-2 ligand that does not contain α2,6-linked sialic acids, is resistant to neuraminidase treatment, and whose expression is maintained on ST6Gal-I sialyltransferase KO DCs, deeming it distinct from the ligands expressed on the surface of B cells, and more likely to contribute to the DC-mediated inhibition of B cells in trans that was demonstrated in the study.
Sialoadhesin and Siglec-7 appear to play reciprocal roles in DCs in the context of T cell activation and polarization. Studies on Campylobacter jejuni lipo-oligosaccharides suggest that α2-3 linked glycans from this microbe that that can serve as mimics for host GD1a and GM1a gangliosides interact with sialoadhesi n in trans and contribute to the induction of a Th1 response, whereas α2-8 linked sialic acid containing moieties mimic host GD1c, interact with Siglec-7 and can drive a Th2 like response (108). Immune responses to sialated C. jejuni lipo-oligosaccharides have been linked to the pathogenesis of Guillain-Barre Syndrome.
Studies in murine models of T. cruzi infection suggest that the heightened pathogenicity of the heavily sialylated T. cruzi strains may also result from the modulation of the function of dendritic cells (DCs) through interactions of the parasite with Siglec-E on DCs (109). Trypomastigotes of a TS-high strain (with increased sialylation), but not TS-low expressors, reduced LPS-induced production of pro-inflammatory IL-12 by bone marrow derived DCs, while increasing the production of anti-inflammatory IL-10 by the same cells. Treatment of the TS-high parasites with neuraminidase to completely remove sialic acid moieties on the cell surface prior to incubation with the bone marrow derived DCs prevented these inhibitory effects on the DCs. Ligation of Siglec-E on DCs by α2-3 linked sialic acid ligands on trypanosomes therefore appears to represent a mechanism whereby the parasite suppresses the host immune response by compromising the ability of DCs to make IL-12, a cytokine required for Th1 responses and for optimal CD8+ T cell activation
Siglec-E is expressed at high levels in murine cDCs, and a number of CD33 related Siglecs including Siglec-9 are prominent in human cDCs. Studies on cDC maturation and T cell activation in Siglec-E knockout mice, especially conditional knockout mice, are likely to provide valuable insights into the function of Siglecs in cDCs.
A direct role for Siglecs in T cells
Although Siglecs expressed in B cells, conventional dendritic cells and plasmacytoid dendritic cells may influence the biology of T cells, there is also some evidence to support the notion that siglecs are expressed in and perhaps have a functional role in T cells. Siglec 9 is expressed on about 2% of CD4+ T cells and 5% of CD8+ T cells (48). Gene expression data from the Immgen consortium reveals a fairly striking elevation of Siglec-9 in central memory T cells. A possible direct function of Siglecs in human memory T cells therefore deserves exploration. Siglec-F, which is expressed at high levels on esoinophils, has been shown to be induced in activated CD4+ T cells in an allergic inflammation model in mice (96). Although a negative regulatory role for Siglec-F in eosinophils has been established using Siglec-F knockout mice (96), conditional deletion of this Siglec in T cells may be required to explore its biological relevance in activated T cells.
Stable expression of Siglecs-7 and-9 in Jurkat cells results in SHP-1 recruitment, partial colocalization with the TCR-CD3 complex, reduced phosphorylation of Tyr-319 on ZAP-70 and a diminution of NFAT activity (110). Mutation of the conserved arginine residue required for sialic acid recognition in Siglec-7 (Arg124) or Siglec-9 (Arg120) decreased the inhibitory function of transfected Siglecs in an in vitro assay. Among a number of non-synonymous SNPs in the SIGLEC-9 gene one polymorphic variant (SIGLEC-9 A391C) actually compromises sialic acid binding. Transient expression of this variant form of Siglec-9 in Jurkat cells followed by TCR engagement led to increased ZAP-70 phosphorylation and IL-2 production compared to wild type Siglec-9 transfectants (111).
There may be a role for Siglec-1 and sialoglycoconjugates during T cell activation and differentiation, and this remains a poorly explored area. Increased sialylation is observed in activated T cells, and the increases seen in α2,3 linked sialoglycoconjugates may reflect an increase in activity of the enzyme ST3GalI. Sialoadhesin binds to glycoconjugates, especialy O-linked mucins found on proteins like CD43, exhibiting α2,3 linked sialic acid. Binding occurs primarily to CD8+ T cells since most of the sialic acid in naïve CD4+ T cells is 9-O-acetylated and cannot bind sialoadhesin (see section in the next section of the review on this post-synthetic modification of sialic acid). Sialoadhesin deficient mice have an increased representation of CD8+ T cells in spleen and LNs (112). This seems to be an effect on the homeostasis of T cells in the periphery since thymic T cell development is normal in these mice. These additional CD8 T cells have not been characterized and it remains to be determined whether the T cell accumulation seen reflects altered recirculation, increased proliferation, or decreased apoptosis. Intriguingly the ST3GalI null mouse exhibits a relative paucity of CD8+ T cells (113), almost the mirror image phenotype of the saloadhesin knockout mouse but the reason for this striking symmetry could lie elsewhere (114).
Naïve murine CD4+ T cells exhibit 9-O-acetylated sialic acids on O-linked mucins. T cell activation results in the loss of these 9-O-acetylated sialic acid moieties (115). Although 9-O acetylation of sialic acid can abrogate binding of sialoadhesin to its ligands, the function of this modification on murine CD4+ T cells has not been elucidated. 9-O-acetylated GD3 has been identified on human T cells characterized in a limited fashion (116, 117). The significance of the expression of this O-acetylated glycolipid, especially in relation to siglec mediated recognition and regulatory events is not known.
Siglecs in B cell biology and the maintenance of immunological tolerance
Siglec-2/CD22 and Siglec-G are expressed at high levels on murine B lymphocytes. Siglec-E is expressed on marginal zone B cells and on a subset of IgMhiIgDhi cells, likely the marginal zone B cell precursor population (27). In humans a number of siglecs are expressed in cells of the B lineage. These include Siglecs-2, -5, -6, -9 and -10 (14, 22). Exhausted B cells in subjects with HIV express increased levels of a number of inhibitory receptors including Siglec-6. Downregulation of this Siglec using siRNA knockown can restore B cell function (118).
Although CD22 knockout and knockin mice have been studied by a number of groups for over a decade and a half, sometimes contradictory conclusions have been arrived at in different studies and there is no consensus in the field regarding the function of siglecs in B cells. Siglec G knockout mice were also generated a few years ago and the study of these mice as well as of cd22−/−/siglecG−/− mice have proved instructive. The interpretation of the existing data on siglecs in B cells that is presented here is an attempt at a synthesis, but not all published reports can be reconciled.
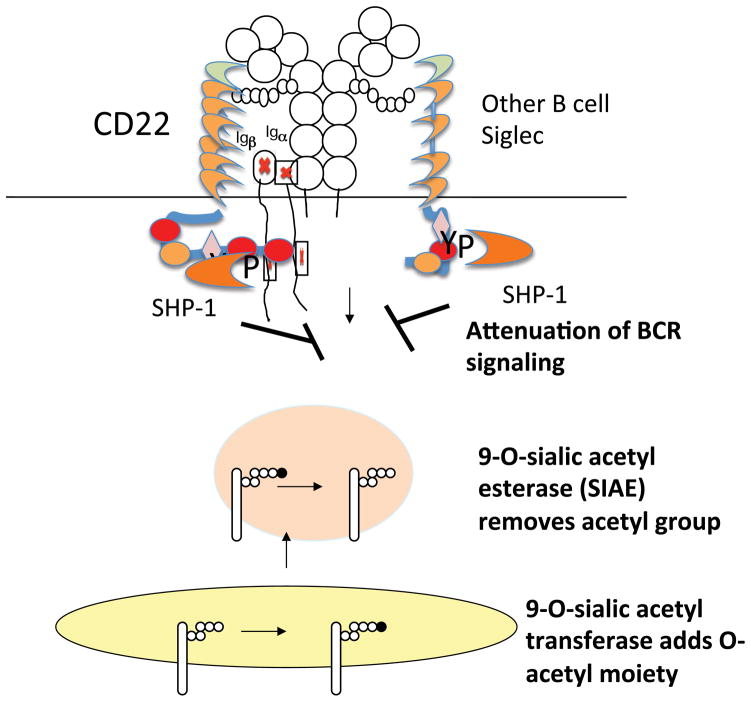
CD22 contains seven extracellular Ig like domains and six cytosolic tyrosines (4). These tyrosine residues contribute to three ITIM motifs, one ITIM like tyrosine, and a motif that can bind the SH2 domain of the Grb2 adaptor. These tyrosine residues can be phosphorylated by Src-family kinases and Lyn is likely the most relevant kinase for CD22 tyrosine phosphorylation. The two most C-terminal ITIM tyrosine residues, tyrosines 843 and 863 contribute to SHP-1 recruitment and inhibitory signaling, while tyrosine 828 can bind the SH2 domain of Grb2 (119,120). Grb2 has one SH2 domain flanked by two SH3 domains and it can potentially recruit other adaptors and signaling enzymes and contribute to activation phenomena. CD22 is capable of both inhibitory and activating signals and it is likely that all the in vivo functions of this Siglec have not yet been fully teased out. Following BCR activation CD22 is tyrosine phosphorylated on ITIM motifs, recruits SHP-1 and dephosphorylates key signaling intermediates (38, 121). This contributes to a diminution of downstream signaling and a decrease in the release of calcium from intracellular stores. Active SHP-1 also contributes to the activation of PMCA-4, a plasma membrane calcium ATPase that causes an efflux of calcium decreasing the intracellular pool (122).
A number of different CD22 knockout mice have been described (121, 123 – 125). The genetic backgrounds of all these mice, housed in different colonies, are likely not identical. CD22 knockout mice all exhibit enhanced release of calcium from intracellular stores upon BCR activation (121, 123 – 125). These knockout mice, however, also exhibit diminished BCR induced B cell proliferation. This proliferation defect might reflect the loss of some activating signals or could alternatively result from defective inhibitory signaling contributing to exaggerated BCR activation and increased B lymphocyte apoptosis (124,125).
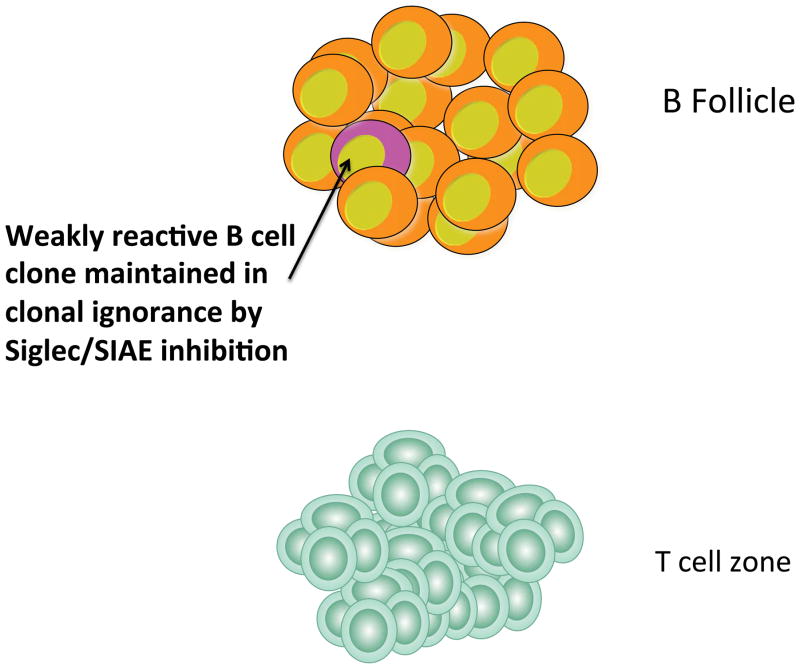
One phenotype observed in CD22 knockout mice is the marked reduction of marginal zone B cells (126). This phenotype might be linked to an increase in BCR signal strength in these mice, though this remains a matter of debate (see discussion in reference 127). The loss of bone marrow perisinusoidal B cells is also likely to be linked to enhanced BCR activation and the consequent premature egress of recirculating mature B cells from the bone marrow compartment, although formal evidence for such a mechanism is lacking (128, 129). Another phenotype that will likely provides important clues regarding the biological function of CD22 and other B cell Siglecs, is the increase in anti-chromatin antibodies seen in older CD22 null mice (130). More striking lupus like phenotypes are observed in mice that lack Lyn (131 – 133) and in mice in which SHP-1 has been conditionally deleted in B cells (134).
CD22 is highly specific for α2–6 linked sialic acid moieties found on N-glycans (135). However the glycoproteins decorated with these ligands that are relevant to the in vivo function of CD22 have not been identified with any certainty. Cell lines in which the critical residues for sialic acid binding are mutated, or in which the ligand binding domain of CD22 has been blocked, exhibit enhanced BCR mediated calcium release from intracellular stores (136, 137). Knockin mice in which the same sialic acid binding residues of CD22 are mutated share some but not all of the phenotypes of cd22−/− mice (138). Most notably, the enhanced calcium release observed in knockout mice was not observed in the knockin animals, and this discrepancy remains to be resolved. It appears possible that CD22 can provide inhibitory signals both in a carbohydrate dependent and carbohydrate independent manner. Carbohydrate independent signals are probably less important than those mediated by sialated N-glycans for reasons discussed below. Some constitutive phosphorylation of the cytosolic tyrosines of CD22 may well occur even in the absence of ligand recognition and contribute to the modulation of tonic BCR signaling.
ST6GalI is the sialyl transferase that adds α2-6 linked sialic acid moieties to the underlying N-acetyl lactosamine moiety on N-glycans and this Siaα2-6Galβ1-4GlcNAc trisaccharide makes up the CD22 “epitope.” ST6GalI knockout mice (139) do not phenocopy cd22 knockout mice – and this led to suggestions that sialic acid binding might not be required for the function of CD22 in vivo. However other studies on more subtle modifications of sialic acid described below make it very likely that the binding of CD22 to sialic acid containing ligands is crucial in vivo. While the absence of ST6GalI might have been predicted to result in defective CD22 activation and enhanced BCR signaling, a marked diminution in BCR and CD40 signaling was observed in this mutant mouse (139). A defect in thymocyte development was also noted in these mice and both T-dependent and T-independent immune responses are defective in the absence of ST6GalI (140). In the cd22/ST6GalI double knockout mouse BCR signaling is enhanced in a manner similar to that seen in cd22 null mice (141, 142). It may be inferred that the loss of CD22 is dominant- in biochemical terms the reduced BCR signaling in the absence of ST6GalI alone likely reflects the reported enhanced co-localization of CD22 and IgM in the absence of this enzyme. (142). However the underlying basis for the co-localization of these molecules in these circumstances remain poorly understood. It is not clear why defects in CD40 signaling, T-dependent immune responses, and thymocyte development are observed in the absence of this sialyl transferase. It is possible that the absence of α2-6 linked sialic acid on glycoconjugates leads to relatively profound changes in vivo - perhaps by contributing to significant differences in the charge and hydrophilicity of some glycoproteins and the aberrant capping of N-acetyllactosamine termini on many N-glycans (143).
One of the modifications of sialic acid that is of relevance to CD22 and to some other Siglec is the O-acetylation of sialic acid in the 9-OH position or the 7-OH position. Enzymatic 7-O-acetylation of sialic acid can result in spontaneous migration of the acetyl group to the 9-OH position. It is known that Siglecs-1,-2, and -3 fail to bind their sialoglycoconjugate ligands if the latter contain 9-O-acetylated sialic acid (144).
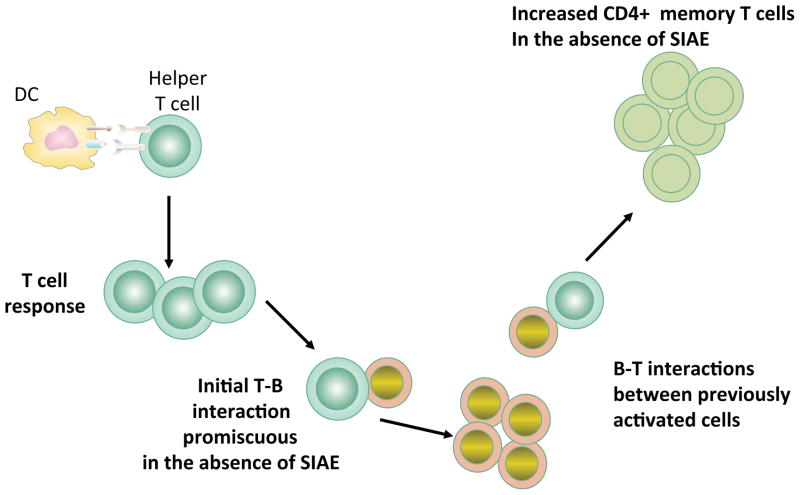
Sialic acid acetylesterase (SIAE), originally known as lysosomal sialic acid acetylesterase or LSE, is an enzyme that removes 9-O-acetyl moieties from 9-O-acetylated sialic acid (145, 146). Originally thought to be lysosomal in location, this enzyme is capable of being secreted, and can access glycosylated membrane proteins that are likely ligands of CD22 either in a post-Golgi vesicular compartment or on the cell surface (147). 9-O-acetyl moieties may be present on sialic acid on N-glycans, on O-linked oligosaccharides of mucins and on gangliosides. However in mice lacking SIAE, enhancement of 9-O-acetylation of sialic acid is observed on B cells, likely on N-glycans, but not on CD4+ T cells which bear 9-O-acetyl sialic acid moieties on O-linked mucins (147). Mice that lack SIAE phenocopy cd22−/− mice in many respects (147). These mice exhibit enhanced BCR signaling in terms of the release of calcium from intracellular stores, have a pronounced deficiency in marginal zone B cells, have a marked deficiency in recirculating bone marrow perisinusoidal B cells, exhibit defective BCR induced proliferation, and spontaneously develop anti-DNA and anti-chromatin autoantibodies. These phenotypes develop in a lymphocyte autonomous fashion suggesting that even though SIAE is capable of being secreted it is required to be expressed in B cells to mediate its biological functions. BCR induced tyrosine phosphorylation of CD22 was markedly diminished as was recruitment of SHP-1.
These data suggest that during their transit to the cell surface, at least in activated B cells, sialated N-glycans on at least some glycoproteins may be 9-O-acetylated by a sialic acid 9-O-acetyl transferase. The identity of this O-acetyl transferase for CD22 ligands is not known. It is unclear if different enzymes or acetylate sialic acid in different linkages to different underlying sugars on N-glycans, mucins, and gangliosides. CASD1 has been identified as a putative 9-O-acetyl transferase for sialic acid on GD3 but further studies need to be carried out on the specificity of this enzyme (148). In wild type B cells the 9-O-acetyl moiety can be removed by SIAE, allowing CD22 ligands to be de-acetylated, and to thus ligate CD22 (Figure 10). Ligation of CD22 presumably facilitates the accessibility of its cytosolic tail to Lyn, which phosphorylates tyrosine residues including the ITIM tyrosines, and thus facilitates recruitment and activation of SHP-1. Active SHP-1 then sets a threshold for BCR activation requiring that a relative high avidity interaction of antigen with the BCR occur to permit B cell activation. The identity of the glycoprotein or glycoproteins that bear the N-glycans that bind to CD22 in vivo remains a subject of debate. Since membrane IgM is heavily sialated and can be brought down with CD22 on immunoprecipitation (149) we have depicted this interaction in Figure 9, but clearly other possibilities must be entertained.
Figure 10.
A 9-O acetyl sialic acid acetyltransferase and a 9-O acetyl sialic acid acetylesterase (SIAE) regulate the availability of siglec ligands in B cells.
In contrast to the absence of ST6GalI which results in fairly drastic alterations in glycoconjugates, the far more subtle alteration in α2-6 linked sialic acids observed in siae mutant mice compromises the ability of CD22 ligands to associate with CD22, and results in enhanced BCR signaling. A further test of this hypothesis involved asking if a distinct subtle alteration of α2-6 linked sialic acid containing CD22 ligands, would also result in enhanced BCR signaling. CMAH is the hydroxylase that converts CMP-Neu5Ac to CMP-Neu5Gc (3). It has been established by a number of groups that murine CD22 (unlike human CD22) binds poorly to Neu5Ac containing ligands but binds relatively well to glycans that contain Neu5G (11, 150 – 152). In Cmah null mice, N-glycans possess terminal α2-6 linked Neu5Ac but not Neu5Gc. BCR induced calcium flux studies demonstrated marked enhancement of BCR signaling in Cmah mutant mice (147). A sizable reduction in MZ B cells was noted in these mice, as was a dramatic reduction in re-circulating perisinusoidal B cells. These data indicate that mice lacking Neu5Gc, and expressing only Neu5Ac, exhibit enhanced BCR signaling similar to that noted in Siae mutant mice. These data support the notion that relatively subtle changes in sialic acid structure that compromise the ability of CD22 ligands to bind CD22 result in enhanced BCR signaling.
Mice with an engineered mutation in CD22 develop autoantibodies only after they are about 9 months old and do not develop glomerulonephritis, unlike Lyn knockout and SHP-1 deficient mice, which develop frank disease. Siae mutant mice present with autoantibodies as early as 5 months after birth and accumulate immune complexes in the kidneys. The slightly stronger autoimmune phenotype observed in siae mutant mice as compared to cd22 null mice suggested that SIAE may be required for the function of more than one inhibitory Siglec (147). Possible support for this notion has been obtained from the study of cd22−/−/siglecg−/− double knockout mice as discussed below.
Siglec-G is a CD33 related Siglec that is expressed in all B cells but at the highest levels in peritoneal B-1a cells. Mice that lack Siglec G (30, 31, 153) exhibit enhanced BCR signaling and accumulate B-1a B cells presumably because of decreased apoptosis that may be linked to the higher expression of the NFATc1 transcription factor in mutant B-1 cells (153) and the uncontrolled activation of NFκB in the absence of this Siglec (31). A similar increase in B-1a B cells is also observed in conditional SHP-1 knockout mice (134) suggesting that Siglec-G probably mediates its inhibitory responses by SHP-1 recruitment to its ITIM motifs. The repertoire of the B-1 cells that accumulate in the absence of Siglec-G resembles the repertoire of B-2 cells suggesting that an alteration in selection occurs during differentiation in the absence of this receptor (153). Siglec-G deficient mice do not exhibit autoimmunity but aged cd22−/−/siglecg−/− mice accumulate autoantibodies and develop a spontaneous glomerulonephritis (154) not unlike that observed in siae−/− mice (147). Siglec-G recognizes sialic acid in certain CD24 glycoforms but there is no information on whether it recognizes only non - 9-O-acetylated sialic acid containing glycans. It remains to be seen whether the recognition of sialoglycoconjugates by Siglec-G is regulated in any way by SIAE. A further potential link between tolerance in B cells and Siglec-G may be inferred from challenge studies conducted by Duong et al. using sialated and non-sialated antigens (155). The presence of sialic acid on synthetic antigens was shown to tolerize B cells in a CD22 and Siglec-G dependent fashion, suggesting that the avid engagement of these Siglecs can dampen B cell activation.
The analysis of mutant mice has convincingly linked the Siglec/SIAE/Lyn/SHP-1 inhibitory pathway to B cell tolerance and the prevention of autoimmunity (130 – 134, 147). The analysis of disease associated rare genetic variants suggests a role for SIAE in preventing human autoimmune and chronic inflammatory disorders (156). How exactly this biochemical pathway functions in cellular terms in order to prevent autoimmunity is not fully understood, but probably involves the maintenance of a form of clonal ignorance in B cells and/or the enhanced generation of CD4+ memory T cells as will be discussed below.
Three major mechanisms of B cell tolerance have been uncovered by studies on B cell receptor (BCR) transgenic and knockin mice. These include receptor editing, deletion, and anergy (157 – 160). These widely studied mechanisms of B cell tolerance all involve the induction of BCR signaling by self-antigen. An inherited defect in BCR signaling would likely result in a defect in this category of B cell tolerance mechanisms and could predispose certain individuals to autoimmunity. Receptor editing and clonal deletion occur in the bone marrow and in transitional B cells in the spleen while anergy likely is induced in mature B cells. The SIAE/Siglec pathway mediates a distinct but important mechanism of peripheral B cell tolerance that involves the repression of BCR signaling when self-antigen is engaged in the periphery by B cells. CD22 is expressed on the cell surface in mature B cells, and SIAE expression increases with B cell maturation. Recent studies examining the role of Lyn in the periphery have helped establish that the CD22 Lyn and SHP-1 are required in the periphery in order to maintain tolerance (161). These data suggest that weakly self-reactive cells may be prevented from being activated unless they encounter a high affinity cognate antigen by the SIAE/Siglec pathway. This pathway presumably seeks to maintain a state of clonal ignorance to prevent weakly self-reactive B cells from being adequately activated to efficiently endocytose antigen, induce CCR7 expression and migrate towards the T cell zone to present antigenic peptides to specific CD4+ T cells (162). A weakly self-reactive B cell that can actually obtain T cell help runs the risk of undergoing somatic mutation and emerging as a strongly auto-reactive B cell (Figure 11). Clonal ignorance in B cells mediated by the SIAE/Siglec pathway may promote bliss by protecting vertebrate B cells from acquiring T cell help. It is widely recognized that activated B cells play crucial roles in the generation of both T follicular helper cells and CD4+ memory T cells. An alternative possibility worth exploring is that in the absence of SIAE, B cells are more promiscuously activated and this results in enhanced CD4+ memory T cells being generated some of which might drive disease processes (Figure 12).
Figure 11.
SIAE may maintain clonal ignorance in B cells preventing the activation of weakly self-reactive B cells. Activation of weakly self-reactive B cells would permit T-B collaboration and consequent somatic mutation of the B cell, potentially making it strongly self-reactive.
Figure 12.
SIAE may permit promiscuous activation of B cells that might in turn facilitate the induction of CD4+ memory T cells that might be linked to disease.
Conclusions
A multitude of enzymes control the availability of sialic acid on sialoglycoconjugates and also contribute to post-synthetic modifications of sialic acid during the development and activation of cells of innate and acquired immunity. These sialic acid containing ligands may be recognized by an array of specific receptors called Siglecs. Sialoadhesin is a Siglec that is primarily expressed on macrophages and dendritic cells and may contribute to cell adhesion events as well as to the internalization of sialic acid expressing pathogens. The majority of ITIM containing Siglecs in innate immune cells primarily attenuate inflammatory responses in different cell types primarily through cis-interactions with sialoglycoconjugates. Pathogens and tumors can subvert these inhibitory responses for their own ends. The biological functions of siglecs which associate with signaling adaptors such as DAP12 are less well understood. They may generate signals that counter TLR generated responses but little is known about the ligands that target these Siglecs. The activity of some Siglecs on B lymphocytes may be regulated by the 9-O-acetylation of sialic acid on their ligands. These siglecs may contribute to B cell clonal ignorance and provide protection against the development of autoimmunity. A more thorough understanding of the regulation of the synthesis and activity of siglecs, of the generation and availability of their ligands, and the consequences of their genetic ablation in specific cell types may provide a better understanding of how the immune system is regulated.
Acknowledgments
We thank Ajit Varki for many helpful discussions over the years, and Marco Colonna, Paul Crocker, Jim Paulson and Ajit Varki for being willing to make preprints of their recent work available. This work was supported by grants AI076505, AR058481, AI090867 and AI064930 from the NIH.
Glossary
- MINI-GLOSSARY
C-Type Lectins, Calcium dependent carbohydrate binding protein
- DAMP
damage associated molecular pattern
- ITAM
immunoreceptor tyrosine based activating motif
- ITIM
immunoreceptor tyrosine based inhibitory motif
- I-type lectin
Ig domain containing carbohydrate binding protein
- PAMP
pathogen associated molecular pattern
- Sialic acid
Nine carbon sugar with a carboxylic carbon in a six-carbon ring and a three carbon exoxyclic chain
- Siglec
sialic acid binding Ig-like lectin
Literature cited
- 1.Sharon N. Advanced Book Program. Reading, Mass: Addison-Wesley Pub. Co; 1975. Complex carbohydrates, their chemistry, biosynthesis, and functions: a set of lecture notes; p. xix.p. 466.p. 7. [Google Scholar]
- 2.Laine RA. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 10(12) structures for a reducing hexasaccharide: the Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology. 1994;4:759–67. doi: 10.1093/glycob/4.6.759. The calculations made here emphasize the structural diversity that carbohydrates can generate. [DOI] [PubMed] [Google Scholar]
- 3.Varki A. Sialic acids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Chapter 15. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- 4.Stamenkovic I, Seed B. The B-cell antigen CD22 mediates monocyte and erythrocyte adhesion. Nature. 1990;345:74–7. doi: 10.1038/345074a0. [DOI] [PubMed] [Google Scholar]
- 5.Stamenkovic I, Sgroi D, Aruffo A, Sy MS, Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991;66:1133–44. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- 6.Crocker PR, Kelm S, Dubois C, Martin B, McWilliam AS, Shotton DM, Paulson JC, Gordon S. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. The EMBO journal. 1991;10:1661–9. doi: 10.1002/j.1460-2075.1991.tb07689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crocker PR, Mucklow S, Bouckson V, McWilliam A, Willis AC, Gordon S, Milon G, Kelm S, Bradfield P. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. The EMBO journal. 1994;13:4490–503. doi: 10.1002/j.1460-2075.1994.tb06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelm S, Pelz A, Schauer R, Filbin MT, Tang S, de Bellard ME, Schnaar RL, Mahoney JA, Hartnell A, Bradfield P, et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Current biology: CB. 1994;4:965–72. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 9.Powell LD, Varki A. I-type lectins. The Journal of biological chemistry. 1995;270:14243–6. doi: 10.1074/jbc.270.24.14243. [DOI] [PubMed] [Google Scholar]
- 10.Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, Kehrl JH, Kelm S, Le Douarin N, Powell L, Roder J, Schnaar RL, Sgroi DC, Stamenkovic K, Schauer R, Schachner M, van den Berg TK, van der Merwe PA, Watt SM, Varki A. Siglecs: a family of sialic-acid binding lectins. Glycobiology. 1998;8:v. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- 11.Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. The Journal of biological chemistry. 2003;278:31007–19. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 12.Yamaji T, Teranishi T, Alphey MS, Crocker PR, Hashimoto Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. The Journal of biological chemistry. 2002;277:6324–32. doi: 10.1074/jbc.M110146200. [DOI] [PubMed] [Google Scholar]
- 13.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–35. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 14.Cao H, Crocker PR. Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation? Immunology. 2011;132:18–26. doi: 10.1111/j.1365-2567.2010.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May AP, Robinson RC, Vinson M, Crocker PR, Jones EY. Crystal structure of the N-terminal domain of sialoadhesin in complex with 3′ sialyllactose at 1.85 A resolution. Molecular cell. 1998;1:719–28. doi: 10.1016/s1097-2765(00)80071-4. [DOI] [PubMed] [Google Scholar]
- 16.Zaccai NR, Maenaka K, Maenaka T, Crocker PR, Brossmer R, Kelm S, Jones EY. Structure-guided design of sialic acid-based Siglec inhibitors and crystallographic analysis in complex with sialoadhesin. Structure. 2003;11:557–67. doi: 10.1016/s0969-2126(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 17.Alphey MS, Attrill H, Crocker PR, van Aalten DM. High resolution crystal structures of Siglec-7. Insights into ligand specificity in the Siglec family. The Journal of biological chemistry. 2003;278:3372–7. doi: 10.1074/jbc.M210602200. [DOI] [PubMed] [Google Scholar]
- 18.Dimasi N, Moretta A, Moretta L, Biassoni R, Mariuzza RA. Structure of the saccharide-binding domain of the human natural killer cell inhibitory receptor p75/AIRM1. Acta crystallographica. Section D, Biological crystallography. 2004;60:401–3. doi: 10.1107/S0907444903028439. [DOI] [PubMed] [Google Scholar]
- 19.Attrill H, Imamura A, Sharma RS, Kiso M, Crocker PR, van Aalten DM. Siglec-7 undergoes a major conformational change when complexed with the alpha(2,8)-disialylganglioside GT1b. The Journal of biological chemistry. 2006;281:32774–83. doi: 10.1074/jbc.M601714200. [DOI] [PubMed] [Google Scholar]
- 20.Attrill H, Takazawa H, Witt S, Kelm S, Isecke R, Brossmer R, Ando T, Ishida H, Kiso M, Crocker PR, van Aalten DM. The structure of siglec-7 in complex with sialosides: leads for rational structure-based inhibitor design. The Biochemical journal. 2006;397:271–8. doi: 10.1042/BJ20060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaccai NR, May AP, Robinson RC, Burtnick LD, Crocker PR, Brossmer R, Kelm S, Jones EY. Crystallographic and in silico analysis of the sialoside-binding characteristics of the Siglec sialoadhesin. Journal of molecular biology. 2007;365:1469–79. doi: 10.1016/j.jmb.2006.10.084. [DOI] [PubMed] [Google Scholar]
- 22.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nature reviews Immunology. 2007;7:255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 23.Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochemical Society transactions. 2008;36:1467–71. doi: 10.1042/BST0361467. [DOI] [PubMed] [Google Scholar]
- 24.Varki A, Angata T. Siglecs--the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 25.Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(Suppl 2):8939–46. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkman-Van der Linden EC, Angata T, Reynolds SA, Powell LD, Hedrick SM, Varki A. CD33/Siglec-3 binding specificity, expression pattern, and consequences of gene deletion in mice. Molecular and cellular biology. 2003;23:4199–206. doi: 10.1128/MCB.23.12.4199-4206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. European journal of immunology. 2004;34:1175–84. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 28.Angata T, Hingorani R, Varki NM, Varki A. Cloning and characterization of a novel mouse Siglec, mSiglec-F: differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. The Journal of biological chemistry. 2001;276:45128–36. doi: 10.1074/jbc.M108573200. [DOI] [PubMed] [Google Scholar]
- 29.Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G) Genomics. 2003;82:521–30. doi: 10.1016/s0888-7543(03)00171-x. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, Winkler TH, Kneitz B, Crocker PR, Nitschke L. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nature immunology. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 31.Ding C, Liu Y, Wang Y, Park BK, Wang CY, Zheng P. Siglecg limits the size of B1a B cell lineage by down-regulating NFkappaB activation. PloS one. 2007;2:e997. doi: 10.1371/journal.pone.0000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blasius A, Vermi W, Krug A, Facchetti F, Cella M, Colonna M. A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion of interferon-alpha. Blood. 2004;103:4201–6. doi: 10.1182/blood-2003-09-3108. [DOI] [PubMed] [Google Scholar]
- 33.Blasius AL, Colonna M. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends in immunology. 2006;27:255–60. doi: 10.1016/j.it.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, Cerundolo V, Crocker PR. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–8. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 35.Mitra N, Banda K, Altheide TK, Schaffer L, Johnson-Pais TL, Beuten J, Leach RJ, Angata T, Varki N, Varki A. SIGLEC12, a Human-specific Segregating (Pseudo)gene, Encodes a Signaling Molecule Expressed in Prostate Carcinomas. The Journal of biological chemistry. 2011;286:23003–11. doi: 10.1074/jbc.M111.244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13251–6. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao H, de Bono B, Belov K, Wong ES, Trowsdale J, Barrow AD. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large-scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics. 2009;61:401–17. doi: 10.1007/s00251-009-0372-0. [DOI] [PubMed] [Google Scholar]
- 38.Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin J, Thomas ML, Fearon DT. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–4. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 39.Taylor VC, Buckley CD, Douglas M, Cody AJ, Simmons DL, Freeman SD. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. The Journal of biological chemistry. 1999;274:11505–12. doi: 10.1074/jbc.274.17.11505. [DOI] [PubMed] [Google Scholar]
- 40.Yu Z, Maoui M, Wu L, Banville D, Shen S. mSiglec-E, a novel mouse CD33-related siglec (sialic acid-binding immunoglobulin-like lectin) that recruits Src homology 2 (SH2)-domain-containing protein tyrosine phosphatases SHP-1 and SHP-2. The Biochemical journal. 2001;353:483–92. doi: 10.1042/0264-6021:3530483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. Journal of immunology. 2004;173:6841–9. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- 42.Avril T, Freeman SD, Attrill H, Clarke RG, Crocker PR. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. The Journal of biological chemistry. 2005;280:19843–51. doi: 10.1074/jbc.M502041200. [DOI] [PubMed] [Google Scholar]
- 43.Freeman SD, Kelm S, Barber EK, Crocker PR. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85:2005–12. [PubMed] [Google Scholar]
- 44.Munday J, Kerr S, Ni J, Cornish AL, Zhang JQ, Nicoll G, Floyd H, Mattei MG, Moore P, Liu D, Crocker PR. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. The Biochemical journal. 2001;355:489–97. doi: 10.1042/0264-6021:3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li N, Zhang W, Wan T, Zhang J, Chen T, Yu Y, Wang J, Cao X. Cloning and characterization of Siglec-10, a novel sialic acid binding member of the Ig superfamily, from human dendritic cells. The Journal of biological chemistry. 2001;276:28106–12. doi: 10.1074/jbc.M100467200. [DOI] [PubMed] [Google Scholar]
- 46.Falco M, Biassoni R, Bottino C, Vitale M, Sivori S, Augugliaro R, Moretta L, Moretta A. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. The Journal of experimental medicine. 1999;190:793–802. doi: 10.1084/jem.190.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicoll G, Ni J, Liu D, Klenerman P, Munday J, Dubock S, Mattei MG, Crocker PR. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. The Journal of biological chemistry. 1999;274:34089–95. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 48.Zhang JQ, Nicoll G, Jones C, Crocker PR. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. The Journal of biological chemistry. 2000;275:22121–6. doi: 10.1074/jbc.M002788200. leukocytes. The Journal of biological chemistry 275: 22121–6. [DOI] [PubMed] [Google Scholar]
- 49.Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D’Alessio KJ, Holmes SD, Abrahamson JA, Erickson-Miller CL, Murdock PR, Tachimoto H, Schleimer RP, White JR. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. The Journal of allergy and clinical immunology. 2000;105:1093–100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 50.Angata T, Kerr SC, Greaves DR, Varki NM, Crocker PR, Varki A. Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. The Journal of biological chemistry. 2002;277:24466–74. doi: 10.1074/jbc.M202833200. [DOI] [PubMed] [Google Scholar]
- 51.Angata T, Hayakawa T, Yamanaka M, Varki A, Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:1964–73. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 52.Angata T, Tabuchi Y, Nakamura K, Nakamura M. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838–46. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- 53.Cao H, Lakner U, de Bono B, Traherne JA, Trowsdale J, Barrow AD. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. European journal of immunology. 2008;38:2303–15. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Gordon RA, Huynh L, Su X, Park Min KH, Han J, Arthur JS, Kalliolias GD, Ivashkiv LB. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity. 2010;32:518–30. doi: 10.1016/j.immuni.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blank U, Launay P, Benhamou M, Monteiro RC. Inhibitory ITAMs as novel regulators of immunity. Immunological reviews. 2009;232:59–71. doi: 10.1111/j.1600-065X.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- 56.Pinheiro da Silva F, Aloulou M, Benhamou M, Monteiro RC. Inhibitory ITAMs: a matter of life and death. Trends in immunology. 2008;29:366–73. doi: 10.1016/j.it.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 57.van den Berg TK, Breve JJ, Damoiseaux JG, Dopp EA, Kelm S, Crocker PR, Dijkstra CD, Kraal G. Sialoadhesin on macrophages: its identification as a lymphocyte adhesion molecule. The Journal of experimental medicine. 1992;176:647–55. doi: 10.1084/jem.176.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delputte PL, Van Gorp H, Favoreel HW, Hoebeke I, Delrue I, Dewerchin H, Verdonck F, Verhasselt B, Cox E, Nauwynck HJ. Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages. PloS one. 2011;6:e16827. doi: 10.1371/journal.pone.0016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones C, Virji M, Crocker PR. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Molecular microbiology. 2003;49:1213–25. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- 60.Monteiro VG, Lobato CS, Silva AR, Medina DV, de Oliveira MA, Seabra SH, de Souza W, DaMatta RA. Increased association of Trypanosoma cruzi with sialoadhesin positive mice macrophages. Parasitology research. 2005;97:380–5. doi: 10.1007/s00436-005-1460-1. [DOI] [PubMed] [Google Scholar]
- 61.Vanderheijden N, Delputte PL, Favoreel HW, Vandekerckhove J, Van Damme J, van Woensel PA, Nauwynck HJ. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. Journal of virology. 2003;77:8207–15. doi: 10.1128/JVI.77.15.8207-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rapoport EM, Sapot’ko YB, Pazynina GV, Bojenko VK, Bovin NV. Sialoside-binding macrophage lectins in phagocytosis of apoptotic bodies. Biochemistry Biokhimiia. 2005;70:330–8. doi: 10.1007/s10541-005-0119-y. [DOI] [PubMed] [Google Scholar]
- 63.Meesmann HM, Fehr EM, Kierschke S, Herrmann M, Bilyy R, Heyder P, Blank N, Krienke S, Lorenz HM, Schiller M. Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. Journal of cell science. 2010;123:3347–56. doi: 10.1242/jcs.066696. [DOI] [PubMed] [Google Scholar]
- 64.Malagolini N, Chiricolo M, Marini M, Dall’Olio F. Exposure of alpha2,6-sialylated lactosaminic chains marks apoptotic and necrotic death in different cell types. Glycobiology. 2009;19:172–81. doi: 10.1093/glycob/cwn122. [DOI] [PubMed] [Google Scholar]
- 65.Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, Paulson JC. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Molecular and cellular biology. 2007;27:5699–710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orr SJ, Morgan NM, Elliott J, Burrows JF, Scott CJ, McVicar DW, Johnston JA. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood. 2007;109:1061–8. doi: 10.1182/blood-2006-05-023556. [DOI] [PubMed] [Google Scholar]
- 67.Orr SJ, Morgan NM, Buick RJ, Boyd CR, Elliott J, Burrows JF, Jefferies CA, Crocker PR, Johnston JA. SOCS3 targets Siglec 7 for proteasomal degradation and blocks Siglec 7-mediated responses. The Journal of biological chemistry. 2007;282:3418–22. doi: 10.1074/jbc.C600216200. [DOI] [PubMed] [Google Scholar]
- 68.Walter RB, Raden BW, Zeng R, Hausermann P, Bernstein ID, Cooper JA. ITIM-dependent endocytosis of CD33-related Siglecs: role of intracellular domain, tyrosine phosphorylation, and the tyrosine phosphatases, Shp1 and Shp2. Journal of leukocyte biology. 2008;83:200–11. doi: 10.1189/jlb.0607388. [DOI] [PubMed] [Google Scholar]
- 69.Walter RB, Hausermann P, Raden BW, Teckchandani AM, Kamikura DM, Bernstein ID, Cooper JA. Phosphorylated ITIMs enable ubiquitylation of an inhibitory cell surface receptor. Traffic. 2008;9:267–79. doi: 10.1111/j.1600-0854.2007.00682.x. [DOI] [PubMed] [Google Scholar]
- 70.O’Reilly MK, Tian H, Paulson JC. CD22 is a recycling receptor that can shuttle cargo between the cell surface and endosomal compartments of B cells. Journal of immunology. 2011;186:1554–63. doi: 10.4049/jimmunol.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shan D, Press OW. Constitutive endocytosis and degradation of CD22 by human B cells. Journal of immunology. 1995;154:4466–75. [PubMed] [Google Scholar]
- 72.O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends in pharmacological sciences. 2009;30:240–8. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyd CR, Orr SJ, Spence S, Burrows JF, Elliott J, Carroll HP, Brennan K, Ni Gabhann J, Coulter WA, Jones C, Crocker PR, Johnston JA, Jefferies CA. Siglec-E is up-regulated and phosphorylated following lipopolysaccharide stimulation in order to limit TLR-driven cytokine production. Journal of immunology. 2009;183:7703–9. doi: 10.4049/jimmunol.0902780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ando M, Tu W, Nishijima K, Iijima S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochemical and biophysical research communications. 2008;369:878–83. doi: 10.1016/j.bbrc.2008.02.111. [DOI] [PubMed] [Google Scholar]
- 75.Pereira-Chioccola VL, Acosta-Serrano A, Correia de Almeida I, Ferguson MA, Souto-Padron T, Rodrigues MM, Travassos LR, Schenkman S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. Journal of cell science. 2000;113 (Pt 7):1299–307. doi: 10.1242/jcs.113.7.1299. [DOI] [PubMed] [Google Scholar]
- 76.Joiner K, Sher A, Gaither T, Hammer C. Evasion of alternative complement pathway by Trypanosoma cruzi results from inefficient binding of factor B. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:6593–7. doi: 10.1073/pnas.83.17.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carlin AF, Lewis AL, Varki A, Nizet V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. Journal of bacteriology. 2007;189:1231–7. doi: 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–6. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carlin AF, Chang YC, Areschoug T, Lindahl G, Hurtado-Ziola N, King CC, Varki A, Nizet V. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. The Journal of experimental medicine. 2009;206:1691–9. doi: 10.1084/jem.20090691. This paper describes how a pathogen may utilize a siglec for immune evasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weiman S, Dahesh S, Carlin AF, Varki A, Nizet V, Lewis AL. Genetic and biochemical modulation of sialic acid O-acetylation on group B Streptococcus: phenotypic and functional impact. Glycobiology. 2009;19:1204–13. doi: 10.1093/glycob/cwp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiman S, Uchiyama S, Lin FY, Chaffin D, Varki A, Nizet V, Lewis AL. O-Acetylation of sialic acid on Group B Streptococcus inhibits neutrophil suppression and virulence. The Biochemical journal. 2010;428:163–8. doi: 10.1042/BJ20100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohta M, Ishida A, Toda M, Akita K, Inoue M, Yamashita K, Watanabe M, Murata T, Usui T, Nakada H. Immunomodulation of monocyte-derived dendritic cells through ligation of tumor-produced mucins to Siglec-9. Biochemical and biophysical research communications. 2010;402:663–9. doi: 10.1016/j.bbrc.2010.10.079. [DOI] [PubMed] [Google Scholar]
- 83.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–5. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y, Chen GY, Zheng P. Sialoside-based pattern recognitions discriminating infections from tissue injuries. Current opinion in immunology. 2011;23:41–5. doi: 10.1016/j.coi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen GY, Chen X, King S, Cavassani KA, Cheng J, Zheng X, Cao H, Yu H, Qu J, Fang D, Wu W, Bai XF, Liu JQ, Woodiga SA, Chen C, Sun L, Hogaboam CM, Kunkel SL, Zheng P, Liu Y. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nature biotechnology. 2011;29:428–35. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, Doherty TA, Varki A, Broide DH. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respiratory research. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Linnartz B, Wang Y, Neumann H. Microglial immunoreceptor tyrosine-based activation and inhibition motif signaling in neuroinflammation. International journal of Alzheimer’s disease 2010. 2010 doi: 10.4061/2010/587463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lunnon K, Teeling JL, Tutt AL, Cragg MS, Glennie MJ, Perry VH. Systemic Inflammation Modulates Fc Receptor Expression on Microglia during Chronic Neurodegeneration. Journal of immunology. 2011;186:7215–24. doi: 10.4049/jimmunol.0903833. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Neumann H. Alleviation of neurotoxicity by microglial human Siglec-11. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:3482–8. doi: 10.1523/JNEUROSCI.3940-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. Glycan array screening reveals a candidate ligand for Siglec-8. The Journal of biological chemistry. 2005;280:4307–12. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 91.Gao PS, Shimizu K, Grant AV, Rafaels N, Zhou LF, Hudson SA, Konno S, Zimmermann N, Araujo MI, Ponte EV, Cruz AA, Nishimura M, Su SN, Hizawa N, Beaty TH, Mathias RA, Rothenberg ME, Barnes KC, Bochner BS. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. European journal of human genetics: EJHG. 2010;18:713–9. doi: 10.1038/ejhg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–20. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 93.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochemical and biophysical research communications. 2005;336:918–24. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 94.von Gunten S, Yousefi S, Seitz M, Jakob SM, Schaffner T, Seger R, Takala J, Villiger PM, Simon HU. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 2005;106:1423–31. doi: 10.1182/blood-2004-10-4112. [DOI] [PubMed] [Google Scholar]
- 95.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–63. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–7. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Avril T, North SJ, Haslam SM, Willison HJ, Crocker PR. Probing the cis interactions of the inhibitory receptor Siglec-7 with alpha2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of alpha2,8-sialyltransferase gene expression. Journal of leukocyte biology. 2006;80:787–96. doi: 10.1189/jlb.1005559. [DOI] [PubMed] [Google Scholar]
- 98.Nicoll G, Avril T, Lock K, Furukawa K, Bovin N, Crocker PR. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. European journal of immunology. 2003;33:1642–8. doi: 10.1002/eji.200323693. [DOI] [PubMed] [Google Scholar]
- 99.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, Moretta A, Mavilio D. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. 2009;114:3822–30. doi: 10.1182/blood-2009-06-226332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–66. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–6. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bjorck P, Leong HX, Engleman EG. Plasmacytoid dendritic cell dichotomy: identification of IFN-alpha producing cells as a phenotypically and functionally distinct subset. Journal of immunology. 2011;186:1477–85. doi: 10.4049/jimmunol.1000454. This paper suggests how Siglec-H expression helps divide plasmacytoid dendritic cells into functionally distinct subsets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bax M, Garcia-Vallejo JJ, Jang-Lee J, North SJ, Gilmartin TJ, Hernandez G, Crocker PR, Leffler H, Head SR, Haslam SM, Dell A, van Kooyk Y. Dendritic cell maturation results in pronounced changes in glycan expression affecting recognition by siglecs and galectins. Journal of immunology. 2007;179:8216–24. doi: 10.4049/jimmunol.179.12.8216. [DOI] [PubMed] [Google Scholar]
- 104.Kirchberger S, Majdic O, Steinberger P, Bluml S, Pfistershammer K, Zlabinger G, Deszcz L, Kuechler E, Knapp W, Stockl J. Human rhinoviruses inhibit the accessory function of dendritic cells by inducing sialoadhesin and B7-H1 expression. Journal of immunology. 2005;175:1145–52. doi: 10.4049/jimmunol.175.2.1145. [DOI] [PubMed] [Google Scholar]
- 105.Seyerl M, Kirchberger S, Majdic O, Seipelt J, Jindra C, Schrauf C, Stockl J. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. European journal of immunology. 2010;40:321–9. doi: 10.1002/eji.200939527. [DOI] [PubMed] [Google Scholar]
- 106.Lock K, Zhang J, Lu J, Lee SH, Crocker PR. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 2004;209:199–207. doi: 10.1016/j.imbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 107.Santos L, Draves KE, Boton M, Grewal PK, Marth JD, Clark EA. Dendritic cell-dependent inhibition of B cell proliferation requires CD22. Journal of immunology. 2008;180:4561–9. doi: 10.4049/jimmunol.180.7.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bax M, Kuijf ML, Heikema AP, van Rijs W, Bruijns SC, Garcia-Vallejo JJ, Crocker PR, Jacobs BC, van Vliet SJ, van Kooyk Y. Campylobacter jejuni Lipooligosaccharides Modulate Dendritic Cell-Mediated T Cell Polarization in a Sialic Acid Linkage-Dependent Manner. Infection and immunity. 2011;79:2681–9. doi: 10.1128/IAI.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Erdmann H, Steeg C, Koch-Nolte F, Fleischer B, Jacobs T. Sialylated ligands on pathogenic Trypanosoma cruzi interact with Siglec-E (sialic acid-binding Ig-like lectin-E) Cellular microbiology. 2009;11:1600–11. doi: 10.1111/j.1462-5822.2009.01350.x. [DOI] [PubMed] [Google Scholar]
- 110.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. The Journal of biological chemistry. 2004;279:43117–25. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- 111.Cheong KA, Chang YS, Roh JY, Kim BJ, Kim MN, Park YM, Park HJ, Kim ND, Lee CH, Lee AY. A novel function of Siglec-9 A391C polymorphism on T cell receptor signaling. International archives of allergy and immunology. 2011;154:111–8. doi: 10.1159/000320225. [DOI] [PubMed] [Google Scholar]
- 112.Oetke C, Vinson MC, Jones C, Crocker PR. Sialoadhesin-deficient mice exhibit subtle changes in B- and T-cell populations and reduced immunoglobulin M levels. Molecular and cellular biology. 2006;26:1549–57. doi: 10.1128/MCB.26.4.1549-1557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Priatel JJ, Chui D, Hiraoka N, Simmons CJ, Richardson KB, Page DM, Fukuda M, Varki NM, Marth JD. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity. 2000;12:273–83. doi: 10.1016/s1074-7613(00)80180-6. [DOI] [PubMed] [Google Scholar]
- 114.Moody AM, Chui D, Reche PA, Priatel JJ, Marth JD, Reinherz EL. Developmentally regulated glycosylation of the CD8alphabeta coreceptor stalk modulates ligand binding. Cell. 2001;107:501–12. doi: 10.1016/s0092-8674(01)00577-3. [DOI] [PubMed] [Google Scholar]
- 115.Krishna M, Varki A. 9-O-Acetylation of sialomucins: a novel marker of murine CD4 T cells that is regulated during maturation and activation. The Journal of experimental medicine. 1997;185:1997–2013. doi: 10.1084/jem.185.11.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kniep B, Flegel WA, Northoff H, Rieber EP. CDw60 glycolipid antigens of human leukocytes: structural characterization and cellular distribution. Blood. 1993;82:1776–86. [PubMed] [Google Scholar]
- 117.Wipfler D, Srinivasan GV, Sadick H, Kniep B, Arming S, Willhauck-Fleckenstein M, Vlasak R, Schauer R, Schwartz-Albiez R. Differentially regulated expression of 9-O-acetyl GD3 (CD60b) and 7-O-acetyl-GD3 (CD60c) during differentiation and maturation of human T and B lymphocytes. Glycobiology. 2011 doi: 10.1093/glycob/cwr050. [DOI] [PubMed] [Google Scholar]
- 118.Kardava L, Moir S, Wang W, Ho J, Buckner CM, Posada JG, O’Shea MA, Roby G, Chen J, Sohn HW, Chun TW, Pierce SK, Fauci AS. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. The Journal of clinical investigation. 2011 doi: 10.1172/JCI45685. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poe JC, Fujimoto M, Jansen PJ, Miller AS, Tedder TF. CD22 forms a quaternary complex with SHIP, Grb2, and Shc. A pathway for regulation of B lymphocyte antigen receptor-induced calcium flux. The Journal of biological chemistry. 2000;275:17420–7. doi: 10.1074/jbc.M001892200. [DOI] [PubMed] [Google Scholar]
- 120.Otipoby KL, Draves KE, Clark EA. CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. The Journal of biological chemistry. 2001;276:44315–22. doi: 10.1074/jbc.M105446200. [DOI] [PubMed] [Google Scholar]
- 121.Sato S, Miller AS, Inaoki M, Bock CB, Jansen PJ, Tang ML, Tedder TF. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551–62. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 122.Chen J, McLean PA, Neel BG, Okunade G, Shull GE, Wortis HH. CD22 attenuates calcium signaling by potentiating plasma membrane calcium-ATPase activity. Nature immunology. 2004;5:651–7. doi: 10.1038/ni1072. [DOI] [PubMed] [Google Scholar]
- 123.O’Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficientmice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 124.Otipoby KL, Andersson KB, Draves KE, Klaus SJ, Farr AG, Kerner JD, Perlmutter RM, Law CL, Clark EA. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634–7. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 125.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Current biology: CB. 1997;7:133–43. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 126.Samardzic T, Marinkovic D, Danzer CP, Gerlach J, Nitschke L, Wirth T. Reduction of marginal zone B cells in CD22-deficient mice. European journal of immunology. 2002;32:561–7. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 127.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nature reviews Immunology. 2009;9:767–77. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 128.Cariappa A, Mazo IB, Chase C, Shi HN, Liu H, Li Q, Rose H, Leung H, Cherayil BJ, Russell P, von Andrian U, Pillai S. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23:397–407. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 129.Pillai S, Cariappa A. The bone marrow perisinusoidal niche for recirculating B cells and the positive selection of bone marrow-derived B lymphocytes. Immunology and cell biology. 2009;87:16–9. doi: 10.1038/icb.2008.89. [DOI] [PubMed] [Google Scholar]
- 130.O’Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. The Journal of experimental medicine. 1999;189:1307–13. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–11. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 132.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–60. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 133.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 134.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 135.Powell LD, Sgroi D, Sjoberg ER, Stamenkovic I, Varki A. Natural ligands of the B cell adhesion molecule CD22 beta carry N-linked oligosaccharides with alpha-2,6-linked sialic acids that are required for recognition. The Journal of biological chemistry. 1993;268:7019–27. [PubMed] [Google Scholar]
- 136.Jin L, McLean PA, Neel BG, Wortis HH. Sialic acid binding domains of CD22 are required for negative regulation of B cell receptor signaling. The Journal of experimental medicine. 2002;195:1199–205. doi: 10.1084/jem.20011796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kelm S, Gerlach J, Brossmer R, Danzer CP, Nitschke L. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. The Journal of experimental medicine. 2002;195:1207–13. doi: 10.1084/jem.20011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Poe JC, Fujimoto Y, Hasegawa M, Haas KM, Miller AS, Sanford IG, Bock CB, Fujimoto M, Tedder TF. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nature immunology. 2004;5:1078–87. doi: 10.1038/ni1121. [DOI] [PubMed] [Google Scholar]
- 139.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4504–9. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marino JH, Tan C, Davis B, Han ES, Hickey M, Naukam R, Taylor A, Miller KS, Van De Wiele CJ, Teague TK. Disruption of thymopoiesis in ST6Gal I-deficient mice. Glycobiology. 2008;18:719–26. doi: 10.1093/glycob/cwn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghosh S, Bandulet C, Nitschke L. Regulation of B cell development and B cell signalling by CD22 and its ligands alpha2,6-linked sialic acids. International immunology. 2006;18:603–11. doi: 10.1093/intimm/dxh402. [DOI] [PubMed] [Google Scholar]
- 142.Collins BE, Smith BA, Bengtson P, Paulson JC. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nature immunology. 2006;7:199–206. doi: 10.1038/ni1283. [DOI] [PubMed] [Google Scholar]
- 143.Martin LT, Marth JD, Varki A, Varki NM. Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. The Journal of biological chemistry. 2002;277:32930–8. doi: 10.1074/jbc.M203362200. [DOI] [PubMed] [Google Scholar]
- 144.Sjoberg ER, Powell LD, Klein A, Varki A. Natural ligands of the B cell adhesion molecule CD22 beta can be masked by 9-O-acetylation of sialic acids. The Journal of cell biology. 1994;126:549–62. doi: 10.1083/jcb.126.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guimaraes MJ, Bazan JF, Castagnola J, Diaz S, Copeland NG, Gilbert DJ, Jenkins NA, Varki A, Zlotnik A. Molecular cloning and characterization of lysosomal sialic acid O-acetylesterase. The Journal of biological chemistry. 1996;271:13697–705. doi: 10.1074/jbc.271.23.13697. [DOI] [PubMed] [Google Scholar]
- 146.Takematsu H, Diaz S, Stoddart A, Zhang Y, Varki A. Lysosomal and cytosolic sialic acid 9-O-acetylesterase activities can Be encoded by one gene via differential usage of a signal peptide-encoding exon at the N terminus. The Journal of biological chemistry. 1999;274:25623–31. doi: 10.1074/jbc.274.36.25623. [DOI] [PubMed] [Google Scholar]
- 147.Cariappa A, Takematsu H, Liu H, Diaz S, Haider K, Boboila C, Kalloo G, Connole M, Shi HN, Varki N, Varki A, Pillai S. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. The Journal of experimental medicine. 2009;206:125–38. doi: 10.1084/jem.20081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arming S, Wipfler D, Mayr J, Merling A, Vilas U, Schauer R, Schwartz-Albiez R, Vlasak R. The human Cas1 protein: a sialic acid-specific O-acetyltransferase? Glycobiology. 2011;21:553–64. doi: 10.1093/glycob/cwq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peaker CJ, Neuberger MS. Association of CD22 with the B cell antigen receptor. European journal of immunology. 1993;23:1358–63. doi: 10.1002/eji.1830230626. [DOI] [PubMed] [Google Scholar]
- 150.Brinkman-Van der Linden EC, Sjoberg ER, Juneja LR, Crocker PR, Varki N, Varki A. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. The Journal of biological chemistry. 2000;275:8633–40. doi: 10.1074/jbc.275.12.8633. [DOI] [PubMed] [Google Scholar]
- 151.Kelm S, Brossmer R, Isecke R, Gross HJ, Strenge K, Schauer R. Functional groups of sialic acids involved in binding to siglecs (sialoadhesins) deduced from interactions with synthetic analogues. European journal of biochemistry/FEBS. 1998;255:663–72. doi: 10.1046/j.1432-1327.1998.2550663.x. [DOI] [PubMed] [Google Scholar]
- 152.Collins BE, Blixt O, Bovin NV, Danzer CP, Chui D, Marth JD, Nitschke L, Paulson JC. Constitutively unmasked CD22 on B cells of ST6Gal I knockout mice: novel sialoside probe for murine CD22. Glycobiology. 2002;12:563–71. doi: 10.1093/glycob/cwf067. [DOI] [PubMed] [Google Scholar]
- 153.Jellusova J, Duber S, Guckel E, Binder CJ, Weiss S, Voll R, Nitschke L. Siglec-G regulates B1 cell survival and selection. Journal of immunology. 2010;185:3277–84. doi: 10.4049/jimmunol.1001792. [DOI] [PubMed] [Google Scholar]
- 154.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. Journal of immunology. 2010;184:3618–27. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 155.Duong BH, Tian H, Ota T, Completo G, Han S, Vela JL, Ota M, Kubitz M, Bovin N, Paulson JC, Nemazee D. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. The Journal of experimental medicine. 2010;207:173–87. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Surolia I, Pirnie SP, Chellappa V, Taylor KN, Cariappa A, Moya J, Liu H, Bell DW, Driscoll DR, Diederichs S, Haider K, Netravali I, Le S, Elia R, Dow E, Lee A, Freudenberg J, De Jager PL, Chretien Y, Varki A, Macdonald ME, Gillis T, Behrens TW, Bloch D, Collier D, Korzenik J, Podolsky DK, Hafler D, Murali M, Sands B, Stone JH, Gregersen PK, Pillai S. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 2010;466:243–7. doi: 10.1038/nature09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–82. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 158.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–6. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 159.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. The Journal of experimental medicine. 1993;177:1009–20. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. The Journal of experimental medicine. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. Journal of immunology. 2009;182:5382–92. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pillai S, Cariappa A, Pirnie SP. Esterases and autoimmunity: the sialic acid acetylesterase pathway and the regulation of peripheral B cell tolerance. Trends in immunology. 2009;30:488–93. doi: 10.1016/j.it.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]