Abstract
Methionine sulfoxide reductase (MSRA) is an antioxidant enzyme implicated in protection against oxidative stress and protein maintenance. We have previously reported the association of marker D8S542, located within the MSRA gene, with schizophrenia in the Central Valley of Costa Rica (CVCR). By performing fine mapping analysis, we have now identified a potential 3-marker at risk haplotype within MSRA in the same CVCR sample, with a global P value slightly above nominal significance (P = 0.0526). By sequencing the MSRA gene in individuals carrying this haplotype, we identified a novel four-base pair deletion 1792 bases upstream of the MSRA transcription start site. This deletion was significantly under-transmitted to schizophrenia patients in the CVCR sample (P = 0.0292) using FBAT, and this was replicated in a large independent sample of 321 schizophrenia families from the Hispanic population (P = 0.0367). These findings suggest a protective effect of the deletion against schizophrenia. Further, MSRA mRNA levels were significantly lower in lymphoblastoid cell lines of individuals homozygous for the deletion compared to carriers of the normal allele (P = 0.0135), although significance was only evident when genotypes were collapsed. This suggests that the deleted sequence may play a role in regulating MSRA expression. In conclusion, this work points towards MSRA as a novel schizophrenia candidate gene. Further studies into the mechanisms by which MSRA is involved in schizophrenia pathophysiology may shed light into the biological underpinnings of this disorder.
Keywords: linkage disequilibrium, Central Valley of Costa Rica, deletion variant, protection, under-transmission
INTRODUCTION
In spite of the difficulty of replicating positive genetic linkages in schizophrenia, several genome-wide scans have shown repeated evidence for a potential schizophrenia gene locus on chromosome 8p (Owen et al., 2005). In two independent meta-analyses, (Badner and Gershon, 2002; Lewis et al., 2003) significant results for chromosomes 8 and 22 were obtained. In several independent studies, the small arm of chromosome 8 (8p) has shown evidence of association with schizophrenia (Blouin et al., 1998; Brzustowicz et al., 1999; Gurling et al., 2001; Kaufmann et al., 1998; Kendler et al., 1996; Pulver et al.,1995). We previously performed a linkage disequilibrium screen of chromosome 8, using families of subjects with schizophrenia from the founder population of the Central Valley of Costa Rica (CVCR) (Walss-Bass et al., 2006). We identified four regions (8p23.1, 8p21.3, 8q13.3 and 8q24.3) showing evidence of potential association to the phenotype of schizophrenia. The strongest evidence of association was found in region 8p23.1, with marker D8S542 (P = 0.008). This same region showed the strongest evidence of association in a genome-wide linkage disequilibrium screen for severe bipolar disorder in the same Costa Rican population (Ophoff et al., 2002) and is also within linkage peaks reported by previous schizophrenia linkage studies (Gurling et al 2001, Kaufmann et al 1998) . D8S542 is located within a gene encoding the antioxidant repair enzyme methionine sulphoxide reductase A (MSRA). MSRA reduces both free and protein-linked oxidized methionine residues, and is considered an important defense mechanism against oxidative damage (Kim and Gladyshev, 2007). It is found in a variety of tissues, especially kidney, liver and brain (Kuschel et al., 1999; Moskovitz et al., 1996). The relatively high expression levels in specific regions of the brain, such as the hippocampus and cerebellum, suggest that MSRA may participate in brain-specific tasks such as learning and memory. MSRA has been implicated in the pathology of neurodegenerative disorders including Alzheimer’s disease (Gabbita et al., 1999) and Parkinsons disease (Wassef et al., 2007), as well as in protection against aging (Cabreiro et al., 2006; Moskovitz et al., 2001; Ruan et al., 2002). Given the preliminary evidence of association of D8S542 with schizophrenia in the CVCR, and the increasing accumulation of evidence pointing towards oxidative stress as an important factor in schizophrenia pathology (Gysin et al., 2007; Prabakaran et al., 2004; Prabakaran et al., 2007; Zhang et al., 2007), we sought to determine whether MSRA could be a gene involved in predisposition for schizophrenia .
MATERIALS AND METHODS
Sample collection
All subjects were recruited after obtaining written informed consent in accordance with the principles of the Declaration of Helsinki and with approval from the Institutional Review Boards of all participating sites. For the first part of this study (fine-mapping and association of the deletion variant), we analyzed 378 subjects from the CVCR (from 151 different families). All probands were diagnosed by a best estimate diagnostic process (Escamilla et al., 1996), using the DSM-IV criteria. Final best estimate consensus diagnoses were as follows: schizophrenia (N=103), schizoaffective disorder (N=22),. bipolar disorder type I (N=14), major depressive episode (N=7) or psychotic disorder not otherwise specified (N=5). Blood was drawn from probands and available parents and/or siblings for DNA extraction and generation of lymphoblastoid cell lines. Given that the strongest finding of association with D8S542 in our previous study was obtained using the diagnosis of schizophrenia (Walss-Bass et al 2006), all analyses in the present study were performed considering only individuals with the phenotype of schizophrenia or schizoaffective disorder (N=125) as affected.
A second schizophrenia population sample of individuals of Mexican or Central American ancestry was used to attempt to replicate the association of the MSRA c.-1796_-1793delATGA with schizophrenia observed in the CVCR subjects. For this independent sample, families with two or more siblings affected with schizophrenia or schizoaffective disorder were recruited from sites in Mexico (49% of the sample), the United States (25%), Costa Rica (22%) and Guatemala (4%). Families were recruited if at least two grandparents were of Mexican or Central American origin. A detailed description of the ascertainment of these families has been recently published (Escamilla et al., 2007). A total of 1086 individuals from 321 different families were genotyped and analyzed statistically in this study. Of the 1086 subjects, 514 had a best estimate consensus diagnosis of schizophrenia or schizoaffective disorder (DSM-IV criteria). Blood was obtained from all affected subjects, as well as available parents and siblings, for generation of lymphoblastoid cell lines.
Generation of lymphoblastoid cell lines
Peripheral blood leucocytes were isolated using LeucoPREP brand cell separation tubes (Becton Dickinson Labware, Lincoln Park, NJ, USA) and transformed using Epstein-Barr virus (EBV) as previously described (Anderson and Gusella, 1984). Cells were grown in RPMI 1640 medium with 2mM L-glutamine and 15% bovine growth serum at 37 °C in a humidified 5% CO2 chamber to a density of approximately 2 × 106 cells/ml, collected by centrifugation and cryo-preserved for future use.
Genotyping
Genomic DNA was extracted from lymphoblastoid cell lines using the Puregene DNA purification kit (Gentra; Minneapolis, MN, USA). Four single nucleotide polymorphisms (SNPs) spanning the MSRA gene were selected from the SNPbrowser database (Applied Biosystems; Foster City, CA, USA) based on location (Table 1) and heterozygosity. Allelic discrimination was performed using the Taqman 5′ nuclease assay (Applied Biosystems). Genotypes were determined using the ABI 7900HT SDS 2.2.2 software adapted in the ABI 7900HT Sequence Detection System (Applied Biosystems). Microsatellite D8S542 had been previously genotyped in this sample (Walss-Bass et al 2006).
Table I.
Association analysis of MSRA individual markers with schizophrenia in the CVCR
| Marker | Base position1 | Alleles2 | Frequency | P-value3 | Z score |
|---|---|---|---|---|---|
| rs592420 | 9,797,829 | G | 0.277 | 0.8755 | 0.157 |
| A | 0.723 | 0.8755 | −0.157 | ||
| MSRA | 9,949,236 | ||||
| rs2409632 | 9,978,104 | A | 0.536 | 0.9676 | −0.041 |
| G | 0.464 | 0.9676 | 0.041 | ||
| D8S542 | 10,195,159 | 12 | 0.457 | 0.7191 | 0.360 |
| 13 | 0.130 | 0.0085 | 2.630 | ||
| 14 | 0.364 | 0.0113 | −2.531 | ||
| rs4260896 | 10,244,873 | G | 0.388 | 0.0916 | −1.687 |
| A | 0.612 | 0.0916 | 1.687 | ||
| MSRA | 10,323,805 | ||||
| rs4410951 | 10,328,717 | C | 0.560 | 0.1278 | −1.523 |
| A | 0.440 | 0.1278 | 1.523 |
According to the National Center for Biotechnology Information (NCBI) SNP database
Only alleles present in at least 10 informative families are shown
P values are for FBAT bi-allelic analysis. For marker D8S542, the global P value (multi-allelic analysis) was P = 0.016980 (Chi square= 10.195).
The location of the MSRA gene is indicated for reference
The Primer3 software (Whitehead Institute, Cambridge, MA, USA) was used to design the primers for genotyping of the c.-1796_-1793delATGA in the MSRA upstream region (see Results): forward-5′ccattggaatactcgagaacg3′ and reverse-5′aacaaatgcagtgtgcgtgt3′. One of the primers was labeled fluorescently and standard PCR was performed using a PTC-200 Peltier Thermal Cycler (MJ Research; Miami, FL, USA). Each reaction was performed in a final volume of 15 μl with 9 μl of ABI PRISM True Allele Premix (Applied Biosystems), 0.5 μl of each primer (10 ng/μl), 2 μl of DNA (20 ng/μl), and 3 μl of H2O. The thermal conditions were 95°C for 12min, followed by 20 cycles of 15s at 94°C, 15s at 55°C, and 15s at 72°C, and a final extension of 10min at 72°C. The amplified fragments were analyzed on the 3100 Genetic Analyzer (Applied Biosystems), and the genotypes were assigned using the GeneMapper v4.0 software (Applied Biosystems). The MSRA deletion was genotyped in 378 individuals from the CVCR population and 1086 individuals from the independent sample of individuals of Mexican and Central American ancestry.
All genotypes were scored separately by two individuals who were blind to diagnosis of the subjects. Genotype discrepancies were discussed and final genotypes agreed upon. Genotypes were checked for violations of Mendelian inheritance by the PEDSYS program INFER (Southwest Foundation for Biomedical Research, San Antonio, TX, USA). When possible, the INFER program infers parental genotypes, using available sibling genotypes. One family was discarded from the analyses due to recurring Mendelian discrepancies.
DNA Sequencing
We sequenced all six coding MSRA exons, including splicing junctions, as well as 2000 base pairs upstream of the MSRA transcription start site, in 30 individuals who had a consensus diagnosis of schizophrenia and carried the 3-marker MSRA haplotype that appeared to be over-transmitted in schizophrenia (see Results). Sequencing was performed by Polymorphic DNA technologies (Alameda, CA, USA).
Preparation of cDNA samples
Lymphoblastoid cell lines of 66 unrelated individuals previously genotyped for the MSRA deletion were selected randomly regardless of affected status (21 homozygous with the deletion, 21 heterozygous, 24 homozygous without the deletion). 37 cell lines were derived from affected individuals (9 S/S, 15 S/L and 13 L/L) and 29 cell lines were derived from unrelated unaffected individuals (12 S/S, 6 S/L and 11 L/L). Total RNA was extracted using TRIzol reagent (Life Technologies, Grand Island, USA), as instructed. Samples were diluted to100 ng/μl and stored at −80 °C until use. Reverse transcription (RT) was performed under the following conditions: 2.5 μl of 10X buffer, 5.5 μl of 25mM MgCl2, 5 μl of 10mM dNTPs mix, 1.25 μl of 50 μM random hexamers, 0.5 μl of 20U/μl RNase inhibitor, 0.625 μl of 50U/μl reverse transcriptase, 4.625 μl of RNase free H2O, and 5 μl of RNA (100ng/ μl). The thermal cycling conditions were 10min at 25°C, 2 h at 37°C and 5min a 95°C. The RT products were determined by electrophoresis on a 1% agarose gel stained with ethidium bromide.
Quantitative RT PCR analysis
The expression of MSRA was analyzed by quantitative real time PCR (qRT-PCR) using Applied Biosystems Assays-on-Demand primer/probe sets specific for MSRA (Hs00737166, Exon boundaries 5-6) and the endogenous control gene 18s (Hs99999901), and an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). Each reaction was performed in a final volume of 30 μl with 15 μl of TaqMan 2X Universal PCR Master Mix, No AmpErase UNG (Applied Biosystems), 1.5 μl of the corresponding gene expression assay, 11.5 μl of H2O, and 2 μl of cDNA (20ng/ μl). The thermal cycling conditions included an initial temperature of 95°C for 10min, followed by 40 cycles of 15 sec at 95°C, and 1min at 60°C. Relative levels of the MSRA and 18s transcripts were determined by interpolation with cDNA standard curves, prepared from serial dilutions of known amounts of cDNA, and amplified in the same plate and under the same conditions as the unknown samples. All reactions were performed in duplicate. The average expression levels of MSRA cDNA for each sample were normalized to the endogenous reference cDNA (18s).
Statistical analysis
All association analyses were performed using the Family-Based Association Test (FBAT) (Horvath et al., 2001) and the phenotype of schizophrenia (which includes schizoaffective individuals). The FBAT software provides methods for a wide range of situations that arise in family-based association studies, such as more than one affected subject per family, and it can test association of individual markers or haplotypes, as well as perform bi-allelic and global tests of association. The bi-allelic test provides asymptotic P values of the Z score function, which looks at the transmitted marker alleles to affected offspring, compared to non-transmitted parental alleles. When both parents were not available, additional siblings of the affected subjects had been genotyped to permit the inference of the parental alleles. Linkage disequilibrium was evaluated between markers using the command hapfreq –d implemented in FBAT. Given the observed pattern of LD between markers, we analyzed haplotypes of 3 markers within MSRA for association using the HBAT test (bi-allelic and global tests, with minimum informative family counts: 10).
Differences in relative MSRA expression (normalized values) between low expressing and high expressing genotypes were evaluated by two-tailed Student t test with P < 0.05 considered significant.
RESULTS
Association analysis of markers within the MSRA gene with schizophrenia
In order to investigate whether the MSRA gene could be a candidate gene for schizophrenia in the Costa Rican population, we genotyped 4 SNPs spanning the MSRA gene in a sample of 378 individuals from the CVCR and analyzed this data in conjunction with marker D8S542, which had been previously genotyped in this same population (Walss-Bass et al 2006). The chromosomal position of the 5 markers, in relation to the MSRA gene, is shown in Table I.
None of the SNPs showed individual evidence of association to the phenotype. D8S542 showed evidence of association with schizophrenia in the CVCR sample, with a multiallelic (global test) significance of p = 0.017 (Table I). The alleles showing evidence of association for marker D8S542 were 13 (over-transmitted, P = 0.0085, Z = 2.630) and 14 (under-transmitted, P= 0.0113, Z= −2.531). These results using FBAT were similar to the evidence of potential association reported in our original study using the CLUMP method for statistical analysis, where T1 is equivalent to the multiallelic mode and T3 is equivalent to the biallelic mode (Walss-Bass et al 2006).
Analysis of LD between the 5 MSRA markers showed that markers D8S542, rs4260896 and rs4410951 are part of an LD block (Table II).
Table II.
Linkage disequilibrium (D’)1 analysis of markers within the MSRA gene
| Marker | rs592420 | rs2409632 | D8S542 | rs4260896 |
|---|---|---|---|---|
| rs2409632 | 0.15 | |||
| D8S542 | 1.00 | 1.00 | ||
| rs4260896 | 0.04 | 0.04 | 1.00 | |
| rs4410951 | 0.15 | 0.04 | 1.00 | 0.52 |
LD (linkage disequilibrium) was calculated using the “hapfreq” command in FBAT
We therefore analyzed haplotypes formed by these markers for association with schizophrenia in the CVCR. Of the 18 possible haplotypes formed by these three markers, only 7 were present in at least 10 informative families. These haplotypes were the only ones included in the HBAT association analysis. The global haplotype test (multiallelic mode) showed a near significant evidence of association, with a P value of 0.0526. Individually, haplotypes 1 and 6 showed a non-significant trend for over-transmission (P = 0.0713) and under-transmission (P = 0.0606) to subjects with the schizophrenia phenotype, respectively (bi-allelic HBAT test, Table III).
Table III.
Association analysis of MSRA haplotypes with schizophrenia in the CVCR
| Haplotypes1 | Markers | P value2 | ||
|---|---|---|---|---|
| D8S542 | rs4260896 | rs4410951 | ||
| H1 | 12 | A | A | 0.0713 |
| H2 | 12 | A | C | 0.2465 |
| H3 | 12 | G | C | 0.2371 |
| H4 | 14 | A | A | 0.5461 |
| H5 | 14 | G | C | 0.5745 |
| H6 | 14 | A | C | 0.0606 |
| H7 | 14 | G | C | 0.1096 |
Only haplotypes present in at least 10 informative families are shown
P values are for HBAT bi-allelic analysis. The global P value (multi-allelic analysis) was P = 0.0526 (Chi square= 13.918).
Identification of a novel 4-base pair MSRA deletion associated with schizophrenia
We then sequenced all 6 MSRA coding exons, including 50 base-pairs of splicing junction, and 2000 base pairs of upstream region, in 30 CVCR subjects carrying the haplotype exhibiting a trend for over-transmission (haplotype 1 in Table III), in order to identify possible causative mutations. We identified 9 known and 12 unknown SNPs in these subjects, none of which were non-synonymous (not shown). In addition, we identified a novel 4 base-pair deletion, c.-1796_-1793delATGA in the promoter region of the MSRA gene, 1792 bases upstream of the transcription start site (Figure 1). This deletion was identified in 3 individuals (1 homozygous and 2 heterozygous). Given that this deletion had not been previously reported and had potential for functional significance, we focused all further association analyses on this previously unknown deletion exclusively, in order to avoid the necessity of corrections due to multiple allele testing.
Figure 1.
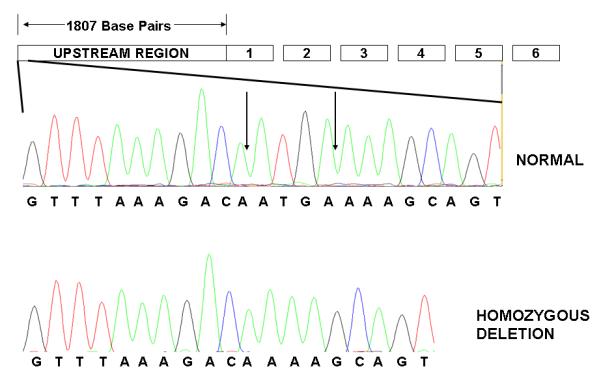
MSRA deletion c.-1796_-1793delATGA. A graphic representation of the MSRA gene is indicated (GenBank accession no.BC054033). Boxes 1-6 represent the MSRA exons. A region of 1807 bases upstream of the transcription start site is represented in the left most box. Within this upstream region, a sequence containing the 4 base pair deletion is expanded. The vertical arrows indicate the position of the 4 bases in an individual with the normal genotype. An individual homozygous for the deletion is shown at the bottom.
To determine whether this novel deletion variant was associated with schizophrenia, we genotyped the deletion in our sample of 378 CVCR individuals. The frequency of the short allele (4 base-pairs deleted) was 14.3% among cases and 23.6% among unaffected relatives. Association tests using FBAT revealed that the MSRA c.-1796_-1793delATGA variant was positively associated with the phenotype of schizophrenia in the CVCR sample (P = 0.0292). We then tested the long (L, wild type) versus short (S, deletion) variant in an independent Hispanic schizophrenia population sample of 1086 subjects, where we found significant evidence of association of the variation in this gene with schizophrenia (P = 0.0367). The frequency of the S allele was 20% among cases and 23.7% among unaffected relatives in this independent sample. In both populations, the long L allele was over-transmitted to schizophrenia patients (Z = 2.179 and 2.089, respectively), while the short S allele appeared to be under-transmitted to schizophrenia offspring (Z = −2.179 and Z = −2.089, respectively).
Linkage disequilibrium analysis of the MSRA deletion using FBAT showed that it was in complete LD with microsatellite marker D8S542 (D’=1.00) in the CVCR sample. Allele 14 of D8S542 was associated with the MSRA S allele (deletion), while allele 13 of D8S542 was associated with the L allele
Expression of MSRA mRNA
In order to determine whether the ATGA MSRA sequence could be important for regulating MSRA expression, the relationship between MSRA deletion genotype and MSRA expression was investigated using cDNA samples of 66 individuals selected on the basis of genotype. The number of L/L, L/S and S/S individuals were 24, 21 and 21, respectively. As seen in Figure 2, the L allele appeared to have a dominant, high-expressing role: samples with the S/S genotype exhibited 32.6% and 39% lower expression compared with S/L and L/L genotypes, respectively. We therefore grouped the L/L and L/S genotypes as “high expressing” L and compared the expression levels with those of the S/S “low expressing” genotype (S/S vs. L) (Figure 2). With this, the mean expressions of the S/S and L groups were 0.7717 ± 0.467 and 1.209 ± 0.932, respectively. Comparison of these two groups using student’s t-test indicated a significant relationship between genotype and MSRA expression (P = 0.0135). Similarly, when comparing only the S/S and L/L groups, the relationship was also significant (P = 0.0294). However, tests using ANOVA did not a reveal significant relationship between genotype and MSRA expression.
Figure 2.
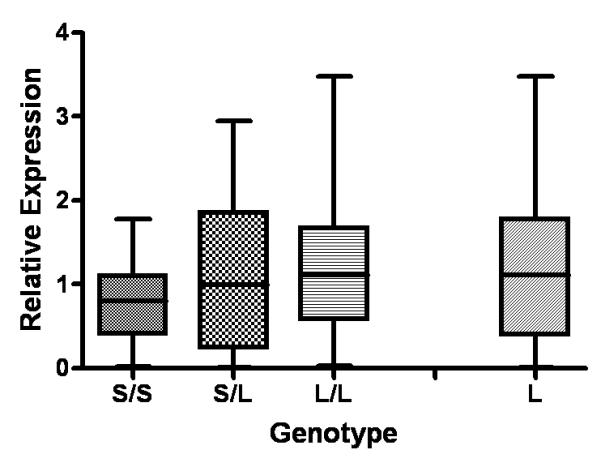
Relative expression of the MSRA gene in relation to MSRA c.-1796_-1793delATGA genotype. S/S = individuals homozygous for the deletion, N=21; S/L = heterozygous, N= 21; L/L= homozygous normal, N= 24. L= high expressing genotypes (L/L and L/S), N= 45. Each box displays the mean (black bar), the 25th and the 75th percentiles (the lower and the upper edges of the box, respectively), and the extreme values. P = 0.0135 comparing the L and S/S groups, and P = 0.0294 comparing the L/L and S/S groups (two-tailed student’s t-test).
DISCUSSION
Following previous evidence of association of D8S542 with schizophrenia, we have identified a novel gene, MSRA, as a putative candidate for schizophrenia susceptibility. The MSRA enzyme repairs oxidative damage to proteins caused by free radicals or reactive oxygen species (ROS) (Kim and Gladyshev, 2007). ROS radicals are highly reactive chemical species generated during normal metabolic processes. A small portion of these play important roles in physiological processes, and the remaining are inactivated by cellular antioxidant defense systems (Mahadik and Mukherjee, 1996). Excessive generation of free radicals or a deficient cellular antioxidant defense system leads to oxidative stress, which may cause modifications of proteins, lipids, and nucleic acids. There is evidence that indicates that this balance is altered in schizophrenia (Mahadik and Scheffer, 1996; Prabakaran et al., 2004; Prabakaran et al.,2007; Ranjekar et al., 2003).
Our present results provide evidence of a deletion in the promoter region of the MSRA gene being under-transmitted to individuals with schizophrenia in two different populations (Z = −2.179 and −2.089). In the CVCR population, the MSRA deletion is in high linkage disequilibrium with an allele of marker D8S542 (allele 14), which is also found to be undertransmitted to schizophrenia individuals. These results suggest that the MSRA deletion may have a protective effect against schizophrenia. However, the evidence of reduced MSRA expression in individuals homozygous for the deletion appears counterintuitive. Due to MSRA function against oxidative stress, and the evidence of oxidative damage in individuals with schizophrenia, it would be expected that a decline in MSRA expression would increase susceptibility to schizophrenia. It is possible that the identified MSRA deletion is not the causative variant but is in linkage disequilibrium with another variant which is the one actually impacting disease pathophysiology. To this respect, several novel synonymous SNPs were identified in the course of this study. Although we chose to not follow-up these SNPs for the present study (due to resource limitations and to avoid problems with multiple testing) it is highly possible that one of these SNPs is the actual causative variant. Four of the identified SNPs were found in the DNA sequence between exons 1 and 3. In humans, three distinct MSRA transcript variants, arising from differential splicing of a single gene, have been identified (Kim and Gladyshev, 2006). Splicing occurs within exons 1-3, and after exon 3 all transcripts are identical. Each of the three different MSRA variants targets distinct intracellular regions (Kim and Gladyshev, 2006). It is possible that one of the SNPs identified is involved in regulation of alternative splicing and this is the subject of future investigations in our laboratory. Similarly, we are investigating whether the c.-1796_-1793delATGA deletion identified in this study is involved in regulating expression of all or specific splice variants.
A putative human MSRA promoter has been identified between nucleotides −309 and +11 of the MSRA gene (De Luca et al., 2006). Whether the ATGA sequence found to be deleted in the present study is a transcription factor binding motif and part of a cis regulatory sequence remains to be determined. However, analysis of the MSRA sequence using TRANSFAC® indicated that the ATGA sequence is a potential binding site for pituitary-specific trans-acting factor (Pit-1) (Peers et al., 1991; Peers et al., 1990).
In conclusion, we report the finding of association of a novel candidate gene, MSRA, with schizophrenia. A 4 nucleotide deletion within the MSRA gene is under-transmitted to schizophrenia patients in two different populations and causes reduced gene expression in individuals homozygous for the deletion. Our findings suggest that there may be a link between the MSRA polymorphism and protection against schizophrenia Further investigations into the mechanisms by which this pccurs may be important for advancing our understanding of the pathophysiology of schizophrenia.
ACKNOWLEDGEMENTS
We acknowledge the contributions of Dr. Salvador Contreras, Dr. Mercedes A. Ramirez, Arturo Camacho, Erasmo Cano, and Dr. Regina Armas, and the investigative teams at each site where samples were collected. This grant was supported by a San Antonio Area Foundation grant, a Friends of Psychiatry (UTHSCSA Dept. of Psychiatry) grant, a NARSAD: The Mental Health Research Association Young Investigator Award, and a Stanley Medical Research Institute research grant to CWB; a National Institute of Mental Health (R01-MH61884 and MH-60881) to MAE; International Center for Genetic Engineering and Biotechnology Project (CRP/COS98-01) to HR.
REFERENCES
- Alamuri P, Maier RJ. Methionine sulphoxide reductase is an important antioxidant enzyme in the gastric pathogen Helicobacter pylori. Mol Microbiol. 2004;53(5):1397–406. doi: 10.1111/j.1365-2958.2004.04190.x. [DOI] [PubMed] [Google Scholar]
- Anderson MA, Gusella JF. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984;20(11):856–8. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7(4):405–11. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Blouin JL, Dombroski BA, Nath SK, Lasseter VK, Wolyniec PS, Nestadt G, Thornquist M, Ullrich G, McGrath J, Kasch L. Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nat Genet. 1998;20(1):70–3. doi: 10.1038/1734. others. [DOI] [PubMed] [Google Scholar]
- Brzustowicz LM, Honer WG, Chow EW, Little D, Hogan J, Hodgkinson K, Bassett AS. Linkage of familial schizophrenia to chromosome 13q32. Am J Hum Genet. 1999;65(4):1096–103. doi: 10.1086/302579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Picot CR, Friguet B, Petropoulos I. Methionine sulfoxide reductases: relevance to aging and protection against oxidative stress. Ann N Y Acad Sci. 2006;1067:37–44. doi: 10.1196/annals.1354.006. [DOI] [PubMed] [Google Scholar]
- De Hert M, Hautekeete M, De Wilde D, Peuskens J. High prevalence of Helicobacter pylori in institutionalized schizophrenic patients. Schizophr Res. 1997;26(2-3):243–4. doi: 10.1016/s0920-9964(97)00069-8. [DOI] [PubMed] [Google Scholar]
- De Luca A, Sacchetta P, Di Ilio C, Favaloro B. Identification and analysis of the promoter region of the human methionine sulphoxide reductase A gene. Biochem J. 2006;393(Pt 1):321–9. doi: 10.1042/BJ20050973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaye L, Becerra A, Orgel L, Lazcano A. Molecular evolution of peptide methionine sulfoxide reductases (MsrA and MsrB): on the early development of a mechanism that protects against oxidative damage. J Mol Evol. 2007;64(1):15–32. doi: 10.1007/s00239-005-0281-2. [DOI] [PubMed] [Google Scholar]
- Dhandayuthapani S, Blaylock MW, Bebear CM, Rasmussen WG, Baseman JB. Peptide methionine sulfoxide reductase (MsrA) is a virulence determinant in Mycoplasma genitalium. J Bacteriol. 2001;183(19):5645–50. doi: 10.1128/JB.183.19.5645-5650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamilla MA, Ontiveros A, Nicolini H, Raventos H, Mendoza R, Medina R, Munoz R, Levinson D, Peralta JM, Dassori A. A genome-wide scan for schizophrenia and psychosis susceptibility loci in families of Mexican and Central American ancestry. Am J Med Genet B Neuropsychiatr Genet. 2007;144(2):193–9. doi: 10.1002/ajmg.b.30411. others. [DOI] [PubMed] [Google Scholar]
- Escamilla MA, Spesny M, Reus VI, Gallegos A, Meza L, Molina J, Sandkuijl LA, Fournier E, Leon PE, Smith LB. Use of linkage disequilibrium approaches to map genes for bipolar disorder in the Costa Rican population. Am J Med Genet. 1996;67(3):244–53. doi: 10.1002/(SICI)1096-8628(19960531)67:3<244::AID-AJMG2>3.0.CO;2-N. others. [DOI] [PubMed] [Google Scholar]
- Ezraty B, Bos J, Barras F, Aussel L. Methionine sulfoxide reduction and assimilation in Escherichia coli: new role for the biotin sulfoxide reductase BisC. J Bacteriol. 2005;187(1):231–7. doi: 10.1128/JB.187.1.231-237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellerhoff B, Laumbacher B, Mueller N, Gu S, Wank R. Associations between Chlamydophila infections, schizophrenia and risk of HLA-A10. Mol Psychiatry. 2007;12(3):264–72. doi: 10.1038/sj.mp.4001925. [DOI] [PubMed] [Google Scholar]
- Gabbita SP, Aksenov MY, Lovell MA, Markesbery WR. Decrease in peptide methionine sulfoxide reductase in Alzheimer’s disease brain. J Neurochem. 1999;73(4):1660–6. doi: 10.1046/j.1471-4159.1999.0731660.x. [DOI] [PubMed] [Google Scholar]
- Gurling HM, Kalsi G, Brynjolfson J, Sigmundsson T, Sherrington R, Mankoo BS, Read T, Murphy P, Blaveri E, McQuillin A. Genomewide genetic linkage analysis confirms the presence of susceptibility loci for schizophrenia, on chromosomes 1q32.2, 5q33.2, and 8p21-22 and provides support for linkage to schizophrenia, on chromosomes 11q23.3-24 and 20q12.1-11.23. Am J Hum Genet. 2001;68(3):661–73. doi: 10.1086/318788. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104(42):16621–6. doi: 10.1073/pnas.0706778104. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze-Selch D, Daubener W, Eggert L, Erdag S, Stoltenberg R, Wilms S. A controlled prospective study of toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophr Bull. 2007;33(3):782–8. doi: 10.1093/schbul/sbm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype--phenotype associations. Eur J Hum Genet. 2001;9(4):301–6. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- Kaufmann CA, Suarez B, Malaspina D, Pepple J, Svrakic D, Markel PD, Meyer J, Zambuto CT, Schmitt K, Matise TC. NIMH Genetics Initiative Millenium Schizophrenia Consortium: linkage analysis of African-American pedigrees. Am J Med Genet. 1998;81(4):282–9. others. [PubMed] [Google Scholar]
- Kendler KS, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F, Shinkwin R, Easter SM, Webb BT, Zhang J. Evidence for a schizophrenia vulnerability locus on chromosome 8p in the Irish Study of High-Density Schizophrenia Families. Am J Psychiatry. 1996;153(12):1534–40. doi: 10.1176/ajp.153.12.1534. others. [DOI] [PubMed] [Google Scholar]
- Kim HY, Gladyshev VN. Alternative first exon splicing regulates subcellular distribution of methionine sulfoxide reductases. BMC Mol Biol. 2006;7:11. doi: 10.1186/1471-2199-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Gladyshev VN. Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J. 2007;407(3):321–9. doi: 10.1042/BJ20070929. [DOI] [PubMed] [Google Scholar]
- Kuschel L, Hansel A, Schonherr R, Weissbach H, Brot N, Hoshi T, Heinemann SH. Molecular cloning and functional expression of a human peptide methionine sulfoxide reductase (hMsrA) FEBS Lett. 1999;456(1):17–21. doi: 10.1016/s0014-5793(99)00917-5. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73(1):34–48. doi: 10.1086/376549. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadik SP, Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia: a review. Schizophr Res. 1996;19(1):1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Scheffer RE. Oxidative injury and potential use of antioxidants in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 1996;55(1-2):45–54. doi: 10.1016/s0952-3278(96)90144-1. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98(23):12920–5. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Jenkins NA, Gilbert DJ, Copeland NG, Jursky F, Weissbach H, Brot N. Chromosomal localization of the mammalian peptide-methionine sulfoxide reductase gene and its differential expression in various tissues. Proc Natl Acad Sci U S A. 1996;93(8):3205–8. doi: 10.1073/pnas.93.8.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr DW, Millikan AM, Cowan DN, Yolken R, Li Y, Weber NS. Selected infectious agents and risk of schizophrenia among u.s. Military personnel. Am J Psychiatry. 2008;165(1):99–106. doi: 10.1176/appi.ajp.2007.06081254. [DOI] [PubMed] [Google Scholar]
- Ophoff RA, Escamilla MA, Service SK, Spesny M, Meshi DB, Poon W, Molina J, Fournier E, Gallegos A, Mathews C. Genomewide linkage disequilibrium mapping of severe bipolar disorder in a population isolate. Am J Hum Genet. 2002;71(3):565–74. doi: 10.1086/342291. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O’Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005;21(9):518–25. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Peers B, Monget P, Nalda MA, Voz ML, Berwaer M, Belayew A, Martial JA. Transcriptional induction of the human prolactin gene by cAMP requires two cis-acting elements and at least the pituitary-specific factor Pit-1. J Biol Chem. 1991;266(27):18127–34. [PubMed] [Google Scholar]
- Peers B, Voz ML, Monget P, Mathy-Hartert M, Berwaer M, Belayew A, Martial JA. Regulatory elements controlling pituitary-specific expression of the human prolactin gene. Mol Cell Biol. 1990;10(9):4690–700. doi: 10.1128/mcb.10.9.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9(7):684–97. doi: 10.1038/sj.mp.4001511. others. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Wengenroth M, Lockstone HE, Lilley K, Leweke FM, Bahn S. 2-D DIGE analysis of liver and red blood cells provides further evidence for oxidative stress in schizophrenia. J Proteome Res. 2007;6(1):141–9. doi: 10.1021/pr060308a. [DOI] [PubMed] [Google Scholar]
- Pulver AE, Lasseter VK, Kasch L, Wolyniec P, Nestadt G, Blouin JL, Kimberland M, Babb R, Vourlis S, Chen H. Schizophrenia: a genome scan targets chromosomes 3p and 8p as potential sites of susceptibility genes. Am J Med Genet. 1995;60(3):252–60. doi: 10.1002/ajmg.1320600316. others. [DOI] [PubMed] [Google Scholar]
- Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121(2):109–22. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci U S A. 2002;99(5):2748–53. doi: 10.1073/pnas.032671199. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasindran SJ, Saikolappan S, Dhandayuthapani S. Methionine sulfoxide reductases and virulence of bacterial pathogens. Future Microbiol. 2007;2:619–30. doi: 10.2217/17460913.2.6.619. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729–36. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walss-Bass C, Montero AP, Armas R, Dassori A, Contreras SA, Liu W, Medina R, Levinson D, Pereira M, Atmella I. Linkage disequilibrium analyses in the Costa Rican population suggests discrete gene loci for schizophrenia at 8p23.1 and 8q13.3. Psychiatr Genet. 2006;16(4):159–68. doi: 10.1097/01.ypg.0000218616.27515.67. others. [DOI] [PubMed] [Google Scholar]
- Wassef R, Haenold R, Hansel A, Brot N, Heinemann SH, Hoshi T. Methionine sulfoxide reductase A and a dietary supplement S-methyl-L-cysteine prevent Parkinson’s-like symptoms. J Neurosci. 2007;27(47):12808–16. doi: 10.1523/JNEUROSCI.0322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizemann TM, Moskovitz J, Pearce BJ, Cundell D, Arvidson CG, So M, Weissbach H, Brot N, Masure HR. Peptide methionine sulfoxide reductase contributes to the maintenance of adhesins in three major pathogens. Proc Natl Acad Sci U S A. 1996;93(15):7985–90. doi: 10.1073/pnas.93.15.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Zhou DF, Cao LY, Wu GY, Haile CN, Kosten TA, Kosten TR. Disrupted antioxidant enzyme activity and elevated lipid peroxidation products in schizophrenic patients with tardive dyskinesia. J Clin Psychiatry. 2007;68(5):754–60. doi: 10.4088/jcp.v68n0513. [DOI] [PubMed] [Google Scholar]
- Zhu S, Guo MF, Feng QC, Fan JM, Zhang LX. Epidemiological evidences from China assume that psychiatric-related diseases may be associated with Toxoplasma gondii infection. Neuro Endocrinol Lett. 2007;28(2):115–20. [PubMed] [Google Scholar]


