Abstract
Quasi-ultrafine PM (PM0.25) and its components were measured in indoor and outdoor environments at four retirement communities in the Los Angeles basin, CA, as part of the Cardiovascular Health and Air Pollution Study (CHAPS). The present paper focuses on the characterization of the sources, organic constituents and indoor and outdoor relationships of quasi-ultrafine PM. The average indoor/outdoor ratios of most of the measured PAHs, hopanes and steranes were close to- or slightly lower than 1, and the corresponding indoor-outdoor correlation coefficients (R) were always positive and, for the most part, moderately strong (median R was 0.60 for PAHs and 0.74 for hopanes and steranes). This may reflect the possible impact of outdoor sources on indoor PAHs, hopanes and steranes. Conversely, indoor n-alkanes and n-alkanoic acids were likely to be influenced by indoor sources.
A Chemical Mass Balance (CMB) model was applied to both indoor and outdoor speciated chemical measurements of quasi-ultrafine PM. Among all apportioned sources of both indoor and outdoor particles, vehicular emissions was the one contributing the most to the PM0.25 mass concentration measured at all sites (24–47% on average).
Keywords: Indoor and outdoor pollution, ultrafine particles, organic compounds, chemical mass balance model, source apportionment
INTRODUCTION
Positive associations between exposure to atmospheric particulate matter (PM) and adverse health effects have been shown by numerous epidemiological and toxicological studies (Pope and Dockery, 2006; Environmental Protection Agency Integrated Science Assessments, 2009). Particle size is an important parameter affecting the percentage of the inhaled aerosol that deposits in the human lungs as well as the deposition site. Fine PM (PM2.5; particles with an aerodynamic diameter smaller that 2.5 µm) has been more strongly associated with mortality and morbidity, although coarse particles (PM2.5–10; aerodynamic diameter between 2.5 and 10 µm) have also been correlated with respiratory hospital admissions (Brunekreef and Forsberg, 2005). Ultrafine particles, tenuously defined as those with diameters less than 0.1 – 0.2 µm (see more discussion on the appropriate cutpoint of the ultrafine PM mode in Sioutas et al., 2005) have the ability to penetrate deep into the alveolar region of the respiratory system, and can translocate in other parts of the human body (Elder et al., 2006). However, for this PM fraction, penetration to and deposition within the alveolar region vary in a complex manner with particle size, and particles smaller than < 30 nm have a greater tendency to deposit in the tracheobronchial region of the lung because of higher diffusivity. Despite this complex behavior, toxicological data suggest that ultrafine particles are more strongly associated with cardiovascular and respiratory health outcomes (Araujo et al., 2007) compared to larger particles. In previous studies conducted in collaborations with researchers at the University of California, Los Angeles we have demonstrated that ambient particles in the ultrafine range are the most potent in terms of toxicological activity. Their toxicity (expressed on a per-PM-mass basis) seems to be directly correlated to the amount of organic material present in the collected samples (Li et al., 2003, Cho et al., 2005, Hu et al., 2008). So far, there is little direct epidemiologic research to support this (reviewed by Delfino et al., 2005 and Weichenthal et al., 2007). Along with particle size, chemical composition influences the toxicity of PM. Thus, exposure to several types of highly toxic organic particle components [including quinones, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls and other organochlorine compounds] may result in acute effects (Li et al., 2003).
Although air quality standards have been established for outdoor / ambient environments, a significant portion of human exposures to PM occurs indoors, where people spend around 85–90% of their time (Klepeis et al., 2001). Hence, it is important to understand the composition and sources of both indoor and outdoor PM and their relationships. Indoor PM consists of outdoor particles that have infiltrated indoors, particles emitted indoors (primary), and particles formed indoors (secondary) from precursors emitted both indoors and outdoors (Weschler, 2004). To the best of our knowledge, only few studies on indoor PM source apportionment have been conducted in the past few years. These were mainly focused on examining the influence of outdoor sources on the measured indoor concentrations of fine PM (without any further size fractionation), although indoor measurements of organic tracers that are typically used for source apportionment outdoors were also conducted (Olson et al., 2008). Positive Matrix Factorization (PMF) is a useful modelling tool that has also been used to apportion the main sources of PM in indoor environments (Hopke et al., 2003; Ogulei et al., 2006). Few studies have demonstrated that indoor sources can contribute up to 50% to the indoor concentrations of fine PM and its components (Wallace, 1996; Meng et al., 2005), whereas other studies reported lower contributions (6–22%) (Yli-Tuomi et al., 2008).
The present paper has been conducted as part of the Cardiovascular Health and Air Pollution Study (CHAPS), a multidisciplinary project funded by the National Institutes of Health (NIH) and designed to investigate the effects of micro-environmental exposures to PM on cardiovascular outcomes in elderly retirees affected by coronary artery disease. The elderly population with coronary artery disease is likely to be among the most vulnerable to the adverse effects of particulate air pollutants. In an earlier investigation, also part of this study (Delfino et al., 2008), we reported that indoor PM of outdoor origin (mostly from combustion sources) was more significantly associated with systemic inflammation, platelet activation, and decreases in erythrocyte antioxidant activity than uncharacterized indoor PM that included particles of indoor origin. The present study focuses on the quasi-ultrafine (quasi-UF) fraction of PM (PM0.25; particles with an aerodynamic diameter smaller that 0.25 µm) in both indoor and outdoor environments at four distinct retirement communities of the Los Angeles Basin, which were study sites of the CHAPS project. The main objectives of this study are: a) to evaluate the organic composition of quasi-ultrafine PM (PM0.25) in both indoor and outdoor environments throughout the calendar year, b) to identify the most important sources of these sub-micrometer particles, and c) to quantify their contribution to the total PM mass concentrations in both indoor and outdoor environments. The study focuses on PM0.25 because we have shown in earlier publications that this size range had the strongest and most significant association with circulating biomarkers of inflammation, antioxidant activity, and platelet activation measured in the study subjects (Delfino et al, 2008). Furthermore, to best of our knowledge, there are no other studies of sources and composition of indoor ultrafine or quasi-UF particles. The results described in this paper will be used by the CHAPS investigators to evaluate associations between indoor and outdoor PM sources and cardiovascular outcomes.
METHODS
Sampling sites and schedule
Indoor and outdoor PM measurements were conducted at four retirement communities in southern California between 2005 and 2007. Three of these communities were in the San Gabriel Valley, CA (sites San Gabriel 1, 2 and 3) and the fourth in Riverside, CA. Site San Gabriel 1 was located in a residential area about 50 km east of downtown Los Angeles, approximately 3 km away from a major freeway. Site San Gabriel 2 was about 8 km east of Los Angeles, approximately 300 m away of a major freeway. Site San Gabriel 3 was situated about 55 km east of downtown Los Angeles, 2.5 km from two busy freeways and 150 m away from a major street. Site Riverside was located about 110 km east of Los Angeles, 15 km southeast of downtown Riverside, 3 km away from the closest freeway and 1 km from a major street (downwind of the site). The abundant vegetation surrounding this last community may be a potential source of precursors of biogenically generated PM (Rogge et al., 1993).
Two identical sampling stations were installed at each site, one indoors and one outdoors. The indoor sampling station at site San Gabriel 1 was set-up in a recreational area of the community’s main building, in close proximity to a construction site. San Gabriel 2 indoor station was located in the dining room of the community’s central building (see Polidori et al., 2007, for further details on sites San Gabriel 1 and 2). The indoor station at site San Gabriel 3 was in a recreational area of the main retirement community complex. The indoor station at site Riverside was in the hallway of the main building with a dining room, activity room and numerous apartment units nearby. Outdoor sampling equipment was set-up inside a movable trailer, positioned within 300 m from the indoor station at all sites.
Two 6-week sampling campaigns were conducted at each location, one during summer and early fall (warmer phase) and one throughout late fall and winter (colder phase); see Arhami et al. (2009) for more details.
Sampling method and chemical analyses
24-h size segregated PM samples were collected daily from Monday to Friday by means of Sioutas™ Personal Cascade Impactors (SKC Inc, Eighty Four, PA). Coarse, accumulation, and quasi-UF mode PM were sampled on Zefluor filters (3 µm pore-size, Pall Life Sciences, Ann Arbor MI); however the present study focuses only in the quasi-UF fraction. The PM mass concentration was determined gravimetrically by weighing filters in a controlled temperature and relative humidity room using a microbalance (Mettler-Toledo, Columbus, OH; weight uncertainty ± 2 µg).
Filters were composited weekly (including 5 daily collected samples-from Monday to Friday) for chemical analyses. Composites were cut into 3 uneven sections: 1 section consisting of 1/2 of the filter and 2 sections consisting of 1/4 of the filter each. The 1/2 section was analyzed for 92 different organic compounds using Gas Chromatography/Mass Spectrometry (GC/MS) (Stone et al., 2008). One of the 1/4 sections of the composited filters were digested with concentrated acid using microwave digestion and then analyzed by high resolution Inductively Coupled Plasma Mass Spectrometer (HR-ICPMS, Finnigan Element 2) to determine 52 trace elements (Herner et al., 2006). The second 1/4 section was analyzed for water soluble organic carbon (WSOC) and used for a reactive organic species (ROS) assay. A General Electric Instrument (Sievers Total Organic Carbon, TOC; GE, Inc.) was used to determine WSOC concentrations (Zhang et al., 2008). ROS data will be used and described in other CHAPS papers.
Hourly elemental and organic carbon (EC and OC, respectively) levels in the fine PM fraction were measured using 2 semi-continuous OC_EC analyzers (Model 3F, Sunset Laboratory Inc), one indoors and one outdoors. Quasi-ultrafine EC was estimated from measured fine EC data using a factor of 0.70 ± 0.18 (quasi-ultrafine EC = fine EC × 0.70), which is based on recent size distribution data obtained over a 3 year period at 10 locations of the Los Angeles Basin (Arhami et al., 2006; Minguillon et al., 2008; Arhami et al., 2009). Dasibi Carbon Monoxide Analyzers (Model 3008, Dasibi Environmental Corp, Glendale, CA) were implemented to measure continuous (1-min) indoor and outdoor CO levels.
Field blanks, laboratory blanks, spiked samples, and small aliquots of standard reference material (NIST Urban Dust SRM 1649a) were analyzed along with the composite quasi-UF samples used for organic tracer compound analysis by GC/MS. Spike recovery after correction for internal standard recoveries range from 96–110% for the reported PAHs, 99–104% for the hopanes and steranes, and 68–136% for the n-alkanes with all but three of the n-alkanes falling within the range of 80–120%. The blank levels of hopanes, steranes, medium molecular weight PAHs, and high molecular weight PAHs were below analytical detection limits of approximately 10 picograms per cubic meter of air sampled. For all other compounds, method detection limits were limited by field and laboratory blanks. All measurements were blank corrected using the average and standard deviation of the blanks. Point-wise estimates of uncertainties for each measurement, which were based on analytical uncertainties and uncertainties associated with blank correction, were used to determine if each measurement was statistically different from zero. Although duplicate samples were not available to evaluate method precision, the precision of the spike and standard reference material analyses was used to estimate method precision, which was less than 20% for all PAHs, hopanes, steranes, and n-alkanes.
Source Apportionment
The Chemical Mass Balance (CMB) model (version CMB8.2 from the US Environmental Protection Agency) was used for the apportionment of the total measured ambient organic carbon (OC). In order to apportion the most important quasi-ultrafine PM sources, all model results were converted to equivalent PM based on the correspondent OC/PM ratio of each of the considered sources. OC/PM ratios were calculated for source profiles assuming that quasi-ultrafine PM = Elemental Carbon (EC) + Organic Matter (OM) and that OM=1.4*OC (Turpin et al., 2000; Polidori et al., 2008). Other contributors to PM considered in this study are: sulfate, estimated from S concentrations assuming that all measured S by ICP-MS was in the form of fully neutralized ammonium sulfate (Arhami et al., 2009); sea spray, calculated based on total Na as an estimate of water soluble Na concentrations and using a multiplication factor of 3.248 (Simoneit, 1986); resuspended dust, calculated from Si, Al, Ca, Fe and K concentrations, assuming they appear predominantly as oxides (Brook et al., 1997) [Si was not measured but estimated assuming Si=3*Al (Sillanpaa et al., 2006)]; and SOA estimations were based on measurements of WSOC. In polluted regions, compounds comprising the WSOC fraction are either mainly emitted from biomass burning sources (Docherty et al., 2008) or formed via secondary atmospheric processes (Weber et al., 2007). Thus, measured WSOC concentrations, minus the OC fraction apportioned to biomass burning (from the CMB output), was multiplied by a factor of 2.5 µgOM/µgOC (Turpin and Lim, 2001; Polidori et al., 2008) to convert it to SOA. The resulting SOA was then adjusted to account for the water-insoluble organic carbon (WISOC) fraction, assuming that 20% of the SOA concentration was water insoluble (Kondo et al., 2007). The uncertainty associated with this method is discussed in the results section.
A careful selection of OC sources is critical for the correct application of the CMB model, as demonstrated in previous sensitivity studies (Subramanian et al., 2006; Sheesley et al., 2007). Hence, after evaluating all potential pollutant emissions in the study area, the sources considered in this work were: light duty and heavy-duty vehicles (LDV and HDV respectively) (Kuhn et al., 2005; Ntziachristos et al., 2007; Phuleria et al., 2007), biomass burning in the Western US (Fine et al., 2004), and ocean vessels (Rogge et al., 1997; Agrawal et al., 2008). Vehicular profiles correspond to roadway data from studies carried out at the CA-110 and I-710 freeways in Los Angeles and, thus, they represent emissions from a mixture of vehicular sources (Phuleria et al., 2007). Other typical OC sources were included in the first modeling attempts, but were found to be non-quantifiable (meat cooking and natural gas) or their contribution was very low (candle smoke and cigarette smoke, with contributions <1% for OC). A set of fitting species was chosen based on: a) their chemical stability (Schauer et al., 1996), b) availability of their concentrations in different source profiles and in ambient data, and c) previous studies that identified markers for different sources (Schauer et al., 1996; Simoneit, 1999; Schauer and Cass, 2000). Thus, the following species were used as fitting species: EC, Benzo(k)fluoranthene, Benzo(e)pyrene, Benzo(b)fluoranthene, Benzo(ghi)perylene, Coronene, 17α(H)-22,29,30-Trisnorhopane, 17α(H)-21β(H)-Hopane, 22S-Homohopane, 22R-Homohopane, Sitostane, Levoglucosan, Vanadium and Nickel.
Each model result was evaluated using the regression statistics parameters accompanying each CMB model output (Table S1 in the Supporting Information): correlation coefficient (R2) and chi-square (χ2), which were within the desired ranges (0.81–1.00 and 0.0–5.7, respectively). The χ2 is the weighted sum of squares of the differences between the calculated and measured fitting species concentrations (for more details and a more in depth description of the statistical parameters, see CMB8.2 manual by the US Environmental Protection Agency). Weekly source contributions were estimated and the results were averaged over each phase of study at each site (weekly source apportionment results are also shown in Table S1). On average, 67 ± 15% of the measured mass was reconstructed by the apportionment results.
Data Analysis
A number of relevant organic species were not detectable at several sites or during particular phases of the study. In order to make a fair comparison between sites and time phases, half of the detection limit for each species was used as its concentration with half of the detection limit as uncertainty (the detection limit varied from 1.7e-5 to 0.06 ng/m3 for different species).
Because one of the main aims of CHAPS is to evaluate the effects of outdoor air pollutants on indoor and personal exposure (Delfino et al., 2008), we estimated air exchange rates (AER) from CO measurements during periods affected by a dominant indoor source (Abt et al., 2000) and infiltration factors (Finf; defined as the equilibrium fraction of the outdoor species of interest that penetrate indoors and remain suspended) at each site. In particular, Finf for OC, EC, PM2.5 and particle number (PN) were calculated using the recursive model (RM) developed by Allen et al. (2003). The average AERs calculated during CHAPS at the 4 retirement communities ranged from 0.21 to 0.4 hr−1. The generally low estimated AERs are consistent with the structural characteristics of the sampling sites, the low number of open windows and doors, and the presence of central air conditioners. The average Finf results were highest for EC (0.64–0.82) and OC (0.60–0.98) compared to those of PM2.5 (0.38–0.57) and PN (0.41–0.78). In general, the Finf results were similar across the warmer phase (summer and fall) and the colder phase (fall and winter), which is consistent with no seasonal changes in home dynamics and ventilation conditions as indicated by the rather constant AERs calculated throughout the study. More details on the Finf and AERs estimation methods and results are described in Polidori et al. (2007).
RESULTS AND DISCUSSION
Overview
The recorded meteorological data highlight the overall climatological stability of the Los Angeles basin, characterized by moderate differences in terms of temperature (T) and relative humidity (RH%) between colder and warmer phases (Table 1). These moderate meteorological variations may cause low variability in pollutants levels throughout the year. The average T and RH% were lower in Riverside than at the three San Gabriel sites. During the warmer phase of the study, the indoor and outdoor areas were generally characterized by similar T, whereas during the colder phases the average T was ~10°C higher indoors than outdoors. The higher differences between indoor and outdoor T values in the colder phase can potentially cause higher variability between indoor and outdoor concentrations compared to the warmer phase, especially for volatile (or semi-volatile) components. The air exchange rates (AER) at different sites were relatively similar to each other throughout the monitoring phases (Table 1), suggesting an overall similarity in home characteristics among different communities (Polidori et al., 2007). The magnitude of the AERs was generally low (0.21–0.4 h−1), and consistent with the low number of open windows and doors, the presence of central air conditioners, and the overall structural characteristics of the studied retirement homes.
Table 1.
Studied sites PM concentrations, meteorology and air exchange rates
| Quasi-UF PM (µg/m3) |
Temperature (°C) |
Outdoor Humidity (%) |
AER* (h−1) |
||||
|---|---|---|---|---|---|---|---|
| outdoor | Indoor | outdoor | Indoor | ||||
| Warmer Phase | San Gabriel 1 | 9.9 ± 2.2 | 10.3 ± 1.6 | 25.1 ± 2.2 | 26.1 ± 1.2 | 60 ± 6 | 0.25 ± 0.04 |
| San Gabriel 2 | 9.3 ± 1.8 | 9.8 ± 1.6 | 21.5 ± 2.1 | 23.6 ± 0.9 | 58 ± 15 | 0.28 ± 0.06 | |
| San Gabriel 3 | 10.4 ± 2.3 | 6.6 ± 1.3 | 25.9 ± 2.8 | 23.3 ± 1.1 | 58 ± 11 | 0.40 ± 0.12 | |
| Riverside | 11.5 ± 3.0 | 8.7 ± 2.3 | 21.1 ± 4.0 | 24.9 ± 1.7 | 53 ± 15 | 0.21 ± 0.06 | |
| Colder Phase | San Gabriel 1 | 8.8 ± 1.8 | 9.6 ± 3.1 | 15.4 ± 2.8 | 23.4 ± 1.2 | 58 ± 19 | 0.33 ± 0.07 |
| San Gabriel 2 | 10.4 ± 2.6 | 9.4 ± 2.6 | 14.9 ± 2.1 | 23.9 ± 0.8 | 49 ± 14 | 0.31 ± 0.10 | |
| San Gabriel 3 | 10.7 ± 2.0 | 7.1 ± 2.6 | 16.6 ± 3.6 | 24.7 ± 1.2 | 55 ± 10 | 0.26 ± 0.08 | |
| Riverside | 7.2 ± 2.2 | 6.0 ± 1.5 | 11.2 ± 2.8 | 25.4 ± 0.7 | 42 ± 22 | 0.31 ± 0.09 | |
Average ± Standard Deviation of weekly data are reported
AER: Air Exchange Rate
The average outdoor quasi-UF mass concentrations at all sites varied from 9.3 to 11.5 µg/m3 in the warmer phases and from 8.9 to 10.7 µg/m3 in the colder phases, thus indicating relatively low seasonal variability. Similar to outdoor quasi-ultrafine PM levels, indoor levels were consistent throughout the year at all sites. Mean indoor concentrations were generally lower than or similar to the corresponding outdoor concentrations (average indoor levels were 63–107% of their outdoor values).
Outdoor Organic Species and Seasonal Variability
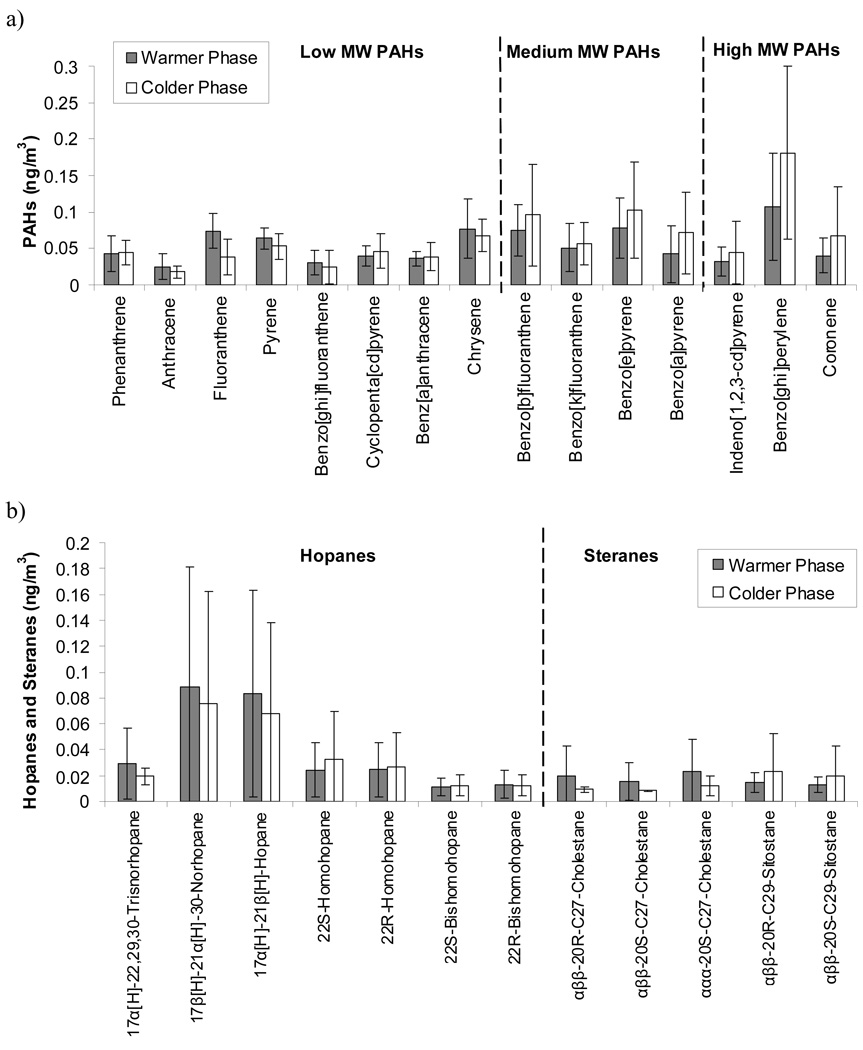
As shown in Figure 1a, the outdoor PAHs concentrations were similar in the warmer and the colder phases. However, medium and high molecular weight PAHs levels were slightly higher in the colder months compared to the warmer periods. PAHs are mainly products of incomplete combustion, including vehicular emissions (Manchester-Neesvig et al., 2003). The higher colder phase levels could be attributed to the influence of cold start spark-ignition from gasoline-powered vehicles, which emit higher amounts of high molecular weight PAHs, such as benzo(ghi)perylene and coronene, than hot-start conditions (Miguel et al., 1998; Fine et al., 2004; Lough et al., 2007).
Figure 1.
Outdoor concentrations of a) PAH’s, b) Hopanes and Steranes, c) n-Alkanes and d) Acids. The presented values are average concentrations across all sites and error bars are standard deviation of these averages at each site.
The average seasonal changes in hopanes and steranes were also quite small (Figure 1b), a result that can be explained by the low seasonal variability in the emission rates of their main source (i.e., engine lubricating oil of mobile sources; Rogge et al., 1993; Rogge et al., 1996; Schauer et al., 1996), which are independent of the driving conditions (e.g. cold-start, hot-start or steady state; Schauer et al., 2002). Moreover, this low seasonal variability may reflect the low volatility of these organic species.
A major portion of the analyzed n-alkanes was characterized by substantially higher and more variable concentrations in the colder months over the studied sites (Figure 1c), possibly because of the enhanced condensation of gas phase n-alkanes onto existing particles (Fraser et al., 1997; Kuhn et al., 2005). Conversely, the lower n-alkanes concentrations observed in the warmer phases could be due to volatilization of their most volatile fraction (Fraser et al., 1997; Kuhn et al., 2005) and to variations in the emission sources of these compounds.
Hexadecanoic, octadecanoic and phthalic acids were the most dominant measured acids in quasi-ultrafine PM (Figure 1d). Phthalic acid concentration in the warmer months was on average more than 2 times higher than in the colder periods. This variability is probably due to relatively higher photo-oxidation rates of organic gases in warmer conditions (Rogge et al., 1991; Pandis et al., 1993; Robinson et al., 2007).
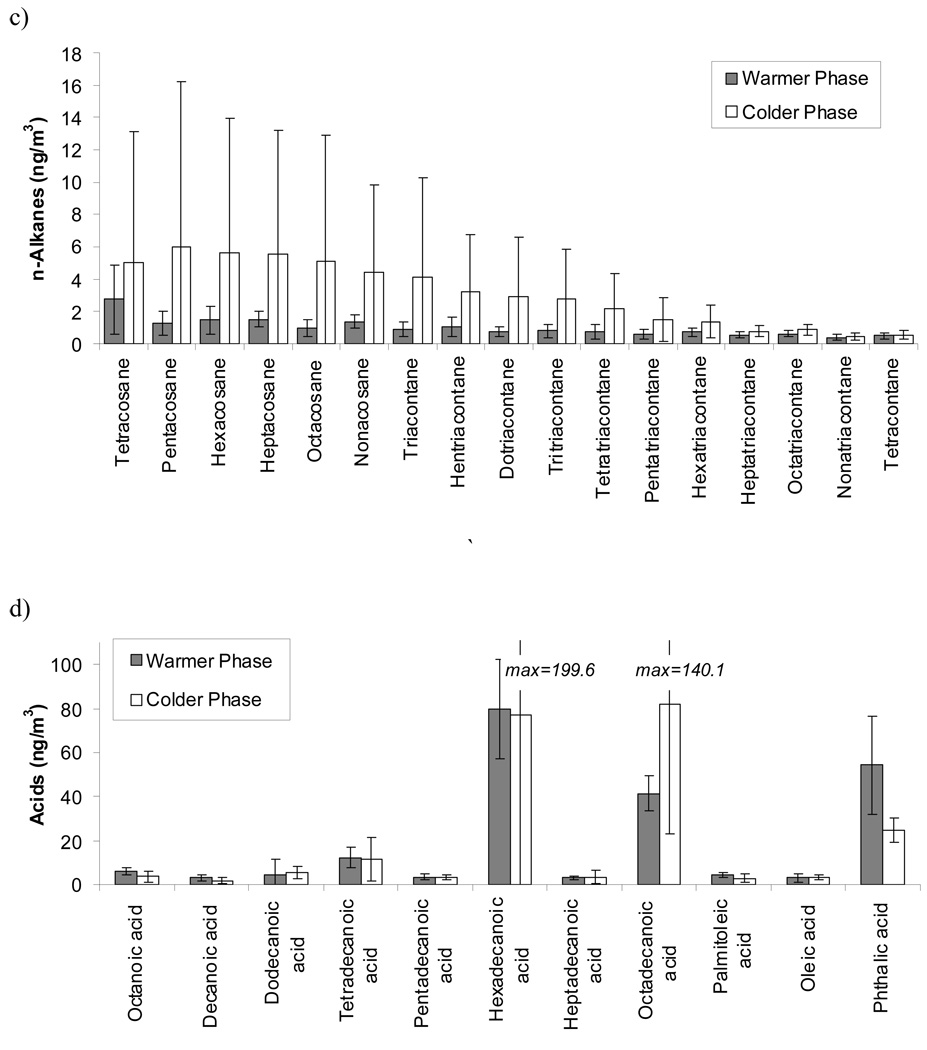
Indoor-Outdoor Organics
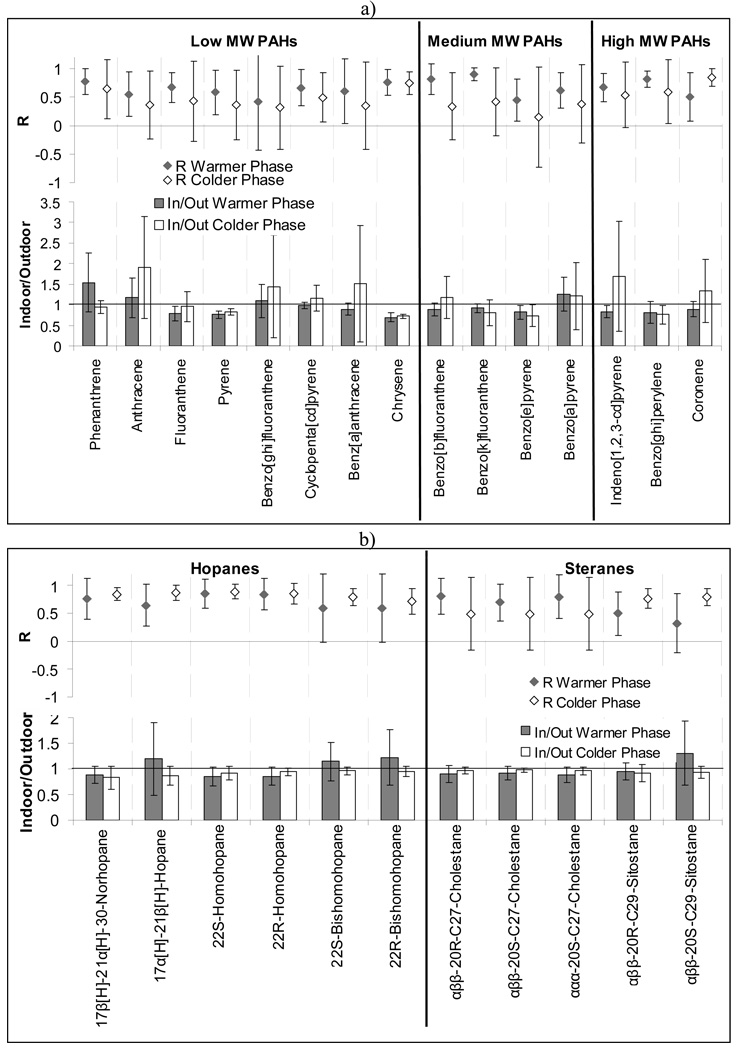
Figure 2 and Figure 3 present the relationship between indoor and outdoor concentrations for the studied organic compounds in the quasi-UF particle range. Figure 2 shows the average indoor and outdoor levels of PAHs, hopanes and steranes, n-alkanes and organic acids at each site and phase of the study. Figure 3 shows the indoor / outdoor (IN / OUT) ratios and correlation coefficients of all measured organic species for different phases of the study. The average outdoor level of the sum of all PAHs were lowest in Riverside (0.5 ng/m3) and highest at San Gabriel 2 site (1.5 ng/m3). The Riverside site was the most distant from any primary combustion sources (i.e. freeways and busy roadways), while the San Gabriel 2 site was the closest to a major freeway (within 300 m) among all sites. Typically, the average concentrations of the sum of all measured PAHs were similar, but slightly lower indoors than outdoors. Accordingly, the average IN / OUT ratio of most of the measured PAHs was close to or lower than 1 and correlation coefficients were always positive and generally high for most of the components (median R for all components = 0.60). These results may reflect the possible impact of outdoor sources (e.g. motor-vehicle emissions) on indoor PAHs in the quasi-UF mode, which is consistent with previous studies (Ohura et al., 2004). PAHs generated by tobacco smoke were not expected indoors, since all of the studied retirement communities were non-smoking residences. Few individual PAH components, such as phenanthrene, anthracene and benz(a)anthracene, showed slightly higher than 1 average IN / OUT ratios and a relatively high standard deviation, hence indicating the possibility of indoor sources (e.g. natural gas appliances) for these species.
Figure 2.
Concentration of total a) PAH’s, b) Hopanes and Steranes, c) n-Alkanes and d) Acids. Dots are average of concentrations across all the sites and error bars are standard deviation of these averages at each site
Figure 3.
Correlation coefficient and indoor and outdoor ratios of a) PAHs, b) Hopanes and Steranes, c) n-Alkanes and d) Acids, values are averaged over the sites and bars are the standard deviation over the sites
Similarly to PAHs, the sum of all measured hopanes and steranes concentrations was slightly higher outdoors than indoors at all sites. Average IN / OUT ratios were close to 1 (min=0.83, max=1.31 and median=0.94), accompanied by relatively high R values (median of R for all components = 0.74). As in the case of PAHs, these results highlight the possible influence of outdoor sources to the measured indoor concentrations of hopanes and steranes. There were no clear seasonal patterns for the corresponding IN / OUT ratios and R values. As hopanes and steranes are more stable species compared to PAHs, the effect of temperature differences between indoor and outdoor environments on their indoor and outdoor associations becomes less significant. Similar relationships between the indoor-outdoor concentrations of hopanes and steranes (and also PAHs) were found in a recent study conducted in Tampa, FL (Olson et al., 2008).
The average indoor concentrations of the sum of all measured n-alkanes were typically higher than the corresponding outdoor levels. Exceptionally high indoor levels with large standard deviation were shown during the colder phase at San Gabriel 3 and Riverside sites. Most of the IN / OUT ratios of n-alkanes were much higher than 1 (up to 23 during the colder phase). R values were not always positive and, on average, were much lower than those found for PAHs, hopanes and steranes (min=−0.14, max=0.79 and median=0.41). The high IN / OUT ratios and low R values are indicative of the significant influence of indoor sources of n-alkanes. Considerably higher indoor n-alkanes levels compared to the outdoor levels were also found in a previous study (Olson et al., 2008). A variety of PM sources such as cooking, household products, dust, smoking and candle burning are known as indoor sources of n-alkanes (Fine et al., 1999; Schauer et al., 1999; Kleeman et al., 2008). The average indoor and outdoor concentrations of n-alkanes at the San Gabriel 3 site were substantially higher than those at the other sites.
Similarly to n-alkanes, average indoor concentrations of the sum of all measured n-alkanoic acids were higher than the corresponding outdoor levels. The indoor concentrations were substantially enriched (i.e, more than 3 times of outdoor levels) with a large variation in indoor concentrations during both phases at San Gabriel 1 and 2 sites. IN / OUT ratios of measured organic acids were higher than 1 (except for octanoic acid in the colder months) with average IN / OUT ratios of 4.8 (IN/OUT ratios of up to ~30 were found for oleic acid in the warmer phases). The high IN / OUT ratios were accompanied by low R-values (median R values = 0.19; lowest R = −0.27 for oleic acid in the warmer phases), indicating the influence of indoor sources for organic acids. Cooking is a major indoor source of these acids, and oleic and palimitoleic acid had often been used as biomarkers of food cooking (Robinson et al., 2006). Other significant sources of organic acids include direct emission from people (e.g. skin oils from heated surfaces). Throughout the study, no specific seasonal trends were observed for the association between indoor and outdoor organic acids. All R values and slopes of the regressions between indoor and outdoor concentrations and their related intercepts are listed for all individual organic species in Table S2 of the Supplemental Information. For any PM species measured, the regression slope and the corresponding intercept are good surrogates for Finf and indoor-generated concentration, respectively, during periods characterized by a relatively high R. A more comprehensive discussion about the outdoor-generated and indoor-infiltrated contributions to the measured indoor concentrations of PM2.5 and its carbonaceous components is presented in Polidori et al., 2007. Scatter plots showing the indoor and outdoor concentrations of representative organic species measured at all sites and during all phases of CHAPS are shown in Figure S1 of the Supplemental Information.
The carbon preference index (CPI), i.e., the ratio of the concentrations of odd-carbon-to-even-carbon n-alkanes, is a parameter used to differentiate between anthropogenic and biogenic source contributions to PM (Simoneit, 1986). Previous studies have shown that n-alkanes originating from anthropogenic sources have a CPI close to 1, whereas the CPI is generally higher than 2 when the biogenic sources are dominant (Simoneit, 1986). The average indoor and outdoor CPIs at sampling sites varied from 0.60 to 0.95, suggesting that anthropogenic emissions (originated from fossil fuel) are the dominating sources in the studied area (see Figure S2 in supplemental information).
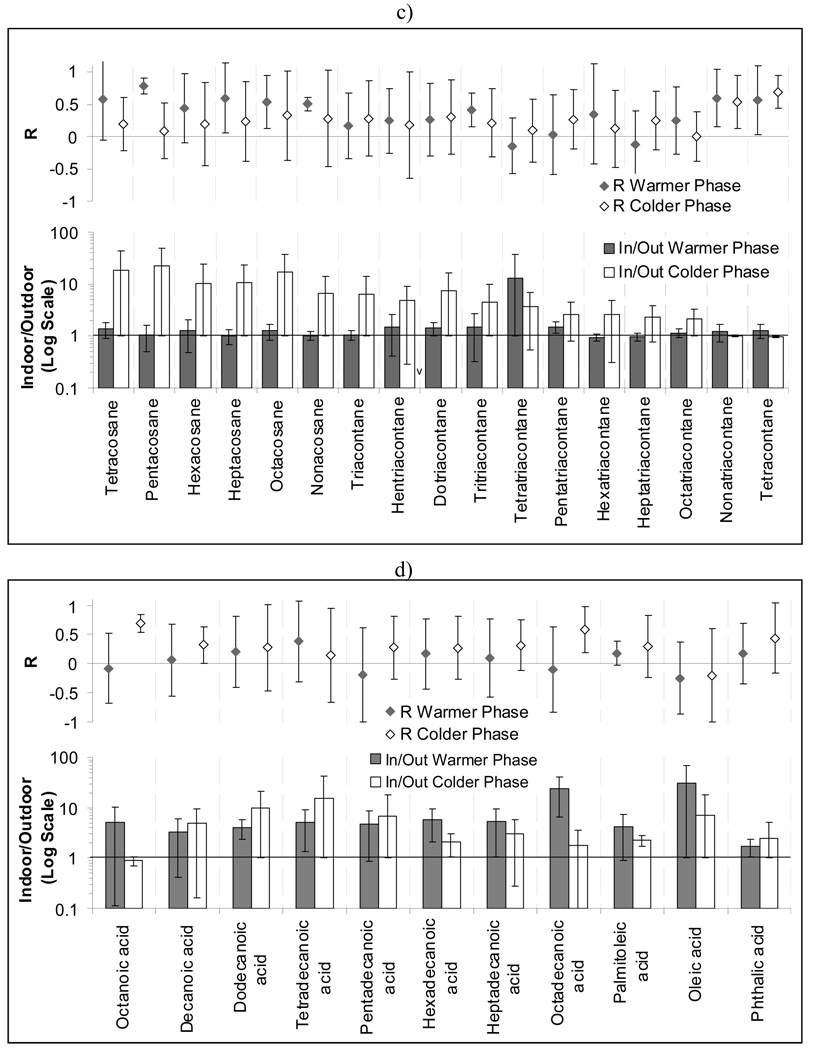
Spatial Variability
The coefficient of variance, also called coefficient of variation (CV = standard deviation / mean), was determined for several measured components to investigate their spatial variation over the studied area (Figure 4). Most of the uncertainty in the calculated coefficient of variations is due to the fact that sampling at the different sites was not concurrent, which can affect the calculated CVs to some extent. However CV was calculated separately for colder and warmer phases to reduce this effect. In general, colder phases were characterized by higher CV values compared to the warmer phases, probably due to the lower regional atmospheric mixing and increased atmospheric stability during cold meteorological conditions. Quasi-UF mass concentrations showed relatively low variability over the studied sites in both indoor and outdoor environments (CV= 0.09 to 0.22). Outdoor WSOC was less variable in the warmer phases compared to colder phases, probably because of the seasonal variability of the main WSOC sources (i.e. secondary atmospheric processes and biomass burning; Weber et al., 2007). Secondary quasi-ultrafine OC formation is expected to be higher in warmer periods due to higher photochemical activity, whereas higher biomass burning emission of OC is expected in colder conditions in the absence of wildfire. Biomass burning has localized effects on OC compared to secondary photo-oxidation formation of OC, which can influence the concentrations of several carbonaceous compounds on a regional scale. Although particulate OC formation by biomass burning is generally not significant (at least in the absence of wildfires) in the polluted Los Angeles basin (Minguillon et al., 2008), it may still affect the spatial variability of WSOC levels. Regarding indoor CVs of WSOC, the relatively high values in the warmer months may be due to the high levels of indoor WSOC at the San Gabriel 1 and 2 sites during these periods. The spatial variance (CV) of outdoor hopanes and steranes was 0.83 and 0.97, respectively, one of the highest among all organic species. This variability originates from differences in the local traffic sources and their influence on each sampling site. Spatial variability in indoor and outdoor PAHs was similar, due to the significant contribution of outdoor originated PAHs to indoor PM.
Figure 4.
Coefficient of variance (CV) for indoor and outdoor organic groups for: a) warmer and b) colder period of the study
Source Contribution Estimations
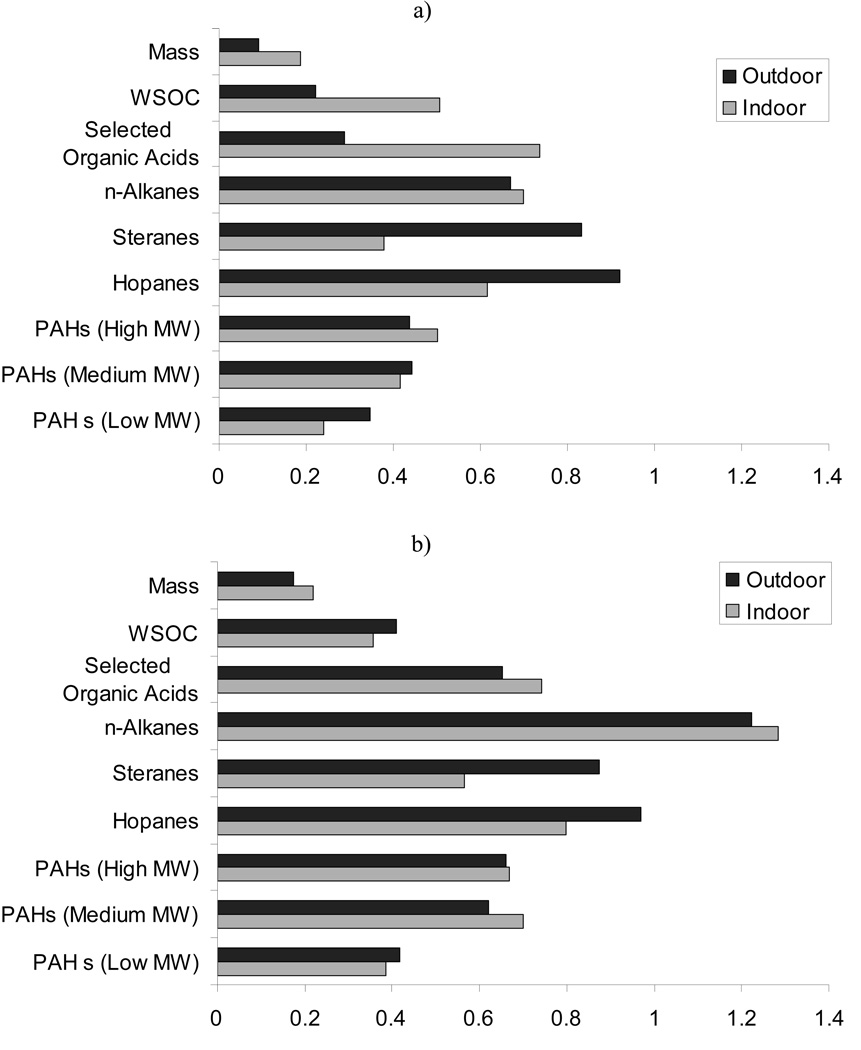
The results of the source apportionment of quasi-UF particle mass are presented in Figure 5. Vehicular sources, including both HDV and LDV, showed the highest contribution for both indoor and outdoor particles at all sites (on average 1.67–4.86 µg/m3 or 24–47% of the quasi-UF mass). Estimations of the HDV contribution were higher than those from LDV. This could be due to the location of the study sites, all situated in the eastern region of Los Angeles, where the traffic fleet has a higher fraction of HDV compared to the typical urban area of Los Angeles. The average percentage contribution of HDV to the total vehicle fleet (HDV + LDV) at the I-10, I-210 and I-60 freeways in eastern Los Angeles counties (San Bernardino and Riverside counties), where most of the sites were located, was ~10 to 15%, which is ~2–3 times higher than the corresponding percentage in the Los Angeles urban area (values were estimated from data obtained from the Caltrans website; http://traffic-counts.dot.ca.gov). As we stated in the methods section, the LDV source profile used for the CMB analysis is from a study conducted near the I-110 freeway, between downtown Los Angeles and Pasadena, CA (Phuleria et al., 2007). However, in the study areas, the emissions of EC from older vehicles can be higher than that at the I-110 (Schauer et al., 2002). This could bias the results obtained by the CMB analysis by underestimating the LDV contribution, because in this model, EC is a key determinant for discriminating between HDV and LDV. Therefore, higher EC levels emitted from old LDV can artificially increase the source contribution estimations for HDV.
Figure 5.
Contribution of different sources to quasi-ultrafine PM in the four sites and during the two sampling periods
The relative contribution of biomass burning to the measured indoor and outdoor quasi-UF mass was low (on average, from 0.06 to 0.87 µg/m3, or about 1 to 9% of the measured mass, across all sites and phases of the study, with the exception of San Gabriel 2 site). The differences between indoor and outdoor contributions were low, except at the San Gabriel 2 site in the warmer phase. The average outdoor biomass burning contribution was 1.4 to 3.8 times higher in the colder phases compared to the warmer phase. Sea spray and ship emission contributions to PM were negligible, as it is expected for sites located far away from the ocean (median sea spray and ship contributions were 0.12 and 0.08 µg/m3, respectively, over all sites and phases, which corresponds to around 1% of the measured mass). Estimated indoor and outdoor non-sea salt sulfate contributions tracked each other at most of sites, suggesting that a substantial portion of indoor sulfate originates from outdoors (median = 0.72µg/m3 indoors and 0.74µg/m3 outdoors). Outdoor non-sea salt sulfate was, on average, 33% higher in warmer phases compared to colder phases, confirming the secondary origin of this pollutant (Rodhe, 1999).
Candle and cigarette smoke sources were also used as an input for the CMB model, but the resulting contributions were negligible (no more than 0.02 µg/m3, or less that 1% of the measured mass, at all sites and during all phases of the study). Our CMB model was not able to apportion the contribution of cooking using common meat cooking source profiles (Schauer et al., 1999; Kleeman et al., 2008). This does not necessarily imply that the contribution of cooking to the quasi-UF particle mass was negligible, but it can indicate that the cooking source profile used is not representative of the specific food cooking emissions at our study sites. The influence of food cooking emissions on indoor PM is evidenced by the elevated levels of indoor organic acids such as oleic acid and palimitoleic acid, frequently used as biomarkers of food cooking (Robinson et al., 2006). The same study also showed that significant inconsistencies exist between ambient data and published source profiles for cooking, which makes it difficult to obtain reliable estimates of the relative contribution of cooking to ambient PM. Resuspended dust contributions were 0.02 to 1.66 µg/m3 (or approximately 0 to 22% of the measured mass) to indoor, and 0.27 to 2.17 µg/m3 to outdoor quasi-ultrafine PM across all sites. Road dust and indoor activities are respectively the main outdoor and indoor sources of resuspended dust. Warmer phase resuspended dust was on average more than 100% higher than the corresponding colder phase levels across all outdoor stations.
Estimated SOA accounted for 0.23 to 1.62 µg/m3 (or around 3 to 19% of the measured mass) of the indoor and outdoor quasi-ultrafine PM at all sites and during all phases. At some sites (e.g. San Gabriel 2, colder phase), estimated SOA concentrations were higher indoors than outdoors (up to ~3 times), which can be partially due to the formation of secondary particles in indoor environments from reactions of household products with ozone and to a lesser extent hydroxyl radicals (Destaillats et al., 2006; Weschler and Nazaroff, 2008); Also, since SOA was estimated here from WSOC, it can be influenced by indoor emissions of water soluble organics (such as those from cooking). The average SOA contribution in the warmer phase was about 2 times higher than during the colder phase at all outdoor sites, which highlights the important role of photo-oxidation in the formation of SOA. The average un-apportioned fraction of quasi-ultrafine PM was 33 ± 15% among all sites and phases. A fraction of this un-apportioned mass could be attributed to ammonium nitrate, which was not measured, but could account for as much as 2–3 µg/m3 of the PM0.25 mass concentrations, especially in the Riverside area (Kleeman et al., 1999; Hughes et al., 2002; Sardar et al., 2005). Moreover, there are uncertainties associated with the calculations of SOA. Part of this uncertainty originates from the multiplication factor used to convert WSOC to SOA and from the assumed fraction of WISOC in SOA (20%), as they both can vary with time and location; (Docherty et al., 2008). A study carried out in Tokyo showed that 6 to 26% of summer oxygenated organic carbon was water-insoluble (Kondo et al., 2007), whereas water-insoluble SOA fractions as large as 60% have recently been reported for urban environments (Favez et al., 2008). In a recent study by Docherty et al., (2008) SOA was estimated to comprise 45 to 90% of the organic fine aerosol mass in the Los Angeles basin.
Lastly, the estimated indoor LDV and HDV source contributions were similar to those calculated outdoors during both phases and at all retirement communities. This is probably due to the significant role of outdoor mobile sources on indoor environments and illustrates the high indoor infiltration of particles generated by mobile sources. This finding has important exposure and health implications considering that in an earlier publication (also part of the CHAPS study) we found that traffic-related particles had much stronger associations with adverse health effects in the elderly retirees of the studied communities compared to uncharacterized indoor particles (Delfino et al., 2008).
To the best of our knowledge, this work and the one conducted by Minguillon et al. (2008) are the only source apportionment studies on quasi-ultrafine PM conducted in the LA area. The results from both investigations are very consistent.
CONCLUSIONS
The mass and chemical composition of indoor and outdoor quasi-ultrafine PM levels did not generally show a clear seasonal pattern. However, the concentrations of most n-alkanes and n-alkanoic acid were higher in the colder periods and in the warmer months, respectively. No major seasonal differences were found for PAHs, hopanes and steranes. High influence of outdoor sources (mainly vehicular sources) and insignificant contributions from indoor sources were observed for PAHs, hopanes and steranes. By contrast, indoor sources (e.g. cooking) seemed to have a significant impact on the measured indoor concentrations of n-alkanes and organics acids. We speculate that inside some studied retirement communities there was evidence of secondary organic aerosol formation, probably from reactions of household products with indoor ozone.
Vehicular sources showed the highest contribution among the apportioned sources for both indoor and outdoor particles at all sites (on average 24–47% of the quasi-UF mass). The contribution of mobile sources to indoor levels was similar to their corresponding outdoor estimates, thus illustrating the importance of these sources on indoor PM concentrations. A major implication of these findings is that, even if the elderly retirees living in the studied homes typically spend most of their time indoors, a sizeable portion of the PM0.25 particles to which they are exposed might come from outdoor mobile sources. The significance of this conclusion is supported by the fact that indoor infiltrated particles from mobile sources were more strongly associated with the adverse health effects observed in the elderly subjects living in the studied retirement communities compared to uncharacterized indoor particles.
PRACTICAL IMPLICATIONS
Although people (particularly the elderly retirees of our study) generally spend most of their time indoors, a major portion of the PM0.25 particles they are exposed to comes from outdoor mobile sources. This is important because an earlier investigation, also conducted within the Cardiovascular Health and Air Pollution Study (CHAPS), showed that indoor-infiltrated particles from mobile sources are more strongly correlated with adverse health effects observed in the elderly subjects living in the studied retirement communities compared to other particles found indoors (Delfino et al., 2008).
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the NIEHS-NIH grant no. ES-12243 and the California Air Resources Board (CARB) contract no. 03-329. We thank Mike Olson, Martin Shafer, and Brandon Shelton from Wisconsin State Lab of Hygiene (WSLH) for the chemical analysis of the PM samples
REFERENCES
- Abt E, Suh HH, Catalano P, Koutrakis P. Relative contribution of outdoor and indoor particle sources to indoor concentrations. Environmental Science & Technology. 2000;34:3579–3587. [Google Scholar]
- Agrawal H, Malloy QGJ, Welch WA, Miller JW, Cocker DR. In-use gaseous and particulate matter emissions from a modern ocean going container vessel. Atmospheric Environment. 2008;42:5504–5510. [Google Scholar]
- Allen R, Larson T, Sheppard L, Wallace L, Liu L. Use of Real-Time Light Scattering Data to Estimate the Contribution of Infiltrated and Indoor-Generated Particles to Indoor Air. Environmental Science & Technology. 2003;37:3484–3492. doi: 10.1021/es021007e. [DOI] [PubMed] [Google Scholar]
- Araujo JA, Barajas B, Kleinman M, Wang XP, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel A. Ambient particulate pollutants in the ultrafine range promote atherosclerosis and systemic oxidative stress. Arteriosclerosis Thrombosis and Vascular Biology. 2007;27:E39–E39. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhami M, Kuhn T, Fine PM, Delfino RJ, Sioutas C. Effects of sampling artifacts and operating parameters on the performance of a semicontinuous particulate elemental carbon/organic carbon monitor. Environmental Science & Technology. 2006;40:945–954. doi: 10.1021/es0510313. [DOI] [PubMed] [Google Scholar]
- Arhami M, Sillanpää M, Hu S, Olson MR, Schauer JJ, Sioutas C. Size-Segregated Inorganic and Organic Components of PM in the Communities of the Los Angeles Harbor. Aerosol Science and Technology. 2009;43:145–160. [Google Scholar]
- Brook JR, Dann TF, Burnett RT. The relationship among TSP, PM(10), PM(2.5), and inorganic constituents of atmospheric particulate matter at multiple Canadian locations. Journal of the Air & Waste Management Association. 1997;47:2–19. [Google Scholar]
- Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. European Respiratory Journal. 2005;26:309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- Cho AK, Sioutas C, Schmitz DA, Kumagai Y, Singh M, Miguel AH, Froines JR. Redox activity of airborne particulate matter (PM) at different sites in the Los Angeles Basin. Environmental Research. 2005;99(1):40–47. doi: 10.1016/j.envres.2005.01.003. 2005. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environmental Health Perspectives. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, Siouta SC. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environmental Health Perspectives. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaillats H, Lunden MM, Singer BC, Coleman BK, Hodgson AT, Weschler CJ, Nazaroff WW. Indoor secondary pollutants from household product emissions in the presence of ozone: A bench-scale chamber study. Environmental Science & Technology. 2006;40:4421–4428. doi: 10.1021/es052198z. [DOI] [PubMed] [Google Scholar]
- Docherty KS, Stone EA, Ulbrich IM, DeCarlo PF, Snyder DC, Schauer JJ, Peltier RE, Weber RJ, Murphy SN, Seinfeld JH, Grover BD, Eatough DJ, Jiimenez JL. Apportionment of Primary and Secondary Organic Aerosols in Southern California during the 2005 Study of Organic Aerosols in Riverside (SOAR-1) Environmental Science & Technology. 2008;42:7655–7662. doi: 10.1021/es8008166. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Finkelstein J, Oberdorster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environmental Health Perspectives. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency Integrated Science Assessments (External Review Draft; 2009) http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=201805.
- Favez O, Sciare J, Cachier H, Alfaro SC, Abdelwahab MM. Significant formation of water-insoluble secondary organic aerosols in semi-arid urban environment. Geophysical Research Letters. 2008;35(15):LL15801.1–L15801.5. [Google Scholar]
- Fine PM, Cass GR, Simoneit BRT. Characterization of fine particle emissions from burning church candles. Environmental Science & Technology. 1999;33:2352–2362. [Google Scholar]
- Fine PM, Cass GR, Simoneit BRT. Chemical characterization of fine particle emissions from the fireplace combustion of wood types grown in the Midwestern and Western United States. Environmental Engineering Science. 2004;21:387–409. [Google Scholar]
- Fine PM, Chakrabarti B, Krudysz M, Schauer JJ, Sioutas C. Diurnal variations of individual organic compound constituents of ultrafine and accumulation mode particulate matter in the Los Angeles basin. Environmental Science & Technology. 2004;38:1296–1304. doi: 10.1021/es0348389. [DOI] [PubMed] [Google Scholar]
- Fraser MP, Cass GR, Simoneit BRT, Rasmussen RA. Air quality model evaluation data for organics .4. C-2-C-36 non-aromatic hydrocarbons. Environmental Science & Technology. 1997;31:2356–2367. [Google Scholar]
- Herner JD, Green PG, Kleeman MJ. Measuring the trace elemental composition of size-resolved airborne particles. Environmental Science & Technology. 2006;40:1925–1933. doi: 10.1021/es052315q. [DOI] [PubMed] [Google Scholar]
- Hopke PK, Ramadan Z, Paatero P, Norris GA, Landis MS, Williams RW, Lewis CW. Receptor modeling of ambient and personal exposure samples: 1998 Baltimore Particulate Matter Epidemiology-Exposure Study. Atmospheric Environment. 2003;37:3289–3302. [Google Scholar]
- Hu S, Polidori A, Schafer M, Cho A, Schauer JJ, Sioutas C. Redox Activity and Chemical Speciation of Size Fractioned PM in the Communities of the Los Angeles - Long Beach Harbor. Atmospheric Chemistry and Physics. 2008;8:6439–6451. 2008. [Google Scholar]
- Hughes LS, Allen JO, Salmon LG, Mayo PR, Johnson RJ, Cass GR. Evolution of nitrogen species air pollutants along trajectories crossing the Los Angeles area. Environmental Science & Technology. 2002;36:3928–3935. doi: 10.1021/es0110630. [DOI] [PubMed] [Google Scholar]
- Kleeman MJ, Hughes LS, Allen JO, Cass GR. Source contributions to the size and composition distribution of atmospheric particles: Southern California in September 1996. Environmental Science & Technology. 1999;33:4331–4341. [Google Scholar]
- Kleeman MJ, Robert MA, Riddle SG, Fine PM, Hays MD, Schauer JJ, Hannigan MP. Size distribution of trace organic species emitted from biomass combustion and meat charbroiling (vol 42, pg 3059, 2008) Atmospheric Environment. 2008;42:6152–6154. [Google Scholar]
- Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of Exposure Analysis and Environmental Epidemiology. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Miyazaki Y, Takegawa N, Miyakawa T, Weber RJ, Jimenez JL, Zhang Q, Worsnop DR. Oxygenated and water-soluble organic aerosols in Tokyo. Journal of Geophysical Research-Atmospheres. 2007;112(D1):D01203.1–D01203.11. [Google Scholar]
- Kuhn T, Biswas S, Sioutas C. Diurnal and seasonal characteristics of particle volatility and chemical composition in the vicinity of a light-duty vehicle freeway. Atmospheric Environment. 2005;39:7154–7166. [Google Scholar]
- Kuhn T, Krudysz M, Zhu Y, Fine PM, Hinds WC, Froines J, Sioutas C. Volatility of indoor and outdoor ultrafine particulate matter near a freeway. Journal of Aerosol Science. 2005;36:291–302. [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang MY, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental Health Perspectives. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough GC, Christensen CG, Schauer JJ, Tortorelli J, Mani E, Lawson DR, Clark NN, Gabele PA. Development of molecular marker source profiles for emissions from on-road gasoline and diesel vehicle fleets. Journal of the Air & Waste Management Association. 2007;57:1190–1199. doi: 10.3155/1047-3289.57.10.1190. [DOI] [PubMed] [Google Scholar]
- Manchester-Neesvig JB, Schauer JJ, Cass GR. The distribution of particle-phase organic compounds in the atmosphere and their use for source apportionment during the southern California children's health study. Journal of the Air & Waste Management Association. 2003;53:1065–1079. doi: 10.1080/10473289.2003.10466265. [DOI] [PubMed] [Google Scholar]
- Meng QY, Turpin BJ, Korn L, Weisel CP, Morandi M, Colome S, Zhang JFJ, Stock T, Spektor D, Winer A, Zhang L, Lee JH, Giovanetti R, Cui W, Kwon J, Alimokhtari S, Shendell D, Jones J, Farrar C, Maberti S. Influence of ambient (outdoor) sources on residential indoor and personal PM2.5 concentrations: Analyses of RIOPA data. Journal of Exposure Analysis and Environmental Epidemiology. 2005;15:17–28. doi: 10.1038/sj.jea.7500378. [DOI] [PubMed] [Google Scholar]
- Miguel AH, Kirchstetter TW, Harley RA, Hering SV. On-road emissions of particulate polycyclic aromatic hydrocarbons and black carbon from gasoline and diesel vehicles. Environmental Science & Technology. 1998;32:450–455. [Google Scholar]
- Minguillon MC, Arhami M, Schauer JJ, Sioutas C. Seasonal and spatial variations of sources of fine and quasi-ultrafine particulate matter in neighborhoods near the Los Angeles-Long Beach harbor. Atmospheric Environment. 2008;42:7317–7328. [Google Scholar]
- Ntziachristos L, Ning Z, Geller MD, Sioutas C. Particle concentration and characteristics near a major freeway with heavy-duty diesel traffic. Environmental Science & Technology. 2007;41:2223–2230. doi: 10.1021/es062590s. [DOI] [PubMed] [Google Scholar]
- Ogulei D, Hopke PK, Wallace LA. Analysis of indoor particle size distributions in an occupied townhouse using positive matrix factorization. Indoor Air. 2006;16:204–215. doi: 10.1111/j.1600-0668.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- Ohura T, Amagai T, Sugiyama T, Fusaya M, Matsushita H. Characteristics of particle matter and associated polycyclic aromatic hydrocarbons in indoor and outdoor air in two cities in Shizuoka, Japan. Atmospheric Environment. 2004;38:2045–2054. [Google Scholar]
- Olson DA, Turlington J, Duvall RV, Vicdow SR, Stevens CD, Williams R. Indoor and outdoor concentrations of organic and inorganic molecular markers: Source apportionment of PM2.5 using low-volume samples. Atmospheric Environment. 2008;42:1742–1751. [Google Scholar]
- Pandis SN, Wexler AS, Seinfeld JH. Secondary Organic Aerosol Formation and Transport .2. Predicting the Ambient Secondary Organic Aerosol-Size Distribution. Atmospheric Environment Part a-General Topics. 1993;27:2403–2416. [Google Scholar]
- Phuleria HC, Sheesley RJ, Schauer JJ, Fine PM, Sioutas C. Roadside measurements of size-segregated particulate organic compounds near gasoline and diesel-dominated freeways in Los Angeles, CA. Atmospheric Environment. 2007;41:4653–4671. [Google Scholar]
- Polidori A, Arhami M, Sioutas C, Delfino RJ, Allen R. Indoor/outdoor relationships, trends, and carbonaceous content of fine particulate matter in retirement homes of the Los Angeles basin. Journal of the Air & Waste Management Association. 2007;57:366–379. doi: 10.1080/10473289.2007.10465339. [DOI] [PubMed] [Google Scholar]
- Polidori A, Turpin BJ, Davidson CI, Rodenburg LA, Maimone F. Organic PM2.5: Fractionation by polarity, FTIR spectroscopy, and OM/OC ratio for the Pittsburgh aerosol. Aerosol Science and Technology. 2008;42:233–246. [Google Scholar]
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: Lines that connect. Journal of the Air & Waste Management Association. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Robinson AL, Donahue NM, Shrivastava MK, Weitkamp EA, Sage AM, Grieshop AP, Lane TE, Pierce JR, Pandis SN. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science. 2007;315:1259–1262. doi: 10.1126/science.1133061. [DOI] [PubMed] [Google Scholar]
- Robinson AL, Subramanian R, Donahue NM, Bernardo-Bricker A, Rogge WF. Source apportionment of molecular markers and organic aerosol. 3. Food cooking emissions. Environmental Science & Technology. 2006;40:7820–7827. doi: 10.1021/es060781p. [DOI] [PubMed] [Google Scholar]
- Rodhe H. Human impact on the atmospheric sulfur balance. Tellus Series a-Dynamic Meteorology and Oceanography. 1999;51:110–122. [Google Scholar]
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Sources of Fine Organic Aerosol .2. Noncatalyst and Catalyst-Equipped Automobiles and Heavy-Duty Diesel Trucks. Environmental Science & Technology. 1993;27:636–651. [Google Scholar]
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Sources of Fine Organic Aerosol .4. Particulate Abrasion Products from Leaf Surfaces of Urban Plants. Environmental Science & Technology. 1993;27:2700–2711. [Google Scholar]
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Mathematical modeling of atmospheric fine particle-associated primary organic compound concentrations. Journal of Geophysical Research-Atmospheres. 1996;101:19379–19394. [Google Scholar]
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Sources of fine organic aerosol .8. Boilers burning No. 2 distillate fuel oil. Environmental Science & Technology. 1997;31:2731–2737. doi: 10.1021/es00056a030. [DOI] [PubMed] [Google Scholar]
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simonelt BRT. Sources of Fine Organic Aerosol .1. Charbroilers and Meat Cooking Operations. Environmental Science & Technology. 1991;25:1112–1125. doi: 10.1021/es00056a030. [DOI] [PubMed] [Google Scholar]
- Sardar SB, Fine PM, Sioutas C. Seasonal and spatial variability of the size-resolved chemical composition of particulate matter (PM10) in the Los Angeles Basin. Journal of Geophysical Research-Atmospheres. 2005;110 [Google Scholar]
- Schauer JJ, Cass GR. Source apportionment of wintertime gas-phase and particle-phase air pollutants using organic compounds as tracers. Environmental Science & Technology. 2000;34:1821–1832. [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 1. C-1 through C-29 organic compounds from meat charbroiling. Environmental Science & Technology. 1999;33:1566–1577. [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 5. C-1-C-32 organic compounds from gasoline-powered motor vehicles. Environmental Science & Technology. 2002;36:1169–1180. doi: 10.1021/es0108077. [DOI] [PubMed] [Google Scholar]
- Schauer JJ, Rogge WF, Hildemann LM, Mazurek MA, Cass GR. Source apportionment of airborne particulate matter using organic compounds as tracers. Atmospheric Environment. 1996;30:3837–3855. [Google Scholar]
- Sheesley RJ, Schauer JJ, Zheng M, Wang B. Sensitivity of molecular marker-based CMB models to biomass burning source profiles. Atmospheric Environment. 2007;41:9050–9063. [Google Scholar]
- Sillanpaa M, Hillamo R, Saarikoski S, Frey A, Pennanen A, Makkonen U, Spolnik Z, Van Grieken R, Branis M, Brunekreef B, Chalbot MC, Kuhlbusch T, Sunyer J, Kerminen VM, Kulmala M, Salonen RO. Chemical composition and mass closure of particulate matter at six urban sites in Europe. Atmospheric Environment. 2006;40:S212–S223. [Google Scholar]
- Simoneit BRT. Characterization of organic-constituents in aerosols in relation to their origin and transports A review. Int. J. Environ. Anal. Chem. 1986;23:207–237. [Google Scholar]
- Simoneit BRT. A review of biomarker compounds as source indicators and tracers for air pollution. Environmental Science and Pollution Research. 1999;6:159–169. doi: 10.1007/BF02987621. [DOI] [PubMed] [Google Scholar]
- Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environmental Health Perspectives. 2005;113:947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Snyder DC, Sheesley RJ, Sullivan AP, Weber RJ, Schauer JJ. Source apportionment of fine organic aerosol in Mexico City during the MILAGRO experiment 2006. Atmospheric Chemistry and Physics. 2008;8:1249–1259. [Google Scholar]
- Subramanian R, Donahue NM, Bernardo-Bricker A, Rogge WF, Robinson AL. Contribution of motor vehicle emissions to organic carbon and fine particle mass in Pittsburgh, Pennsylvania: Effects of varying source profiles and seasonal trends in ambient marker concentrations. Atmospheric Environment. 2006;40:8002–8019. [Google Scholar]
- Turpin BJ, Lim HJ. Species contributions to PM2.5 mass concentrations: Revisiting common assumptions for estimating organic mass. Aerosol Science and Technology. 2001;35:602–610. [Google Scholar]
- Turpin BJ, Saxena P, Andrews E. Measuring and simulating particulate organics in the atmosphere: problems and prospects. Atmospheric Environment. 2000;34:2983–3013. [Google Scholar]
- Wallace L. Indoor particles: A review. Journal of the Air & Waste Management Association. 1996;46:98–126. doi: 10.1080/10473289.1996.10467451. [DOI] [PubMed] [Google Scholar]
- Weber RJ, Sullivan AP, Peltier RE, Russell A, Yan B, Zheng M, de Gouw J, Warneke C, Brock C, Holloway JS, Atlas EL, Edgerton E. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. Journal of Geophysical Research-Atmospheres. 2007;112 [Google Scholar]
- Weichenthal S, Dufresne A, Infante-Rivard C. Indoor ultrafine particles and childhood asthma: exploring a potential public health concern. Indoor Air. 2007;17:81–91. doi: 10.1111/j.1600-0668.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- Weschler CJ. Chemical reactions among indoor pollutants: what we've learned in the new millennium. Indoor Air. 2004;14:184–194. doi: 10.1111/j.1600-0668.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- Weschler CJ, Nazaroff WW. Semivolatile organic compounds in indoor environments. Atmospheric Environment. 2008;42:9018–9040. [Google Scholar]
- Yli-Tuomi T, Lanki T, Hoek G, Brunekreef B, Pekkanen J. Determination of the sources of indoor PM2.5 in Amsterdam and Helsinki. Environmental Science & Technology. 2008;42:4440–4446. doi: 10.1021/es0716655. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schauer JJ, Shafer MM, Hannigan MP, Dutton SJ. Source Apportionment of in vitro Reactive Oxygen Species Bioassay Activity from Atmospheric Particulate Matter. Environmental Science and Technology. 2008;42:7502–7509. doi: 10.1021/es800126y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.