Abstract
The EcoCyc database is an online scientific database which provides an integrated view of the metabolic and regulatory network of the bacterium Escherichia coli K-12 and facilitates computational exploration of this important model organism. We have analysed the occurrence of dead end metabolites within the database – these are metabolites which lack the requisite reactions (either metabolic or transport) that would account for their production or consumption within the metabolic network. 127 dead end metabolites were identified from the 995 compounds that are contained within the EcoCyc metabolic network. Their presence reflects either a deficit in our representation of the network or in our knowledge of E. coli metabolism. Extensive literature searches resulted in the addition of 38 transport reactions and 3 metabolic reactions to the database and led to an improved representation of the pathway for Vitamin B12 salvage. 39 dead end metabolites were identified as components of reactions that are not physiologically relevant to E. coli K-12 – these reactions are properties of purified enzymes in vitro that would not be expected to occur in vivo. Our analysis led to improvements in the software that underpins the database and to the program that finds dead end metabolites within EcoCyc. The remaining dead end metabolites in the EcoCyc database likely represent deficiencies in our knowledge of E. coli metabolism.
Introduction
Symbolic systems biology is the application of logic-based computational methods to the systems-level analysis of an organism. Previously, several types of symbolic systems biology approaches have provided novel biological insights. For example, metabolic pathway analysis of genomes can be used to identify reactions within metabolic pathways that have no associated enzyme (“pathway holes”) [1], thus motivating a search for gene(s) within the organism that code for the missing enzyme. Conversely, orphan enzymes are enzymes whose biochemical function has been demonstrated experimentally, but for which the associated gene has not been identified [2]. In both cases, the explicit identification of holes in our knowledge spurs a whole series of new investigations.
A dead-end metabolite (DEM) is defined as a metabolite that is produced by the known metabolic reactions of an organism and has no reactions consuming it, or that is consumed by the metabolic reactions of an organism and has no known reactions producing it, and in both cases has no identified transporter (Figure 1). DEMs are thus isolated compounds within a metabolic network. In some cases, DEMs reflect a deficit or an error in how a metabolic database represents knowledge from the scientific literature, and alerts us to the need for further curation of the database. In other cases this systems-level analysis alerts us to areas where more experimental research is required. In the latter case DEMs act as signposts to the ‘known unknowns’ of metabolism.
Figure 1. Representation of generic dead end metabolites (A, B, C and D) within a metabolic network.
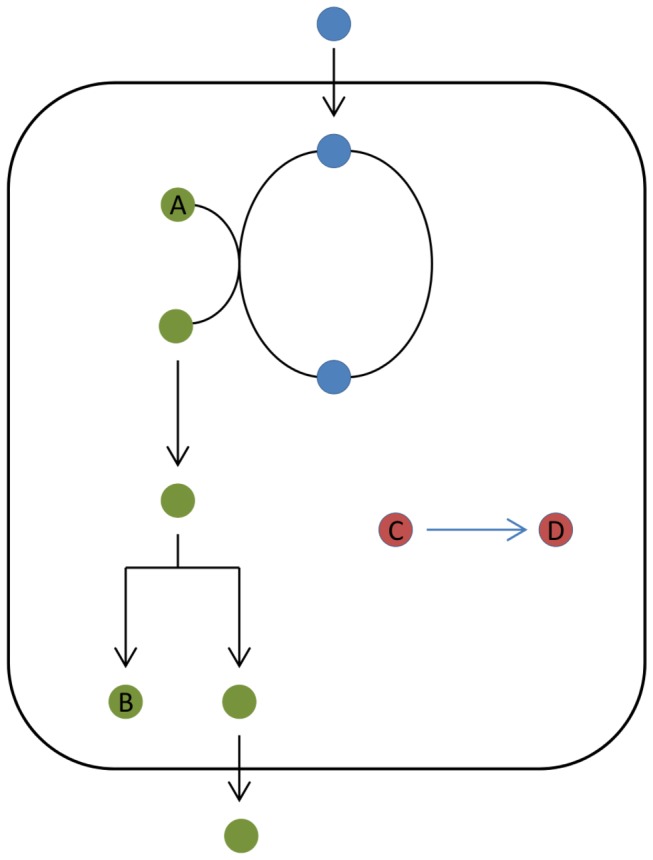
The compounds labeled A and C are neither produced nor transported by any other reaction within the network while the compounds B and D are neither consumed nor transported by any other reaction within the network.
Our DEM analysis of Escherichia coli K-12 MG1655 was conducted using EcoCyc (http://EcoCyc.org), an online encyclopedia of E. coli K-12 biology that provides an integrated view of the genome, genes and gene products, and the metabolic and regulatory networks of this important model organism [3]. EcoCyc combines computable representations of these biological features of E. coli K-12, along with detailed summaries from manual literature curation. In release version 17.0 (March, 2013), EcoCyc contained 1497 metabolic enzymes and 268 transporters catalysing a total of 2175 reactions. The database contains 2392 compounds of which 995 are directly involved in reactions (the remainder being, for example, enzyme cofactors or inhibitors). EcoCyc version 17.0 also cites 24,391 publications from the literature. In addition to being a comprehensive reference resource, EcoCyc also provides tools that can be used for computational exploration within the database including multiple search tools and the identification of DEMs [4] (see EcoCyc website command Tools → Dead-end metabolites).
This project was undertaken to identify and analyse the dead end metabolites within the EcoCyc database. Our analysis led to the improved curation of many compounds within the database and also to improvements within the Pathway Tools software that underpins the database. We were able to resolve the dead end status of a large number of compounds through the addition of previously missing metabolic or transport reactions. As a result we are able to more accurately define the true DEMs within the EcoCyc database and by extension the ‘known unknowns’ within the metabolic machinery of the model organism E. coli K-12.
Results
Identification of DEMs in EcoCyc
DEMs within the EcoCyc database were identified using the DEM finder tool. In EcoCyc, metabolites may be reactants or products of reactions that occur within metabolic pathways defined within the database, or metabolites may form part of isolated reactions that are not contained within defined pathways. The DEM finder tool in EcoCyc can be customised to identify only those compounds that exist within metabolic pathways (pathway DEMs) or to include DEMs that come from reactions occurring outside pathways (non-pathway DEMs). Participation in metabolic pathways may make pathway DEMs rare, but also more likely to be physiologically relevant. A search of 271 metabolic pathways yielded 32 DEMs while a search of 393 isolated reactions within EcoCyc returned a further 123 compounds. 28 of these compounds were lacking proper classification within the EcoCyc database and could be resolved by correcting this omission. For example, correct classification of the compound “methylphosphonate” as a child of the class “alkylphosphonates” meant that it was recognised by the EcoCyc software as a substrate of the phosphonate ABC transporter, thus resolving its dead end status. The remaining 127 compounds (Table 1) were the subject of further analysis.
Table 1. Dead end metabolites from the EcoCyc database.
| (2R,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran (AI-2)p | allantoin p | maltopentaose |
| (E)-3-(methoxycarbonyl)pent-2-enedioate | aminoacetaldehyde p | methanol p |
| (R)-beta-lysine | aminomethylphosphonate | methyl red |
| (R)-malate | an unknown C3 fragment | methyl-1,4-benzoquinol |
| (R)-pantolactone | benzaldehyde | methyl-1,4-benzoquinone |
| 1-chloro-2,4-dinitrobenzene (CDNB) | CDP-choline | N,N'-dimethyl-p-phenylenediamine |
| 1-deoxy-D-xylulose | cholate | N-alpha-acetyl lysine methyl ester |
| 2,3-diaminopropionate | cis-vaccenate p | N-ethylmaleimide |
| 2,4-dinitrophenyl-S-glutathione | CO | N-ethylsuccinimide |
| 2-aminobutyrate | cobinamide p | nicotinamide riboside |
| 2-aminomalonate-semialdehyde | cofactor | nigerose |
| 2-dehydropantolactone | corresponding carbamoyl amino acid | N-methyltryptophan |
| 2-deoxy-D-glucose | Cr3+ | NMNH |
| 2-deoxy-D-glucose 6-phosphate | Cr6+ | octanoate p |
| 2-deoxygluconate | curcumin p | oxalate |
| 2-protocatechuoylphloroglucinolcarboxylate | D-4-hydroxy-2-keto-glutarate (KHG) | oxamate p |
| 3,4-dihydroxyphenylacetate | d-biotin d-sulfoxide | phenylethylamine p |
| 3,4-dihydroxyphenylacetyl-CoA | D-galactono-1,4-lactone | phenylhydantoin |
| 3,5-tetradecadienoatep | diacetyl | pre-cofactor |
| 3-alpha,12-alpha-dihydroxy-7-oxo-5-beta-cholanate | diacylglycerol pyrophosphate | pseudouridine p |
| 3-chloro-D-alanine | dihydrolipoamide | psicoselysine p |
| 3-dehydro-2-deoxy-D-gluconate | ethanolamine p | pyrazinamide |
| 3-hydroxy-5-cis-tetradecenoyl-CoAp | ethyl-(2R)-methyl-(3S)-hydroxybutanoate | pyrazinoate |
| 3-hydroxypropionatep | ethyl-2-methylacetoacetate | queuine |
| 3-hydroxy-trans-cinnamatep | fructoselysine p | quinate |
| 3-mercaptopyruvate | GDP-alpha-D-glucose | S2- p |
| 3-methylcrotonyl-CoA | glycerol 2-phosphate | S-adenosyl-4-methylthio-2-oxobutanoate p |
| 3-phenylpropanoatep | GMP-lysine | salicyl alcohol |
| 3-sulfinoalanine (AKA cysteine sulfinic acid) | heptosyl-KDO2-lipid IVA | S-carboxymethyl-D-cysteine |
| 4-(2-aminophenyl)-2,4-dioxobutanoate | hydroxylamine | selenate1,3-propanediol |
| 4-coumarate | hydroxymethylpyrimidine (HMP)p | selenite |
| 4-coumaroyl-CoA | hydroxypropionaldehyde | S-methyl-5-thio-D-ribose p |
| 4-methyl-5-(beta-hydroxyethyl)thiazole (THZ)p | isovaleryl-CoA | S-methyl-L-methionine |
| 4-nitrobenzaldehyde | kynurenine | tetrahydrocurcumin p |
| 4-nitrobenzyl alcohol | L-glyceraldehyde 3-phosphate | tetrahydromonapterin p |
| 5', 5'-diadenosine triphosphate | L-idarate | thioglycolate |
| 5,6,7,8-tetrahydropteridine | linear dimeric GMP | trans-aconitate |
| 5,6-dimethylbenzimidazole (DMB)p | lipoamide | trans-cinnamate p |
| 6,7-dihydropteridine | lipoate p | UDP-[N-acetyl-D-glucosamine]n |
| acetoacetate p | L-methionine sulfoxide | urate p |
| acetylmaltose | L-rhamnonate | urea p |
| adenosine thiamine triphosphate | L-selenocysteine | |
| adenosylcobalamin-5'-phosphate | L-threo-3-phenylserine |
p DEMs derived from within EcoCyc metabolic pathways.
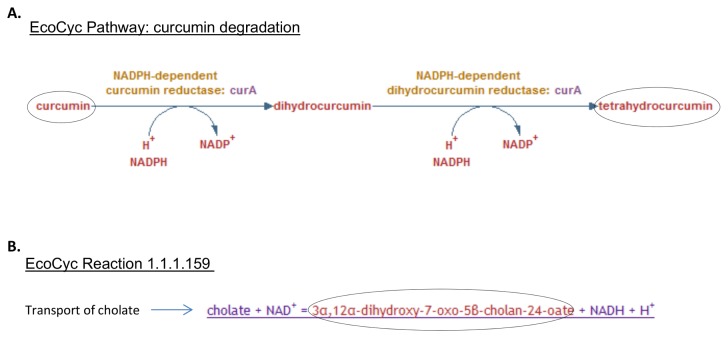
Examples of DEMs and the EcoCyc reactions from which they derive are shown in Figure 2. Both curcumin (a secondary metabolite produced in turmeric and other plant species, see http://www.metacyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-6432) and tetrahydrocurcumin are considered to be pathway DEMs in EcoCyc because the database contains no other reactions for these molecules – it does not describe the production nor transport of curcumin, nor the fate of tetrahydrocurcumin. In contrast, the compound 3α,12α-dihydroxy-7-oxo-5β-cholan-24-oate (product of an E. coli 7-α-hydroxysteroid dehydrogenase, HdhA) is a DEM from an isolated reaction in EcoCyc. HdhA acts on cholate, which is thought to cross the inner membrane of E. coli [5]; the fate of 3α,12α-dihydroxy-7-oxo-5β-cholan-24-oate in the E. coli cell is unknown although it is known to be further metabolised by other anaerobic and facultative anaerobic bacteria in the human intestine (see http://metacyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-6518).
Figure 2. Dead end metabolites in EcoCyc.
A. The EcoCyc curcumin degradation pathway in EcoCyc produces two dead end metabolites (circled). B. An isolated EcoCyc reaction (catalysed by E. coli 7-α-hydroxysteroid dehydrogenase) produces the single dead end metabolite, 3α,12α-dihydroxy-7-oxo-5β-cholan-24-oate.
DEM analysis and resolution
The 127 DEMs identified in the EcoCyc database encompass a wide variety of compounds. As a necessary first step to understand the biochemistry of each compound and its metabolic context, the reactions containing the DEMs were identified within EcoCyc and any associated literature was reviewed. Extensive literature searches were then conducted to see if further information relating to the compound could be uncovered.
Resolution of DEMs through the addition of transport reactions
38 DEMs were resolved through the addition of transport reactions to the database (Table 2). 29 new transport reactions were created to represent the import of metabolites across the inner membrane (Table S1) and 9 reactions were created to represent export of metabolites from the cytoplasm (Table S2). Transport reactions were only added to EcoCyc in cases where we found substantiating evidence in the experimental literature (Table 2). Several of the references describing transport date back more than 30 years. For example, the experimental work reporting the production and excretion of urea into the culture media dates back to studies of putrescine biosynthesis in E. coli K-12 carried out in 1967 [6]. Similarly the ability of E. coli K-12 to use acetoacetate as a sole carbon source was reported by Pauli and Overath in 1972 [7]. For 15 of the 38 reactions, a specific transport protein had been characterised and reported in the literature, while in a further 4 cases the identity of membrane proteins responsible for transport had been predicted. For the remaining metabolites, no information on the mechanism of transport was located in the literature during this study, suggesting that these transport systems remain to be characterised. In these latter cases a transport reaction was added to the EcoCyc database but it was not associated with a specific transport protein.
Table 2. DEMs resolved by the addition of transport reactions to the EcoCyc database.
| Metabolite | Transport system | Literature reference |
|---|---|---|
| acetoacetate | predicted short chain fatty acid transporter AtoE | [7] |
| phenylacetaldehyde | - | [25] |
| psicoselysine | psicoselysine transporter FrlA | [32] |
| fructoselysine | fructoselysine transporter FrlA | [33] |
| octanoate | - | [34] |
| 3-phenylpropanoate | predicted 3-phenylpropanoate transporter HcaT | [35] |
| lipoate | - | [36] |
| cobinamide | vitamin B12 transport system | [37,38] |
| 3-hydroxy-trans-cinnamate | 3-hydroxycinnamate:H+ symporter MhpT | [39] |
| trans-cinnamate | - | [35,39] |
| (2R,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran (AI-2) | autoinducer-2 ABC transporter LsrACDB | [40] |
| 4-methyl-5-(β-hydroxyethyl)thiazole (THZ) | - | [41] |
| 5,6-dimethylbenzimidazole (DMB) | - | [8] |
| hydroxymethylpyrimidine (HMP) | - | [42] |
| pseudouridine | predicted pseudouridine transporter PsuT | [43] |
| ethanolamine | - | [44,45] |
| allantoin | predicted transporter YbbW | [46] |
| R-malate | C4 dicarboxylate/orotate transporter DctA | [47] |
| 1-deoxy-D-xylulose | - | [48] |
| d-biotin-d-sulfoxide | - | [27,49] |
| glycerol-2-phosphate | glycerol-3-phosphate/glycerol-2-phosphate ABC transporter | [50] |
| nicotinamide riboside | nicotinamide riboside ABC transporter | [51] |
| S-methyl-L-methionine | S-methyl-L-methionine transporter | [52] |
| selenite | sulfate/thiosulfate/selenite/selenate ABC transporter | [53] |
| selenate | sulfate/thiosulfate/selenite/selenate ABC transporter | [53] |
| L-selenocysteine | - | [54] |
| cholate | - | [5] |
| L-glyceraldehyde 3-phosphate | glycerol 3-phosphate transport systems | [55,56] |
| aminomethylphosphonate | phosphonate ABC transporter | [57,58] |
| 3,5-tetradecadienoate | - | [10,59] |
| 3-hydroxypropionate | - | [60] |
| S-methyl-5-thio-D-ribose | - | [61] |
| urea | glycerol channel GlpF; passive diffusion | [6] |
| methanol | - | [62] |
| (2R,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran (AI-2) | quorum signal AI-2 exporter TqsA | [63] |
| acetylmaltose | - | [64] |
| salicyl alcohol | - | [65] |
| hydroquinone | - | [65] |
Resolution of DEMs through the addition of metabolic reactions
The number of metabolic reactions added to EcoCyc as a result of DEM analysis was small. One case worth mentioning involved the DEM adenosylcobinamide-5-phosphate, whose presence alerted us to inaccuracies in our representation of the pathway of adenosylcobalamin (coenzyme B12) salvage in EcoCyc. Experimental work on this pathway is largely carried out in Salmonella typhimurium , which can synthesize adenosylcobalamin de novo, unlike E. coli K-12, which can synthesize cobalamin only when supplied with the intermediate compound cobinamide [8]. Despite this, the enzymes of the salvage pathway in E. coli are homologous to those from S . typhimurium and our DEM analysis revealed that the final steps of the pathway in S . typhimurium had recently been experimentally characterised [9]. Based on this we were able to correct our representation of the final two steps in the pathway of adenosylcobalamin salvage in EcoCyc (see http://ecocyc.org/ECOLI/NEW-IMAGE?type=PATHWAY&object=COBALSYN-PWY) and in the process resolve the dead end status of adenosylcobinamide-5-phosphate.
Another DEM, 3-hydroxy-5-cis-tetradecenoyl-CoA (product of a reaction catalysed by the FadB subunit of the fatty acid oxidation complex in E. coli K-12), revealed a deficiency in our link between two related pathways, oleate β-oxidation and fatty acid β-oxidation. This was remedied by the addition of the following two metabolic reactions [10]:
i) 3-hydroxy-5-cis-tetradecenoyl-CoA + NAD → 3-keto-5-cis-tetradecenoyl-CoA + NADH + H+ (an instance reaction of EC 1.1.1.211, catalysed by E. coli K-12 FadB)
ii) 3-keto-5-cis-tetradecenoyl-CoA + coenzyme A → 3-cis-dodecenoyl-CoA + acetyl-CoA (an instance reaction of EC 2.3.1.16, catalysed by E. coli K-12 FadA)
The product of EC 2.3.1.16, 3-cis-dodecenoyl-CoA, feeds into fatty acid oxidation (after conversion to a trans isomer) and thus the two reactions provide the direct link between oleate β-oxidation and fatty acid oxidation that had previously been lacking in the EcoCyc database.
One spontaneous reaction (trans-aconitate ↔ cis-aconitate; EC 5.3.3.7), representing formation of the DEM trans-aconitate from the citric acid cycle intermediate cis-aconitate [11], was also added to EcoCyc as a result of our DEM analysis.
DEMs that are not physiologically relevant
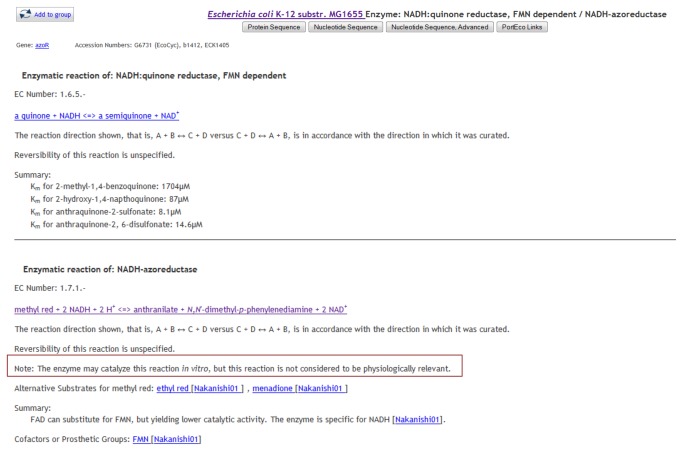
A large number of DEMs were associated with reactions that are not part of the normal physiology of E. coli K-12 or are of uncertain physiological relevance (Table 3). These reactions are properties of the purified enzyme in vitro that probably do not occur in vivo. Some of these reactions represent standard laboratory assays used to test for enzyme activity. For example, the compound 1-chloro-2,4-dinitrobenzene (CDNB) is used to assay glutathione S-transferase activity (EC 2.5.1.18) in vitro but CDNB is not membrane permeable [12] and thus is highly unlikely to be present within an E. coli cell. In many cases an enzyme catalysing a reaction containing non-physiological DEMS is also associated with a reaction that has physiological relevance to E. coli. For example, D-cysteine desulfhydrase (encoded by dcyD) is a cysteine-specific carbon-sulfur lyase with a role in the utilization of D-cysteine as a source of sulfur and is associated in EcoCyc with the physiologically relevant reaction EC4.4.1.15. However, the purified enzyme is also an effective 3-chloro-D-alanine dehydrochlorinase (EC 4.5.1.2) in vitro although it does not contribute to detoxification in vivo [13]. Association of DcyD with EC 4.5.1.2 creates two non-pathway DEMs in EcoCyc. In other cases, the non-pathway DEMs and their specific reactions are associated with enzymes whose true physiological role is unknown. E. coli proteins encoded by ygeX, solA and hyuA are examples of enzymes with associated reactions in EcoCyc, whose physiological role has not been characterised. One enzyme (AzoR) which had been characterised as an azoreductase in vitro was reannotated after a literature search revealed its physiologically relevant role to be an NADH: quinone reductase with a role in the protection against thiol stress [14].
Table 3. DEMs that are non-physiological or of uncertain physiological relevance in EcoCyc.
| Metabolites that are non-physiological | EcoCyc reaction and associated enzyme (if known) |
|---|---|
| 2-deoxy-D-glucose 6-phosphate; 2-deoxy-D-glucose | 2-deoxy-D-glucose 6-phosphate + H2O ↔ phosphate + 2-deoxy-D-glucose(YniC) |
| (R)-pantolactone; 2-dehydropantolactone | (R)-pantolactone + NADP+ ↔ 2-dehydropantolactone + NADPH + H+ |
| 1-chloro-2,4-dinitrobenzene (CDNB); 2,4-dinitrophenyl-S-glutathione | 1-chloro-2,4-dinitrobenzene + glutathione ↔ 2,4-dinitrophenyl-S-glutathione + chloride + H+ (YfcF, GstA) |
| 3-chloro-D-alanine; S-carboxymethyl-D-cysteine; thioglycolate | 3-chloro-D-alanine + thioglycolate ↔ S-carboxymethyl-D-cysteine + chloride + H+ (DcyD) |
| hydroxypropionaldehyde; 1,3-propanediol | 3-hydroxypropionaldehyde + NADPH + H+ = 1,3-propanediol + NADP+(YqhD) |
| 2,3-diaminopropionate | 2,3-diaminopropanoate + H2O <=> 2 ammonia + pyruvate + H+ (YgeX) |
| Cr3+; Cr6+ | 2 NAD(P)H + Cr6+ + oxygen → 2NAD(P)+ + Cr3 + hydrogen peroxide (YieF) |
| methyl red; N,N'-dimethyl-p-phenylenediamine | methyl red + 2NADH + 2H+ = anthranilate + N,N'-dimethyl-p-phenylenediamine + 2NAD+ (AzoR) |
| N-methyltryptophan | N-methyltryptophan + oxygen + H2O → L-tryptophan + hydrogen peroxide + formaldehyde (SolA) |
| phenylhydantoin | phenylhydantoin + H2O ↔ corresponding carbamoyl amino acid (HyuA) |
| pyrazinamide; pyrazinoate | pyrazinamide + H2O = pyrazinoate + ammonia + H+ (PncA) |
| Metabolites of uncertain physiological significance | EcoCyc reaction and associated enzyme (if known) |
| 3,4-dihydroxyphenylacetate; 3,4-dihydroxyphenylacetyl-CoA | 3,4-dihydroxyphenylacetyl-CoA + H2O <=> 3,4-dihydroxyphenylacetate + coenzyme A + H+ (PaaI) |
| quercetin; 2-protocatechuoylphloroglucinolcarboxylate | quercetin + oxygen → 2-protocatechuoylphloroglucinolcarboxylate + carbon monoxide (YhhW) |
| 4-(2-aminophenyl)-2,4-dioxobutanoate; kynurenine | 2-oxoglutarate + kynurenine = L-glutamate + 4-(2-aminophenyl)-2,4-dioxobutanoate(AspC) |
| 4-nitrobenzaldehyde; 4-nitrobenzyl alcohol | 4-nitrobenzaldehyde + NADPH + H+ = 4-nitrobenzyl alcohol + NADP+(DkgB) |
| benzaldehyde; L-threo-3-phenylserine | L-threo-3-phenylserine = benzaldehyde + glycine(LtaE) |
| GMP-lysine; N-alpha-acetyl lysine methyl ester | GMP-N-ε-(N-α-acetyl lysine methyl ester) 5'-phosphoramidate + H2O → GMP + N- α-acetyl lysine methyl ester (HinT) |
| N-ethylmaleimide; N-ethylsuccinimide | N-ethylmaleimide + 2H+ = N-ethylsuccinimide (NemA) |
| methyl-1,4-benzoquinol; methyl-1,4-benzoquinone | methyl-1,4-benzoquinone + NADPH + 3H+ = methyl-1,4-benzoquinol + NADP+(YtfG) |
| nigerose | nigerose + H2O = 2α-D-glucose(YgjK) |
| 5,6,7,8-tetrahydropteridine; 6,7-dihydropteridine | NAD(P)+ + 5,6,7,8-tetrahydropteridine ↔ NAD(P)H + 6,7-dihydropteridine + H+ (NfsB, Hmp) |
The identification of non-physiological DEMs has resulted in improvements to the Pathway Tools software, which now enables reactions to be tagged as non-physiological in order to clarify their role (Figure 3). In addition, the software program for identifying DEMs in EcoCyc was modified to exclude reactions that are tagged as non-physiological ensuring that the compounds involved in these reactions will not be returned by the DEM finder. Compounds of uncertain physiological relevance will continue to be returned by the DEM finder in EcoCyc.
Figure 3. Reactions that are not part of the normal physiology of E. coli K-12 MG1655 can now be identified as such to EcoCyc users.
The two reactions associated with AzoR (NADH: quinone reductase, FMN dependent / NADH-azoreductase) as represented in EcoCyc are shown below. The top reaction is the physiologically relevant reaction catalysed by AzoR, the bottom reaction is the non-physiological reaction catalysed by the enzyme in vitro.
A small number of compounds identified as DEMs were deleted from EcoCyc. In several cases, enzyme-catalysed reactions had been added to EcoCyc based on putative functional assignments for E. coli open reading frames reported in the literature [15]. For example, the uncharacterised YnbA protein was annotated in EcoCyc as a diacylglycerol cholinephosphotransferase, catalysing the reaction CDP-choline + 1,2-diacylglycerol <=> CMP + a phosphatidylcholine (E.C 2.7.8.2). This reaction produces phosphatidylcholine, which is a major constituent of eukaryotic membrane phospholipids but is present in only about 10% of all bacterial species and is not present in E. coli [16]. The reaction was removed from EcoCyc, but information regarding the initial bioinformatic prediction was retained in the text summary associated with the YnbA protein. Similarly, the annotation of E. coli acs as an hydroxycinnamate-CoA ligase (E.C 6.2.1.12) was removed, as no evidence for the presence of the common plant metabolites 4-coumarate and 4-coumaroyl-CoA in E. coli could be uncovered.
DEMs remaining in the EcoCyc database
The physiologically relevant DEMs remaining in the EcoCyc database are listed in Table 4, and likely represent deficiencies in our knowledge of E. coli metabolism. 9 of these compounds lack reactions accounting for their production within the network while a further 17 compounds lack reactions that would account for their consumption. 5 compounds (sulfinoalanine, curcumin, L-rhamnonate, quinate and urate) are possible nutrient sources in E. coli K-12. Sulfinoalanine, quinate and urate are included on Biolog phenotype assay plates [17] and growth observations from studies using this system have been collated on the EcoCyc website (see EcoCyc website command Search → Growth Media) while L-rhamnose and curcumin utilization have been documented in the literature. The EcoCyc reactions containing the DEMs and the relevant enzymes (Table 4) have all been experimentally characterised in E. coli K-12; literature references are available on the EcoCyc website under the enzyme of interest.
Table 4. Dead end metabolites remaining in EcoCyc.
| Dead end metabolite | EcoCyc reaction | Enzyme |
|---|---|---|
| (E)-3-(methoxycarbonyl)pent-2-enedioate | trans-aconitate + S-adenosyl-L-methionine → (E)-3-(methoxycarbonyl)pent-2-enedioate + S-adenosyl-L-homocysteine | trans-aconitate methyltransferase (tam) |
| 2-aminobutyrate | an aminated amine donor + 2-oxobutanoate → 2-aminobutyrate + a deaminated amine donor | valine-pyruvate aminotransferase (avtA) |
| 2-aminomalonate-semialdehyde | L-serine + NADP+ → 2-aminomalonate-semialdehyde + NADPH + 2H+ | 3-hydroxy acid dehydrogenase (ydfG) |
| 2-deoxygluconate; 3-dehydro-2-deoxy-D-gluconate | NAD+ + 2-deoxygluconate → NADH + 3-dehydro-2-deoxy-D-gluconate + H+ | 2-deoxy-D-gluconate 3-dehydrogenase (kduD) |
| 3-methylcrotonyl-CoA; isovaleryl-CoA | isovaleryl-CoA + an oxidized electron-transfer flavoprotein → 3-methylcrotonyl-CoA + a reduced electron-transfer flavoprotein | isovaleryl-CoA dehydrogenase (aidB) |
| 3-sulfinoalanine | 3-sulfinoalanine + H2O → L-alanine + sulfite + H+ | L-cysteine desulfurase (sufS) / cysteine sulfinate desulfinase (csdA) |
| 3-α,12-α-dihydroxy-7-oxo-5-β-cholanate | cholate + NAD+ → 3α,12α-dihydroxy-7-oxo-5β-cholan-24-oate + NADH + H+ | 7-α-hydroxysteroid dehydrogenase (hdhA) |
| 4-hydroxy-2-oxoglutarate | 4-hydroxy-2-oxoglutarate ← glyoxylate + pyruvate | multifunctional 2-keto-3-deoxygluconate 6-phosphate aldolase (eda) |
| aminoacetaldehyde | taurine + 2-oxoglutarate + oxygen → aminoacetaldehyde + sulfite + succinate + CO2 + H+ | taurine dioxygenase (tauD) |
| curcumin | curcumin + NADPH + H+ → dihydrocurcumin + NADP+ | NADPH-dependent curcumin/dihydrocurcumin reductase (curA) |
| tetrahydrocurcumin | dihydrocurcumin + NADPH + H+ → tetrahydrocurcumin + NADP+ | NADPH-dependent curcumin/dihydrocurcumin reductase (curA) |
| diacetyl | (S)-2-acetolactate + an oxidized electron acceptor + H+ → diacetyl + CO2 + a reduced electron acceptor acetoin + NADP+ → diacetyl + NADPH + H+ | yohF (predicted oxidoreductase) |
| ethyl-(2R)-methyl-(3S)-hydroxybutanoate; ethyl-2-methylacetoacetate | ethyl-(2R)-methyl-(3S)-hydroxybutanoate + NADP+ → ethyl-2-methylacetoacetate + NADPH + H+ | β-keto ester reductase (dkgA) |
| GDP-α-D-glucose | GDP-α-D-glucose + H2O → β-D-glucose + GDP + H+ | GDP-mannose mannosyl hydrolase (gmm) |
| hydroxylamine | pyruvate + hydroxylamine → pyruvic oxime + H2O hydroxylamine + a reduced electron acceptor → ammonia + an oxidized electron acceptor + H2O | hybrid-cluster protein (hcp) |
| linear dimeric GMP | cyclic di-3',5'-guanylate + H2O → linear dimeric GMP + H+ | cyclic di-GMP phosphodiesterase |
| L-rhamnonate | L-rhamnonate → 2-keto-3-deoxy-L-rhamnonate + H2O | L-rhamnonate dehydratase (yfaW) |
| nicotinamide mononucleotide (reduced) (NMNH) | NADH + H2O → NMNH + AMP + 2H+ | NADH pyrophosphatase (nudC) |
| oxamate | oxamate + carbamoyl-phosphate ← oxalurate + phosphate | - |
| quinate | NAD(P)+ + L-quinate → NAD(P)H + 3-dehydroquinate + H+ | quinate dehydrogenase (ydiB) |
| S-adenosyl-4-methylthio-2-oxobutanoate | S-adenosyl-L-methionine + 7-keto-8-aminopelargonate → S-adenosyl-4-methylthio-2-oxobutanoate + 7,8-diaminopelargonate | 7,8-diaminopelargonic acid synthase (bioA) |
| tetrahydromonopterin | tetrahydromonapterin + NADP+ ← 7,8-dihydromonapterin + NADPH + H+ | dihydromonapterin reductase (folM) |
| urate | xanthine + NAD+ + H2O → urate + NADH + H+ | xanthine dehydrogenase (xdhABC) |
For a number of DEMs the literature record provides some hint as to possible solutions for resolution. For example, it has recently been suggested that the dead end metabolite oxamate may be further degraded by a pathway that begins with the action of an oxamate:CoA ligase encoded by the gene fdrA [18]. Similarly, it has been suggested that the DEM S-adenosyl-4-methylthio-2-oxobutanoate - a by-product of biotin biosynthesis - could decompose non-enzymatically [19]. Several of the remaining DEMs are already the focus of current research efforts. Linear dimeric GMP is the product of the degradation of the signaling molecule cyclic di-GMP (E.C.3.1.4.52), and five enzymes from E. coli K-12 are annotated with this activity. Early research showing that the extremely slow degradation of linear dimeric GMP to GMP in E. coli K-12 was unlikely to be relevant in vivo [20] has led to the more recent suggestion that linear dimeric GMP may itself have signaling capacity which contributes additional complexity to cellular signaling [21]. Likewise, current research into the E. coli AidB protein (annotated in EcoCyc as an isovaleryl-CoA dehydrogenase - E.C.1.3.8.4 – and associated with the DEMs isovaleryl-CoA and methylcrotonyl-CoA) is uncovering a role in the protection from alkylating agents [22].
The DEMs remaining at the completion of this study were also queried within the latest version of the Palsson group’s model of E. coli K-12 metabolism – known as iJO1366 [23]. Six metabolites are also DEMs in iJO1366. (E) -3-(methoxycarbonyl) pent-2-enedioate, aminoacetaldehyde, oxamate, S-adenosyl-4-methylthio-2-oxobutanoate and tetrahydromonapterin are metabolites with producing reactions but no consuming reactions (called root no consumption gaps in iJO1366) while sulfinoalanine has a consuming reaction but no producing reaction (root no production gap). The EcoCyc DEMs quinate and urate are not DEMs in iJO1366 which includes orphan reactions (enzymatic activity believed to be present in an organism but without any known coding genes) for uricase activity and quinate transport. The EcoCyc metabolic network has neither of these reactions because we do not yet feel there is compelling evidence that these activities occur in E. coli K-12. In iJO1366 the reaction producing 2-aminomalonate-semialdehyde is specified as reversible whereas in EcoCyc the reversibility is unspecified, although the text summary in EcoCyc notes that kinetic analysis [24] suggests the reaction would proceed in the reductive direction (that is, towards the production of 2-aminomalonate-semialdehyde). Finally, neither linear dimeric GMP nor NMNH are present in iJO1366 due to differences in the funtional annotation of dosP (a cyclic-di-GMP phosphodiesterase in EcoCyc and a 3’ 5’ cyclic nucleotide phosphodiesterase in iJO1366) and nudC (an NADH diphosphatase with NAD+ as an alternative substrate in EcoCyc and an NAD+ diphosphatase in iJO1366).
Discussion
The DEM finder tool is able to identify dead end metabolites in the biochemical network of E. coli K-12 as represented in the EcoCyc database. Using this program, we identified 127 DEMs within the database, analysed their context and undertook a literature search to see if any could be further resolved. The model organism E. coli K-12 is an intensively studied bacterium, much of its biochemical network has been experimentally elucidated, and many of the biochemical pathways represented in EcoCyc are complete – this is reflected in the considerably smaller number of DEMs derived from within pathways (32 compounds from 932 reactions, a rate of .035 DEM per reaction) compared to those derived from isolated reactions (95 compounds from 393 reactions, a rate of .24 DEM per reaction). Our analyses resulted in the direct resolution of the dead-end status of just under half these metabolites.
38 new transport reactions were added to the EcoCyc database. Over half of these could not be assigned to a specific transport protein. In the majority of cases where a transporter was identified, the transport capability reflected an expanded substrate range for a previously characterised protein rather than the assignment of new function to a transporter of unknown substrate specificity. The identification of compounds that are imported into E. coli K-12 but are lacking known transport proteins (Table 2) may be of interest to researchers in this field. For example, E. coli K-12 can use phenylethylamine as a sole carbon and energy source [25] and a pathway for the degradation of this compound has been characterised (http://ecocyc.org/ECOLI/NEW-IMAGE?type=PATHWAY&object=PWY-6071). A periplasmic amine oxidase (encoded by tynA) converts phenylethylamine to phenylacetaldehyde which is the substrate of a cytoplasmic dehydrogenase (encoded by feaB) but an inner membrane transport system for phenylacetaldehyde has not been characterised. Transport proteins specific for aromatic compounds such as hydroxyphenylpropionic acid and 3-phenylpropionate and for aromatic amino acids have been characterised in E. coli K-12, and perhaps one of these also transports phenylacetaldehyde. The existence of an uncharacterised open reading frame - ydbH – predicted to contain beta-barrel structure [26] and located just upstream of the genes encoding proteins involved in phenylethylamine degradation is also noted.
Several compounds with unknown transport systems are substrates of scavenging reactions. E. coli can salvage the compounds 4-methyl-5-(β-hydroxyethyl)thiazole (THZ) and hydroxymethylpyrimidine (HMP) for use in thiamine synthesis (http://metacyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-6897). A predicted thiamine transporter encoded by the genes tbpA, thiP and thiQ exists in E. coli K-12 but has not been functionally characterised and thus its substrate range is unknown. Similarly, biotin-d-sulfoxide can be used as an alternative source of biotin by E. coli mutant strains that are unable to synthesize biotin [27]. Active transport of biotin is thought to occur in E. coli but no transporter has been identified [28,29].
Our analysis identified reactions within the EcoCyc database that are not physiologically relevant; these reactions and compounds are not part of normal E. coli metabolism, however their inclusion in EcoCyc provides useful data regarding enzyme specificity and kinetics. Again, the status of E. coli K-12 as a model organism is a contributing factor to the large number of non-physiological compounds that are included in the EcoCyc database. Many of its enzymes have been purified and characterised in vitro, sometimes using standard assays that are not relevant in vivo or in other cases focusing on enzyme activity and compounds that may be of industrial importance. Our DEM analysis indicated a clear need to better identify these reactions in EcoCyc and to modify the software program that is used to identify DEMs in order to exclude these reactions from future analyses.
DEMs have the potential to affect metabolic modelling, for instance DEM-driven corrections to a metabolic database will improve the accuracy of flux-balance analysis (FBA) models [30] derived from that database (e.g., the flux-balance analysis metabolic model derived from EcoCyc [3]). In FBA models, the fluxes of reactions that produce a metabolite must be balanced by the fluxes of reactions that consume that metabolite. Thus, reactions involving DEMs can never carry flux in FBA models, which decreases model fidelity since under some growth conditions these reactions do carry flux in living cells. For example, the addition of the transport reaction for 1-deoxy-D-xylulose allows the metabolic model to now correctly predict growth of E. coli under that substrate, and correction of a DEM in the EcoCyc pathway for adenosylcobalamin (coenzyme B12) salvage will allow that pathway to carry flux.
In theory, DEMs reflect areas where our knowledge of metabolism is lacking and thus may be a useful tool for identifying potential research targets. Achieving this goal relies on accurate identification of physiologically relevant DEMs and our analysis of the output of the DEM finder in EcoCyc has enabled us to improve on the ability to do that for E. coli K-12. The improvements and insights we have gained by analysing the DEMs within EcoCyc are likely to prove useful when applied to Pathway/Genome Databases of other organisms. Manual curation of the EcoCyc database is an ongoing process and thus the list of dead end metabolites is not static. As new enzyme functions are characterised and biochemical pathways elucidated, the output of the dead end finder will vary. Evaluation of the DEMs in the database will thus be an ongoing task and an important check on the quality and overall coherence of the information in EcoCyc.
Methods
The computational definition of DEMs used by the EcoCyc DEM finder is given below. The definition references a specified compartment, including the reactions and transporters present in that compartment, which for the purpose of this study was always the cytoplasm. Substrates in EcoCyc reactions can be specific compounds or they can be compound classes. The term “C” refers to a specific compound while “a parent of C” refers to a compound class to which compound C belongs. For example, the specific compound “cytosine” belongs to the compound class “a pyrimidine base”.
A compound C is a DEM if
-
1
C or a parent of C is only produced by small-molecule metabolic (SMM) reactions in Compartment, and C or a parent of C is not transported out of Compartment, OR
-
2
C or a parent of C is only consumed by SMM reactions in Compartment, and C or a parent of C is not transported into Compartment
The DEM finder was also instructed to ignore compounds that are recorded in EcoCyc as enzyme cofactors, since we would expect synthesized cofactors to be used by the cell but not necessarily consumed by metabolic reactions.
Compounds returned by the DEM finder were initially analysed within the context of the EcoCyc database in order to locate the reactions (and associated enzymes) producing or consuming them and to review the literature that led to their inclusion in the database. Comprehensive literature searches were then undertaken to see if further information relating to the metabolism or transport of the compound of interest could be uncovered. Specific searches of E. coli-related literature were done using the text mining system, Textpresso [31]. In Textpresso for E. coli (see EcoCyc website command Search → Search Full-Text Articles) a user can search, via specific keywords and/or categories, a literature database that currently contains just over 30,000 full text articles and 7000 abstracts. More general searches were also undertaken using publicly available internet search engines.
Supporting Information
Transport reactions representing the import of a metabolite added to EcoCyc.
(DOCX)
Transport reactions representing the export of a metabolite added to EcoCyc.
(DOCX)
Funding Statement
Work on this project was supported by National Institute of General Medical Sciences of the National Institutes of Health [U24GM077678]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Green ML, Karp PD (2004) A Bayesian method for identifying missing enzymes in predicted metabolic pathway databases. BMC Bioinformatics 5: 76. doi:10.1186/1471-2105-5-76. PubMed: 15189570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karp PD (2004) Call for an enzyme genomics initiative. Genome Biol 5: 401. doi:10.1186/gb-2004-5-8-401. PubMed: 15287973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keseler IM, Mackie A, Peralta-Gil M, Santos-Zavaleta A, Gama-Castro S et al. (2013) EcoCyc: fusing model organism databases with systems biology. Nucleic Acids Res 41: D605-D612. doi:10.1093/nar/gks1027. PubMed: 23143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karp PD, Keseler IM, Shearer A, Latendresse M, Krummenacker M et al. (2007) Multidimensional annotation of the Escherichia coli K-12 genome. Nucleic Acids Res 35: 7577-7590. doi:10.1093/nar/gkm740. PubMed: 17940092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thanassi DG, Cheng LW, Nikaido H (1997) Active efflux of bile salts by Escherichia coli. J Bacteriol 179: 2512-2518. PubMed: 9098046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris DR, Koffron KL (1967) Urea production and putrescine biosynthesis by Escherichia coli. J Bacteriol 94: 1516-1519. PubMed: 4862195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pauli G, Overath P (1972) ato Operon: a highly inducible system for acetoacetate and butyrate degradation in Escherichia coli. Eur J Biochem 29: 553-562. doi:10.1111/j.1432-1033.1972.tb02021.x. PubMed: 4563344. [DOI] [PubMed] [Google Scholar]
- 8. Lawrence JG, Roth JR (1995) The cobalamin (coenzyme B12) biosynthetic genes of Escherichia coli. J Bacteriol 177: 6371-6380. PubMed: 7592411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zayas CL, Escalante-Semerena JC (2007) Reassessment of the late steps of coenzyme B12 synthesis in Salmonella enterica: evidence that dephosphorylation of adenosylcobalamin-5'-phosphate by the CobC phosphatase is the last step of the pathway. J Bacteriol 189: 2210-2218. doi:10.1128/JB.01665-06. PubMed: 17209023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ren Y, Aguirre J, Ntamack AG, Chu C, Schulz H (2004) An alternative pathway of oleate beta-oxidation in Escherichia coli involving the hydrolysis of a dead end intermediate by a thioesterase. J Biol Chem 279: 11042-11050. doi:10.1074/jbc.M310032200. PubMed: 14707139. [DOI] [PubMed] [Google Scholar]
- 11. Cai H, Clarke S (1999) A novel methyltransferase catalyzes the methyl esterification of trans-aconitate in Escherichia coli. J Biol Chem 274: 13470-13479. doi:10.1074/jbc.274.19.13470. PubMed: 10224113. [DOI] [PubMed] [Google Scholar]
- 12. Iizuka M, Inoue Y, Murata K, Kimura A (1989) Purification and some properties of glutathione S-transferase from Escherichia coli B. J Bacteriol 171: 6039-6042. PubMed: 2553668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagasawa T, Ishii T, Yamada H (1988) Physiological comparison of D-cysteine desulfhydrase of Escherichia coli with 3-chloro-D-alanine dehydrochlorinase of Pseudomonas putida CR 1-1. Arch Microbiol 149: 413-416. doi:10.1007/BF00425580. PubMed: 3132906. [DOI] [PubMed] [Google Scholar]
- 14. Liu G, Zhou J, Fu QS, Wang J (2009) The Escherichia coli azoreductase AzoR Is involved in resistance to thiol-specific stress caused by electrophilic quinones. J Bacteriol 191: 6394-6400. doi:10.1128/JB.00552-09. PubMed: 19666717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed JL, Vo TD, Schilling CH, Palsson BO (2003) An expanded genome-scale model of Escherichia coli K-12 (iJR904 GSM/GPR). Genome Biol 4: R54. doi:10.1186/gb-2003-4-9-r54. PubMed: 12952533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aktas M, Wessel M, Hacker S, Klüsener S, Gleichenhagen J et al. (2010) Phosphatidylcholine biosynthesis and its significance in bacteria interacting with eukaryotic cells. Eur J Cell Biol 89: 888-894. doi:10.1016/j.ejcb.2010.06.013. PubMed: 20656373. [DOI] [PubMed] [Google Scholar]
- 17. Bochner BR, Gadzinski P, Panomitros E (2001) Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res 11: 1246-1255. doi:10.1101/gr.186501. PubMed: 11435407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith AA, Belda E, Viari A, Medigue C, Vallenet D (2012) The CanOE strategy: integrating genomic and metabolic contexts across multiple prokaryote genomes to find candidate genes for orphan enzymes. PLOS Comput Biol 8: e1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoner GL, Eisenberg MA (1975) Purification and properties of 7, 8-diaminopelargonic acid aminotransferase. J Biol Chem 250: 4029-4036. PubMed: 1092681. [PubMed] [Google Scholar]
- 20. Schmidt AJ, Ryjenkov DA, Gomelsky M (2005) The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol 187: 4774-4781. doi:10.1128/JB.187.14.4774-4781.2005. PubMed: 15995192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jenal U, Malone J (2006) Mechanisms of cyclic-di-GMP signaling in bacteria. Annu Rev Genet 40: 385-407. doi:10.1146/annurev.genet.40.110405.090423. PubMed: 16895465. [DOI] [PubMed] [Google Scholar]
- 22. Rippa V, Duilio A, di Pasquale P, Amoresano A, Landini P et al. (2011) Preferential DNA damage prevention by the E. coli AidB gene: A new mechanism for the protection of specific genes. DNA Repair (Amst) 10: 934-941. doi:10.1016/j.dnarep.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orth JD, Conrad TM, Na J, Lerman JA, Nam H et al. (2011) A comprehensive genome-scale reconstruction of Escherichia coli metabolism--2011. Mol Syst Biol 7: 535 PubMed: 21988831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fujisawa H, Nagata S, Misono H (2003) Characterization of short-chain dehydrogenase/reductase homologues of Escherichia coli (YdfG) and Saccharomyces cerevisiae (YMR226C). Biochim Biophys Acta 1645: 89-94. doi:10.1016/S1570-9639(02)00533-2. PubMed: 12535615. [DOI] [PubMed] [Google Scholar]
- 25. Parrott S, Jones S, Cooper RA (1987) 2-Phenylethylamine catabolism by Escherichia coli K12. J Gen Microbiol 133: 347-351. PubMed: 3309152. [DOI] [PubMed] [Google Scholar]
- 26. Zhai Y, Saier MH Jr. (2002) The beta-barrel finder (BBF) program, allowing identification of outer membrane beta-barrel proteins encoded within prokaryotic genomes. Protein Sci 11: 2196-2207. PubMed: 12192075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pierson DE, Campbell A (1990) Cloning and nucleotide sequence of bisC, the structural gene for biotin sulfoxide reductase in Escherichia coli. J Bacteriol 172: 2194-2198. PubMed: 2180922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hebbeln P, Rodionov DA, Alfandega A, Eitinger T (2007) Biotin uptake in prokaryotes by solute transporters with an optional ATP-binding cassette-containing module. Proc Natl Acad Sci U S A 104: 2909-2914. doi:10.1073/pnas.0609905104. PubMed: 17301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prakash O, Eisenberg MA (1974) Active transport of biotin in Escherichia coli K-12. J Bacteriol 120: 785-791. PubMed: 4616949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orth JD, Thiele I, Palsson BO (2010) What is flux balance analysis? Nat Biotechnol 28: 245-248. doi:10.1038/nbt.1614. PubMed: 20212490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Müller HM, Kenny EE, Sternberg PW (2004) Textpresso: an ontology-based information retrieval and extraction system for biological literature. PLOS Biol 2: e309. doi:10.1371/journal.pbio.0020309. PubMed: 15383839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wiame E, Delpierre G, Collard F, Van Schaftingen E (2002) Identification of a pathway for the utilization of the Amadori product fructoselysine in Escherichia coli. J Biol Chem 277: 42523-42529. doi:10.1074/jbc.M200863200. PubMed: 12147680. [DOI] [PubMed] [Google Scholar]
- 33. Wiame E, Van Schaftingen E (2004) Fructoselysine 3-epimerase, an enzyme involved in the metabolism of the unusual Amadori compound psicoselysine in Escherichia coli. Biochem J 378: 1047-1052. doi:10.1042/BJ20031527. PubMed: 14641112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao X, Miller JR, Jiang Y, Marletta MA, Cronan JE (2003) Assembly of the covalent linkage between lipoic acid and its cognate enzymes. Chem Biol 10: 1293-1302. doi:10.1016/j.chembiol.2003.11.016. PubMed: 14700636. [DOI] [PubMed] [Google Scholar]
- 35. Burlingame R, Chapman PJ (1983) Catabolism of phenylpropionic acid and its 3-hydroxy derivative by Escherichia coli. J Bacteriol 155: 113-121. PubMed: 6345502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris TW, Reed KE, Cronan JE Jr. (1995) Lipoic acid metabolism in Escherichia coli: the lplA and lipB genes define redundant pathways for ligation of lipoyl groups to apoprotein. J Bacteriol 177: 1-10. PubMed: 8002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bradbeer C, Kenley JS, Di Masi DR, Leighton M (1978) Transport of vitamin B12 in Escherichia coli. Corrinoid specificities of the periplasmic B12-binding protein and of energy-dependent B12 transport. J Biol Chem 253: 1347-1352. PubMed: 342526. [PubMed] [Google Scholar]
- 38. Kenley JS, Leighton M, Bradbeer C (1978) Transport of vitamin B12 in Escherichia coli. Corrinoid specificity of the outer membrane receptor. J Biol Chem 253: 1341-1346. PubMed: 342525. [PubMed] [Google Scholar]
- 39. Díaz E, Ferrández A, Prieto MA, García JL (2001) Biodegradation of aromatic compounds by Escherichia coli. Microbiol Mol Biol Rev 65: 523-569. doi:10.1128/MMBR.65.4.523-569.2001. PubMed: 11729263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xavier KB, Bassler BL (2005) Regulation of uptake and processing of the quorum-sensing autoinducer AI-2 in Escherichia coli. J Bacteriol 187: 238-248. doi:10.1128/JB.187.1.238-248.2005. PubMed: 15601708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamasaki H, Sanemori H, Yamada K, Kawasaki T (1973) Hydroxyethylthiazole uptake in Escherichia coli: general properties and relationship between uptake and thiamine biosynthesis. J Bacteriol 116: 1280-1286. PubMed: 4584810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bellion E, Lash TD (1983) Transport of 2-methyl-4-amino-5-hydroxymethylpyrimidine by Salmonella typhimurium. Biochim Biophys Acta 735: 337-340. doi:10.1016/0005-2736(83)90147-5. PubMed: 6357279. [DOI] [PubMed] [Google Scholar]
- 43. Solomon LR, Breitman TR (1971) Pseudouridine kinase of escherichia coli: a new enzyme. Biochem Biophys Res Commun 44: 299-304. doi:10.1016/0006-291X(71)90599-7. PubMed: 4334133. [DOI] [PubMed] [Google Scholar]
- 44. Blackwell CM, Scarlett FA, Turner JM (1976) Ethanolamine catabolism by bacteria, including Escherichia coli. Biochem Soc Trans 4: 495-497. PubMed: 793895. [DOI] [PubMed] [Google Scholar]
- 45. Jones PW, Turner JM (1984) Interrelationships between the enzymes of ethanolamine metabolism in Escherichia coli. J Gen Microbiol 130: 299-308. PubMed: 6374020. [DOI] [PubMed] [Google Scholar]
- 46. Cusa E, Obradors N, Baldomà L, Badía J, Aguilar J (1999) Genetic analysis of a chromosomal region containing genes required for assimilation of allantoin nitrogen and linked glyoxylate metabolism in Escherichia coli. J Bacteriol 181: 7479-7484. PubMed: 10601204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reed JL, Patel TR, Chen KH, Joyce AR, Applebee MK et al. (2006) Systems approach to refining genome annotation. Proc Natl Acad Sci U S A 103: 17480-17484. doi:10.1073/pnas.0603364103. PubMed: 17088549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Putra SR, Lois LM, Campos N, Boronat A, Rohmer M (1998) Incorporation of [2,3-13C2]- and [2,4-13C2]-D-1-deoxyxylulose into ubiquinone of Escherichia coli via the mevalonate-independent pathway for isoprenoid biosynthesis. Tetrahedron Lett 39: 23-26. doi:10.1016/S0040-4039(97)10458-0. [Google Scholar]
- 49. Dykhuizen D (1973) Genetic analysis of the system that reduces biotin-d-sulfoxide in Escherichia coli. J Bacteriol 115: 662-667. PubMed: 4579877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang K, Wang M, Metcalf WW (2009) Uptake of glycerol-2-phosphate via the ugp-encoded transporter in Escherichia coli K-12. J Bacteriol 191: 4667-4670. doi:10.1128/JB.00235-09. PubMed: 19429609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sauer E, Merdanovic M, Mortimer AP, Bringmann G, Reidl J (2004) PnuC and the utilization of the nicotinamide riboside analog 3-aminopyridine in Haemophilus influenzae. Antimicrob Agents Chemother 48: 4532-4541. doi:10.1128/AAC.48.12.4532-4541.2004. PubMed: 15561822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thanbichler M, Neuhierl B, Böck A (1999) S-methylmethionine metabolism in Escherichia coli. J Bacteriol 181: 662-665. PubMed: 9882684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lindblow-Kull C, Kull FJ, Shrift A (1985) Single transporter for sulfate, selenate, and selenite in Escherichia coli K-12. J Bacteriol 163: 1267-1269. PubMed: 3897189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lacourciere GM (2002) Selenium is mobilized in vivo from free selenocysteine and is incorporated specifically into formate dehydrogenase H and tRNA nucleosides. J Bacteriol 184: 1940-1946. doi:10.1128/JB.184.7.1940-1946.2002. PubMed: 11889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guth A, Engel R, Tropp BE (1980) Uptake of glycerol 3-phosphate and some of its analogs by the hexose phosphate transport system of Escherichia coli. J Bacteriol 143: 538-539. PubMed: 6995450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang CT, Engel R, Tropp BE (1977) L-Glyceraldehude 3-phosphate, a bactericidal agent. Antimicrob Agents Chemother 11: 147-153. doi:10.1128/AAC.11.1.147. PubMed: 319747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Iqbal S, Parker G, Davidson H, Moslehi-Rahmani E, Robson RL (2004) Reversible phase variation in the phnE gene, which is required for phosphonate metabolism in Escherichia coli K-12. J Bacteriol 186: 6118-6123. doi:10.1128/JB.186.18.6118-6123.2004. PubMed: 15342581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rizk SS, Cuneo MJ, Hellinga HW (2006) Identification of cognate ligands for the Escherichia coli phnD protein product and engineering of a reagentless fluorescent biosensor for phosphonates. Protein Sci 15: 1745-1751. doi:10.1110/ps.062135206. PubMed: 16751609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nie L, Ren Y, Schulz H (2008) Identification and characterization of Escherichia coli thioesterase III that functions in fatty acid beta-oxidation. Biochemistry 47: 7744-7751. doi:10.1021/bi800595f. PubMed: 18576672. [DOI] [PubMed] [Google Scholar]
- 60. Loh KD, Gyaneshwar P, Markenscoff Papadimitriou E, Fong R, Kim KS et al. (2006) A previously undescribed pathway for pyrimidine catabolism. Proc Natl Acad Sci U S A 103: 5114-5119. doi:10.1073/pnas.0600521103. PubMed: 16540542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hughes JA (2006) In vivo hydrolysis of S-adenosyl-L-methionine in Escherichia coli increases export of 5-methylthioribose. Can J Microbiol 52: 599-602. doi:10.1139/w06-008. PubMed: 16788729. [DOI] [PubMed] [Google Scholar]
- 62. Toews ML, Adler J (1979) Methanol formation in vivo from methylated chemotaxis proteins in Escherichia coli. J Biol Chem 254: 1761-1764. PubMed: 370114. [PubMed] [Google Scholar]
- 63. Herzberg M, Kaye IK, Peti W, Wood TK (2006) YdgG (TqsA) controls biofilm formation in Escherichia coli K-12 through autoinducer 2 transport. J Bacteriol 188: 587-598. doi:10.1128/JB.188.2.587-598.2006. PubMed: 16385049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boos W, Ferenci T, Shuman HA (1981) Formation and excretion of acetylmaltose after accumulation of maltose in Escherichia coli. J Bacteriol 146: 725-732. PubMed: 7012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schaefler S (1967) Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol 93: 254-263.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transport reactions representing the import of a metabolite added to EcoCyc.
(DOCX)
Transport reactions representing the export of a metabolite added to EcoCyc.
(DOCX)