Abstract
Campylobacter concisus is an oral bacterium. A number of studies detected a significantly higher prevalence of C . concisus in the intestinal tract of patients with inflammatory bowel disease (IBD) as compared to controls. The prevalence of zonula occluden toxin (zot) gene, which encodes a toxin known to increase intestinal permeability, in oral C . concisus strains is unknown. Increased intestinal permeability is a feature of IBD. A total of 56 oral C . concisus strains isolated from 19 patients with IBD and 20 controls were examined (some individuals were colonized with multiple strains). A filtration method was used for isolation of C . concisus from saliva samples. SDS-PAGE was used to define strains. PCR was used to amplify zot from C . concisus strains. Positive PCR products were sequenced and the nucleotides and amino acids were compared. Of the 56 oral C . concisus strains examined, 17 strains (30.4%) were positive for zot. The prevalence of zot-positive oral C . concisus strains was 54.5% in patients with active IBD, which was not significantly different from that in healthy controls (40%). Polymorphisms of C . concisus zot were revealed. zot 808T , zot 350-351AC and zot Multiple were detected only in patients with IBD, but not in healthy controls. Both zot 808T and zot Multiple alleles resulted in substitution of valine at position 270, which occurred in 36.4% of patients with active IBD but not in healthy controls (P = 0.011). Furthermore, the prevalence of multiple oral C . concisus strains in patients with active IBD was significantly higher than that in healthy controls (P = 0.013). This is the first study reporting the prevalence of zot in human oral C . concisus strains and the polymorphisms of C . concisus zot gene. The data suggest that the possible role of C . concisus strains containing specific polymorphic forms of zot gene in human IBD should be investigated.
Introduction
Increased Campylobacter concisus intestinal colonization has been associated with inflammatory bowel disease (IBD). IBD is a chronic inflammatory disease of the gastrointestinal tract with unknown aetiology. Crohn’s disease (CD) and ulcerative colitis (UC) are the two major clinical forms of IBD [1]. A number of studies have detected a significantly higher prevalence of C . concisus in fecal samples and intestinal biopsies collected from patients with IBD as compared to controls [2,3,4,5]. However, a recent study by Hansen et al. found a similar prevalence of C . concisus in intestinal biopsies collected from children with IBD and controls [6]. The difference in biopsy collection site (inflamed area vs macroscopically non-inflamed area at the edge of the inflamed site) may have contributed to the inconsistent results between the study from Hansen et al. and the other study in pediatric population [2].
C . concisus is a flagellated Gram-negative bacterium that requires H2-enriched microaerobic conditions for growth [7]. Humans are the main natural host of C . concisus , with the oral cavity being the primary colonization site [8,9]. We previously isolated C . concisus from 75% (44/59) of saliva samples from healthy individuals using a filtration method and detected C . concisus in 97% (57/59) of these samples using PCR [8]. In addition to the human oral cavity, C . concisus was detected by PCR in 12.5% of saliva sample of domestic cats [10]. C . concisus was also isolated from 10% (18/185) of chicken meat and 3% of beef meat (6/186) samples [11].
Using multilocus analysis of housekeeping genes, we showed C . concisus colonizing the human oral cavity to be a source of C . concisus that colonizes the human intestinal tract in some patients with IBD [12]. We also found that some patients with IBD are colonized with multiple C . concisus strains in the oral cavity and intestinal tract [12].
The mechanisms by which C . concisus may contribute to enteric diseases have been investigated. Both oral and enteric C . concisus strains have been shown to induce the production of IL-8 in HT-29 cells [13,14,15]. Some oral C . concisus strains isolated from patients with IBD were found to be invasive to Caco2 cells and more effective in upregulating surface expression of Toll like receptor 4 in HT-29 cells [12,15]. Furthermore, increased intestinal epithelial apoptosis and permeability by some C . concisus strains have been previously reported [13,14,16]. These data suggest that some oral C . concisus strains may have the potential to cause enteric diseases in individuals whose intestinal environment is suitable for C . concisus colonization.
Zonula occluden toxin (zot) gene has been detected in some C . concisus strains isolated from diarrheal and non-diarrheal stool samples [14]. The zot gene was first detected in Vibrio cholerae, the pathogen that causes cholera [17]. The zot gene in V. cholerae is part of a chromosomally integrated filamentous phage genome [18]. The protein encoded by
V. cholerae zot gene has been shown to increase intestinal permeability by affecting the tight junctions through actin reorganization [19]. Furthermore V. cholerae zot gene is related to induction of mild to moderate diarrhea [20]. Evidence suggests that increased intestinal permeability is a possible etiologic factor of IBD [21,22,23,24].
Currently, the prevalence of zot gene in human oral C . concisus strains is unknown. In this study, we have examined the prevalence of the zot gene in multiple C . concisus strains isolated from saliva samples of patients with IBD and controls. Furthermore, the polymorphisms of C . concisus zot gene were examined.
Materials and Methods
Ethics statement
Written informed consent was obtained from the adult subjects and the guardians on behalf of the minors/children involved in this study. Ethics approval for this study was granted by the Ethics Committees of the University of New South Wales and the South East Sydney Local Health District, Australia (HREC 09237/SESIAHS 09/078, HREC08335/SESIAHS (CHN) 07/48) and HREC 06233/SESAHS (ES) 06/164).
Clinical information of patients with IBD and controls
Nineteen patients with IBD (13 CD and six UC) and 20 healthy controls recruited from Sydney area in Australia were included in this study. The patients were aged 5-73 years old and the controls were 4-67 years old. The age of patients with IBD (mean ± SD, 33 ± 5.7) and the healthy controls (26 ± 4.7) was not statistically different. Eleven patients had active disease (ten new cases and one relapsed case) and eight patients were in remission (Table 1). Patients with active disease were not receiving any treatment for IBD at the time of saliva sample collection and had not received antibiotics in the three month prior to sample collection. The relapsed patient with active disease (patient No. 2) had antibiotic treatment (metronidazole + ciprofloxacin) two years earlier, but not at the time of relapse. Five of the eight patients in remission had previously received antibiotics and all patients in remission were receiving immunosuppressive therapies at the time of sample collection. The details of the drugs received by patients in remission were listed in Table 2. The healthy controls had not received antibiotics in the three month prior to sample collection.
Table 1. Clinical information of patients with IBD included in this study.
| Patient ID | Age(y)/sex | Diagnosis | Disease activity |
|---|---|---|---|
| Patient No. 1 | 5/M | CD | Remission |
| Patient No. 2 | 19/M | CD | Relapse, active |
| Patient No. 3 | 23/M | UC | New case, active |
| Patient No. 4 | 16/F | CD | Remission |
| Patient No. 5 | 13/M | CD | Remission |
| Patient No. 6 | 13/M | CD | Remission |
| Patient No. 7 | 65/M | UC | New case, active |
| Patient No. 8 | 16/M | CD | Remission |
| Patient No. 9 | 17/M | CD | Remission |
| Patient No. 10 | 19/M | CD | New case, active |
| Patient No. 11 | 33/M | CD | New case, active |
| Patient No. 12 | 55/F | CD | New case, active |
| Patient No. 13 | 22/M | UC | New case, active |
| Patient No. 14 | 34/M | UC | New case, active |
| Patient No. 15 | 39/M | UC | New case, active |
| Patient No. 16 | 67/M | UC | New case, active |
| Patient No. 17 | 73/M | CD | New case, active |
| Patient No. 18 | 14/F | CD | Remission |
| Patient No. 19 | 9/M | CD | Remission |
Disease activity refers to the disease activity at the time of saliva sample collection.
Table 2. Treatment details of patients in remission.
| Patient ID | Diagnosis | Previous antibiotics | Current Treatment |
|---|---|---|---|
| Patient No. 1 | CD | No | Mesalazine |
| Azathioprine | |||
| Iron supplements | |||
| Patient No. 4 | CD | No | Azathioprine |
| Patient No. 5 | CD | Metronidazole | Mesalazine |
| Ciprofloxacin | Azathioprine | ||
| 10 months prior | |||
| Patient No. 6 | CD | No | Mesalazine |
| Patient No. 8 | CD | Metronidazole | Sulfasalazine |
| 2 months prior | |||
| Patient No. 9 | CD | Cotrimoxazole | Cotrimoxazole |
| 3 months prior | Tacrolimus | ||
| Calcium | |||
| Fish oil | |||
| Patient No. 18 | CD | Metronidazole | Azathioprine |
| Ciprofloxacin | |||
| 1 year prior | |||
| Patient No. 19 | CD | Metronidazole | Mesalazine |
| 3 months prior | Azathioprine | ||
| Iron supplements |
Current treatment is the treatment that the patients were receiving at the time of sample collection.
Isolation of multiple C . concisus strains from saliva samples
Isolation of C . concisus from saliva samples was carried out using a previously described filtration method [8]. For each saliva sample, 12 putative C . concisus isolates were collected. The putative C . concisus isolates were subjected to a previously described C . concisus PCR to confirm the identity of C . concisus and then subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) for whole cell protein profile analysis to define the strains as previously described [12]. Isolates with identical SDS-PAGE pattern were defined as the same strain.
Detection of zot gene in C . concisus strains and sequencing the amplified zot gene
C . concisus DNA was prepared using the Puregene DNA Extraction kit (Gentra, Minneapolis, USA) following the manufacturer’s instructions. The forward primer FCCC13826_2075 (5’-TGCAAACCCTTTGTGATGAA-3’) has been previously described [14]. The reverse primer Ccon_zotR_257 (5’-TCGGTCCTCCACGATCTG-3’) was designed in this study using Primer 3 plus software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi/) based on the genome sequence of C . concisus strain 13826 (Accession No. CP000792.1). PCR product size is 1055 base pairs (bp).
To amplify the zot gene, hot start PCR reactions were performed in a 25 µl reaction mixture containing PCR buffer, 200 nM of deoxynucleotide triphosphate, 2.5 mM MgCl2, 5.5 U of Taq polymerase (Fisher Biotech, Subiaco, Australia), 10 pmol of each primer and 10 ng of bacterial DNA extracted from each C . concisus strains. The thermal cycling conditions consist of denaturing at 96°C for 5 minutes, followed by 35 cycles of 95 °C for 10 seconds, annealing at 55–58 °C for 10 seconds and 72 °C for 45 seconds. All positive PCR products were sequenced from both ends using BigDyeTM reagents version 3.2 (Applied Biosystems, Foster City, CA) and analyzed on an ABI Capillary DNA Sequencer ABI3730 (Applied Biosystems). DNA extracted from C . concisus strain 13826 as the positive control and PCR mixture without bacterial DNA was used as the negative control.
Analysis of zot gene sequences
Molecular evolutionary genetics analysis (MEGA) software version 5.0 was used for zot gene sequence alignment [25]. PHYLogeny Inference Packge (PHYLIP) was used to generate the neighbour-joining dendrogram of the zot gene amplified from different C . concisus strains [26]. Translation of nucleotide sequences was performed using Expasy translate tool [27]. Alignment of amino acid sequences was conducted using Clustalw multiple alignment tool [28].
GenBank sequence submission
The sequences of the zot gene amplified from C . concisus strains were submitted to Genbank.
Statistical analysis
Fisher’s exact test (two tailed) was used to compare the prevalence of multiple C . concisus strains in patients with IBD and controls and the prevalence of zot gene in C . concisus strains isolated from patients with IBD and control. Unpaired t test was used to compare the age of patients and controls. Statistical analysis was performed using GraphPad Prism 6 software (San Diego, CA).
Results
Isolation of multiple C . concisus strains from saliva samples of patients with IBD and controls
Of the 420 putative C . concisus isolates collected from saliva samples of 16 patients with IBD and 19 controls, 401 isolates were confirmed to be C . concisus by the C . concisus specific PCR. These 401 C . concisus isolates were shown to represent 50 different strains, with each strain showing a distinct whole cell protein profile on SDS-PAGE. These 50 oral C . concisus strains and the six oral C . concisus strains previously isolated from three patients with IBD and a healthy control were included in this study [12].
More than one oral C . concisus strains were isolated from each of nine patients with IBD and three controls (Table 3). The prevalence of multiple oral C . concisus strains in patients with active IBD was 63.6% (7/11), which was significantly higher than that in healthy controls 15% (3/20) in healthy controls (P = 0.013). Of the eight patients in remission, patients without antibiotics treatment had a prevalence of multiple oral C . concisus strains of 66.7% (2/3), none of the five patients who had antibiotics treatment were colonized with multiple oral C . concicus strains (0/5) (Tables 2 and 3).
Table 3. Individuals who were colonized with multiple oral C . concisus strains.
| Individual | C . concisus strains |
|---|---|
| Patient No. 1# | P1CDO2, P1CDO3 |
| Patient No. 2@ | P2CDO3, P2CDO4 |
| Patient No. 4# | P4CDO-S1, P4CDO-S2, P4CDO-S3 |
| Patient No. 10@ | P10CDO-S1, P10CDO-S2 |
| Patient No. 12@ | P12CDO-S1, P12CDO-S2 |
| Patient No. 13@ | P13UCO-S1, P13UCO-S2, P13UCO-S3 |
| Patient No. 14@ | P14UCO-S1, P14UCO-S2, P14UCO-S3 |
| Patient No. 15@ | P15UCO-S1, P15UC-SO2, P15UC-SO3 |
| Patient No. 16@ | P16UCO-S1, P16UCO-S2 |
| Healthy No. 8 | H8O-S1, H8O-S2 |
| Healthy No. 9 | H9O-S1, H9O-S2, H9O-S3 |
| Healthy No. 11 | H11O-S1, H11O-S2 |
@ IBD patients with active disease. # IBD patients in remission. Nine patients with IBD and three healthy controls were colonized with multiple oral C . concisus strains. The remaining 10 patients with IBD and 17 healthy controls were colonized with a single C . concisus strain in the oral cavity. The strains were defined by C . concisus specific PCR and SDS-PAGE patterns. The prevalence of multiple oral C . concisus strains was 63.6% (7/11) in patients with active IBD, which was significantly higher than that in healthy controls 15% (3/20) (P = 0.013). In patients in remission, the prevalence of multiple oral C . concisus strains was 66.7% (2/3) in patients without antibiotics treatment and was zero (0/5) in patients received antibiotics treatment.
The prevalence of zot gene in oral C . concisus strains isolated from patients with IBD and controls
Of the total 56 oral C . concisus strains examined in this study, 17 strains (30.4%) were positive for zot gene. In individuals colonized with multiple oral C . concisus strains, usually only one strain was positive for zot gene except for patient No. 2 and patient No. 13. The two oral C . concisus strains isolated from patient No. 2 were positive for zot gene and two of the three strains isolated from patient No. 13 were positive for zot gene.
The prevalence of zot-positive C . concisus strains in the oral cavity of healthy controls was 40% (8/20). The prevalence of zot-positive C . concisus strains in healthy children was 50% (4/8), which was not statistically different from that in the adult healthy individuals (33%, 4/12). The prevalence of zot-positive C . concisus strains in the oral cavity of patients with active IBD was 54.5% (6/11), which was not significantly higher than that in healthy controls (40%, 8/20). Of the eight patients in remission, patients without antibiotics treatment for IBD had a prevalence of zot-positive oral C . concisus strains of 33.3% (1/3), none of the five patients who had antibiotics treatment were colonized with zot-positive oral C . concicus strains (0/5) (Figure 1).
Figure 1. Prevalence of zot-positive C . concisus strains in the oral cavity of patients with IBD and controls.

The prevalence of zot-positive C . concisus strains in active IBD and healthy controls was not statistically different. zot-positive C . concisus strains were not detected in patients in remission who received antibiotics treatment for IBD.
Comparison of the sequences of zot gene amplified from oral C . concisus strains isolated from patients with IBD and controls
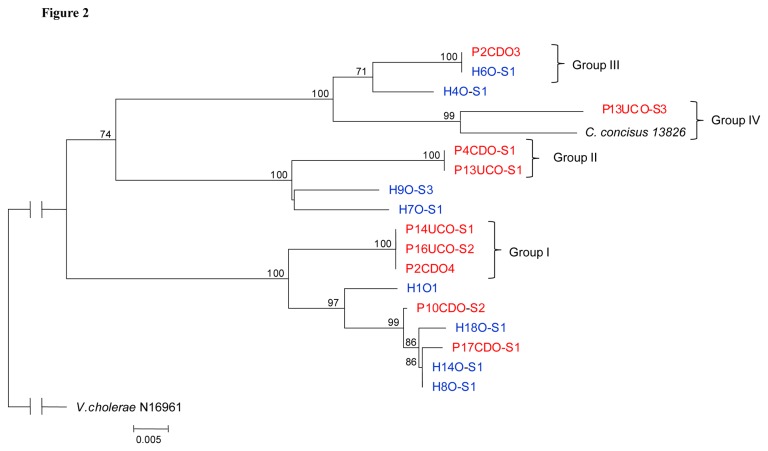
The neighbour- joining dendrogram generated based on the DNA sequences of the sequences of zot gene amplified from the 17 zot-positive C . concisus strains (670 bp) is shown in Figure 2. The whole genome sequenced C . concisus 13826 was also included in the analysis. Three groups of C . concisus zot with identical zot gene sequences (Group I, Group II and Group III) were revealed. C . concisus strains in Group I and Group II were from patients with IBD only. In Group III, one strain was from a patient with IBD and one strain was from a healthy control.
Figure 2. Neighbour-joining dendrogram based on the zot sequences of 17 oral C . concisus strains.
Strains from patients with IBD are coloured red. Strains from healthy controls are coloured blue. Groups I, II and III are identical. Vibrio cholerae is used as an outgroup. C . concisus strain 13826 is the whole genome sequenced strain (Accession No. CP000792.1). P2CDO3 and P2CDO4 were from patient No. 2. P13UCO-S1 and P13UCO-S3 were from patient No. 13. The remaining strains were from individual patients and controls.
In addition to the three groups of C . concisus with identical zot gene sequences, another group of zot (Group IV), which contained C . concisus strains from individuals with enteric diseases, was identified. Group IV consisted of one oral strain from a patient with UC and one enteric strain from a patient with bloody diarrhea ( C . concisus strain 13826), the similarity of these two zot gene sequences was 95%.
Genetic polymorphisms of the zot gene in different oral C . concisus strains
The nucleotide sequences of the zot gene from the 17 zot-positive C . concisus strains were aligned to examine the nucleotide polymorphisms (Table 4). Group I C . concisus zot had a unique nucleotide polymorphism at position 808bp (zot 808T) and Group II C . concisus zot had unique nucleotide polymorphisms at positions 350bp and 351bp (zot 350-351AC) ( C . concisus strain 13826 nucleotide position). Group III C . concisus zot did not show any unique polymorphisms. Group IV C . concisus zot had nucleotide polymorphisms at ten positions including 747 bp, 769 bp, 786-789 bp, 805-806 bp, 809 bp and 816 bp (zot Multiple). The zot genes amplified from oral C . concisus strains isolated from healthy controls did not show any unique nucleotide polymorphisms (Table 4).
Table 4. Nucleotide polymorphisms of Group I, Group II and Group IV C . concisus zot.
| 350-351a | 747 | 769 | 786-789 | 805-806 | 808-809 | 816 | |
|---|---|---|---|---|---|---|---|
| C . concisus strains | |||||||
| Group I | CA | T | A | GCCT | AG | TT | T |
| Group II | AC | T | A | GCCT | AG | GT | T |
| Group III | CG | T | A | GCCT | AG | GT | T |
| Group IV | CG | A | G | TATA | GA | GC | A |
| H9O-S3 | CG | T | A | GCCT | AG | GT | T |
| H7O-S1 | CG | T | A | GCCT | AG | GT | T |
| P17CDO-S1 | CA | T | A | ACCT | AG | GT | T |
| H1O1 | CA | T | A | ACCT | AG | GT | T |
| H8O-S1 | CA | T | A | ACCT | AG | GT | T |
| H14O-S1 | CA | T | A | ACCT | AG | GT | T |
| P10CDO-S1 | CA | T | A | GCCT | AG | GT | T |
| H18O-S1 | CA | T | A | GCCT | AG | GT | T |
| H4O-S1 | CG | T | A | GCCT | AG | GT | T |
Genetic polymorphisms are in bold and underlined. a The numbers indicate the nucleotide positions of C . concisus zot gene. Group I C . concisus zot had a unique nucleotide polymorphism at position 808 bp (zot 808T). Group II C . concisus zot had unique nucleotide polymorphisms at positions 350 bp and 351 bp (zot 350-351AC). Group IV C . concisus zot had nucleotide polymorphisms at ten positions including 747 bp, 769 bp, 786-789 bp, 805-806 bp, 809 bp and 816 bp (zot Multiple). The zot genes amplified from the remaining
C . concisus strains did not show any unique nucleotide polymorphisms.
Amino acid polymorphisms of different alleles of C . concisus zot gene
The amino acids encoded by the zot gene of the 17 zot-positive C . concisus strains were aligned to examine whether the nucleotide polymorphisms of the above three zot alleles (zot 808T, zot 350-351AC and zot Multiple) have resulted in changes of amino acids. zot 808T resulted in the change of valine to leucine at position 270 ( C . concisus strain 13826 amino acid position). zot 350-351AC resulted in the change of threonine to asparagine at position 117. zot Multiple allele resulted in unique amino acids at seven positions, including aspartic acid to glutamic acid at position 249, threonine to alanine at position 257, having an asparagine at position 262 which was different from all other C . concisus strains, proline to isoleucine at position 263, serine to aspartic acid at position 269, valine to alanine at position 270 and asparagine to lysine at position 272. Both zot 808Tand zot Multiple resulted in substitution of valine at position 270 (Table 5).
Table 5. Amino acid polymorphisms encoded by Group I, Group II and Group IV C . concisus zot.
| 117 a | 249 | 257 | 262 | 263 | 269 | 270 | 272 | |
|---|---|---|---|---|---|---|---|---|
| C . concisus strains | ||||||||
| Group I | T | D | T | K | P | S | L | N |
| Group II | N | D | T | E | P | S | V | N |
| Group III | T | D | T | E | P | S | V | N |
| Group IV | T | E | A | N | I | D | A | K |
| H9O-S3 | T | D | T | E | P | S | V | N |
| H7O-S1 | T | D | T | E | P | S | V | N |
| P17CDO-S1 | T | D | T | T | P | S | V | N |
| H1O1 | T | D | T | T | P | S | V | N |
| H8O-S1 | T | D | T | T | P | S | V | N |
| H14O-S1 | T | D | T | T | P | S | V | N |
| P10CDO-S1 | T | D | T | K | P | S | V | N |
| H18O-S1 | T | D | T | K | P | S | V | N |
| H4O-S1 | T | D | T | E | P | S | V | N |
Amino acid polymorphisms are in bold and underlined. a The numbers indicate the amino acid positions of C . concisus Zot protein. zot 808T resulted in the change of valine to leucine at position 270. zot 350-351AC resulted in the change of threonine to asparagine at position 117. zot Multiple allele resulted in unique amino acids at seven positions.
The association between C . concisus zot polymorphisms and IBD
zot 808T , zot 350-351AC and zot Multiple alleles were detected only in patients with IBD, not in healthy controls (Table 6). The prevalence of zot 808T allele in patients with active IBD (27.2%, 3/11) was significantly different from that in healthy controls (0/20) (P = 0.037) (Table 6). zot 350-351AC allele was detected in 9% of patient with active IBD (1/11) and none of the healthy controls. zot Multiple was detected in 9% of patient with active IBD (1/11) and none of the healthy controls.
Table 6. Prevalence of zot 808T, zot 350-351AC and zot Multiple in patients with IBD and controls.
| Active IBD n=11 | Healthy controls n=20 | IBD in remission without antibiotics treatment n=3 | IBD in remission received antibiotics treatment n=5 | |
|---|---|---|---|---|
| zot808T | 3/11 (27.2%)*@ | 0/20 | 0/3 | 0/5 |
| zot350-351AC | 1/11 (9%) | 0/20 | 1/3 (33%) | 0/5 |
| zotMultiple | 1/11 (9%)@ | 0/20 | 0/3 | 0/5 |
zot 808T , zot 350-351AC and zot Multiple were detected only in patients with IBD, not in healthy controls. * The prevalence of zot 808T allele in patients with active IBD (27.2%) was significantly higher compared to healthy controls (0/20) (P = 0.037). @ Polymorphisms of zot that have resulted in substitution of valine at position 270, which was detected only in patients with active IBD (36.4%, 4/11) but not in healthy controls (0/20) (P = 0.011).
Of the eight patients in remission, one of the three patients who received no antibiotics treatment for IBD was colonized with a zot 350-351AC strain. zot 808T , zot 350-351AC and zot Multiple alleles were not detected in any of the five patients who received antibiotics treatment for IBD (Table 6).
At the amino acid level, substitution of valine at position 270 occurred in 36.4% of patients with active IBD (4/11) and none of the healthy controls (0/20) (P = 0.011). Substitution of valine at position 270 was not detected in C . concisus strains isolated from patients in remission (Tables 5 and 6).
Sequence accession numbers
The accession numbers for the sequences of zot gene submitted to GenBank were KC935342-KC935358.
Discussion
In this study, we investigated the presence of zot gene in multiple C . concisus strains isolated from saliva samples of patients with IBD and healthy controls and the polymorphisms of C . concisus zot gene.
The toxin encoded by V. cholerae zot gene affects the tight junctions through activation of proteinase activated receptor 2, which results in an increased intestinal epithelial permeability [29]. Patients with IBD have an increased intestinal permeability in comparison to healthy controls [21,22,23,24]. The increase in intestinal permeability has been found to precede the development of inflammatory changes, suggesting that increased intestinal permeability is a possible etiologic factor of IBD [21,22,23]. Zeissig et al. found that there was a change in expression and distribution of tight junction proteins in patients with active CD as compared to controls [30]. In this study we detected three C . concisus zot alleles (zot 808T, zot 350-351AC and zot Multiple alleles) only in patients with IBD, but not in healthy controls. Interestingly, both zot 808T and zot Multiple alleles resulted in substitution of valine at position 270 and substitution of valine at position 270 was detected only in patients with active IBD. These data suggest that specific mutations have occurred in the zot gene of C . concisus strains isolated from patients with active IBD. It is possible that these mutations have changed the function of C . concisus Zot toxin or increased the interaction between the C . concisus Zot toxin with human intestinal epithelial cells, which contributes to the initiation of IBD. These speculations remain to be investigated in future studies.
In addition to its association with IBD, C . concisus has been frequently isolated from diarrheal stool samples [31,32,33,34,35]. Recently, Nielsen et al. found that gastroenteritis caused by C . concisus was milder than that caused by Campylobacter jejuni and Campylobacter coli [34]. The level of fecal calprotecin, a marker of inflammation, in individuals colonized with C . concisus was similar to the level seen in patients with viral gastroenteritis [35]. In addition to causing an increase in intestinal epithelial permeability, toxin encoded by Vibrio cholerae zot has also been associated with mild to moderate diarrhea in humans [20]. It is possible that some polymorphic forms of C . concisus zot may play a role in human diarrheal disease, which requires further investigation.
We performed bioinformatics analysis of the whole genome sequenced C . concisus strain 13826 and found that the zot gene is a component of a prophage genome (unpublished data). Our finding in this study that approximately 30% of C . concisus strains colonizing the human oral cavity were positive for zot suggests that these C . concisus strains were infected with bacterial phage. Of the multiple C . concisus strains isolated from individual patients with IBD and controls, usually only one C . concisus strain was positive for zot (except for patient No. 2 and patient No. 13). This suggests that zot-positive C . concisus strains may have specific receptors that predispose them to bacterial phage infection.
A further interesting finding from this study was that a significantly higher number of IBD patients with active disease were colonized with multiple oral C . concisus strains in comparison to healthy controls (63.6% vs 15%). It is possible that patients with active IBD have acquired more virulent C . concisus strains from other sources in addition to the existing C . concisus strains that colonize the oral cavity. An alternative explanation is that C . concisus strains colonizing the oral cavity of patients with IBD may have gone through genetic changes and resulted in new C . concisus strains.
In our study, we have included eight IBD patients who were in remission. Data from this group of patients suggest that antibiotics used in treatment of IBD have effects on oral
C . concisus colonization. This view is supported by the findings that none of the five patients who received antibiotics treatment for IBD were colonized with multiple oral C . concisus strains and that there were no zot-positive C . concisus strains isolated from these patients. However, whether the antibiotics used in these five patients with IBD have eradicated zot-positive C . concisus strains from the oral cavity or greatly inhibited the zot-positiveC. concisus strains is unknown.
The prevalence of zot-positive C . concisus in the human intestinal tract has not been systematically investigated. A study from Kalischuk et al. detected zot in 80% (4/5) of C . concisus strains isolated from stool samples of healthy controls and in 22% (2/9) of C . concisus strains isolated from stool samples of patients with diarrhea [14]. However, this study did not isolate multiple C . concisus isolates from individual patients or controls and detected only five C . concisus strains from healthy controls. Given this, it is difficult to draw a convincing conclusion regarding the prevalence of zot-positive intestinal C . concisus strains based on this study. Future studies examining larger numbers of enteric samples are required to determine the prevalence of zot-positive C . concisus strains in the intestinal tract of healthy individuals and patients with enteric diseases including IBD as well as the polymorphisms of zot gene in enteric C . concisus strains.
In addition to IBD, increased gut permeability has been seen in a number of other chronic human diseases such as diabetes [36]. Our findings that about 30% of oral C . concisus strains in the human oral cavity were positive for zot and C . concisus zot has polymorphic forms suggest that future studies should be conducted to investigate whether C . concisus zot contributes to the initiation of these chronic human diseases.
In summary, this is the first study examining the prevalence of zot-positive C . concisus strains in the human oral cavity and the polymorphisms of C . concisus zot gene. We found that about 30% of oral C . concisus strains in the human oral cavity were positive for zot and that C . concisus zot gene has polymorphic forms. zot 808T, zot 350-351AC and zot Multiple alleles were detected only in patients with IBD, but not in the healthy controls. Both zot 808T and zot Multiple alleles resulted in substitution of valine at position 270, which occurred only in patients with active IBD. Furthermore, a significantly higher number of patients with active IBD were colonized with multiple oral C . concisus strains as compared to healthy controls. These data suggest that future studies are required to investigate the effects of polymorphic C . concisus Zot proteins on human gastrointestinal tract permeability and their potential involvement in initiating a subgroup of human IBD. Despite the very interesting findings in this study, the sample size of patients with IBD was relatively small. Future studies examining a larger number of patients with IBD should be conducted to verify the findings of this study.
Funding Statement
This work was supported by a Faculty Research Grant of the University of New South Wales awarded to Dr LZ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347: 417-429. doi:10.1056/NEJMra020831. PubMed: 12167685. [DOI] [PubMed] [Google Scholar]
- 2. Zhang L, Man SM, Day AS, Leach ST, Lemberg DA et al. (2009) Detection and isolation of Campylobacter species other than C. jejuni from children with Crohn’s disease. J Clin Microbiol 47: 453-455. doi:10.1128/JCM.01949-08. PubMed: 19052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Man SM, Zhang L, Day AS, Leach ST, Lemberg DA et al. (2010) Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn’s disease. Inflamm Bowel Dis 16: 1008-1016. doi:10.1002/ibd.21157. PubMed: 19885905. [DOI] [PubMed] [Google Scholar]
- 4. Mukhopadhya I, Thomson JM, Hansen R, Berry SH, El-Omar EM et al. (2011) Detection of Campylobacter concisus and other Campylobacter species in colonic biopsies from adults with ulcerative colitis. PLOS ONE 6: e21490: e:21490. PubMed: 21738679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahendran V, Riordan SM, Grimm MC, Tran TA, Major J et al. (2011) Prevalence of Campylobacter species in adult Crohn’s disease and the preferential colonization sites of Campylobacter species in the human intestine. PLOS ONE 6: e25417. doi:10.1371/journal.pone.0025417. PubMed: 21966525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansen R, Berry SH, Mukhopadhya I, Thomson JM, Saunders KA et al. (2013) The microaerophilic microbiota of de-novo paediatric inflammatory bowel disease: The BISCUIT Study. PLOS ONE 8: e58825. doi:10.1371/journal.pone.0058825. PubMed: 23554935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vandamme P, Dewhirst FE, Paster BJ, On SLW (2005) Genus I. Campylobacter . In: Garrity GM, Brenner DJ, Krieg NR, Staley JT. Bergey’s Manual of Syst. J Bacteriol 2 ed. New York: Springer Verlag; pp. 1147-1160. [Google Scholar]
- 8. Zhang L, Budiman V, Day AS, Mitchell H, Lemberg DA et al. (2010) Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J Clin Microbiol 48: 2965-2967. doi:10.1128/JCM.02391-09. PubMed: 20519479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanner ACR, Badger S, Lai CH, Listgarten MA, Visconti RA et al. (1981) Wolinella gen-nov, Wolinella-succinogenes (Vibrio-succinogenes-wolin et-al) comb-nov, and description of Bacteroides-gracilis sp-nov, Wolinella-recta sp-nov, Campylobacter-concisus sp-nov, and Eikenella-corrodens from humans with periodontal-disease. Int J Syst Bacteriol 31: 432-445. doi:10.1099/00207713-31-4-432. [Google Scholar]
- 10. Petersen RF, Harrington CS, Kortegaard HE, On SLW (2007) A PCR-DGGE method for detection and identification of Campylobacter, Helicobacter, Arcobacter and related Epsilobacteria and its application to saliva samples from humans and domestic pets. J Appl Microbiol 103: 2601-2615. doi:10.1111/j.1365-2672.2007.03515.x. PubMed: 17916160. [DOI] [PubMed] [Google Scholar]
- 11. Lynch OA, Cagney C, McDowell DA, Duffy G (2011) Occurrence of fastidious Campylobacter spp. in fresh meat and poultry using an adapted cultural protocol. Int J Food Microbiol 150: 171-177. doi:10.1016/j.ijfoodmicro.2011.07.037. PubMed: 21855156. [DOI] [PubMed] [Google Scholar]
- 12. Ismail Y, Mahendran V, Octavia S, Day AS, Riordan SM et al. (2012) Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains Isolated from patients with inflammatory bowel disease. PLOS ONE 7: e38217. doi:10.1371/journal.pone.0038217. PubMed: 22666490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Man SM, Kaakoush NO, Leach ST, Nahidi L, Lu HK et al. (2010) Host attachment, invasion, and stimulation of proinflammatory cytokines by Campylobacter concisus and other non-Campylobacter jejuni Campylobacter species. J Infect Dis 202: 1855-1865. doi:10.1086/657316. PubMed: 21050118. [DOI] [PubMed] [Google Scholar]
- 14. Kalischuk LD, Inglis GD (2011) Comparative genotypic and pathogenic examination of Campylobacter concisus isolates from diarrheic and non-diarrheic humans. Bmc Microbiol 11: 53-66. doi:10.1186/1471-2180-11-53. PubMed: 21406111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ismail Y, Lee H, Riordan SM, Grimm MC, Zhang L (2013) The Effects of oral and enteric Campylobacter concisus strains on expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 Cells. PLOS ONE 8: e56888. doi:10.1371/journal.pone.0056888. PubMed: 23437263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nielsen HL, Nielsen H, Ejlertsen T, Engberg J, Günzel D et al. (2011) Oral and fecal Campylobacter concisus strains perturb barrier function by apoptosis induction in HT-29/b6 intestinal epithelial cells. PLOS ONE 6: e23858. doi:10.1371/journal.pone.0023858. PubMed: 21887334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD et al. (1991) Vibrio-cholerae produces a 2nd enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci U S A 88: 5242-5246. doi:10.1073/pnas.88.12.5242. PubMed: 2052603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waldor MK, Mekalanos JJ (1996) Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272: 1910-1914. doi:10.1126/science.272.5270.1910. PubMed: 8658163. [DOI] [PubMed] [Google Scholar]
- 19. Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper JB et al. (1995) Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest 96: 710-720. doi:10.1172/JCI118114. PubMed: 7635964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levine MM, Kaper JB, Herrington D, Losonsky G, Morris JG et al. (1988) Volunteer studies of deletion mutants of Vibrio-cholerae O1 prepared by recombinant techniques. Infect Immun 56: 161-167. PubMed: 3335402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T et al. (1986) Increased intestinal permeability in patients with Crohn’s Disease and their relatives: a possible etiologic factor. Ann Intern Med 105: 883-885. doi:10.7326/0003-4819-105-6-883. PubMed: 3777713. [DOI] [PubMed] [Google Scholar]
- 22. May GR, Sutherland LR, Meddings JB (1993) Is small-intestinal permeability really increased in relatives of patients with Crohns disease? Gastroenterology 104: 1627-1632. PubMed: 8500719. [DOI] [PubMed] [Google Scholar]
- 23. Irvine EJ, Marshall JK (2000) Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology 119: 1740-1744. doi:10.1053/gast.2000.20231. PubMed: 11113095. [DOI] [PubMed] [Google Scholar]
- 24. Welcker K, Martin A, Kölle P, Siebeck M, Gross M (2004) Increased intestinal permeability in patients with inflammatory bowel disease. Eur J Med Res 9: 456-460. PubMed: 15546811. [PubMed] [Google Scholar]
- 25. Tamura K, Peterson D, Peterson N, Stecher G, Nei M et al. (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731-2739. doi:10.1093/molbev/msr121. PubMed: 21546353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felsenstein J (1989) PHYLIP -- Phylogeny Inference Package (Version 3.2). Cladistics 5: 164-166. [Google Scholar]
- 27. Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G et al. (2012) ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res 40: W597-W603. doi:10.1093/nar/gks400. PubMed: 22661580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA et al. (2007) Clustal W and clustal X version 2.0. Bioinformatics 23: 2947-2948. doi:10.1093/bioinformatics/btm404. PubMed: 17846036. [DOI] [PubMed] [Google Scholar]
- 29. Goldblum SE, Rai U, Tripathi A, Thakar M, De Leo L et al. (2011) The active Zot domain (aa 288-293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J 25: 144-158. doi:10.1096/fj.10-158972. PubMed: 20852064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J et al. (2007) Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56: 61-72. doi:10.1136/gut.2006.094375. PubMed: 16822808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lindblom GB, Sjögren E, Hansson-Westerberg J, Kaijser B (1995) Campylobacter upsaliensis, C. sputorum sputorum and C. concisus as common causes of diarrhea in swedish children. Scand J Infect Dis 27: 187-188. doi:10.3109/00365549509019006. PubMed: 7660089. [DOI] [PubMed] [Google Scholar]
- 32. Engberg J, On SLW, Harrington CS, Gerner-Smidt P (2000) Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J Clin Microbiol 38: 286-291. PubMed: 10618103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lastovica AJ (2006) Emerging Campylobacter spp.: the tip of the iceberg. Clin Microbiol Newsl 28: 49-56. doi:10.1016/j.clinmicnews.2006.03.004. [Google Scholar]
- 34. Nielsen HL, Engberg J, Ejlertsen T, Bücker R, Nielsen H (2012) Short-term and medium-term clinical outcomes of Campylobacter concisus infection. Clin Microbiol Infect 18: E459-E465. doi:10.1111/j.1469-0691.2012.03869.x. PubMed: 22882347. [DOI] [PubMed] [Google Scholar]
- 35. Nielsen HL, Ejlertsen T, Engberg J, Nielsen H (2013) High incidence of Campylobacter concisus in gastroenteritis in North Jutland, Denmark: a population-based study. Clin Microbiol Infect 19: 445-450. doi:10.1111/j.1469-0691.2012.03852.x. PubMed: 22512739. [DOI] [PubMed] [Google Scholar]
- 36. Arrieta MC, Bistritz L, Meddings JB (2006) Alterations in intestinal permeability. Gut 55: 1512-1520. doi:10.1136/gut.2005.085373. PubMed: 16966705. [DOI] [PMC free article] [PubMed] [Google Scholar]