Abstract
Background
Use of continuous glucose monitoring (CGM) systems can improve glycemic control, but widespread adoption of CGM utilization has been limited, in part because of real and perceived problems with accuracy and reliability. This study compared accuracy and performance metrics for a new-generation CGM system with those of a previous-generation device.
Subjects and Methods
Subjects were enrolled in a 7-day, open-label, multicenter pivotal study. Sensor readings were compared with venous YSI measurements (blood glucose analyzer from YSI Inc., Yellow Springs, OH) every 15 min (±5 min) during in-clinic visits. The aggregate and individual sensor accuracy and reliability of a new CGM system, the Dexcom® (San Diego, CA) G4™ PLATINUM (DG4P), were compared with those of the previous CGM system, the Dexcom SEVEN® PLUS (DSP).
Results
Both study design and subject characteristics were similar. The aggregate mean absolute relative difference (MARD) for DG4P was 13% compared with 16% for DSP (P<0.0001), and 82% of DG4P readings were within ±20 mg/dL (for YSI ≤80 mg/dL) or 20% of YSI values (for YSI >80 mg/dL) compared with 76% for DSP (P<0.001). Ninety percent of the DG4P sensors had an individual MARD ≤20% compared with only 76% of DSP sensors (P=0.015). Half of DG4P sensors had a MARD less than 12.5% compared with 14% for the DSP sensors (P=0.028). The mean absolute difference for biochemical hypoglycemia (YSI <70 mg/dL) for DG4P was 11 mg/dL compared with 16 mg/dL for DSP (P<0.001).
Conclusions
The performance of DG4P was significantly improved compared with that of DSP, which may increase routine clinical use of CGM and improve patient outcomes.
Introduction
Continuous glucose monitoring (CGM) has evolved from an early-stage technology to an emerging standard of care for diabetes patients managed with intensive insulin therapy.1–4 A key driver of this evolution is a growing realization among patients, clinicians, and payers of the potential clinical value of CGM in motivating patients and helping them reduce their exposure to hypoglycemia and hyperglycemia.
Numerous studies have shown that use of real-time CGM can improve glycemic control in both children and adults with type 1 diabetes5–11 and in patients with type 2 diabetes who are managed with or without intensive insulin treatment.12–14 The clinical benefit of CGM, however, is directly related to the frequency of use of the technology. In many CGM studies, significant clinical improvements were seen only in those patients who regularly wore their CGM devices for approximately 6 days or more per week.6,7,9–11,15,16
Despite the demonstrated benefits of CGM, widespread adoption of this technology has been slow, and sustained use has been limited compared with its potential benefit on glycemic control. In a recent analysis of a type 1 diabetes registry in the United States, it was found that only 6% of patients surveyed used CGM as a regular component of their diabetes management.17 Although lack of reimbursement is often cited as an obstacle,18,19 cost alone does not fully explain the low percentage of CGM use. In fact, commercial insurance coverage for CGM in type 1 diabetes is now common in the United States, although it is subject to various degrees of clinician documentation of medical necessity, depending on the insurance carrier.20 Nevertheless, many patients who are started on CGM discontinue it shortly thereafter.21 Even in clinical trials, in which patients receive greater physician and nurse support than in routine clinical practice, the number of patients who discontinued the studies because of sensor-related issues or noncompliance with the protocol was significant, ranging from 5.6%7 to 56%.9
The deleterious impact of poor accuracy, reliability, and usability on sustained use of the technology was seen beginning with the earliest real-time CGM systems. A study that compared the first Food and Drug Administration (FDA)-approved real-time CGM system, the Cygnus (Redwood City, CA) GlucoWatch Biographer, with standard glucose monitoring showed that CGM use declined throughout the study with participants citing skin irritation (76%), frequent gaps in the data (56%), excessive alarms (47%), and inaccurate readings (33%) as the main reasons for declining sensor use.22 Additionally, in studies of children and adolescents, a significant number of subjects reported difficulties with CGM use and/or alarms,21,23,24 and patients often ignored their alarms.25 Moreover, inadequate sensor accuracy, compared with capillary fingerstick or laboratory reference blood glucose measurements, and poor system reliability have been noted as a hindrance to widespread adoption of this technology for current adjunctive use and future use in artificial pancreas systems.26,27 These same issues may also negatively impact clinician acceptance of CGM.28
Although current real-time CGM systems differ considerably from earlier-generation devices in terms of accuracy, reliability, and usability, they also differ from each other in terms of sensor size and geometry, duration of sensor life, and calibration requirements, as well as in accuracy and reliability. All of these factors impact patient and clinician confidence in use of the technology. Findings from a recent survey of current and previous CGM users revealed that continued use of this technology is related to trust in the accuracy and reliability of the data, the usability of the device, and patients' confidence in their ability to use the data to augment information obtained from capillary fingerstick testing.29
A common assessment metric is the aggregate mean absolute relative difference (MARD) (expressed as a percentage) between all temporally matched sensor and reference measurements. MARD is defined as the average of the absolute error between all CGM values and matched reference values; the smaller the difference, the closer the CGM reading is to the reference glucose value. CGM systems with high aggregate MARD values have larger individual measurement discrepancies between the CGM device and blood glucose determinations made with blood glucose meters or laboratory reference devices. The accuracy and reliability of real-time CGM systems, as measured by aggregate MARD, have undergone significant improvement in the relatively short period of time since the first devices were approved by the FDA. The first three systems made by different manufacturers and approved by the FDA in 2001, 2005, and 2006 had MARD values of 22.0%,30 19.7%,31 and 26.0%,32 respectively. One system approved in 2007, but not currently available in the United States, reported a MARD of 12.8%.33 However, this system required a prolonged warm-up period (10 h), and the accuracy of this system in the hypoglycemic range was relatively poor.33 More recently, second- and third-generation CGM systems approved by the FDA in 2007 and 2009 had MARD values of 16.7%34 and 15.9%,35 respectively.
The fear of hypoglycemia is widely recognized as a major barrier to achieving and sustaining optimal glycemic control.36 Accuracy of CGM devices in the hypoglycemic range is important in order for the technology to provide timely warnings of real or impending hypoglycemia, build user trust in their devices, and help users reduce their fear of hypoglycemia. Conversely, poor accuracy of CGM systems in the hypoglycemic range is detrimental to building trust in the technology and overcoming fear of hypoglycemia. In a recent study that looked at short-term use of real-time CGM, the authors attributed the study's failure to show a reduction of the fear of hypoglycemia to the CGM system's inaccuracy at low blood glucose levels.37
In addition to accuracy, patients must perceive their CGM devices to be reliable and easy to use. Studies of earlier CGM systems reported numerous technical difficulties with the devices, including lengthy warm-up periods in which data were unavailable to the patient, receiver signal interruptions (data gaps) lasting several hours or days, device errors that prevented data capture, and frequent erroneous alarms.38
A fourth-generation CGM system, the Dexcom® G4™ Platinum CGM system (DG4P), was approved by the FDA in October 2012 and may address many of the concerns seen in both previous and current CGM systems. The purpose of this study was to compare the accuracy and reliability of the new DG4P system with a previous-generation CGM system, the Dexcom SEVEN® PLUS (DSP) system.
Research Design and Methods
The DG4P system was studied in an open-label, single-arm, multicenter, pivotal study involving subjects older than 17 years of age with type 1 and type 2 diabetes who were willing and thought able to comply with the study protocol. Subjects were excluded if they had hematocrit levels outside the range of the study blood glucose meter, were pregnant or on dialysis, had a condition such as cardiovascular or cerebrovascular disease or epilepsy that would pose a risk from hypoglycemia, had significant hypoglycemia unawareness or recent severe hypoglycemia, or had a known, chronic infectious disease that could pose a risk to the study staff handling blood samples. The study protocol was reviewed by the FDA through the Investigational Device Exemption process and approved by the institutional review boards of all participating centers. All subjects provided witnessed, written informed consent prior to enrollment.
Study device
The DG4P system utilizes an advanced version of the glucose oxidase sensor technology used in previous electrochemical glucose sensor technologies. Similar to its predecessor, the DSP system, the new device consists of a 7-day transcutaneous sensor, a transmitter, and a receiver and measures glucose in the interstitial fluid at 5-min increments. The transmitter sends an electrical signal to the receiver, where it is processed into a glucose value and adjusted based on twice-daily calibration using self-monitoring of blood glucose (SMBG).
DG4P study procedures
After training, subjects inserted their own sensors into subcutaneous tissue in their abdomen. Subjects were instructed to calibrate their CGM device twice daily per labeling recommendation. All subjects used the OneTouch® Ultra® 2 meter (LifeScan, Inc., Milpitas, CA) for calibration of the CGM device and for routine blood glucose testing during the study. Subjects were asked to come to the clinic on Days 1, 4, and 7 for a 12-h period of glucose monitoring using both venous samples and capillary fingersticks for comparison. During each session, subjects had venous blood draws approximately once every 15±5 min to allow for comparison against the YSI (Yellow Springs, OH) blood glucose analyzer and SMBG values. The venous samples were arterialized with a heating pad at the venous sample catheter site. SMBG was performed twice per hour and as indicated for diabetes management. These additional SMBG measurements were not used for further calibration of the CGM device. Carbohydrate consumption, insulin dosing, and meal timing were manipulated to obtain a wide range of glucose values (from <60 mg/dL up to 400 mg/dL) per precise instructions in the protocol and under close direction and observation of the study investigator staff. Adverse event screening and sensor insertion site assessments were performed at each clinic visit and documented. Digital data from all study receivers and SMBG meters were downloaded via personal computer for analysis. Data from all CGM systems were masked to the study staff and subjects for the duration of each in-clinic session. During home use, CGM data were unmasked. Subjects were instructed to manage their glucose level as per their routine diabetes management guidelines.
Evaluation of CGM performance
The accuracy of both the GDP4 and the DSP systems was assessed using the MARD overall, the mean absolute difference (MAD) (in mg/dL) in hypoglycemia, and the clinically accurate Clarke error grid (CEG) A zone39 with reference to temporally matched measurements made with the venous samples tested on a YSI analyzer. The MARD was determined as an aggregate value from the total number of paired points compared with the reference value. The MARD was also assessed individually for each sensor in the study and plotted as a histogram distribution to permit evaluation of a sensor performance metric that may better reflect users' experience with their sensors. In addition, the system point accuracy was measured as the proportion of all sensor values that were within ±20 mg/dL of the YSI reference value (obtained during in-clinic days) for glucose levels ≤80 mg/dL and ±20% at YSI glucose levels >80 mg/dL (hereafter referred to as %20/20).
Evaluation of sensor precision and reliability
Approximately one-half of DG4P study subjects and approximately one-third of DSP study subjects wore two sensors, simultaneously, to assess precision using the percentage coefficient of variation, defined as the standard deviation (SD) of the two systems worn divided by the average of the two sensors. During home use, CGM data from one system were masked, whereas data from the other system were unmasked. The number of sensor measurements per day was evaluated relative to the maximum possible readings per day. Sensor life was also evaluated as the time from the first CGM reading after insertion to the time of sensor failure prior to the removal.
Statistical analysis
Univariate analyses were performed to obtain all descriptive statistics of evaluated variables. Goodness of fit tests were used to evaluate the distributions. The comparisons included accuracy overall, accuracy in hypoglycemic range, clinical accuracy, precision, and reliability.
Two-sample nonparametric tests were used for the comparison of continuous variables, and χ2 tests were used for the comparison of categorical variables such as the percentage of values in the CEG A zone. Histogram plots and empirical distribution plots were used to virtually assess the differences of the systems' MARDs. All comparisons were conducted at α=0.05 level of significance using two-tailed tests. Analyses were performed using SAS version 9.3 software (SAS Institute, Inc., Cary, NC).
Results
In the DG4P pivotal study conducted in 2011, 72 subjects were enrolled at four clinical centers in the United States. In the DSP pivotal study conducted in 2008, 53 subjects were enrolled at three sites in the United States. In total, 108 sensors were worn by subjects in the DG4P study compared with 67 sensors in the DSP study. Over the measurement range of 40–400 mg/dL, there were in total 13,538 matched pairs in the DG4P study compared with 1,827 matched pairs in the DSP study. Baseline characteristics of subjects in both groups were comparable. In the DG4P study, 60 (83%) subjects had type 1 diabetes, and 12 (17%) subjects had type 2 diabetes; in the DSP study, 43 (81%) subjects had type 1 diabetes, and 10 (19%) had type 2 diabetes. In the DG4P population, 44 (61%) were male, and the mean age was 42.2±14.0 years (range, 18–74 years). In the DSP population, 31 (58.5%) were male, and the mean age was 47.3±12.4 years (range, 22–71 years). In the DG4P study, subjects had a mean baseline glycated hemoglobin (A1C) level of 7.7±1.3%, with a mean body mass index of 28.7±5.8 kg/m2. In the DSP study, subjects had a mean baseline A1C level of 7.4±1.3%, with a mean body mass index of 27.9±7.7 kg/m2. Subjects in both studies were predominantly non-Hispanic white (76.3% for DG4P and 98.1% for DSP).
The DG4P system performance was significantly better than that of the DSP system in all parameters assessed, including MAD, MARD, CEG, %20/20, low glucose ranges, paired sensor coefficient variability, sensor display rate, and sensor life (Table 1). The aggregate MARD value for the DG4P system was significantly less than that for the DSP system on Days 4 and 7 (P<0.0001). Accuracy in the hypoglycemia range was also significantly better with the DG4P compared with the DSP (P≤0.001). There was greater consistency in DG4P performance as measured in the subset of 36 subjects who wore two sensors simultaneously. The coefficient of variation among these subjects was 7% on average, compared with 11% on average observed in the DSP study. Lastly, more DG4P sensors lasted 7 days compared with DSP sensors and provided significantly more CGM daily readings (P<0.001).
Table 1.
Comparisons of Performance Metrics Between the Dexcom G4 Platinum and SEVEN PLUS Systems
| Parameter | DG4P | DSP | P value |
|---|---|---|---|
| Sensors (n) | 108 | 67 | — |
| Number of samples paired with reference (YSI) | 13,538 | 1,827 | — |
| %20/20 mg/dL | 82% | 76% | <0.0001 |
| MAD (mg/dL) | 21 | 25 | <0.0001 |
| MARD | 13% | 16% | <0.0001 |
| Within biochemical hypoglycemia (YSI ≤70 mg/dL) | |||
| MAD (mg/dL) | 11 | 16 | <0.0001 |
| MARD | 18% | 27% | <0.0001 |
| Percentage of CGM values that were ≤70 mg/dL when severe hypoglycemia (YSI ≤55 mg/dL) | 88% | 73% | 0.015 |
| CEG Zone A | |||
| Day 1 | 70% | 73% | 0.0003 |
| Day 4 | 85% | 75% | <0.0001 |
| Day 7 | 83% | 68% | <0.0001 |
| Overall | 79% | 73% | <0.0001 |
| Aggregate MARD | |||
| Day 1 | 16.7% | 16.2% | 0.534 |
| Day 4 | 11.4% | 14.3% | <0.0001 |
| Day 7 | 11.9% | 17.7% | <0.0001 |
| CEG Zone A within biochemical hypoglycemia (YSI <70 mg/dL) | 70% | 53% | <0.0001 |
| Mean paired sensor coefficient of variation | 7% | 11% | <0.001 |
| Sensor daily display rate | 97% | 90% | <0.001 |
| Sensor duration (% of 7 days) | 94% | 89% | 0.278 |
CEG, Clarke error grid; CGM, continuous glucose monitoring; DG4P, Dexcom G4 Platinum; DSP, Dexcom SEVEN PLUS; MAD, mean absolute difference; MARD, mean absolute relative difference; YSI, reference value measured with the YSI blood glucose analyzer.
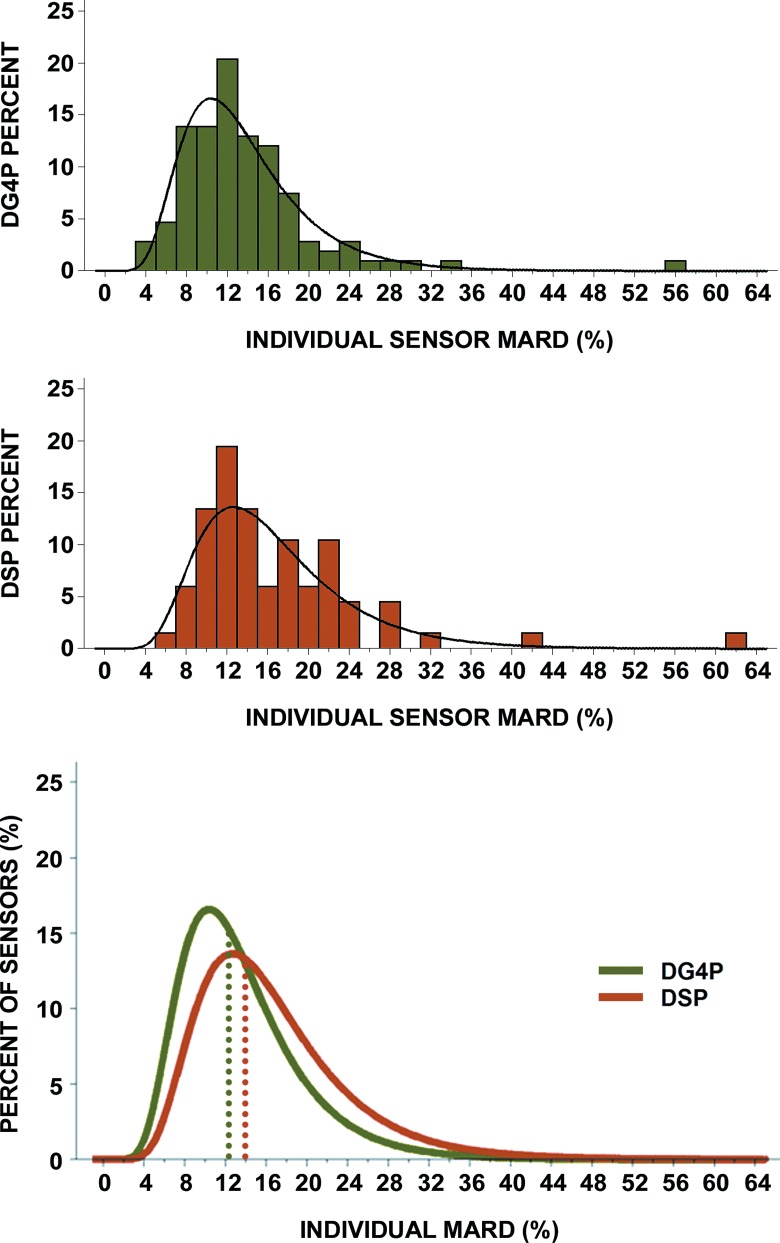
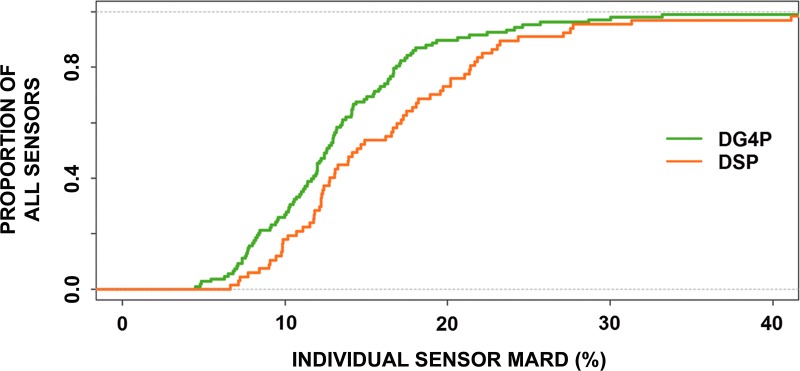
The MARD of all matched pairs (CGM reading to YSI value) for each individual sensor was evaluated and plotted as a histogram (Fig. 1) and empirical distribution (Fig. 2). The presence of outlier sensor MARD and the asymmetrical distribution of MARD values necessitate the use of logarithmic scale for proper statistical analysis. The log-normal distribution of the sensor MARD values for both the DG4P and DSP data is shown in Figure 1. The median of the individual DG4P sensor MARD values was 12.5% with an interquartile range from 9% to 16%, whereas the median of the individual DSP sensor MARD values was 14% with an interquartile range from 12% to 20%. Using a standard statistical test method, the nonparametric two-sample test, the differences between the DG4P and DSP distributions were found to be statistically significant (P=0.028). The bottom graph in Figure 1 shows the two distributions with clear separation between the median MARD for the DG4P and DSP systems. The cumulative distribution function is a method for calculating and displaying the proportion of sensors with a given MARD or less. The cumulative distribution function for the individual sensor MARDs for the DG4P and DSP systems shown in Figure 2 demonstrates a clear separation and statistically significant better performance between the DG4P and the DSP. Comparison of the two cumulative distribution function curves shows there is a higher proportion of DG4P sensors with lower MARDs than of DSP sensors. In addition, the t test for the log-transformed MARD variables also demonstrated a statistical significant smaller MARD for the DG4P sensors (P=0.003). Furthermore, 97 of the 108 (90%) DG4P sensors had a MARD less than 20% compared with 51 of the 67 (76%) DSP sensors (P=0.01).
FIG. 1.
Histograms of individual sensor mean absolute relative difference (MARD) for the Dexcom (top) G4 Platinum (DG4P) and (middle) SEVEN PLUS (DSP), with a log-normal curve superimposed on each distribution. (Bottom) The median of the values of the individual DG4P sensor MARD values was 12.5% compared with 14% for the DSP individual sensor MARD values. The interquartile range for the individual sensor MARD for the DG4P was 9–16% compared with 12–20% for the DSP. In brief, the histograms show that the individual sensor MARD distribution for the DG4P has been narrowed and shifted toward the left compared with the DSP.
FIG. 2.
The cumulative distribution functions of the individual sensor mean absolute relative difference (MARD) for the Dexcom G4 Platinum (DG4P) and SEVEN PLUS (DSP) showing the difference in individual sensor MARD between the two systems. Comparison of the two cumulative distribution function curves shows there are a higher proportion of DG4P sensors with lower MARDs than of the DSP sensors.
In the CEG analysis, the overall percentage of matched pairs in the clinically accurate CEG A zone was significantly greater for the DG4P system compared with the DSP sensor (P<0.0001) (Table 1). However, the DSP system had a small but statistically significant higher CEG A zone percentage than the DG4P on the first day (P=0.0003). After the first day, the DG4P system was more clinically accurate than the DSP system with statistically significantly higher percentages of values in the CEG A zone on Days 4 (P<0.0001) and 7 (P<0.0001). In addition, the top quartile of DG4P sensors with the lowest MARDs exhibited 95% of their readings in the CEG A zone and had a mean individual sensor MARD of 7%. The top two quartiles of DG4P sensors exhibited 90% of their readings in the CEG A zone and had a mean individual sensor MARD of 9%. The top three quartiles of DG4P sensors exhibited 86% of their readings in the CEG A zone and had a mean individual sensor MARD of 11%. The comparison of interquartile performance of DG4P and DSP is shown in Table 2.
Table 2.
Clarke Error Grid Zone A and Mean Sensor Mean Absolute Relative Difference (MARD) Comparison Between the Dexcom G4 Platinum and SEVEN PLUS Systems Based on Quartiles of MARD Values for Each Individual Sensor
| |
Sensor MARD quartiles |
|
||
|---|---|---|---|---|
| System, comparison | Top 25% | Top 50% | Top 75% | All sensors |
| DG4P | ||||
| Number of sensors | 28 | 56 | 84 | 108 |
| Average sensor MARD | 7% | 9% | 11% | 13% |
| Number of of matched pairs per quartile | 3,316 | 7,032 | 1,0191 | 13,538 |
| CEG Zone A | 96% | 90% | 86% | 80% |
| DSP | ||||
| Number of sensors | 17 | 34 | 51 | 67 |
| Average sensor MARD | 9% | 11% | 13% | 16% |
| Number of matched pairs per quartile | 471 | 957 | 1,427 | 1,827 |
| CEG Zone A | 92% | 88% | 80% | 73% |
CEG, Clarke error grid; DG4P, Dexcom G4 Platinum; DSP, Dexcom SEVEN PLUS; MARD, mean absolute relative difference.
Discussion
In this report, significant improvements in the accuracy and reliability of a new-generation CGM device, the DG4P, were demonstrated after the first day of use and sustained over the duration of sensor wear compared with a previous-generation CGM device, the DSP. Improvements were most clinically relevant in the system's accuracy within the hypoglycemia range. When the actual glucose level, as determined by the YSI analyzer value, was in marked clinical hypoglycemia (<55 mg/dL), the DG4P system detected biochemical hypoglycemia (<70 mg/dL) 88% of the time, compared with 73% of the time with DSP.
Furthermore, a significant reduction in the number of outlier sensors (those with an individual sensor MARD >20%) was observed in the clinical study reported here. There was also greater consistency in performance. Although patients will not wear two CGM systems concurrently in routine clinical practice, the decrease in variance between the two systems in half the subjects in the present study demonstrated a higher level of precision than has been seen with CGM systems historically. The increased range of the DG4P transmitter resulted in significantly fewer gaps in the CGM readings, providing a nearly uninterrupted data stream to the receiver (97% or 279 of 288 possible measurements per day).
There are several changes in the DG4P system compared with the DSP system that may be responsible for the improvement in the performance reported here. Both the DSP and DG4P systems use a small, flexible sensor wire (12 mm in length), inserted by the patient into the subcutaneous area of the abdomen at a 45° angle via the same-size 26-gauge introducer needle. This results in a depth of placement of approximately 8 mm into the adipose tissue. The diameter of the sensor wire was decreased from 180 μm in the DSP system to 130 μm in the DG4P. The 60% reduction of the DG4P sensor wire volume compared with the DSP sensor may help attenuate the tissue trauma associated from the in-dwelling sensor wire, thereby facilitating more consistent performance and improved sensor accuracy over time. In addition, modifications in the sensor membrane material in the DG4P sensor were designed to further reduce the foreign body response, achieve improved biocompatibility over a wider range of patients using the device, and decrease data lost because of the reduction of physiological interferences transmitted across the previous sensor membrane compared with the DSP sensor. Furthermore, the glucose oxidase system used in the DG4P was optimized to operate at low oxygen and low current density with a high signal-to-noise ratio in order to improve performance across the full duration of wear and at low glucose. The DG4P also incorporates changes in the algorithm to compensate for known sensor behavior as a function of time during the 7-day duration of use. The change in the DG4P transmission frequency from 0.4 GHz to 2.4 GHz extended the transmitter range from 5 feet with the DSP to 20 feet and improved data capture and overall system reliability.
A key strength of our study was utilization of metrics that may better reflect the true experience users might expect from their CGM device. Although it is customary to cite the aggregate MARD value as a characterization of sensor performance from a given clinical study, this metric may not adequately reflect the user experience with a particular CGM system. Patients using CGM as part of their routine diabetes management experience CGM performance according to the accuracy of each individual sensor, not by aggregate data. Accordingly, in addition to the aggregate MARD, we have reported the distribution of individual sensor MARD values. In our study, device calibration was performed twice daily with an SMBG meter, as stated in the FDA-approved labeling. More frequent calibration with an accurate SMBG system may result in even greater accuracy but is not necessary to achieve the high level of performance reported here. The increase in accuracy seen overall with the DG4P compared with the DSP was not manifest on the first day of sensor use. There are multiple reasons for this, most notably that both systems used the same gauge introducer needle and are both dependent on the accuracy of multiple fingerstick calibrations on Day 1 in order to establish proper calibration of the sensor.
A weakness of the current study was its short duration, which was intended for assessment of the systems' accuracy and reliability and not for evaluation of the sustained use of CGM or of clinical end points, such as A1C change or incidence of hypoglycemia. More studies are needed to evaluate the long-term impact of the improved accuracy and reliability of the latest generation of real-time CGM devices on patient utilization of the technology.
Although CGM does not yet have the point accuracy of the current generation of blood glucose monitors, it can provide patients with timely alerts of real or impending hypoglycemia and hyperglycemia. It also provides additional valuable information on the direction and rate of glucose change, which may assist patients in making better diabetes treatment decisions. Numerous clinical studies to date have shown that the clinical benefit of CGM is directly correlated with regular use of the technology for an average of at least 6 days per week.6,7,9–11,15,16 In these studies, sustained CGM use was associated with reduced duration of hypoglycemia and statistically significant reductions in A1C.6,7 In addition, reduction of the incidence of hypoglycemia has also been observed.11 The full potential clinical benefits of CGM systems have not, however, been fully realized either in clinical studies or in routine clinical use. Despite its potential impact on glycemic control, CGM use has been limited because of real and perceived problems with sensor accuracy and reliability.
The enhanced accuracy and performance of the latest-generation CGM device, the DG4P, may lead to greater clinician and patient acceptance, more sustained use of the technology, and improved glycemic control. Improvements in sensor accuracy and reliability may also be important in facilitating further advances in the development of artificial pancreas systems.
Acknowledgments
This study was funded by Dexcom, Inc., San Diego, CA. The authors would also like to acknowledge the editorial assistance of Christopher G. Parkin, CGParkin Communications, Inc., and Lucas Bohnett, Dexcom, Inc., who assisted with the statistical analysis of the data. All authors reviewed and provided edits and comments on manuscript drafts. The authors had the following responsibilities: M.C., T.B., E.W., and D.L. were co-investigators on the clinical study; D.P. was responsible for the design of the clinical study and participated in the drafting of the manuscript; K.N. developed the statistical analysis plan, was responsible for the statistical data analysis, and participated in the design of the clinical study and drafting of the manuscript; R.B. and T.P. participated in the design of the clinical study, the data analysis, and drafting of the manuscript; and D.P., K.N., and T.P. are responsible for the integrity of the data.
Author Disclosure Statement
M.C., T.B., E.W., and D.L. or their institutions received research grants from Dexcom, Inc. for conducting the trial. D.P., K.N., R.B., and T.P. are employees of Dexcom, Inc.
References
- 1.American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillip M. Battelino T. Rodriguez H. Danne T. Kaufman F. Use of insulin pump therapy in the pediatric age-group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2007;30:1653–1662. doi: 10.2337/dc07-9922. [DOI] [PubMed] [Google Scholar]
- 3.Blevins TC. Bode BW. Garg SK. Grunberger G. Hirsch IB. Jovanovic L. Nardacci E. Orzeck EA. Roberts VL. Tamborlane WV. Rothermel C. Statement by the American Association of Clinical Endocrinologists Consensus Panel on Continuous Glucose Monitoring. Endocr Pract. 2010;16:730–745. doi: 10.4158/EP.16.5.730. [DOI] [PubMed] [Google Scholar]
- 4.Klonoff DC. Buckingham B. Christiansen JS. Montori VM. Tamborlane WV. Vigersky RA. Wolpert H. Continuous glucose monitoring: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:2968–2979. doi: 10.1210/jc.2010-2756. [DOI] [PubMed] [Google Scholar]
- 5.The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 6.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) Trial. Diabetes Care. 2010;33:17–22. doi: 10.2337/dc09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deiss D. Bolinder J. Riveline JP. Battelino T. Bosi E. Tubiana-Rufi N. Kerr D. Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch IB. Abelseth J. Bode BW. Fischer JS. Kaufman FR. Mastrototaro J. Parkin CG. Wolpert HA. Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized Treat-to-Target Study. Diabetes Technol Ther. 2008;10:377–383. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 9.Raccah D. Sulmont V. Reznik Y. Guerci B. Renard E. Hanaire H. Jeandidier N. Nicolino M. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the Realtrend Study. Diabetes Care. 2009;32:2245–2250. doi: 10.2337/dc09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connell MA. Donath S. O'Neal DN. Colman PG. Ambler GR. Jones TW. Davis EA. Cameron FJ. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52:1250–1257. doi: 10.1007/s00125-009-1365-0. [DOI] [PubMed] [Google Scholar]
- 11.Battelino T. Phillip M. Bratina N. Nimri R. Oskarsson P. Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795–800. doi: 10.2337/dc10-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg S. Jovanovic L. Relationship of fasting and hourly blood glucose levels to HbA1c values: safety, accuracy, and improvements in glucose profiles obtained using a 7-day continuous glucose sensor. Diabetes Care. 2006;29:2644–2649. doi: 10.2337/dc06-1361. [DOI] [PubMed] [Google Scholar]
- 13.Garg S. Zisser H. Schwartz S. Bailey T. Kaplan R. Ellis S. Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29:44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 14.Vigersky RA. Fonda SJ. Chellappa M. Walker MS. Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32–38. doi: 10.2337/dc11-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey TS. Zisser HC. Garg SK. Reduction in hemoglobin A1c with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9:203–210. doi: 10.1089/dia.2007.0205. [DOI] [PubMed] [Google Scholar]
- 16.Beck RW. Buckingham B. Miller K. Wolpert H. Xing D. Block JM. Chase HP. Hirsch I. Kollman C. Laffel L. Lawrence JM. Milaszewski K. Ruedy KJ. Tamborlane WV. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32:1947–1953. doi: 10.2337/dc09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck RW. Tamborlane WV. Bergenstal RM. Miller KM. DuBose SN. Hall CA. The T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2012;97:4383–4389. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 18.Hermanides J. Phillip M. DeVries JH. Current application of continuous glucose monitoring in the treatment of diabetes. Diabetes Care. 2011;34(Suppl 2):S197–S201. doi: 10.2337/dc11-s219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartelme A. Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3:992–995. doi: 10.1177/193229680900300449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CGM resource center. Practice management: CGM reimbursement. http://thecgmresourcecenter.com/practice-management/cgm-reimbursement. [Aug;2012 ]. http://thecgmresourcecenter.com/practice-management/cgm-reimbursement
- 21.Ramchandani N. Arya S. Ten S. Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J Diabetes Sci Technol. 2011;5:860–870. doi: 10.1177/193229681100500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chase HP. Beck R. Tamborlane W. Buckingham B. Mauras N. Tsalikian E. Wysocki T. Weinzimer S. Kollman C. Ruedy K. Xing D. A randomized multicenter trial comparing the Glucowatch Biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care. 2005;28:1101–1106. doi: 10.2337/diacare.28.5.1101. [DOI] [PubMed] [Google Scholar]
- 23.Buckingham B. Beck RW. Tamborlane WV. Xing D. Kollman C. Fiallo-Scharer R. Mauras N. Ruedy KJ. Tansey M. Weinzimer SA. Wysocki T. Continuous glucose monitoring in children with type 1 diabetes. J Pediatr. 2007;151:388–393. doi: 10.1016/j.jpeds.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinzimer S. Xing D. Tansey M. Fiallo-Scharer R. Mauras N. Wysocki T. Beck R. Tamborlane W. Ruedy K. Prolonged use of continuous glucose monitors in children with type 1 diabetes on continuous subcutaneous insulin infusion or intensive multiple-daily injection therapy. Pediatr Diabetes. 2009;10:91–96. doi: 10.1111/j.1399-5448.2008.00476.x. [DOI] [PubMed] [Google Scholar]
- 25.Buckingham B. Block J. Burdick J. Kalajian A. Kollman C. Choy M. Wilson DM. Chase P. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. 2005;7:440–447. doi: 10.1089/dia.2005.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chia CW. Saudek CD. Glucose sensors: toward closed loop insulin delivery. Endocrinol Metab Clin North Am. 2004;33:175–195. doi: 10.1016/j.ecl.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23:1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 28.Mazze RS. Strock E. Borgman S. Wesley D. Stout P. Racchini J. Evaluating the accuracy, reliability, and clinical applicability of continuous glucose monitoring (CGM): is CGM ready for real time? Diabetes Technol Ther. 2009;11:11–18. doi: 10.1089/dia.2008.0041. [DOI] [PubMed] [Google Scholar]
- 29.Hessler DP. Polonsky WH. Bowman F. Price D. The subjective experience of CGM-RT use: comparing current users with ex-users [abstract] Diabetes. 2012;61:A215. [Google Scholar]
- 30.Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the Glucowatch G2 Biographer in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5:791–800. doi: 10.1089/152091503322526996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration. Summary of safety and effectiveness data. www.Fda.Gov/ohrms/dockets/dockets/05m0454/05m-0454-aav0001-03-ssed-vol1.Pdf. [Aug;2012 ]. www.Fda.Gov/ohrms/dockets/dockets/05m0454/05m-0454-aav0001-03-ssed-vol1.Pdf
- 32.U.S. Food and Drug Administration. Summary of safety and effectiveness data. 2006. www.Accessdata.Fda.Gov/cdrh_docs/pdf5/p050012b.Pdf. [Aug;2012 ]. www.Accessdata.Fda.Gov/cdrh_docs/pdf5/p050012b.Pdf
- 33.Weinstein RL. Schwartz SL. Brazg RL. Bugler JR. Peyser TA. McGarraugh GV. Accuracy of the 5-day Freestyle Navigator continuous glucose monitoring system: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30:1125–1130. doi: 10.2337/dc06-1602. [DOI] [PubMed] [Google Scholar]
- 34.Zisser HC. Bailey TS. Schwartz S. Ratner RE. Wise J. Accuracy of the seven continuous glucose monitoring system: comparison with frequently sampled venous glucose measurements. J Diabetes Sci Technol. 2009;3:1146–1154. doi: 10.1177/193229680900300519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey T. Zisser H. Chang A. New features and performance of a next-generation SEVEN-day continuous glucose monitoring system with short lag time. Diabetes Technol Ther. 2009;11:749–755. doi: 10.1089/dia.2009.0075. [DOI] [PubMed] [Google Scholar]
- 36.Cryer PE. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14:750–756. doi: 10.4158/EP.14.6.750. [DOI] [PubMed] [Google Scholar]
- 37.Davey RJ. Stevens K. Jones TW. Fournier PA. The effect of short-term use of the Guardian RT continuous glucose monitoring system on fear of hypoglycaemia in patients with type 1 diabetes mellitus. Prim Care Diabetes. 2012;6:35–39. doi: 10.1016/j.pcd.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Wojciechowski P. Ryś P. Lipowska A. Gawęska M. Małecki MT. Efficacy and safety comparison of continuous glucose monitoring and self-monitoring of blood glucose in type 1 diabetes: systematic review and meta-analysis. Pol Arch Med Wewn. 2011;121:333–343. [PubMed] [Google Scholar]
- 39.Clarke WL. Cox D. Gonder-Frederick LA. Carter W. Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10:622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]