Abstract
Background
‘You are what you eat’ is an accurate summary for humans and animals when it comes to carbon isotope abundance. In biological material, natural13C/12C ratio is subject to minute variations due to diet composition (mainly from ingestion of C3 and C4 metabolism plants) and to the discrimination between ‘light’ and ‘heavy’ isotopes during biochemical reactions (isotope effects and isotopic fractionation).
Methodology/Principal Findings
Carbon isotopic abundance was measured in ZDF (fa/+) and ZDF (fa/fa), (lean and obese-diabetic rats respectively) fed the same diet. By analysing plasma metabolites (glucose and non-esterified fatty acids), breath and liver tissue by high-precision isotope ratio mass spectrometry, we demonstrate for the first time statistically distinguishable metabolic carbon isotope abundance between ZDF (fa/+) and ZDF (fa/fa) rats based on plasma glucose, palmitic, oleic, linoleic, arachidonic acids and bulk analysis of liver tissue (P<0.005) resulting into clear isotopic fingerprints using principal component analysis. We studied the variation of isotopic abundance between both groups for each metabolite and through the metabolic pathways using the precursor/product approach. We confirmed that lipids were depleted in 13C compared to glucose in both genotypes. We found that isotopic abundance of linoleic acid (C18: 2n-6), even though both groups had the same feed, differed significantly between both groups. The likely reason for these changes between ZDF (fa/+) and ZDF (fa/fa) are metabolic dysregulation associated with various routing and fluxes of metabolites.
Conclusion/Significance
This work provides evidence that measurement of natural abundance isotope ratio of both bulk tissue and individual metabolites can provide meaningful information about metabolic changes either associated to phenotype or to genetic effects; irrespective of concentration. In the future measuring the natural abundance δ13C of key metabolites could be used as endpoints for studying in vivo metabolism, especially with regards to metabolic dysregulation, and development and progression of metabolic diseases.
Introduction
In medicine and biology, metabolite concentration or metabolic profiling is often used to diagnose disease state and provide insights into metabolic processes [1]. In the diagnosis of diabetes mellitus type 2 (T2DM), fasting plasma or blood glucose concentrations have long been the first indicator of the development of the disease. Recently, other biomarkers such as acylcarnitines and amino acids have also been suggested for the diagnosis of T2DM [2]. One of the goals of biomarker discovery is to detect the onset and development of diseases such as T2DM before they result in biochemically significant changes in systemic biochemistry. Present screening biomarkers for T2DM generally rely on measuring biomarker concentrations, which occurs relatively late in the cascade of the development of the disease. One different approach to disease diagnosis is to measure fluxes through relevant pathways using stable isotopes. Thus, phenotype (defined by the interaction of the gene, nutrients and environment) and its perturbation (e.g. fasting, postprandial and disease conditions) can be more accurately described through the flux measurements of pathways than measuring concentrations alone [3–5].
In the field of ecology, the measurement of the natural ratio of heavy to light atomic isotopes (e.g.13C/12C15, N/14N, 2H 2/H2 and18O/16O) in mammalian tissues is a powerful tool to study trophic ecology and energy pathways [6–8]. In chemical archaeology, it has been demonstrated that the isotopic composition of the diet (as15N/14N from protein and 13C/12C from protein, carbohydrates and lipids) is reflected in animal or human tissues with some additional in situ isotopic discrimination [9]. This in situ isotopic discrimination (isotopic effect and isotopic fractionation) is due to either some enzymes having a slightly different affinity and reaction rate for heavier molecules (enriched in heavy isotopes) compared to normal or lighter weight molecules (fewer heavy isotopes) [10]. Over time, this slight difference in metabolism of heavier molecules can lead to a measurable difference in isotope ratio. The most widespread example of this difference in ‘isotopic fractionation’ is the carbon isotopic abundance of plants with C3 and C4 photosynthetic pathways [11] (average natural abundance of 13C in all matter is about 1.1%, 1.081% in C3 plants (e.g. soybean) and 1.0975% in C4 plants (e.g. corn). Differences in isotopic enrichment of mammals is not solely dictated by diet (between C3-C4 or between marine and terrestrial diets), and studies looking at changes due to dietary differences in isotopic composition find that different tissues and animals incorporate the isotopic shift at various rates [12]. Isotopic turnover can vary from few days to several months within a single species depending on many physiological parameters including protein turnover and animal growth [13,14]. Other studies in humans have demonstrated that nitrogen isotope abundance of tissues samples (e.g. hair and liver) can also reflect nutritional stress or eating disorders such as anorexia nervosa and bulimia [15,16]. This relationship is explained by the variation of nitrogen isotope abundance δ15N during negative nitrogen balance (associated with isotopic fractionation of nitrogen during deamination and transamination reactions) [17]. Other explorative studies showed that slight natural variation of isotopic abundance of deuterium and 18O can have biological meaning in human and mice [18,19].
The isotopic data generated in such studies are generally measured ‘in bulk’ in tissues, plasma or macromolecules (such as lipids and proteins) using an elemental analyzer coupled to isotope ratio mass spectrometer (EA-IRMS) for 13C and 15N isotopes. However, in plants, changes to natural isotopic abundance have been studied at the intramolecular level [20]. This type of approach is yet to be applied to studies on mammalian metabolism. Several studies have highlighted that “isotopic routing” between various tissues can make the interpretation of isotopic data in mammals a challenging task [21].
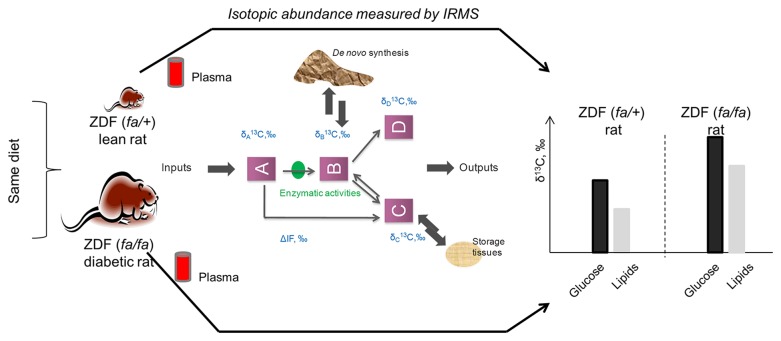
We present here an exploratory study on the relative impact of a pathophysiological condition (diabetics) on the natural carbon isotopic abundance (δ13C) for individual metabolites measured in plasma, in breath and liver tissue samples (total liver isotope ratio) from Zucker Diabetic Fatty (ZDF) rats. To our knowledge, there is little published information on how intrinsic physiological and biochemical factors (as opposed to external factors such as diet) can affect natural isotopic abundance. Knowing that the variability of in vivo isotopic signatures is an index of combined effects of the diet (e.g. natural enrichment via ingested carbon sources), and other inherent variability related to physiology, we compared the natural abundance isotope ratio of rats that spontaneously develop diabetes (ZDF-Lepr fa/Crl; ZDF (fa/fa)) and their non-diabetic littermates (ZDF fa/+) fed the same diet (Figure 1 ). Because of the dietary control, any isotopic difference observed results from expression of the recessive leptin resistance trait (and other possible associated mutations) and the consequent development of diabetes through overeating. If differences in natural isotopic abundance due to metabolic disorders can be detected, this may ultimately shed light on the relevant dysregulated pathways and specific isotopic signatures signalling the presence or onset of metabolic dysregulation.
Figure 1. Synopsys of the exploratory study studying the relative impact of the pathophysiological conditions (ZDF (fa/+) and ZDF (fa/fa) rats) on the natural carbon isotopic abundance (δ13C) for individual metabolites measured in plasma, in breath and liver tissue samples by isotope ratio mass spectrometry.
Results
Physiological characteristics and changes to circulating metabolites
ZDF (fa/fa) develop diabetes due to being homozygous for a spontaneous mutation that leads to a non-functional leptin receptor as well as a second mutation that impairs β-cell function [22]. The rats are leptin resistant, obese and develop hyperlipidemia, insulin resistance and hyperglycemia in a pattern similar to T2DM [23,24]. Their heterozygous (ZDF, fa/+) siblings are non-obese and are phenotypically lean and normoglycaemic. Both groups received the same diets from weaning (commercial rodent feed). Although food intake is more than 1.5 times higher for ZDF (fa/fa) rats compared to ZDF (fa/+) rats [25], this is not known to influence the isotopic abundance of plasma metabolites or body tissues under these conditions.
As expected, at fifteen weeks of age, ZDF (fa/fa) rats had significantly higher body, liver and adipose tissue weights (P<0.01) than ZDF (fa/+) rats (Table 1 ). Basal plasma level of glucose, triglycerides and free fatty acids were all also significantly higher in ZDF (fa/fa) than in ZDF (fa/+) rats (P=0.002). Plasma fatty acid composition also differed, with arachidonic (C20: 4n-6) and stearic acid (C18: 0) concentrations at 8.4% and 2.6% lower in ZDF (fa/fa) rats compared to ZDF (fa/+) respectively, whereas oleic acid (C18: 1) increased by 7.6% (Table 2 ). In both genotypes, the most abundant fatty acid was palmitic acid (C16: 0), 24.9 and 30.3% for ZDF (fa/+) and ZDF (fa/fa) rats respectively.
Table 1. Physiological characteristics of ZDF (fa/+) and ZDF (fa/fa) groups studied at 16 weeks old.
| ZDF (fa/+) rat | ZDF (fa/fa) rat | P value | ||
|---|---|---|---|---|
| Plasma | Glucose (mmol/L) | 3.31 ± 0.19 | 7.25 ± 0.81 | 0.002 |
| Lactate (mmol/L) | 4.06 ± 0.65 | 7.82 ± 2.43 | 0.009 | |
| Triglycerides (mmol/L) | 0.3 ± 0.07 | 2.62 ± 0.35 | 0.002 | |
| Palmitic acid (mmol/L) | 2.67 ± 0.06 | 7.31 ± 0.59 | 0.002 | |
| Stearic acid (mmol/L) | 1.42 ± 0.03 | 2.62 ± 0.09 | 0.002 | |
| Oleic acid (mmol/L) | 0.78 ± 0.08 | 3.66 ± 0.34 | 0.002 | |
| Linoleic acid (mmol/L) | 2.41 ± 0.07 | 5.22 ± 0.39 | 0.002 | |
| Arachidonic acid (mmol/L) | 3.39 ± 0.12 | 5.72 ± 0.35 | 0.002 | |
| Tissues | Body weight (g) | 283 ± 8 | 327 ± 14 | 0.002 |
| Liver weight (g) | 6.6 ± 0.07 | 14.4 ± 0.6 | 0.002 | |
| Adipose weight (g) | 1.33 ± 0.07 | 4.89 ± 0.29 | 0.002 |
The data are expressed as median ± SEM.
Table 2. Plasma fatty acid composition.
| Fatty Acid | ZDF (fa/+) rat | ZDF (fa/fa) rat | P value |
|---|---|---|---|
| Palmitic acid, % | 24.95 ± 0.34 | 30.35 ± 0.32 | < 0.001 |
| Stearic acid, % | 13.19 ± 0.20 | 10.83 ± 0.26 | < 0.001 |
| Oleic acid, % | 7.03 ± 0.43 | 15.21 ± 0.41 | < 0.001 |
| Linoleic acid, % | 22.0 ± 0.40 | 22.84 ± 0.38 | 0.086 |
| Arachidonic acid, % | 32.16 ± 0.91 | 21.91 ± 0.67 | < 0.001 |
All values are expressed as mol % ± SEM. Fatty acid names: C16 (palmitic acid), C18 (stearic acid), C18: 1 (oleic acid), C18: 2 (linoleic acid), and C20: 4 (arachidonic acid).
Variation in isotopic abundance between ZDF (fa/+) and ZDF (fa/fa) rats
Measurement of isotopic abundance of plasma palmitic, stearic, oleic, linoleic and arachidonic acids, glucose, breath carbon dioxide (CO2) and liver tissue from both groups of rats were carried out using high-precision isotope ratio mass spectrometry hyphenated to different peripherals [26,27] (IRMS) (see Materials and Methods).
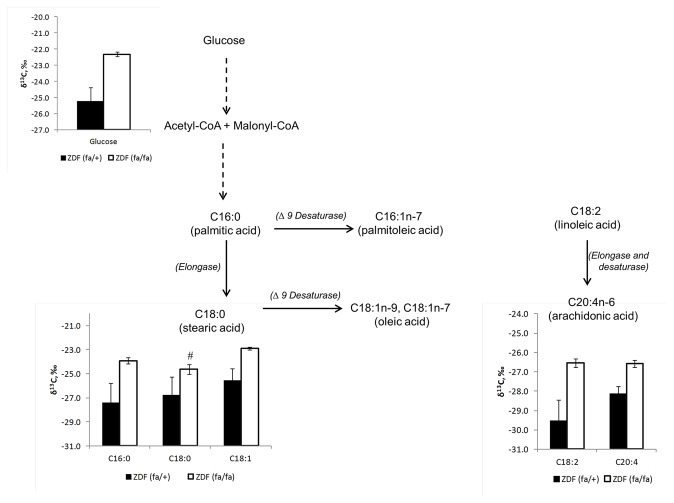
Isotopic abundance (δ13C) was not significantly different between ZDF (fa/+) and ZDF (fa/fa) for breath CO2, whereas liver tissue was significantly different (P=0.004) (Table 3 ). The difference of carbon isotope abundance for liver tissue (Δ13C) between ZDF (fa/fa) and ZDF (fa/+) was 5.06‰, and was the largest natural isotopic difference observed in this study. Similar observations were reported for specific compounds measured in plasma: glucose, palmitic, oleic, linoleic and arichidonic acids had significantly different δ13C (P<0.005) with Δ13C calculated at 2.91, 3.30, 2.45, 3.07 and 1.56‰ respectively. The observed range of isotopic variation indicated that the isotope abundance was always greater (enriched in 13C atoms) in ZDF (fa/fa) rats compared to lean rats. One exception was stearic acid, which did not differ in isotopic abundance between both genotypes (P > 0.2).
Table 3. Carbon isotopic abundance (δ13C, ‰) metabolites, breath and liver samples measured by isotope ratio mass spectrometer hyphenated to various peripherals for ZDF.
(fa/+) and ZDF (fa/fa) rats.
| ZDF (fa/+) rat | ZDF (fa/fa) rat | Δ 13C, ‰ | P value | |
|---|---|---|---|---|
| δ13C, ‰ | δ13C, ‰ | |||
| Breath CO2 | -23.24 ± 0.35 | -22.73 ± 0.11 | 0.59 | 0.266 |
| Glucose | -25.23 ± 0.87 | -22.32 ± 0.15 | 2.91 | 0.002 |
| Palmitic acid | -27.37 ± 1.61 | -23.91 ± 0.25 | 3.30 | 0.004 |
| Stearic acid | -26.76 ± 1.51 | -24.63 ± 0.42 | 1.80 | 0.394 |
| Oleic acid | -25.58 ± 1.01 | -22.91 ± 0.11 | 2.45 | 0.004 |
| Linoleic acid | -29.52 ± 1.08 | -26.53 ± 0.22 | 3.07 | 0.002 |
| Arachidonic acid | -28.13 ± 0.38 | -26.58 ± 0.18 | 1.56 | 0.004 |
| Liver tissue | -21.43 ± 0.31 | -16.37 ± 0.53 | 5.06 | 0.004 |
Δ13C is the isotopic difference for each metabolite between ZDF (fa/+ and ZDF (fa/fa) rats.
Isotopic fractionation within each genotype
The biochemical relationships between glucose and fatty acids or between each fatty acid are not straightforward within an in vivo model due to various metabolic fluxes of fatty acids and glucose between organs and the existence of many intermediates or branching points. However, it is possible to gain additional information by considering the precursor/product relationship between two metabolites through the calculation of absolute isotopic difference (in ‰) as an index of isotopic fractionation. The isotopic fractionation between plasma glucose and palmitic acid for ZDF (fa/fa) and ZDF (fa/+) was calculated at 2.01 and 2.39‰ respectively (P=0.7) (Table 4 ) indicating that no isotopic perturbation of this pathway was observed in these conditions. Similarly, no statistical differences between the two genotypes were measured for biochemical reactions between stearic and oleic acids and between polyunsaturated fatty acids (linoleic to arichidonic acids). Conversely, for the conversion of palmitic to stearic acid, there was a significant difference in isotopic fractionation between the two genotypes (P=0.007). This difference between both groups (for ZDF (fa/+) and ZDF (fa/fa) rats) in isotopic abundance for palmitic to stearic acids (δ13Cpalmitic -δ13Cstearic) was -1.04 ± 0.40‰ and 0.76 ± 0.19‰ respectively.
Table 4. Isotopic fractionation (ΔIF, in ‰) measured for key metabolic transitions between a precursor and its product in lean (ZDF, (fa/+)) and diabetic (ZDF (fa/fa)) rats.
| Precursor/product | ZDF (fa/+) rat | ZDF (fa/fa) rat | P value | |
|---|---|---|---|---|
| ΔIF,‰ | ΔIF,‰ | |||
| Glucose/C16:0 | 2.39 ± 0.38 | 2.01 ± 0.36 | 0.877 | |
| C16:0/C18:0 | -1.04 ± 0.4 | -0.76 ± 0.18 | 0.007 | |
| C18:0/C18:1 | - 0.04 ± 0.64# | -1.67 ± 0.28 | 0.103 | |
| C18:2/C20:4 | -1.31 ± 0.45 | - 0.006 ± 0.53# | 0.232 |
ΔIF, ‰ (δA - δB) where A/B is the precursor/product ratio. Based on the Wilcoxon Signed Rank Test, all the data were significantly different from zero except for those marked with “#”.
Discussion
Potential sources of isotopic changes between genotype
The present data suggest that natural isotopic abundance at the molecular level could be a new approach to obtain signatures and information about the onset of metabolic dysregulation. In contrast to other studies on the effect of past diet on tissues (e.g. bone and hair), we have detected differences that are not due to diet, with a different disease state appearing to be responsible for the difference in observed in vivo molecular isotopic abundance. As the system is pushed out of balance (loss of homeostasis), a dysregulation of the storage and transport of many nutrients such as glucose, lipids and amino acids occur. Although the cause-effect relationship behind the difference in δ13C signature is complex when multiple compartments are modelled [9], we can then assume that isotope discrimination can occur at various steps between the plasma and the different tissues, including 1) isotope fractionation associated with the uptake and utilization of substrate (such as the gluconeogenic precursors); 2) isotopic fractionation associated during elongation and desaturation of fatty acids, fatty acid oxidation and cellular transport between cytoplasma and mitochondria; 3) isotopic fractionation associated to fatty acid mobilization from adipose tissue; 4) isotopic fractionation associated to de novo synthesis; 5) isotopic fractionation associated to changes in the feed composition intake (i.e. the amount of macronutrients ingested) leading to further modulation of enzymatic activity through changes in substrate concentration.
13C isotopic changes between genotype
Using an in vitro model, it was established that the natural isotopic abundance of carbon is not uniformly distributed among the various classes of metabolites [28]. For example, lipids are generally depleted in 13C relative to carbohydrates. This isotope fractionation effect occurs during the decarboxylation of pyruvate by pyruvate deshydrogenase, resulting in depleted acetyl-CoA at the carbon 2 position. The low levels of enrichment at natural abundance mean that only total 13C-enrichment, and not the intramolecular 13C distribution, can be determined. However, we confirmed that the lipids measured were depleted in δ13C compared to glucose in both genotypes. This result indicates a consistent relationship in the isotopic fractionation at the branching points for the acetyl-CoA used for both genotypes.
All plasma metabolites were more enriched in 13C (Δ13C) in ZDF (fa/fa) rats (P < 0.005) except for stearic acid (Figure 2 ). A previous study on dogs found that that carbon 1 in plasma glucose was slightly enriched in diabetic fasting dogs compared to healthy dogs (δ13C= -23.2 ± 2.3‰ vs. -17.8± 2.1‰ for normal versus diabetic dogs) [29], a similar pattern to that observed for total glucose in this study. The cause-effect relationship for the greater isotopic abundance of plasma glucose in the ZDF (fa/fa) rats is difficult to demonstrate. However, it is possible to surmise that the source and routing of glucose (i.e. incomplete suppression of hepatic glucose production and a decreased efficiency of the liver and muscle glucose uptake) differing between both groups may lead to changes in glucose concentration which also impact the concentration of light and heavy isotope. But a clear link between glucose transports, various uptake- of glucose and isotopic differences need to be clarified.
Figure 2. Schematic representation of the variation of carbon isotopic abundance (expressed in δ13C, ‰) of plasma glucose, plasma lipids in ZDF (fa/+) and ZDF (fa/fa) rats.
All the data were significantly different between both groups except for stearic acid (indicated by ‘#’).
In our study, the fatty acid composition of the plasma of the ZDF (fa/fa) rats was distinctly different from the plasma of the ZDF (fa/+) with higher % of palmitic and oleic acids whereas the percentage of stearic and arachidonic acids were higher for ZDF (fa/+). Isotopically, we found that palmitic, oleic, linoleic and arachidonic were significantly different between ZDF (fa/fa) and ZDF (fa/+) with the exception of stearic acid. These data suggest that the source of such fatty acids is different between both groups. Assuming that plasma fatty acids are a mixture of newly synthesized fatty acids (de novo lipogenesis), products of lipolysis and dietary lipids, the significant isotopic results at natural abundance between both groups can be explained by the rate of de novo synthesis and the rate of lipolysis and the influx of dietary lipids. In several studies, when leptin and/or leptin receptor are unregulated, as in the ZDF (fa/fa) rat, lipogenesis is also unregulated, leading to an increase of the percentage of de novo synthesis for palmitate (around 20%), stearate (around 26%) and oleate (around 44%) between ZDF and Zucker lean fed with a low fat diet [30]. Circulating fatty acids also come from lipolysis of pre-existing adipose stores. Therefore, we cannot exclude that this was an important source of fatty acids in our study that could explain the difference in δ13C. It has been reported in 13C-tracer studies that the isotope ratio of plasma stearate may be altered due to variation in membrane phospholipase activity and circulating phospholipids, as well as stearate derived from lipolysis occurring in adipose tissue [31].
Linoleic acid (C18: 2n6) is an essential polyunsaturated fatty acid (PUFA) and the starting substrate for n6 PUFA metabolism. Therefore it would be expected that linoleic acid would mainly be derived from dietary sources. Surprisingly, in our study, the Δ13C between both genotypes was measured at 3.07‰ (P=0.0004), suggesting that its source was not similar between both groups. Arachidonic acid, which is further downstream in the n6 pathway, was also isotopically different between both genotypes (P=0.002). Rhee et al. observed that linoleic acid isotopic abundance in human healthy plasma behaved differently from the other circulating fatty acids with a lower isotopic ratio (about 2‰) compared to the same dietary fatty acid [32]. Although direct evidence remains to be demonstrated, we interpret this result as indicating an increased production and utilization of this essential fatty acid in both genotypes, though we cannot exclude a small contribution of de novo synthesis of C18: 2n6 from other lipids such as C16: 2n6 [33].
Theoretically, under steady state conditions there should be no difference in the precursor/product relationship in a metabolic pathway, even if pool sizes or fluxes differ, unless there is isotopic fractionation specific for that precursor to product reaction [34]. This theoretical consideration appears to hold for the precursor/product ratio of fatty acid synthesis measured in this study (Table 4). Of the ratios measured, only the conversion of palmitic to stearic acid differed (P=0.007) between ZDF (fa/fa) and ZDF (fa/+) rats. This is in spite of around 86-91% of plasma stearate being derived from palmitate elongation in both Zucker lean and ZDF rats fed on low fat diet [35]. Further studies are needed to decipher the cause-effect relationship of isotopic abundance of fatty acids based on measuring information at the level of individual carbon atoms requiring the development of natural abundance intramolecular isotope measurements [36].
Isotopic signature of ZDF (fa/fa) versus ZDF (fa/+) rats
Further analysis using principal component analysis (PCA), based on carbon isotopic abundance of plasma metabolites, showed that ZDF (fa/fa) and ZDF (fa/+) rats were separated along the first component (Figure 3 ). The separation of both genotypes is explained by all the plasma metabolites measured, with glucose, palmitic acid, oleic acid and linoleic acid having the most influence on the model. The separation of the two groups along that the first component suggests that the δ13C measurements of these metabolites could serve as an isotopic fingerprint or signature to distinguish these two groups, though if this difference is due to metabolic or direct genetic effects remains to be determined. Of note, δ13C variation was greater for ZDF (fa/+) rats compared to ZDF (fa/fa) rats for all the metabolites measured with the reverse observed for the metabolites concentrations. As the group size used in this study is low, further works with larger populations are needed before conclusions can be made about the importance of intra and inter-group variability for δ13C values.
Figure 3. Principal component analysis (PCA) score plots of isotopic abundance of plasma metabolites measured by isotope ratio mass spectrometers (i.e. glucose, palmitic, oleic, stearic, linolenic and arachidonic acids) between ZDF (fa/+) and ZDF (fa/fa) rats.
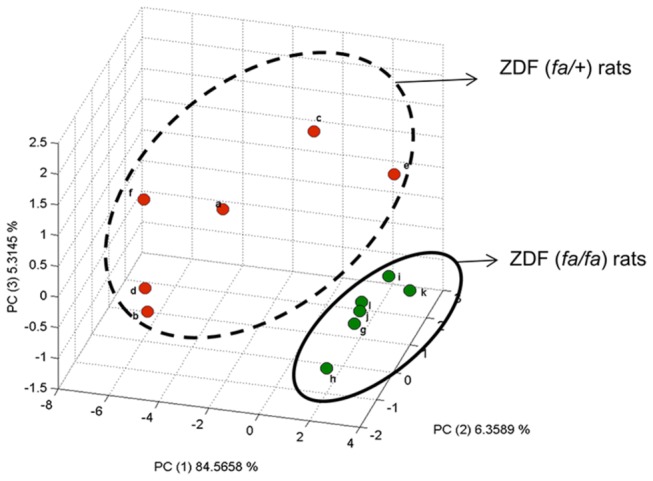
Red dots are for ZDF (fa/+) whereas green ones stand for ZDF (fa/fa) rats. Letters are the identification code of the rat. Based on the PCA, there is a clear separation of both groups along PC1 and PC3, confirming that there is an isotopic signature at natural abundance that separates ZDF (fa/+) and ZDF (fa/fa) rats.
Metabolic flexibility
The ability to adaptively partition metabolic fuel among various tissues has crucial impact on health and diseases [37]. This concept is often referred to as metabolic flexibility, and is in part based on the ability of skeletal muscle to switch from lipid to carbohydrate oxidation under various conditions and is closely associated with the development of insulin resistance. For example, obesity and T2D are associated with an impaired fat oxidation during the fasting state [38]. Although no changes were found in breath 13CO2, the slightly higher carbon isotopic abundance in metabolites related to energy metabolism in diabetic rats may be an indicator that pathways to metabolise energy substrates has been compromised, possibly by an increased flux through these pathways for an extended period of time. We speculate here that the isotopic information gathered in both physiological conditions can represent a proxy of metabolic flexibility characterizing the two groups studied. However, further studies are needed to assess if impaired fat oxidation may have an impact on the isotopic signature of oxidation that may correlate with the natural abundance isotopic pattern of plasma metabolites.
Further work
Although isotopic variation in plasma metabolites confirmed the existence of natural abundance signatures between ZDF (fa/+) and ZDF (fa/fa) rats, additional isotopic measurements of specific components in the diets and in metabolically important tissues such as liver, pancreas and adipose tissue are needed. Mass balance modelling could then be used to shed further light on whole body mechanisms behind changes to natural abundance of individual metabolites.
In conclusion, we showed distinct natural abundance 13C fingerprints between ZDF (fa/+) and ZDF (fa/fa) rats for individual metabolites in plasma and liver tissue. This difference may result from the exchange of fluxes of metabolites between organs, and/or changing of pathways for de novo synthesis associated to isotopic fractionation between light and heavy isotopes. The use of isotopic fingerprints associated with metabolic modelling could lead to the isotopic abundance of key metabolites being a new dimension in understanding the genesis and progression of metabolic diseases. Further studies are also needed to determine if the changes in carbon isotopic abundance occurs before there is a difference in concentration to know if isotope fingerprinting could be a potential approach for early stage diagnostics.
Materials and Methods
Animal study
The pre-clinical study was carried out under the Swiss national guidelines and the protocol approved by the local Cantonal Veterinary Office of Vaud, Switzerland. ZDF (fa/fa) (diabetic) and (fa/+) (lean controls) rats (Charles River SA, France) were used in this study. Both groups of rats (n=6) were fed the same diet from weaning (Purina 5008 (St Louis, MO) from weeks 4-13 and Kliba Nafag 3434 (Provimi Kliba AG, Kaiseraugst, Switzerland) for weeks 13-15). Both diets are grain-based and formulated to be a complete rodent diet. Access to standard pelletized diets and water were ad libitum. At age 16 weeks, rats were weighed and then sacrificed under isofluorane anaesthesia and tissues (liver) were collected, weighed, frozen in liquid nitrogen and then stored at -80°C until analysis. Blood was collected from the carotid artery into Li-heparin tubes and plasma obtained after centrifugation at 1000 g for 10 min. Plasma samples were snap frozen in liquid nitrogen and stored at -80°C before analysis. Breath samples were collected by placing rats in a glass chamber and collecting expired air via a syringe after 5 min. The syringe was equilibrated with two volumes, before a third sample was collected and injected into an expired air analysis vial and analysed within one week of sampling.
Stable isotope notation
The delta notation is defined as follows: δ13C sample, ‰ = (Rs / Rref -1) × 1000, where Rs is the ratio of13C/12C in the sample and Rref is the13C/12C ratio of the international standard used (Vienna PeeDee Belemnite).
Analyses and analytical methods
Breath 13CO2 analysis was carried out using a GC-IRMS (Delta V, Advantage high-precision IRMS Thermo, Bremen, Germany) using a Poraplot GC column heated to 50°C. The GC-IRMS system, used to measure the breath CO2, was calibrated using three reference gases (Eurisotop, France) with certified values: δ13C at -22.8, -13.2 and -7.3‰. Typical precision (1σ) of breath isotopic analysis was 0.15‰.
Glucose concentration was measured using a commercial kit (Roche, Basel, Switzerland). Glucose isotopic analysis was carried out on a MAT252 IRMS (Finnigan MAT, Bremen, Germany) coupled to an LC Isolink® interface (Thermo Electron, Bremen, Germany). The LC-IRMS system was calibrated using two international reference materials IAEA-CH6 and USGS-40, with δ13C at -10.6 and -28.69‰ respectively. Typical precision (1σ) based on inter-day repeatability of a standard glucose sample at natural abundance was 0.4‰ [39].
Liver isotopic analyses (performed on approximately 100 mg of wet tissue) were carried out with a flash elemental analyzer (EA) coupled to a Delta V advantage high-precision IRMS (Thermo, Bremen, Germany). The liver tissue was not homogenised.
Lipid analyses (concentration and isotopic analysis) were carried out with a gas chromatography-combustion-isotope ratio mass spectrometer (GC-C-IRMS) (Thermo, Bremen, Germany). Briefly, a gas chromatograph (GC) was connected to an 850°C combustion furnace coupled to a Delta V advantage high-precision IRMS. The GC carrier gas was helium and was diverted to IRMS and FID devices with a split ratio of 1/10. Fatty acids were transesterified to their methyl esters before analysis [40]. The GC-C-IRMS system was calibrated using a mix of three methylated fatty acids (C15: 0 with a δ13C at -30.22‰; C20: 0 with a δ13C at -33.06‰ and C25: 0 with a δ13C at -28.21‰) (Chiron A/S, Trondheim, Norway). In each sample, an internal standard (i.e. C23: 0 as FAME) was spiked. Typical precision (1σ) based on inter-day repeatability on plasma samples was 0.6‰ (n=57). Fatty acid isotopic analyses were carried out in splitless mode on a DB-23 capillary column (60m x 0.25mm x 0.25µm film thickness, J&W Scientific, Folsom, USA). The GC oven was programmed as follows: 50°C held for 1 min, increased to 175°C at 25 °C/min, increased to 210°C at 10 °C/min and finally increased until 235°C at 5 °C/min and held 8 min.
Statistical analyses
Data were not normally distributed so median and standard error of the median (based on the robust standard deviation Sn of Rousseeuw) are used throughout. The Wilcoxon rank sum test was used to determine whether groups were different and the Hodges-Lehmann estimator used to quantify the median difference between the two groups. Univariate analysis was performed with R 2.6.1 (http://www.R-project.org).
Data pre-treatment, correlation analysis and principal component analysis (PCA) were performed on Matlab™ 7.9 (The Mathworks, Inc., MA, USA). In-house routines were used for importing data and visualization, while pre-processing and PCA modelling were done using the PLS-Toolbox v 5.8 (Eigenvector Research Inc., WA, USA). Coefficients of determination (r2) were calculated using Excel (Microsoft Corp., Redmond, WA).
Acknowledgments
The authors are grateful for the skilled technical assistance of Jose Garcia Sanchez and Christophe Maubert and the expert advice of Massimo Marchesini. The authors gratefully acknowledge the reviewers for their constructive comments.
Funding Statement
Nestlé has funded the work. The funder had no role in the study design, data collection and analysis or preparation of the manuscript.
References
- 1. Jones DP, Park Y, Ziegler TR (2012) Nutritional Metabolomics: Progression Addressing Complexity in Diet and Health. Annu Rev Nutr 10: 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP et al. (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17: 448-453. doi:10.1038/nm.2307. PubMed: 21423183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee WN, Go VL (2005) Nutrient-gene interaction: tracer-based metabolomics. J Nutr 135: 3027S-3032S. PubMed: 16317166. [DOI] [PubMed] [Google Scholar]
- 4. Hellerstein MK (2004) New stable isotope-mass spectrometric techniques for measuring fluxes through intact metabolic pathways in mammalian systems: introduction of moving pictures into functional genomics and biochemical phenotyping. Metab Eng 6: 85-100. doi:10.1016/j.ymben.2003.10.005. PubMed: 14734258. [DOI] [PubMed] [Google Scholar]
- 5. Godin JP, Ross AB, Rezzi S, Poussin C, Martin FP et al. (2010) Isotopomics: a top-down systems biology approach for understanding dynamic metabolism in rats using [1,2- 13 C2] acetate. Anal Chem 82: 646-653. doi:10.1021/ac902086g. PubMed: 20028023. [DOI] [PubMed] [Google Scholar]
- 6. Thompson DR, Bury SJ, Hobson KA, Wassenaar LI, Shannon JP (2005) Stable isotopes in ecological studies. Oecologia 144: 517-519. doi:10.1007/s00442-005-0171-8. PubMed: 16001214. [DOI] [PubMed] [Google Scholar]
- 7. West JB, Bowen GJ, Cerling TE, Ehleringer JR (2006) Stable isotopes as one of nature’s ecological recorders. Trends Ecol Evol 21: 408-414. doi:10.1016/j.tree.2006.04.002. PubMed: 16753238. [DOI] [PubMed] [Google Scholar]
- 8. Cherel Y, Hobson KA, Hassani S (2005) Isotopic discrimination between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiol Biochem Zool 78: 106-115. doi:10.1086/425202. PubMed: 15702469. [DOI] [PubMed] [Google Scholar]
- 9. Schoeller DA (1999) Isotopic fractionation: Why aren’t we what we eat? J Archeological Sciences 26: 667-673. doi:10.1006/jasc.1998.0391. [Google Scholar]
- 10. O’Leary MH (1989) Multiple isotope effects on enzyme-catalyzed reactions. Annu Rev Biochem 58: 377-401. doi:10.1146/annurev.bi.58.070189.002113. PubMed: 2673014. [DOI] [PubMed] [Google Scholar]
- 11. O’Leary MH (1981) Carbon isotope fractionation in plants. Phytochemistry 20: 553-657. doi:10.1016/0031-9422(81)85134-5. [Google Scholar]
- 12. Phillips DL, Eldridge PM (2006) Estimating the timing of diet shifts using stable isotopes. Oecologia 147: 195-203. doi:10.1007/s00442-005-0292-0. PubMed: 16341714. [DOI] [PubMed] [Google Scholar]
- 13. Macavoy SE, Arneson LS, Bassett E (2006) Correlation of metabolism with tissue carbon and nitrogen turnover rate in small mammals. Oecologia 150: 190-201. doi:10.1007/s00442-006-0522-0. PubMed: 16967272. [DOI] [PubMed] [Google Scholar]
- 14. Poupin N, Bos C, Mariotti F, Huneau JF, Tomé D et al. (2011) The nature of the dietary protein impacts the tissue-to-diet 15N discrimination factors in laboratory rats. PLOS ONE 6: e28046. doi:10.1371/journal.pone.0028046. PubMed: 22132207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuller BT, Fuller JL, Sage NE, Harris DA, O’Connell TC et al. (2005) Nitrogen balance and delta 15N: why you’re not what you eat during nutritional stress. Rapid Commun Mass Spectrom 19: 2497-2506. doi:10.1002/rcm.2090. PubMed: 16106342. [DOI] [PubMed] [Google Scholar]
- 16. Fuller BT, Fuller JL, Sage NE, Harris DA, O’Connell TC et al. (2004) Nitrogen balance and delta 15N: why you’re not what you eat during pregnancy. Rapid Commun Mass Spectrom 18: 2889-2896. doi:10.1002/rcm.1708. PubMed: 15517531. [DOI] [PubMed] [Google Scholar]
- 17. Macko SA, Estep MLF, Engel MH, Hare PE (1986) Kinetic fractionation of stable nitrogen isotopes during amino-acid transamination. Geochemistry Cosmochim. Acta 50: 2143-2146. [Google Scholar]
- 18. O’Grady SP, Wende AR, Remien CH, Valenzuela LO, Enright LE et al. (2010) Aberrant water homeostasis detected by stable isotope analysis. PLOS ONE 5: e11699. doi:10.1371/journal.pone.0011699. PubMed: 20657736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuo TC, Wang CH, Lin HC, Lin YH, Lin M et al. (2012) Assessment of renal function by the stable oxygen and hydrogen isotopes in human blood plasma. PLOS ONE 7: e32137. doi:10.1371/journal.pone.0032137. PubMed: 22348150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tcherkez G, Mahe A, Hodges M (2011) 12C/13C fractionations in plant primary metabolism. Trends Plant Sci 16: 499-506, S1360-1385 [DOI] [PubMed]
- 21. McMahon KW, Fogel ML, Elsdon TS, Thorrold SR (2010) Carbon isotope fractionation of amino acids in fish muscle reflects biosynthesis and isotopic routing from dietary protein. J Anim Ecol 79: 1132-1141. doi:10.1111/j.1365-2656.2010.01722.x. PubMed: 20629794. [DOI] [PubMed] [Google Scholar]
- 22. Griffen SC, Wang J, German MS (2001) A genetic defect in beta-cell gene expression segregates independently from the fa locus in the ZDF rat. Diabetes 50: 63-68. doi:10.2337/diabetes.50.1.63. PubMed: 11147796. [DOI] [PubMed] [Google Scholar]
- 23. Clark JB, Palmer CJ, Shaw WN (1983) The diabetic Zucker fatty rat. Proc Soc Exp Biol Med 173: 68-75. doi:10.3181/00379727-173-41611. PubMed: 6344096. [DOI] [PubMed] [Google Scholar]
- 24. Leonard BL, Watson RN, Loomes KM, Phillips AR, Cooper GJ (2005) Insulin resistance in the Zucker diabetic fatty rat: a metabolic characterisation of obese and lean phenotypes. Acta Diabetol 42: 162-170. doi:10.1007/s00592-005-0197-8. PubMed: 16382303. [DOI] [PubMed] [Google Scholar]
- 25. Pickavance L, Widdowson PS, King P, Ishii S, Tanaka H et al. (1998) The development of overt diabetes in young Zucker Diabetic Fatty (ZDF) rats and the effects of chronic MCC-555 treatment. Br J Pharmacol 125: 767-770. doi:10.1038/sj.bjp.0702158. PubMed: 9831913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brenna JT, Corso TN, Tobias HJ, Caimi RJ (1997) High-precision continuous-flow isotope ratio mass spectrometry. Mass Spectrom Rev 16: 227-258. doi:10.1002/(SICI)1098-2787(1997)16:5. PubMed: 9538528. [DOI] [PubMed] [Google Scholar]
- 27. Godin JP, Hopfgartner G, Fay L-B (2007) Liquid Chromatography combined with Mass Spectrometry for 13 C isotopic analysis in Life Science Research. Mass Spectrom Rev 26: 751-774. doi:10.1002/mas.20149. PubMed: 17853432. [DOI] [PubMed] [Google Scholar]
- 28. Abelson PH, Hoering TC (1961) Carbon isotope fractionation in formation of amino acids by photosynthetic organisms. Proc Natl Acad Sci U S A 47: 623-632. doi:10.1073/pnas.47.5.623. PubMed: 13681011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalhan SC, Savin SM, Adam PA (1977) Estimation of glucose turnover with stable tracer glucose 1-13C. J Lab Clin Med 89: 285-294. [PubMed] [Google Scholar]
- 30. Lee WN, Bassilian S, Lim S, Boros LG (2000) Loss of regulation of lipogenesis in the Zucker diabetic (ZDF) rat. Am J Physiol Endocrinol Metab 279: E425-E432. PubMed: 10913044. [DOI] [PubMed] [Google Scholar]
- 31. Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S (2003) What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes 52: 1641-1648. doi:10.2337/diabetes.52.7.1641. PubMed: 12829627. [DOI] [PubMed] [Google Scholar]
- 32. Rhee SK, Reed RG, Brenna JT (1997) Fatty acid carbon isotope ratios in humans on controlled diets. Lipids 32: 1257-1263. doi:10.1007/s11745-006-0161-6. PubMed: 9438235. [DOI] [PubMed] [Google Scholar]
- 33. Cunnane SC, Ryan MA, Craig KS, Brookes S, Koletzko B et al. (1995) Synthesis of linoleate and alpha-linolenate by chain elongation in the rat. Lipids 30: 781-783. doi:10.1007/BF02537807. PubMed: 7475996. [DOI] [PubMed] [Google Scholar]
- 34. Hayes JM (2001) Fractionation of the isotopes of carbon and hydrogen in biosynthetic processes. Rev Mineral Geochem 43: 225-277. doi:10.2138/gsrmg.43.1.225. [Google Scholar]
- 35. Bassilian S, Ahmed S, Lim SK, Boros LG, Mao CS et al. (2002) Loss of regulation of lipogenesis in the Zucker diabetic rat. II. Changes in stearate and oleate synthesis. Am J Physiol Endocrinol Metab 282: E507-E513. [DOI] [PubMed] [Google Scholar]
- 36. Brenna JT (2001) Natural intramolecular isotope measurements in physiology: elements of the case for an effort toward high-precision position-specific isotope analysis. Rapid Commun Mass Spectrom 15: 1252-1262. doi:10.1002/rcm.325. PubMed: 11466780. [DOI] [PubMed] [Google Scholar]
- 37. Friedman MI (1998) Fuel partitioning and food intake. Am J Clin Nutr 67: 513S-518S. PubMed: 9497162. [DOI] [PubMed] [Google Scholar]
- 38. Corpeleijn E, Saris WH, Blaak EE (2009) Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev 10: 178-193. doi:10.1111/j.1467-789X.2008.00544.x. PubMed: 19207879. [DOI] [PubMed] [Google Scholar]
- 39. Godin JP, Stellingwerff T, Actis-Goretta L, Mermoud AF, Kochhar S et al. (2011) The role of liquid chromatography and flow injection analyses coupled to isotope ratio mass spectrometry for studying human in vivo glucose metabolism. Rapid Commun Mass Spectrom 25: 2989-2994. doi:10.1002/rcm.5179. PubMed: 21953953. [DOI] [PubMed] [Google Scholar]
- 40. Masood A, Stark KD, Salem N Jr (2005) A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J Lipid Res 46: 2299-2305. doi:10.1194/jlr.D500022-JLR200. PubMed: 16061957. [DOI] [PubMed] [Google Scholar]