Abstract
Background
The ability to simulate in silico experiments is crucial for fast and cost-effective preliminary studies prior to clinical trials. We present an in silico approach to the design of optimal pramlintide-to-insulin (P/I) ratios, using our computer simulator of the human metabolic system, with a population of virtual adult type 1 diabetes mellitus patients and with individual parameters modified to account for the dynamic effects of pramlintide.
Materials and Methods
A model of pramlintide action on gastric emptying was built using data of 15 type 1 diabetes mellitus subjects studied twice with a standardized dual-tracer meal on placebo and pramlintide, which was incorporated in our type 1 diabetes simulator. Extensive in silico experiments on 100 virtual subjects were performed to optimize the co-administration of pramlintide and insulin prior to its submission to clinical trials; several P/I ratios were tested in terms of efficacy, in attenuating postprandial hyperglycemia, and in hypoglycemia safety.
Results
In silico experiments estimated the optimal P/I ratio to be 9 μg of pramlintide per unit (U) of insulin. Additional simulations narrowing the investigated range indicated that P/I ratios of 8 and 10 μg/U would achieve similar performance. Moreover, simulation results suggested that in clinical trials, insulin boluses should be reduced by approximately 21% at a P/I ratio of 9 μg/U to account for the effects of pramlintide and avoid postprandial hypoglycemia.
Conclusions
We can assert that a valid simulation model of pramlintide action was developed, leading to in silico estimation of optimal pramlintide:insulin co-administration ratio. Clinical trials will confirm (or adjust) this initial estimation.
Introduction
In the past few decades, computer simulation and computer-aided design have made dramatic progress in all areas of development of complex engineering systems. In medicine, realistic computer simulation is capable of providing valuable information about the effectiveness, the safety, and the limitations of various treatments. The emerging field of in silico drug design is based on the recognition that the traditional route to drug discovery and development is a very time- and resource-consuming process.1 As a result, there is a growing effort to apply computational power to streamlining drug discovery, design, development, and optimization.2 Simulation allows experiments with extreme situations that are unrealistic in animals or clinically impossible in humans. Various treatment scenarios can be efficiently tested and either rejected or accepted for inclusion in subsequent clinical studies, which allows for rapid and cost-effective clinical trial design.3 For instance, accurate prediction of clinical outcomes was done by the Archimedes diabetes model.4,5
Typically, the in silico models of the human metabolism are compartmental (e.g., they represent the human body as a set of homogeneous compartments of specific concentrations and volumes linked by diffusion or rate-limited pathways). Classical examples include the Widmark6 model of ethanol pharmacokinetics, which offers a straightforward interpretation with a constant ethanol clearance rate and the human body modeled as one compartment, or the more complex minimal model of glucose kinetics suggested by Bergman et al.7 more than 30 years ago to assess insulin sensitivity in health and diabetes. Deeper understanding of the processes involved and novel measuring tools have allowed more precise measurement of glucose–insulin dynamics and the development of sophisticated nonlinear models, such as the vastly complex meal model,8 which now serves as the basis for the most comprehensive computer simulator of the human metabolic system available to date.9–11
This simulator was developed with the specific goal to facilitate the preclinical testing of treatment strategies in diabetes. The simulation environment encompasses several metabolic subsystems, including gastrointestinal tract, renal function, hepatic glucose production, and others.8,9 Furthermore, the simulation system was equipped with in silico images of the metabolic systems of 300 subjects with type 1 diabetes in three age groups: children, adolescents, and adults. Each in silico image is a set of 26 parameters uniquely identifying the metabolic system of a person. The distribution of these parameters across the general population provides the variability needed for defining the in silico population.10 When the in silico model and the virtual population were built, the computer simulation of the observed biosystem became possible, leading to in silico trials involving virtual “subjects” rather than real people. Because such in silico trials can serve as cost-effective precursors guiding expensive and time-consuming clinical investigations by ruling out ineffective treatment approaches, in January 2008 this simulator received Food and Drug Administration (FDA) acceptance for the preclinical testing of control strategies in artificial pancreas studies.12 The simulator was immediately put to its intended use: since 2008 several scientific publications have reported in silico results from the testing of various insulin treatment strategies,8–11 and several Investigational Device Exemptions were granted by the FDA for closed-loop clinical trials, using solely in silico experiments to test and validate their control systems. Two reviews3,11 have presented the wide array of modeling approaches developed by us and others and their translation into the clinical practice. Thus, we were able to assert that (1) in silico modeling can produce credible preclinical results and (2) in silico testing yields results in a fraction of the time and resources required for animal trials.
Pramlintide is a synthetic analog of human amylin: a 37-amino-acid peptide hormone that is co-located with insulin in secretory granules and co-secreted by pancreatic β-cells in response to nutrient ingestion. Amylin has been shown to contribute to glucose control during the postprandial period by inhibiting glucagon secretion, slowing gastric emptying and reducing food intake via increased satiety, resulting in decreased appetite.13 However, like insulin, amylin is deficient in individuals with diabetes requiring insulin therapy.14 In these patients, maintaining postprandial glucose concentrations within the desired range remains challenging despite the introduction of rapid-acting insulin analogs and enhanced insulin delivery systems. Even so, it has been shown that pramlintide administration restores the effects of amylin and, in turn, lowers average postprandial blood glucose levels, substantially reduces postprandial blood glucose excursions, and decreases food intake, resulting in a reduction in mealtime insulin requirements.15–22 Pramlintide has been approved by the FDA, for use by patients with type 1 and type 2 diabetes who use mealtime insulin. The therapeutic effects and safety profile of pramlintide, when used with various rapid-acting insulin formulations, are well characterized. In pilot experiments, continuous subcutaneous co-administration of insulin and pramlintide appeared to be safe and well tolerated and reduced both fasting and postprandial glucose levels.23–25 However, an optimal pramlintide-to-insulin (P/I) ratio is yet to be determined.
In this article, in an effort to guide the development of a co-formulated pramlintide/insulin product, we present an in silico approach to the identification of optimal P/I ratios for use preprandially. We use our computer simulation environment with the population of virtual adults with type 1 diabetes and with individual parameters modified to account for the dynamic effects of pramlintide as observed in the clinic.26,27
Materials and Methods
Dataset
A previously published dataset27 was used to model the effect of pramlintide on glucose meal rate of appearance. The data included 15 subjects with type 1 diabetes (eight men and seven women, 37±2 years of age, body weight 76±3 kg). The study design included a standardized meal test containing 50 g of glucose enriched with 3 g of 6,6-dideuteroglucose, administered with and without 30 μg of pramlintide injected subcutaneously in the lower abdominal wall. Over the initial 90 min of the postprandial period, blood samples were taken at 15-min intervals and thereafter at 30-min intervals until completion of the experiment at 330 min. Concentrations of plasma glucose, tracer glucose, and insulin were measured. A detailed description of the subjects, protocol, and methods can be found in Woerle et al.27
Modeling the effect of pramlintide
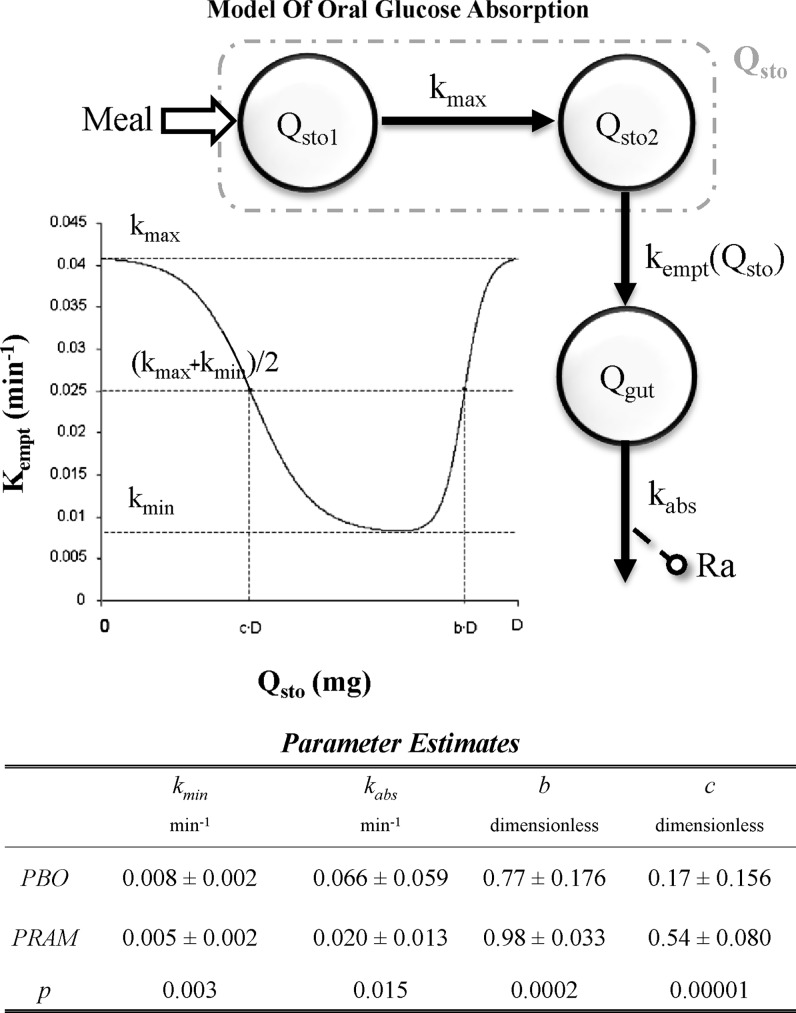
A model of oral glucose absorption (Fig. 1, upper panel) based on the previously introduced meal model8 was used to quantify the effect of pramlintide on meal glucose rate of appearance (Ra). In brief, glucose transit through the stomach (Qsto) and intestine was modeled by two compartments, one for solid (Qsto1) and one for liquid (Qsto2) phase, whereas a single compartment was used to describe the gut (Qgut). kgri (min−1) is the rate of grinding, kempt(Qsto) (min−1) the rate constant of gastric emptying (a nonlinear function of Qsto), and kabs (min−1) the rate constant of intestinal absorption. Ra (mg/kg/min) is the rate of appearance of glucose in plasma. The parameter kgri was assumed equal to kmax.8 The model was numerically identified on placebo and pramlintide, yielding the parameter estimates presented in Figure 1, lower panel.
FIG. 1.
(Upper panel) Scheme of the oral glucose absorption model. kabs, the rate constant of intestinal absorption; kempt(Qsto), the rate constant of gastric emptying; kmax and kmin, maximum and minimum rate constant, respectively; Qgut, glucose transit through the gut; Qsto, glucose transit through the stomach, one for solid (Qsto1) and one for liquid (Qsto2); Ra, rate of appearance. (Lower panel) Parameter estimates of the oral glucose absorption model. Data are average±SD values. PBO, placebo.
The relative variation of gastrointestinal tract parameters due to pramlintide was calculated as:
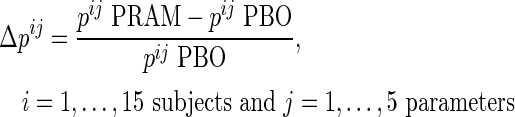 |
where 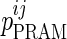 is the value of parameter j estimated in the presence of pramlintide (PRAM) and
is the value of parameter j estimated in the presence of pramlintide (PRAM) and 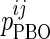 is the value of parameter j estimated in the absence of pramlintide (placebo [PBO]) for subject i.
is the value of parameter j estimated in the absence of pramlintide (placebo [PBO]) for subject i.
Assuming normality, joint parameter distribution was derived from mean and the covariance of Δpij. To achieve intersubject variability, we used this parameter distribution to randomly generate n=100 distinct sets of parameters (i.e., n=100 in silico “adults” with type 1 diabetes responding to pramlintide). Thus, the effect of pramlintide on Ra was incorporated in the simulator via modification of the parameters of the virtual subjects corresponding to the empirically determined effects of administration of pramlintide.27
In silico experiments
The simulation scenario included a meal with a carbohydrate content of 50 g given at 8:00 h to n=100 in silico adults with type 1 diabetes. Concurrent with the meal, placebo and several different P/I ratios were administered: P/I=3, 6, 8, 9, 10, and 12 μg of pramlintide/unit (U) of insulin (μg/U). On placebo, the virtual subjects received a premeal insulin bolus based on each individual's insulin-to-carbohydrate ratio (ICR). With pramlintide, two in silico experiments were performed.
Experiment 1
The virtual subjects received a premeal insulin bolus identical to the bolus used on placebo, without adjustment for the effects of pramlintide. As seen in the next section this resulted in postprandial hypoglycemia, which necessitated Experiment 2.
Experiment 2
The virtual subjects received premeal insulin bolus lowered to account for the effects of pramlintide. The adjustment of the insulin dose (i.e., of each individual's ICR) was done iteratively for each subject, until postprandial hypoglycemia due to insulin overdose was avoided. Then the same P/I ratios were administered again.
Data analysis
Efficacy (attenuation of postprandial hyperglycemia) and safety (reduction of hypoglycemia) of different P/I ratios have been quantitatively evaluated using control variability-grid analysis (CVGA).28 In addition, to select the best P/I candidates, a CVGA Zone Quotient index was calculated using the formula:
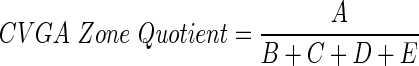 |
where A, B, C, D, and E are the percentages of subjects in the CVGA Zones A–E.28
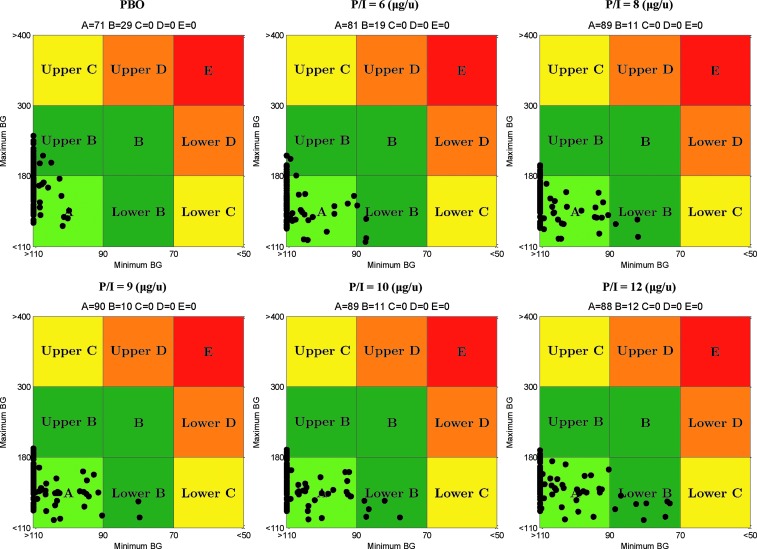
CVGA provides a simultaneous visual and numerical assessment of the overall quality of glycemic regulation in the entire population. In particular, CVGA zones represent accurate control (Zone A), benign deviation into hyper- or hypoglycemia (Zone B), overcorrection of hyper- or hypoglycemia (Zone C), failure to deal with hyper- or hypoglycemia (Zone D), and erroneous control (Zone E).28
Results
Prediction of glucose Ra
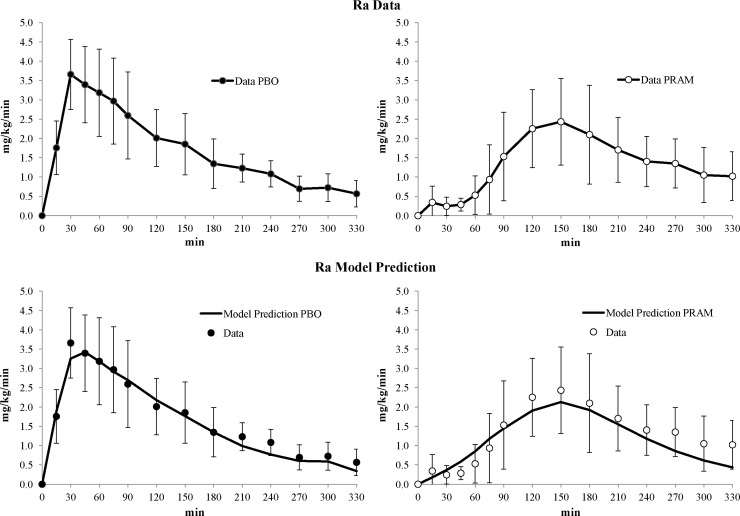
Figure 2 compares the experimentally observed Ra of glucose on placebo and pramlintide (upper panel) and the model-predicted Ra on placebo and pramlintide (lower panel), indicating good agreement between experimental data and model prediction, and therefore model validity.
FIG. 2.
(Upper panels) Average glucose meal rate of appearance (Ra) data with (left) placebo (PBO) and (right) pramlintide (PRAM). (Lower panels) Average oral glucose absorption model prediction (solid line) versus data with (left) PBO (solid dots) and (right) PRAM (white dots). Vertical bars represent SD within the 15 type 1 diabetes mellitus study subjects.27
A comparison of the rate parameters kmin (gastric emptying) and kabs (intestinal absorption) off and on pramlintide indicated a significantly slower gastric emptying and intestinal absorption rates on pramlintide (P=0.003 and P=0.015, respectively) (Fig. 1, lower panel). Comparing parameters b and c shows a significantly increased quantity of glucose retained by the stomach on pramlintide (P=0.0002 and P=0.0001), respectively.
Experiment 1: no adjustment of insulin for pramlintide effect
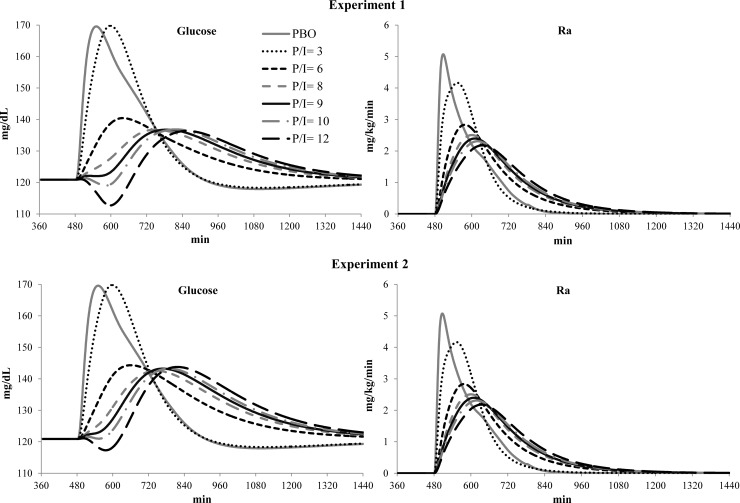
Figure 3, upper panel, presents the average simulated profiles of plasma glucose concentration and glucose Ra for placebo and all tested P/I ratios. The traces indicate that a P/I ratio of 3 was not better than insulin alone (placebo)—it delayed slightly the postprandial blood glucose peak but did not attenuate its magnitude. For P/I ratios of 6, 8, 9, 10, and 12 μg/U, hypoglycemic events occurred in 9%, 10%, 11%, 12%, and 15% of the in silico subjects; thus subjects' ICRs had to be adjusted to compensate for the effects of pramlintide.
FIG. 3.
(Upper panels) Experiment 1 and (lower panels) Experiment 2 average simulated (left) plasma glucose concentrations and (right) rate of appearance (Ra) for placebo (PBO) and pramlintide-to-insulin (P/I) ratios of 3, 6, 8, 9, 10, and 12 μg/unit.
Experiment 2: individual ICR adjusted for pramlintide effects
Figure 3, lower panel, presents average simulated profiles of plasma glucose concentrations and meal Ra, after ICR adjustment. As seen in Figure 3, lower-left panel, hypoglycemia, typically occurring 2 h after premeal insulin, was significantly attenuated, which allowed further evaluation of the relative effectiveness of the various P/I ratios in Experiment 2. We found that:
A P/I ratio of 3 was no more effective than placebo, with 62% versus 71% in CVGA Zone A.
P/I ratios of 6, 8, 9, 10, and 12 μg/U resulted in significant improvements of postprandial glucose control: 81%, 89%, 90%, 89%, and 88% of subjects in CVGA Zone A, respectively, and no hypoglycemia, as shown in Figure 4.
FIG. 4.
Control variability-grid analysis for placebo and pramlintide-to-insulin (P/I) ratios of 6, 8, 9, 10, and 12 μg/unit. Maximum and minimum blood glucose (BG) units are in mg/dL.
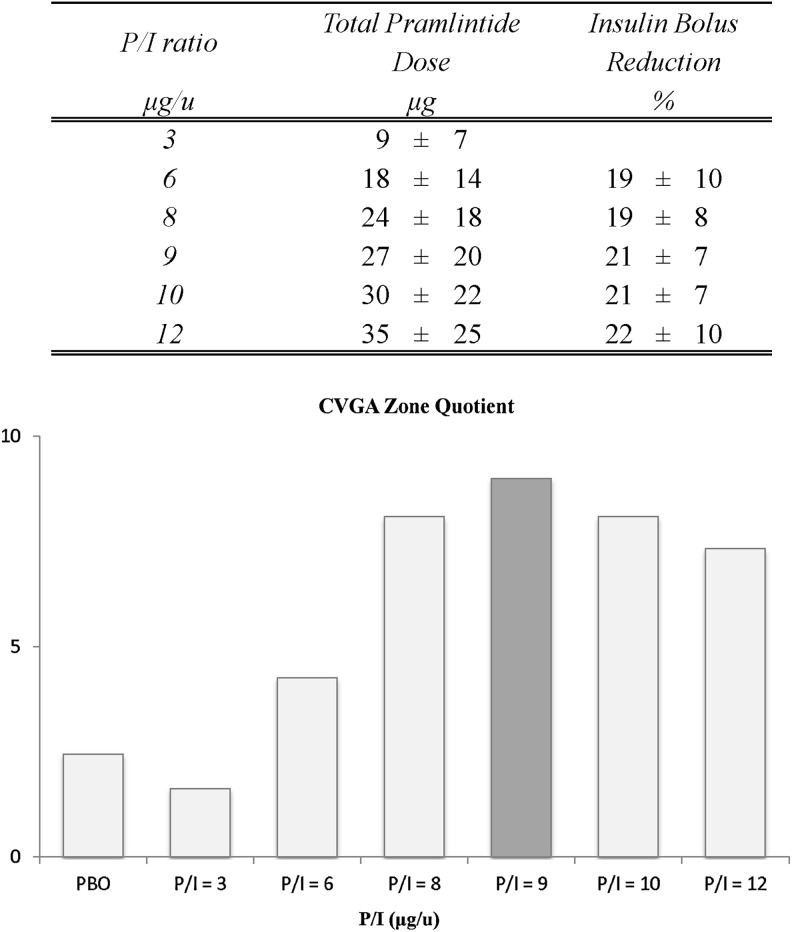
During Experiment 2, the ICR ratio was increased on average by a mean of 26±2%, which resulted in an average reduction in insulin dosing of 20±2% for P/I ratios of 6–12. In Figure 5, upper panel, the total average dose expressed in micrograms of pramlintide administered with the meal and the average percentage reduction in insulin bolus needed to minimize hypoglycemia at different P/I ratios are reported for the different P/I ratios.
FIG. 5.
(Upper panel) Total average dose of pramlintide administered with the meal for the different pramlintide-to-insulin (P/I) ratios and average percentage reduction in insulin bolus needed to minimize hypoglycemia at different P/I ratios. (Lower panel) Control variability-grid analysis (CVGA) Zone Quotient indexes. The darker column indicates the optimal P/I ratio in terms of efficacy and safety.
The CVGA Zone Quotient is shown in Figure 5, lower panel, for placebo, and P/I ratios ranging from 3 to 12 μg/U. The Zone Quotient peaked at a P/I ratio of 9 μg/U, indicating that this would be an optimal mixture of pramlintide and insulin in terms of safety and efficacy.
Discussion
Despite advances in insulin formulations and improvements in insulin delivery and glucose monitoring devices, as well as robust data demonstrating the importance of good glycemic control, multiple recent clinical studies have shown that the majority of patients with type 1 diabetes are not achieving A1C goals with insulin alone; this holds true for observational studies, randomized controlled trials, and registries.29–33 Given that we are now over 20 years past the publication of the landmark results from the Diabetes Control and Complications Trial,34 these data are a powerful indicator that insulin alone is not adequate to achieve the desired level of control in the general population. Major obstacles to achieving this goal include the fear of hypoglycemia and weight gain, which limit the willingness of patients and/or their providers to use more insulin. Furthermore, for approximately 20% of patients who do achieve goal, they do so by maintaining a constant awareness of the blood glucose levels, analyzing the impact of what they have done recently, what they plan to eat, and what they anticipate doing during the next 12-h period. Patients who are not able to practice this level of diligence, for whatever reason, are unlikely to succeed.29
The difficulty in controlling glucose concentration with insulin alone is somewhat expected because glucose regulation in health is achieved by complex feedback mechanisms involving several other hormones (e.g., counterregulatory hormones and amylin). Amylin, a second peptide hormone secreted with insulin by pancreatic β-cells, suppresses glucagon secretion during the postprandial period,16 slows gastric emptying,35 which decreases the rate of appearance of ingested nutrients in the peripheral circulation, and reduces food intake by a centrally mediated mechanism.19 These effects, which work to improve glucose control during the postprandial period, resulting in a reduction in the dose of short-acting mealtime insulin required, are restored by preprandial administration of pramlintide, an analog of human amylin. Pramlintide was approved by the FDA as an adjunct to insulin to improve glycemic control in patients who use mealtime insulin. However, in its current formulation, pramlintide must be administered as a separate injection, increasing the patient's injection burden, and can only be given as a fixed dose regardless the insulin dose. Because amylin and insulin are secreted in a fixed molar ratio by the healthy human pancreas,14 the potential to co-formulate pramlintide with short-acting insulin is attractive as it may come closer to mimicking normal physiology and it would reduce the number of injections required. A fixed-ratio product could also potentially be administered by a subcutaneous infusion similar to today's insulin pumps.
Designing a clinical study that would identify an appropriate fixed ratio for pramlintide and insulin would be prohibitively expensive because countless dose ratios would need to be tested. A cost-efficient alternative is in silico experimentation—an increasing trend in drug design, typically including bioinformatics techniques that allow rapid selection of drug candidates based on high-throughput search in massive databases containing molecules, molecular fragments, or molecular structures.1 In this article the meaning of in silico drug optimization is different: a model of the human metabolic system is used to study the co-dynamics of a combination of compounds with separately known kinetic properties. In that sense, the approach presented here is new and only possible because of recent advances in the in silico modeling of insulin–glucose dynamics in diabetes.3,10 The availability of data from sophisticated tracer studies26,27 allowed the reconstruction of the effects of pramlintide on the glucose rate of appearance following standardized meals. In turn, this allowed for dynamic modeling of the action of pramlintide on the gastrointestinal function and for the formal description of its dynamics via differential equations. A key element of this description is the assembly of an in silico population—a comprehensive set of virtual individuals with widespread characteristics (individual parameters) approximating the variability of pramlintide action observed in vivo.
Thus, deriving from previous technology developments and recent studies of pramlintide dynamics, we have updated our simulator of type 1 diabetes to incorporate the possibility of “administering” pramlintide to our virtual “subjects.” Using this technology, extensive in silico experiments have been performed to optimize the co-administration of pramlintide and insulin prior to its submission to clinical trials. The optimal P/I ratio was estimated to be 9 μg of pramlintide/U of insulin. Additional simulations narrowing the investigated range indicated that P/I ratios of 8 and 10 μg/U would achieve similar performance.
During the simulations, a well-known clinical observation was reproduced: to avoid postprandial hypoglycemia, insulin doses needed to be reduced by approximately 21% at the P/I ratio of 9 μg/U, which is consistent with the reduction of insulin during the titration of pramlintide in clinical practice.21,22 This in silico effect was not preprogrammed—it was a result from the simulation experiments, further confirming the validity of our model.
Although modeling can certainly provide insight into individualization of the P/I ratio and pramlintide doses, the purpose of this study was to suggest a generally optimal ratio, usable in a future co-formulation of the two compounds to be delivered as a single mixture. We can therefore conclude that a valid simulation model of the action of pramlintide was developed, leading to in silico estimation of optimal pramlintide–insulin co-administration ratio. Clinical trials are needed to confirm (or adjust) this initial estimation.
Acknowledgments
This work was supported by grant 15-2011-45 (“Insulin-Pramlintide Combination for the Treatment of Type I Diabetes”) from the JDRF to Amylin.
Author Disclosure Statement
O.K., E.C., and K.H are employees of Bristol-Myers Squibb. F.M., C.D.M., B.K., and C.C. hold a pending patent on the simulation model. J.S. declares no competing financial interests exist.
References
- 1.Van De Waterbeemd H. Gifford E. ADMET in silico modelling: towards prediction paradise? Nat Rev Drug Discov. 2003;2:192–204. doi: 10.1038/nrd1032. [DOI] [PubMed] [Google Scholar]
- 2.Kapetanovic IM. Computer-aided drug discovery and development (CADDD): in silico-chemico-biological approach. Chem Biol Interact. 2008;171:165–176. doi: 10.1016/j.cbi.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobelli C. Dalla Man C. Sparacino G. Magni L. Nicolao G. Kovatchev BP. Diabetes: models, signals, and control. IEEE Rev Biomed Eng. 2009;2:54–96. doi: 10.1109/RBME.2009.2036073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddy DM. Schlessinger L. Archimedes: a trial-validate model of diabetes. Diabetes Care. 2003;26:3093–3101. doi: 10.2337/diacare.26.11.3093. [DOI] [PubMed] [Google Scholar]
- 5.Eddy DM. Schlessinger L. Validation of the Archimedes diabetes model. Diabetes Care. 2003;26:3102–3110. doi: 10.2337/diacare.26.11.3102. [DOI] [PubMed] [Google Scholar]
- 6.Widmark EMP. Die Theoretischen Grundlagen und die Praktische Verwendbarkeit der Gerichtlich-Medizinischen Alkoholbestimmung. Berlin: Urban & Schwarzenberg; 1932. [Google Scholar]
- 7.Bergman RN. Ider YZ. Bowden CR. Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 8.Dalla Man C. Rizza RA. Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007;54:1740–1749. doi: 10.1109/TBME.2007.893506. [DOI] [PubMed] [Google Scholar]
- 9.Dalla Man C. Raimondo DM. Rizza RA. Cobelli C. GIM, simulation software of meal glucose-insulin model. J Diabetes Sci Technol. 2007;1:323–330. doi: 10.1177/193229680700100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovatchev BP. Breton M. Man CD. Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3:44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobelli C. Renard E. Kovatchev BP. Perspectives in diabetes: artificial pancreas: past, present, future. Diabetes. 2011;60:2672–2682. doi: 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovatchev BP. Breton MD. Dalla Man C. Cobelli C. Food and Drug Administration Master File MAF 1521. Rockville, MD: Food and Drug Administration; 2008. Silico Model and Computer Simulation Environment Approximating the Human Glucose/Insulin Utilization. [Google Scholar]
- 13.Pullman J. Darsow T. Frias JP. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vasc Health Risk Manag. 2006;2:203–220. doi: 10.2147/vhrm.2006.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruger DF. Gatcomb PM. Owen SK. Clinical implications of amylin and amylin deficiency. Diabetes Educ. 1999;25:389–397. doi: 10.1177/014572179902500310. [DOI] [PubMed] [Google Scholar]
- 15.Vella A. Lee JS. Camilleri M. Szarka LA. Burton DD. Zinsmeister AR. Rizza RA. Klein PD. Effects of pramlintide, an amylin analogue, on gastric emptying in type 1 and 2 diabetes mellitus. Neurogastroenterol Motil. 2002;14:123–131. doi: 10.1046/j.1365-2982.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 16.Fineman MS. Koda JE. Shen LZ. Strobel SA. Maggs DG. Weyer C. Kolterman OG. The human amylin analog, pramlintide, corrects postprandial hyperglucagonemia in patients with type 1 diabetes. Metabolism. 2002;51:636–641. doi: 10.1053/meta.2002.32022. [DOI] [PubMed] [Google Scholar]
- 17.Levetan C. Want LL. Weyer C. Strobel SA. Crean J. Wang Y. Maggs DG. Kolterman OG. Chandran M. Mudaliar SR. Henry RR. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care. 2003;26:1–8. doi: 10.2337/diacare.26.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Weyer C. Gottlieb A. Kim D. Lutz K. Swartz S. Gutierrez M. Wang Y. Ruggles J. Kolterman O. Maggs D. Pramlintide reduces postprandial glucose excursions when added to regular insulin or insulin lispro in subjects with type 1 diabetes. Diabetes Care. 2003;26:3074–3079. doi: 10.2337/diacare.26.11.3074. [DOI] [PubMed] [Google Scholar]
- 19.Chapman I. Parker B. Doran S. Feinle-Bisset C. Wishart J. Strobel S. Wang Y. Burns C. Lush C. Weyer C. Horowitz M. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia. 2005;48:838–848. doi: 10.1007/s00125-005-1732-4. [DOI] [PubMed] [Google Scholar]
- 20.Fineman MS. Koda JE. Shen LZ. Strobel SA. Maggs DG. Weyer C. Kolterman OG. The human amylin analog, pramlintide, corrects postprandial hyperglucagonemia in patients with type 1 diabetes. Metabolism. 2002;51:636–641. doi: 10.1053/meta.2002.32022. [DOI] [PubMed] [Google Scholar]
- 21.Edelman S. Garg S. Frias J. Maggs D. Wang Y. Zhang B. Strobel S. Lutz K. Kolterman O. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care. 2006;29:2189–2195. doi: 10.2337/dc06-0042. [DOI] [PubMed] [Google Scholar]
- 22.Karl D. Philis-Tsimikas A. Darsow T. Lorenzi G. Kellmeyer T. Lutz K. Wang Y. Frias JP. Pramlintide as an adjunct to insulin in patients with type 2 diabetes in a clinical practice setting reduced A1C, postprandial glucose excursions, and weight. Diabetes Technol Ther. 2007;9:191–199. doi: 10.1089/dia.2006.0013. [DOI] [PubMed] [Google Scholar]
- 23.Huffman DM. McLean GW. Seagrove MA. Continuous subcutaneous pramlintide infusion therapy in patients with type 1 diabetes: observations from a pilot study. Endocr Pract. 2009;15:689–695. doi: 10.4158/EP09044.ORR1. [DOI] [PubMed] [Google Scholar]
- 24.Heptulla RA. Rodriguez LM. Mason KJ. Haymond MW. Twenty-four-hour simultaneous subcutaneous basal-bolus administration of insulin and amylin in adolescents with type 1 diabetes decreases postprandial hyperglycemia. J Clin Endocrinol Metab. 2009;94:1608–1611. doi: 10.1210/jc.2008-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schorr AB. Ofan R. Simultaneous use of two external subcutaneous pumps delivering insulin and SYMLIN: use of a double-pump system. J Diabetes Sci Technol. 2012;6:1507–1508. doi: 10.1177/193229681200600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woerle HJ. Albrecht M. Linke R. Zschau S. Neumann C. Nicolaus M. Gerich J. Goke B. Schirra J. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab. 2008;294:E103–E109. doi: 10.1152/ajpendo.00514.2007. [DOI] [PubMed] [Google Scholar]
- 27.Woerle HJ. Albrecht M. Linke R. Zschau S. Neumann C. Nicolaus M. Gerich JE. Göke B. Schirra J. Impaired hyperglycemia-induced delay in gastric emptying in patients with type 1 diabetes deficient for islet amyloid polypeptide. Diabetes Care. 2008;31:2325–2331. doi: 10.2337/dc07-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magni L. Raimondo DM. Man CD. Breton M. Patek S. Nicolao GD. Cobelli C. Kovatchev BP. Evaluating the efficacy of closed-loop glucose regulation via control-variability grid analysis. J Diabetes Sci Technol. 2008;2:630–635. doi: 10.1177/193229680800200414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Nathan DM. Zinman B. Cleary PA. Backlund JY. Genuth S. Miller R. Orchard TJ. Modern-day clinical course of type 1 diabetes mellitus after 30 years' duration: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications experience. Arch Intern Med. 2009;27:1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eeg-Olofsson K. Cederholm J. Nilsson PM. Gudbjörnsdóttir S. Eliasson B. Steering Committee of the Swedish National Diabetes Register: Glycemic and risk factor control in type 1 diabetes: results from 13,612 patients in a national diabetes register. Diabetes Care. 2007;30:496–502. doi: 10.2337/dc06-1406. [DOI] [PubMed] [Google Scholar]
- 31.Bergenstal RM. Tamborlane WV. Ahmann A. Buse JB. Dailey G. Davis SN. Joyce C. Peoples T. Perkins BA. Welsh JB. Willi SM. Wood MA STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 32.JDRF CGM Study Group: Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 33.Beck RW. Tamborlane WV. Bergenstal RM. Miller KM. Dubose SN. Hall CA T1D Exchange Clinic Network. The T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2012;97:4383–4389. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 34.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 35.Kong MF. Stubbs TA. King P. Macdonald IA. Lambourne JE. Blackshaw PE. Perkins AC. Tattersall RB. The effect of single doses of pramlintide on gastric emptying of two meals in men with IDDM. Diabetologia. 1998;41:577–583. doi: 10.1007/s001250050949. [DOI] [PubMed] [Google Scholar]