Abstract
Background
Short-term continuous subcutaneous insulin infusion (CSII) in patients with newly diagnosed type 2 diabetes has been proved effective in improving metabolic control and β-cell function, thus inducing long-term drug-free remission. A randomized controlled trial was conducted to investigate whether CSII in combination with rosiglitazone, metformin, or α-lipoic acid separately brings about extra benefits.
Patients and Methods
One hundred sixty patients with newly diagnosed type 2 diabetes were randomized to one of four treatment groups: CSII alone, CSII in combination with rosiglitazone or metformin for 3 months, or CSII with α-lipoic acid intravenous infusion for 2 weeks. Duration of CSII treatment was identical in the four groups. Glucose and lipid profiles, homeostasis model assessment (HOMA) indices, acute insulin response (AIR), intramyocellular lipid (IMCL) level, and malondialdehyde level were compared before and after intervention.
Results
The near-normoglycemia rate at the third month in CSII alone and that in combination with rosiglitazone, metformin, or α-lipoic acid was 72.5%, 87.5%, 90%, and 75%, respectively (metformin group vs. CSII alone, P=0.045). The metformin group achieved euglycemia in a shorter time (2.6±1.3 vs. 3.7±1.8 days, P=0.020) with less daily insulin dosage and was more powerful in lowering total cholesterol, increasing AIR and HOMA β-cell function, whereas reduction of IMCL in the soleus was more obvious in the rosiglitazone group but not in the metformin group. The efficacy of combination with α-lipoic acid was similar to that of CSII alone.
Conclusions
Short-term CSII in combination with rosiglitazone or metformin is superior to CSII alone, yet the efficacy of the two differs in some way, whereas that with α-lipoic acid might not have an additive effect.
Introduction
Since Ilkova et al.1 first reported the use of short-term continuous subcutaneous insulin infusion (CSII) in patients with newly diagnosed type 2 diabetes mellitus in 1997, their idea of transient intensive insulin treatment inducing long-term drug-free glycemic remission has been put into clinical practice, and evidence has accumulated for its merit.2–5 Although the idea was questioned by some for its feasibility and laboriousness in practice,6 we showed in our previous studies that short-term CSII treatment not only restores part of the β-cell function and insulin sensitivity, but also has positive impact on patients' attitudes toward diabetes, which in turn is beneficial to the maintenance of long-term drug-free remission.7 In a multicenter randomized parallel-group trial, we demonstrated that in newly diagnosed type 2 diabetes patients treated with short-term CSII, 51.1% of them maintained drug-free remission after 1 year, a value significantly higher than that in the oral hypoglycemic agents group (26.7%; P=0.0012).3 However, nearly half of the patients receiving the same treatment relapsed in less than 1 year. The principle of short-term intensive CSII treatment is to reverse β-cell deficiency and insulin resistance by rapid correction of glucotoxicity and lipotoxicity, the latter of which was associated with chronic oxidative stress. We hypothesize that combination therapy to further enhance β-cell function as well as insulin sensitivity might be warranted to improve the long-term drug-free remission rate.
Rosiglitazone and metformin (MET) are both well-documented insulin sensitizers with different working pathways. We conjectured that combination of the two drugs with short-term CSII separately might demonstrate different effects on improving metabolic control and insulin sensitivity and thus might have different additive effect on long-term remission. An emerging body of evidence has indicated that oxidative stress induced by chronic hyperglycemia plays an important role in the etiology of β-cell damage and peripheral insulin resistance by impairing mitochondrial function as well as insulin signaling. α-Lipoic acid is a potent antioxidant with anti-inflammatory and AMP-activated protein kinase-activating properties, which exhibits therapeutic value in type 2 diabetes and its complications.8,9 Combination of α-lipoic acid with intensive insulin treatment to normalize hyperglycemia rapidly might have an extra effect on alleviating oxidative stress as well as improving β-cell function and insulin sensitivity.
We therefore did a randomized controlled trial of short-term CSII in combination with the insulin sensitizer rosiglitazone or MET or the antioxidant α-lipoic acid separately using CSII alone as a control to compare the efficacy and remission rate at 1 year. The rationale of study design and preliminary data of the first 3 months are reported.
Subjects and Methods
Subjects
Newly diagnosed patients with type 2 diabetes mellitus, according to the 1999 World Health Organization diagnostic criteria, who had not previously received any antidiabetes medication were recruited. The inclusion patients were between 25 and 70 years old, with fasting plasma glucose (FPG) between 7.0 and 16.7 mmol/L and had a body mass index of 21–35 kg/m2. Fundus photographs, urine tests for albumin excretion rate, routine Semmes–Weinstein monofilament, and tuning fork vibration tests were performed to screen for microvascular complications. Patients were excluded if they had severe acute complications or chronic complications of kidney disease due to diabetes (albumin excretion rate >20 μg/min) or diabetic retinopathy, which indicated that the disease was not of recent onset. Those patients with intercurrent illness, who tested positive for autoimmune antibodies to islet, or with a history of macrovascular complications such as stroke or heart attack were also excluded.
Study design
In a 3–7-day run-in period before hospitalization, a preliminary evaluation on diet and exercise habits was performed; as well, a Diabetes Care Profile questionnaire on attitudes toward diabetes was completed by each patient. Inclusion and exclusion criteria were reviewed. Informed consent was signed. Then inclusion patients were randomly assigned to one of four treatment groups. The first three groups received CSII alone or CSII combined with either rosiglitazone (4 mg) once daily (CSII+thiazolidinedione [TZD]) or MET (0.5 g) three times daily (CSII+MET). The two drugs were initiated with pump therapy and were administered consecutively for 3 months. The fourth group was the combination of CSII and 2 weeks of α-lipoic acid (600 mg) intravenous infusion once daily (CSII+ALA). The short-term regimen of CSII was identical in the four treatment groups as described previously.2,3,7 In brief, rapid-acting insulin analogs (NovoRapid® [Novo Nordisk A/S, Bagsvaerd, Denmark] or HumaLog® [Eli Lilly, Indianapolis, IN]) were administered with an insulin pump (Minimed 712; Medtronic, Northridge, CA). The initial insulin dosage was 0.5–0.6 IU/kg/day, with total daily doses divided 50/50 for basal and bolus infusion. Capillary blood glucose was monitored eight times per day (before and 2 h after each meal, at bedtime, and at 3 a.m). The doses were titrated every day in order to achieve euglycemia (fasting blood glucose ≤6.0 mmol/L and postprandial blood glucose ≤8.0 mmol/L) within 3–5 days. CSII treatment was maintained for another 2 weeks and then suspended. No antihyperlipidemic agents were used during the intervention.
During hospitalization, all patients were invited to participate in routine education program on diabetes self-management, including advice on lifestyle, dietary counseling, self-monitoring of blood glucose, and recognition and treatment of hypoglycemia. Regular physical exercises, such as trotting, jogging, or stair-climbing for more than 30 min post-meals, were recommended.
Patients in the four treatment groups maintaining near-normoglycemic targets (fasting blood glucose <7 mmol/L, plasma blood glucose <10 mmol/L) after the suspension of short-term CSII were followed up as outpatients by specially assigned medical staff members. They were encouraged to record all self-monitoring of blood glucose results and maintain diet control and physical exercise as instructed. Whenever blood glucose exceeded a fasting blood glucose of >7.0 mmol/L or plasma blood glucose >10.0 mmol/L, patients were asked to report for examination of venous plasma glucose, even if it was not a scheduled visit. Those not meeting with near-normoglycemic criteria would be confirmed 1 week later with a repeated venous examination. Relapsed patients were treated with either oral hypoglycemic agents or insulin according to guidelines.
The protocol was approved by the Medical Research and Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, People's Republic of China). All subjects had given their written informed consent.
Measurements
Anthropometric and laboratory data, such as height, weight, waist and hip circumferences, blood pressure, FPG, 2-h postprandial plasma glucose (PPG), glycated hemoglobin (HbA1c), serum insulin, lipid profiles, and nonesterified fatty acids (NEFAs), were measured before and after suspension of CSII treatment. Homeostasis model assessment was used to estimate basal β-cell function (HOMA-B) and insulin resistance (HOMA-IR). The acute insulin response (AIR) was assessed during the intravenous glucose tolerance test before and after suspension of CSII, which was calculated as the incremental trapezoidal area during the first 10 min of the intravenous glucose tolerance test. All these measurements were to repeat every 3 months during the follow-up period. Plasma malondialdehyde (MDA), measured by chemical colorimetry, and intramyocellular lipid (IMCL) content, quantified using 1H-magnetic resonance spectroscopy, were performed before and after suspension of CSII.
Statistical analyses
SPSS for Windows version 18.0 software (SPSS, Inc., Chicago, IL) was used for data analysis. Baseline characteristics among the four groups, treatment efficacy, and safety profile between treatment modules were compared after CSII suspension and at the third month's visit using the CSII alone group as the control. One-way analysis of variance was used to compare the differences among groups. Scheffé and Tamhane's T2 post-tests were performed for multiple comparisons between groups. The paired t test was done to compare the differences before and after intervention. The χ2 test was used to analyze the differences of proportions. Logarithmic transformation was performed before comparison for non-normally distributed variables. A two-sided value of P<0.05 was considered statistically significant.
Results
Baseline characteristics and general treatment efficacy
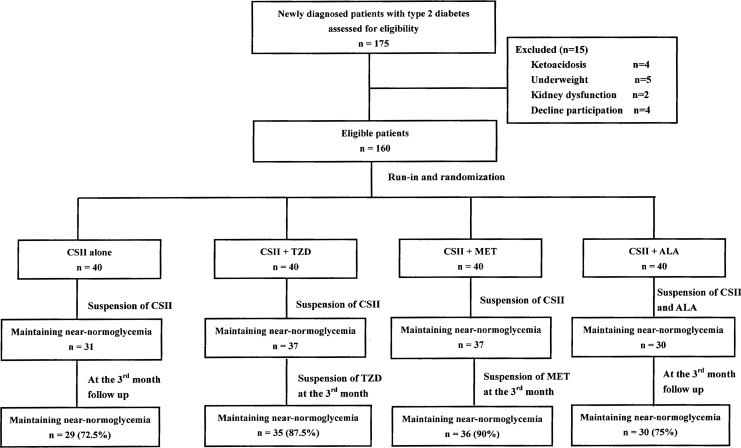
In total, 175 patients with newly diagnosed type 2 diabetes were recruited for the study. Of these, 15 were excluded because of ketoacidosis, underweight, kidney dysfunction, or declining participation. The remaining 160 patients were eligible and were randomly assigned to one of the four treatment groups. The enrolled patients were 49.8±10.4 years of age, with a body mass index of 25.2±3.3 kg/m2, FPG of 11.8±3.2 mmol/L, and HbA1c of 11.0±2.1%. In them, 54 patients were found hyperglycemia during routine physical examinations, and the diagnoses were made when referring to diabetes specialists. The other 106 patients were symptomatic with disease duration of 3 months to 2 years before diagnosis. Baseline features before intervention including anthropometric data, glucose level, lipid profile, indices of β-cell secretion (AIR, HOMA-B, and fasting proinsulin to insulin ratio), insulin sensitivity (HOMA-IR), IMCL content in soleus and tibialis, and plasma MDA level were comparable among the four treatment groups (Table 1).
Table 1.
Baseline Characteristics of Patients in the Four Treatment Groups
| |
Group |
|
|||
|---|---|---|---|---|---|
| Characteristic | CSII alone | CSII+TZD | CSII+MET | CSII+ALA | P value |
| Number | 40 | 40 | 40 | 40 | — |
| Gender (F/M) | 25/15 | 26/14 | 26/14 | 26/14 | 0.993 |
| Age (years) | 50.8±9.7 | 49.9±10.2 | 49.2±11.3 | 49.6±10.5 | 0.921 |
| Familial history (%) | 50 | 55 | 50 | 65 | 0.489 |
| Smoking (%) | 42.5 | 40 | 32.5 | 40 | 0.814 |
| Blood pressure (mm Hg) | |||||
| Systolic | 123±18 | 124±20 | 130±21 | 128±16 | 0.222 |
| Diastolic | 81±11 | 80±9 | 82±10 | 80±10 | 0.905 |
| BMI (kg/m2) | 25.0±3.1 | 25.4±3.5 | 25.8±3.1 | 24.9±3.5 | 0.613 |
| Waist circumference (cm) | 90.2±8.3 | 89.3±9.2 | 91.5±10.0 | 90.6±9.1 | 0.779 |
| Waist to hip ratio (%) | 93.3±6.0 | 91.9±5.9 | 93.5±7.6 | 93.9±5.5 | 0.520 |
| Ketosis at diagnosis (%) | 17.5 | 27.5 | 37.5 | 32.5 | 0.234 |
| HbA1c (%) | 10.9±1.8 | 10.7±2.3 | 11.1±1.8 | 11.2±2.4 | 0.716 |
| FPG (mmol/L) | 11.9±3.2 | 11.6±3.3 | 12.2±3.0 | 11.5±3.4 | 0.798 |
| PPG (mmol/L) | 18.4±5.5 | 17.6±6.3 | 18.2±4.7 | 18.1±6.2 | 0.928 |
| Cholesterol (mmol/L) | 5.8±1.2 | 5.9±0.9 | 5.9±1.3 | 5.9±1.4 | 0.970 |
| Triglycerides (mmol/L) | 1.60 (0.66) | 1.69 (1.45) | 1.99 (1.48) | 1.53 (1.09) | 0.207 |
| HDL-C (mmol/L) | 1.11±0.24 | 1.12±0.24 | 1.08±0.27 | 1.22±0.30 | 0.082 |
| LDL-C (mmol/L) | 4.06±1.12 | 3.79±0.98 | 4.01±1.20 | 3.95±1.31 | 0.751 |
| NEFA (mEq/L) | 0.66±0.20 | 0.66±0.21 | 0.55±0.14 | 0.66±0.36 | 0.476 |
| AIR (pmol/L·10 min) | −80.8 (143) | −110 (141) | −57.8 (197) | −43.0 (151) | 0.375 |
| HOMA-B | 18.7 (16.4) | 17.3 (29.2) | 16.4 (29.7) | 22.3 (32.1) | 0.866 |
| Proinsulin/insulin ratio | 0.66 (0.58) | 0.58 (0.34) | 0.63 (0.43) | 0.54 (0.81) | 0.991 |
| HOMA-IR | 3.72 (2.36) | 4.21 (2.85) | 3.87 (1.88) | 3.17 (2.68) | 0.095 |
| IMCL (mmol/kg) in | |||||
| Soleus | 12.1±3.4 | 13.8±5.4 | 12.5±4.8 | 11.5±4.7 | 0.289 |
| Tibialis | 3.45±1.91 | 3.38±1.74 | 3.94±2.67 | 3.39±1.42 | 0.663 |
| MDA (μmol/mL) | 11.2±4.1 | 14.3±8.9 | 14.3±7.7 | 13.1±7.2 | 0.187 |
Data are mean±SD values. Non-normally distributed variants such as triglyceride levels, acute insulin response (AIR), homeostasis model assessment of β-cell function (HOMA-B), proinsulin to insulin ratio, and homeostasis model assessment of insuin resistance (HOMA-IR) were expressed as median (interquartile range) values and were logarithmically transformed before comparison.
ALA, α-lipoic acid; BMI, body mass index; CSII, continuous subcutaneous insulin infusion; F, female; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IMCL, intramyocellular lipid; LDL-C, low-density lipoprotein cholesterol; M, male; MDA, malondialdehyde; MET, metformin; NEFA, nonesterified fatty acid; PPG, postprandial plasma glucose; TZD, thiazolidinedione.
Compared with baseline, significant reductions in HbA1c, FPG, PPG, proinsulin to insulin ratio, HOMA-IR, and MDA level were observed in all four treatment groups after CSII suspension. Both AIR and HOMA-B increased markedly as well. A similar proportion of patients treated with CSII alone or with α-lipoic acid combination maintained the near-normoglycemic goal after suspension of the insulin pump (77.5% and 75%, respectively; P=0.793), whereas only three patients each in the two insulin sensitizer treatment groups failed the target. At the month 3 visit, two more patients in the CSII alone group relapsed, whereas the proportion in the CSII+ALA group remained the same. Patients in the CSII+TZD and CSII+MET groups stopped the oral medication for 3 days and came back for examination. A similar proportion of patients in the two groups maintained near-normoglycemia (87.5% and 90%, respectively; P=1.000); both were higher than in the CSII alone group with marginal significance (72.5% for CSII alone) (P=0.094 and P=0.045, respectively) (Fig. 1).
FIG. 1.
Flow chart of randomized grouping and numbers of patients maintaining near-normoglycemia in each group after continuous subcutaneous insulin infusion (CSII) suspension and at month 3. ALA, α-lipoic acid; MET, metformin; TZD, thiazolidinedione.
Comparison of treatment efficacy among groups after CSII suspension
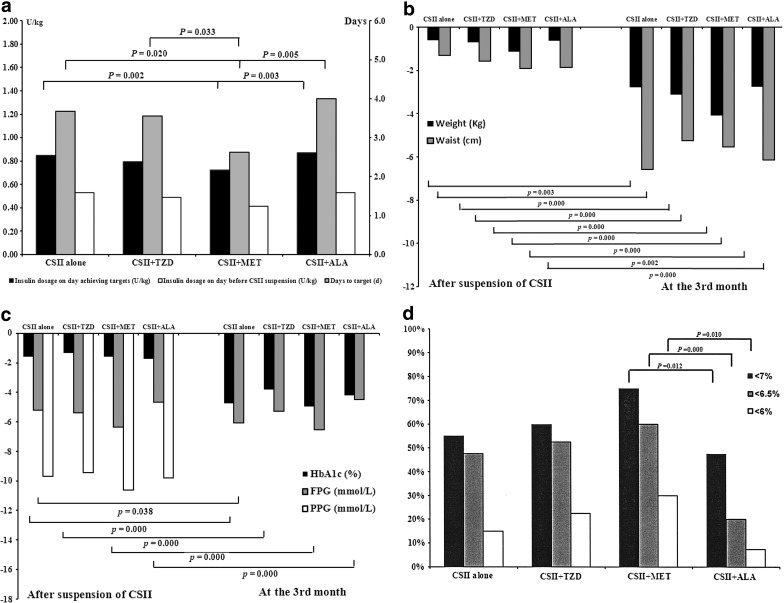
Most patients in the four groups achieved euglycemia within the first week of intervention: 87.3% of them were within 5 days, and 56.9% were within 3 days. The days to target interval was significantly shorter, and daily dosage of insulin (U/kg) on the day achieving euglycemia was significantly less in the CSII+MET group, whereas daily dosage of insulin (U/kg) on the day before suspension of insulin pump was comparable in the four groups. Significant reduction in body weight and waist circumference was recorded in most of the patients, and the effect was not different among groups. Glucose level, including HbA1c, FPG, and PPG, was markedly decreased from baseline, and the decrement was similar among groups. Lipid profile improved to some extent, with CSII+MET treatment being more superior to CSII alone in reducing total cholesterol (P=0.010) and low-density lipoprotein cholesterol (P=0.067). Significant suppression of fasting NEFA level was shown in the CSII alone (P=0.004) and CSII+TZD (P=0.021) groups compared with baseline, but not in the CSII+MET (P=0.425) and CSII+ALA (P=0.886) groups. However, the changes in fasting NEFAs were not significantly different among the groups (Table 2 and Fig. 2).
Table 2.
Insulin Dosage and Clinical Features in the Four Treatment Groups After Continuous Subcutaneous Insulin Infusion Suspension
| |
Group |
|
|||
|---|---|---|---|---|---|
| Characteristic | CSII alone | CSII+TZD | CSII+MET | CSII+ALA | P value |
| Days to target | 3.7±1.8 | 3.6±1.6 | 2.6±1.3 | 4.0±2.1 | 0.004a |
| Daily insulin dosage (units/kg) | |||||
| On day achieving targets | 0.85±0.16 | 0.79±0.15 | 0.72±0.16 | 0.87±0.22 | 0.001a |
| Before CSII suspension | 0.53±0.19 | 0.49±0.17 | 0.41±0.23 | 0.53±0.21 | 0.043a |
| Episodes of hypoglycemia | 4.0±3.4 | 3.1±2.9 | 3.5±2.7 | 5.1±3.9 | 0.052 |
| Body weight (kg) | 68.4±10.9 | 69.0±11.8 | 70.1±11.5 | 67.8±12.6 | 0.853 |
| Waist circumference (cm) | 88.8±8.5 | 87.9±8.1 | 88.3±8.5 | 89.2±9.8 | 0.925 |
| Waist/hip ratio (%) | 93.3±6.8 | 91.7±5.4 | 92.3±7.6 | 93.1±6.2 | 0.709 |
| HbA1c (%) | 9.4±1.5 | 9.3±1.9 | 9.5±1.3 | 9.4±1.9 | 0.984 |
| FPG (mmol/L) | 6.7±1.3 | 6.2±1.0 | 5.8±1.0 | 6.8±2.1 | 0.006a |
| PPG (mmol/L) | 8.8±3.0 | 8.5±2.5 | 7.6±2.5 | 8.6±3.0 | 0.218 |
| Cholesterol (mmol/L) | 5.5±1.1 | 5.6±0.8 | 5.0±0.9 | 5.5±1.3 | 0.073 |
| Triglyceride (mmol/L) | 1.18 (0.59) | 1.43 (0.95) | 1.27 (0.62) | 1.13 (0.92) | 0.067 |
| HDL-C (mmol/L) | 1.23±0.25 | 1.22±0.26 | 1.18±0.20 | 1.28±0.26 | 0.339 |
| LDL-C (mmol/L) | 3.79±0.90 | 3.63±0.72 | 3.43±0.86 | 3.66±1.13 | 0.366 |
| NFFA (mEq/L) | 0.50±0.15 | 0.55±0.18 | 0.52±0.14 | 0.64±0.37 | 0.279 |
| AIR (pmol/L·10 min) | 316 (360) | 329 (943) | 419 (912) | 472 (650) | 0.012a |
| HOMA-B | 53.3 (40.4) | 48.6 (62.6) | 62.6 (57.5) | 50.6 (49.0) | 0.002a |
| Proinsulin/insulin ratio (%) | 30.5 (12) | 30.0 (19) | 23.0 (15) | 38.5 (56) | 0.010a |
| HOMA-IR | 2.00 (1.10) | 2.11 (1.11) | 1.85 (1.61) | 1.57 (1.47) | 0.948 |
| IMCL (mmol/kg) in | |||||
| Soleus | 10.0±2.5 | 10.0±3.5 | 11.7±3.0 | 9.4±3.9 | 0.065 |
| Tibialis | 2.64±1.39 | 2.51±1.23 | 3.50±1.31 | 2.46±0.94 | 0.008a |
| MDA (μmol/mL) | 8.3±3.1 | 11.8±6.1 | 12.3±5.6 | 11.0±7.4 | 0.012a |
Data are mean±SD values. Non-normally distributed variants such as triglycerides, acute insulin response (AIR), homeostasis model assessment of β-cell function (HOMA-B), proinsulin to insulin ratio, and homeostasis model assessment of insulin resistance (HOMA-IR) were expressed as median (interquartile range) values and were logarithmically transformed before comparison.
One-way analysis of variance was used to compare the differences among groups. P<0.05 was considered significant.
ALA, α-lipoic acid; BMI, body mass index; CSII, continuous subcutaneous insulin infusion; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IMCL, intramyocellular lipid; LDL-C, low-density lipoprotein cholesterol; MDA, malondialdehyde; MET, metformin; NEFA, nonesterified fatty acid; PPG, postprandial plasma glucose; TZD, thiazolidinedione.
FIG. 2.
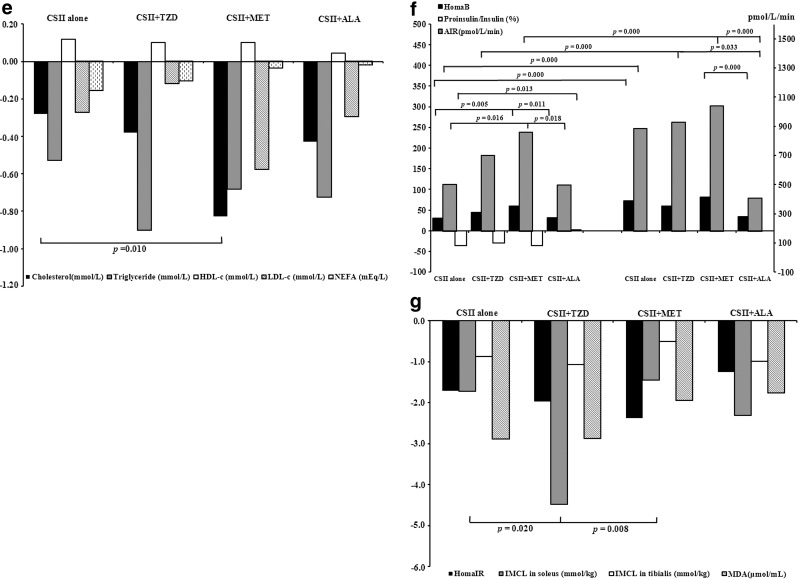
Comparisons of treatment efficacy in the four treatment groups. ALA, α-lipoic acid; MET, metformin; TZD, thiazolidinedione. (a) Insulin dosages and days to target during continuous subcutaneous insulin infusion (CSII) treatment period. (b) Changes from baseline after CSII suspension and at month 3 in body weight and waist circumference. (c) Changes from baseline after CSII suspension and at month 3 in glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and postprandial plasma glucose (PPG). (d) Proportions of patients achieving HbA1c <7%, 6.5%, and 6% in each treatment group. (e) Changes from baseline after CSII suspension in lipid profile. HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; NEFA, nonesterified fatty acid. (f) Changes from baseline after CSII suspension and at month 3 in homeostasis model assessment of β-cell function (HomaB), acute insulin response (AIR), and proinsulin to insulin ratio. (g) Changes from baseline after CSII suspension in homeostasis model assessment of insulin resistance (HomaIR) and intramyocellular lipid (IMCL) and malondialdehyde (MDA) levels.
Both HOMA-B and AIR were significantly elevated from baseline in the four treatment groups. The proinsulin to insulin ratio decreased in most patients except for those in the CSII+ALA group. Combination therapy with MET had extra effect on improving HOMA-B and AIR compared with CSII alone. Significant reduction in HOMA-IR from baseline was documented in the four groups with similar efficacy. The IMCL contents in soleus and tibialis were significantly reduced from baseline in the groups (P<0.05) other than the CSII+MET group (P=0.096 for soleus and P=0.366 for tibialis). The decline of IMCL content in soleus was more obvious in the CSII+TZD group than in the CSII alone group (4.45±4.15 mmol/kg vs. 1.72±3.26 mmol/kg, P=0.022), whereas the decline of IMCL content in tibialis was not different among groups (P=0.240). The MDA level decreased in all the four groups with comparable efficacy (Table 2 and Fig. 2).
Comparison of treatment efficacy between groups at month 3
It is notable that at the month 3 visit, there was further reduction in body weight, waist circumference, and waist to hip ratio in the four groups of patients. For example, patients in the CSII alone group lost about 2.61±3.32 kg in body weight and 3.88±3.50 cm in waist circumference compared with those after CSII suspension. The waist to hip ratio decreased by 3.46±4.33%. The reduction was similar between CSII alone and the other combination treatment groups (Fig. 2).
HbA1c was further reduced to near normal at month 3 with comparable efficacy in the four groups; the reduction was significant compared with that after CSII suspension. The proportions of patients achieving HbA1c below 7%, 6.5%, and 6% were significantly higher in the CSII+MET group than in the CSII+ALA group, yet similar to the other two groups. FPG was maintained near the normal level in all the four treatment groups, and only the reduction from that after CSII suspension was significant in the CSII alone group. PPG was not repeated at this visit (Fig. 2).
AIR improved considerably in the CSII alone group and the two insulin sensitizer groups at month 3 compared with those after CSII suspension. The elevation of HOMA-B achieved significance only in the CSII alone group. Insulin sensitivity decreased slightly from that after CSII suspension as indicated by the small increment of HOMA-IR, even in the two insulin sensitizer groups, but the worsening was not significant. HOMA-IR at month 3 after suspension of all the medication was similar in the four treatment groups (Table 3).
Table 3.
Comparisons at Month 3 in the Four Treatment Groups
| |
Group |
|
|||
|---|---|---|---|---|---|
| Characteristic | CSII alone | CSII+TZD | CSII+MET | CSII+ALA | P value |
| Weight (kg) | 65.7±8.3 | 69.0±12.0 | 68.0±11.5 | 64.2±10.5 | 0.306 |
| Waist (cm) | 83.1±6.0 | 85.3±9.7 | 85.8±7.1 | 84.0±7.7 | 0.429 |
| Waist/hip ratio (%) | 87.8±4.4 | 88.7±7.5 | 89.8±4.0 | 90.9±5.0 | 0.193 |
| HbA1c (%) | 6.4±0.5 | 6.5±0.8 | 6.2±0.5 | 6.9±0.8 | 0.001a |
| FPG (mmol/L) | 5.9±0.9 | 6.1±1.2 | 5.6±1.0 | 6.7±1.4 | 0.006a |
| AIR (pmol/L·10 min) | 88.2 (83.7) | 85.6 (193.0) | 134.7 (137.3) | 42.9±54.6 | 0.004a |
| HOMA-B | 80.6 (55.4) | 68.2 (74.1) | 93.9 (66.9) | 56.9 (36.4) | 0.011a |
| HOMA-IR | 2.32 (2.16) | 2.05 (1.55) | 1.99 (2.32) | 2.24 (1.90) | 0.819 |
Data are mean±SD values. Non-normally distributed variants such as acute insulin response (AIR), homeostasis model assessment of β-cell function (HOMA-B), and homeostasis model assessment of insulin resistance (HOMA-IR) were expressed as median (interquartile range) values and were logarithmically transformed before comparison.
One-way analysis of variance was used to compare the differences among groups. P<0.05 was considered significant.
ALA, α-lipoic acid; CSII, continuous subcutaneous insulin infusion; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; MET, metformin; TZD, thiazolidinedione.
Safety issues
The most common adverse event was hypoglycemia (capillary blood glucose <3.9 mmol/L) during the short-term intensive CSII treatment period. Of the eight times daily capillary blood glucose monitoring for the consecutively 2–3-week period, an average of three to five episodes of hypoglycemia occurred in each patient within the whole CSII treatment period, and the frequency of hypoglycemic episodes was similar among groups. The majority of the hypoglycemic events were documented symptomatic hypoglycemia and probable symptomatic hypoglycemia occurred during the daytime, especially after exercises. They were mild to moderate and were soon corrected by ingestion of a snack of carbohydrate. Relative hypoglycemia was present in some patients in the first few days of diet restriction during CSII treatment. Neither asymptomatic hypoglycemia nor severe hypoglycemia was documented in either group. No hypoglycemic events were reported after CSII suspension.
Of those treated with MET, two patients reported loss of appetite and moderate diarrhea at the beginning, but the drug regimen was tolerated after replacement with enteric-coated tablets and gradual dosage titration. One patient treated with rosiglitazone was allergic to the drug and switched to pioglitazone. No treatment-related edema, liver dysfunction, or cardiovascular events were recorded.
Discussion
In this study, we compared the differences in the amelioration of glucose and lipid metabolism, β-cell function, and insulin sensitivity after intervention between CSII treatment with or without combination. The design rationale for treatment duration and dosage of medication in combination therapy was based on clinical experience as well as scientific hard data. As for the treatment period of the two insulin sensitizers, data from A Diabetes Outcome Progression Trial (ADOPT) demonstrated that for patients with rosiglitazone or MET monotherapy, a marked reduction in FPG and HbA1c was observed at the first 2–4 months, followed by a much gradual descending trend to touch bottom at 1 year.10 On the other hand, TZDs increase insulin action through stimulation of the expression of genes that increase fat oxidation and the expression, synthesis, and release of adiponectin, as well as the stimulation of adipocyte differentiation, resulting in more and smaller fat cells. Thus, glycemic changes may be apparent 2–4 weeks after initiating treatment, but clinically meaningful effects generally occur 8–12 weeks into treatment.11,12 Thus, 3 months might be eligible for short-term as well as effective treatment duration. The dosage and duration of α-lipoic acid intravenous infusion are the standard regimen used in clinical practice for the treatment of diabetic polyneuropathy, which were also shown to have potential cytoprotective effects on β-cells under oxidative stress.13
In accordance with our previous studies, short-term CSII-based intensive treatment was very effective on improving glycemic control, lipid profile, β-cell function, and insulin sensitivity, resulting in immediate good glycemic control freed of medication in a large proportion of patients. The near-normoglycemia rate at month 3 in the CSII alone group and those in combination with rosiglitazone, MET, or α-lipoic acid were 72.5%, 87.5%, 90%, and 75%, respectively. Combination with insulin sensitizers did better in the short run, whereas CSII combined with α-lipoic acid was similar to CSII alone. Because it takes a much longer time for TZDs to have an effect and because previous long-term clinical trials also indicate that the decrease in HbA1c level is sustainable with TZD treatment,10,14 we could not rule out the possibility that there would be progressive improvement in glycemic control that extends to a longer period after cessation of the medication. Likewise, the waning of the effect of TZDs after cessation takes weeks, so that testing just 3 days after discontinuing the drug may limit any conclusions about how it compares with MET, whose biologic effects are of much shorter duration.
Although the efficacy seemed comparable between the two insulin sensitizer treatment groups, there were some delicate differences with respect to the advantage of combination. First, the MET group achieved euglycemia faster with a lower daily insulin dosage, indicating a faster action property, whereas rosiglitazone did not spare the amount of insulin in a 2–3-week CSII treatment period. Second, while still on medication (after suspension of CSII), the MET group was superior to CSII alone in improving HOMA-B and AIR, yet the effect of combination with rosiglitazone was equivalent to CSII alone. The greater improvement of MET on HOMA-B was attributable to a relatively lower FPG and fasting insulin concentration due to its effect on suppressing hepatic gluconeogenesis and reducing hepatic insulin resistance, the latter of which was shown to be closely and inversely correlated to AIR.15–17 However, the ameliorative effect seemed not sustainable because the differences in HOMA-B and AIR was no more significant with the discontinuance of oral medication at month 3. Third, MET treatment had an additional effect on lowering total cholesterol and low-density lipoprotein cholesterol, whereas the favorable effect on lipids was comparable between CSII alone and with the rosiglitazone combination. The United Kingdom Prospective Diabetes Study was the first to manifest MET's vascular protective effect independent of its glucose-lowering action, which was confirmed by several other randomized controlled trials.18–20 The probable explanation was attributable to its moderate anti-atherosclerosis effect on reducing total and low-density lipoprotein cholesterol levels, along with other beneficial effects on the endothelial dysfunction, pro-thrombotic, and pro-inflammatory state.21 The aim of our study was not to investigate the relationship between short-term intensive treatment and macrovascular complications, but we did show an additional effect of MET on the lipid profile even in such a short treatment period. In contrast, previous studies consistently showed that chronic rosiglitazone treatment significantly increased total, low-density lipoprotein, and high-density lipoprotein cholesterol levels, which was at variance with our observation.22–24 We speculate that a short-term treatment, as well as the relatively mild dyslipidemia in newly diagnosed patients, could possibly be offset by significant weight reduction during treatment and rapid normalization of blood glucose. Finally, and interestingly, although HOMA-IR decreased significantly and comparably in the two insulin sensitizer groups after suspension of the insulin pump, the decline of IMCL content in soleus differed. The reduction was more obvious in the rosiglitazone group than in the CSII alone and MET groups. The change was not close to significance in the MET group compared with baseline. Similarly, the previous study by Tiikkainen et al.25 indicated that 16 weeks of treatment with either rosiglitazone or MET improved hepatic insulin sensitivity with comparable efficacy, but only rosiglitazone but not MET decreased liver fat content. IMCL is now regarded as a tissue marker for peripheral insulin resistance, showing a close yet inverse correlation with measurable indices of insulin sensitivity, including data from hyperinsulinemic euglycemic clamp studies.26,27 Soleus is rich in oxidative, slow-twitch type I fibers and contains more IMCL than tibialis and thus has an even closer relation to insulin resistance.28 Rosiglitazone, as a peroxisome proliferator-activated receptor-γ agonist, promotes fatty acid uptake and storage in adipocytes as well as increasing subcutaneous adipose tissue mass, thus sparing other insulin-sensitive tissues, such as skeletal muscle and liver, from NEFA deposition.29 Moreover, TZDs facilitate normal adipocyte differentiation by shifting large adipocytes into new, small, and more insulin-sensitive ones; the latter are more efficient at storing lipids.30 In contrast, MET, serving as an AMP-activated protein kinase activator, inhibits lipolysis at least in part by inhibiting hormone-sensitive lipase translocation to the lipid droplet.31 However, as shown in our study, the reduction of IMCL content in the MET treatment group was not significant or even worse than with CSII alone. Our result is in accordance with two studies from Japan showing a null effect of MET on IMCL in overweight subjects and type 2 diabetes patients.32,33 A recent study by Malin et al.34 showed that 12 weeks of treatment with MET (2,000 mg/day) in prediabetes subjects blunted the full effect of exercise training by 25–30% on insulin sensitivity as measured by the hyperinsulinemic euglycemic clamp. The negligible effect of MET on IMCL content reduction needs further investigation. Correlation analysis in our study showed that the reduction of IMCL content in soleus was positively correlated to the average daily insulin dosage per kilogram (r=0.274, P=0.007) (authors' unpublished data). The insulin-economizing effect of MET might in part be responsible for the lesser reduction of IMCL content.
α-Lipoic acid did not seem to increase the near-normoglycemia targeting rate, nor did it augment the effect of CSII treatment in elevating HOMA-B and AIR or reducing HOMA-IR and IMCL content. It was not superior to CSII alone in decreasing the MDA level either. In a study by Xiao et al.35 examining the effect of 2 weeks of oral α-lipoic acid (1,800 mg/day) on insulin sensitivity and secretion in overweight and obese subjects without diabetes during a 24-h lipid infusion protocol, α-lipoic acid did not provide protection against the detrimental effects of prolonged elevation of NEFA levels on insulin action and secretion as assessed by hyperglycemic and euglycemic clamp techniques. On the other hand, the rapid normalization of glucotoxicity and lipotoxity along with physical exercises to reduce weight per se in patients treated with short-term CSII might occur with a strong antioxidative effect that exceeded the impact of α-lipoic acid itself, the latter of which was used in a relatively short period with a small dose. Because oxidative stress is a complex process involving a great many elements that might interfere with its link to the etiology and development of diabetes, we are not intending to negate α-lipoic acid as a potent antioxidant, which has been proved valid in previous in vitro and in vivo studies for its effect on β-cell function and insulin sensitivity,13,36,37 but the combination with α-lipoic acid might not be advantageous over CSII alone from a cost-effectiveness point of view.
There are some limitations of the study that need to be addressed. The sample size in each group is relatively small. Assuming that the sample size provides 90% power to detect a 10% difference between a CSII combination group and that of CSII alone, while also allowing for 10% loss to follow-up over 1 year, the sample size should be approximately 154 patients in each group, which is far larger than that available at the present study. Thus the marginal results of the effects of MET and rosiglitazone in this study might be due to underpowering. A multicenter clinical trial to include more patients is needed. On the other hand, effective life-style interventions including diet and exercises are very helpful in short-term intensive CSII-based therapies. Detailed forms should be designed and used to evaluate and balance the compliance with life-style intervention in each group to achieve the net effects of the medications.
In conclusion, our randomized controlled trial on combination therapy with the insulin sensitizers rosiglitazone and MET or with the antioxidant α-lipoic acid shows that CSII-based short-term intensive treatment in patients with newly diagnosed type 2 diabetes is very effective in improving glycemic and lipid control, β-cell function, and insulin sensitivity. Combination with α-lipoic acid might not have an additive effect, whereas near-normoglycemia rates in the two insulin sensitizer groups are relatively higher than CSII alone in the short run. The efficacy of rosiglitazone and MET differs on the aspect of day to target interval during CSII treatment, daily insulin dosage, amelioration on lipid profile, reduction of IMCL content in soleus, etc. Their effects on long-term drug-free remission remain to be determined.
Acknowledgments
The research was supported by the Key Science and Technique Research Project of Guangdong Province (grant 2012A030400006), the Guangdong Medical Science Research Fund (grant 2012B060300010), the Natural Science Fund of China (grant 81070659), the Research Fund for the Doctoral Program of Higher Education of China (grant 2009171110054), and National Clinical Key Development Project (grant 2010).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ilkova H. Glaser B. Tunçkale A. Bagriaçik N. Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–1356. doi: 10.2337/diacare.20.9.1353. [DOI] [PubMed] [Google Scholar]
- 2.Li Y. Xu W. Liao Z. Yao B. Chen X. Huang Z. Hu G. Weng J. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of β-cell function. Diabetes Care. 2004;27:2597–2602. doi: 10.2337/diacare.27.11.2597. [DOI] [PubMed] [Google Scholar]
- 3.Weng J. Li Y. Xu W. Shi L. Zhang Q. Zhu D. Hu Y. Zhou Z. Yan X. Tian H. Ran X. Luo Z. Xian J. Yan L. Li F. Zeng L. Chen Y. Yang L. Yan S. Liu J. Li M. Fu Z. Cheng H. Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 4.Park S. Choi SB. Induction of long-term normoglycemia without medication in Korean type 2 diabetes patients after continuous subcutaneous insulin infusion therapy. Diabetes Metab Res Rev. 2003;19:124–130. doi: 10.1002/dmrr.343. [DOI] [PubMed] [Google Scholar]
- 5.Ryan EA. Imes S. Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–1032. doi: 10.2337/diacare.27.5.1028. [DOI] [PubMed] [Google Scholar]
- 6.Davidson MB. Pro's and con's of the early use of insulin in the management of type 2 diabetes: A clinical evaluation. Curr Opin Endocrinol Diabetes Obes. 2009;16:107–112. doi: 10.1097/MED.0b013e328322f92e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen A. Huang Z. Wan X. Deng W. Wu J. Li L. Cai Q. Xiao H. Li Y. Attitudes toward diabetes affects maintenance of drug-free remission in patients with newly diagnosed type 2 diabetes mellitus after short-term continuous subcutaneous insulin infusion treatment. Diabetes Care. 2012;35:474–481. doi: 10.2337/dc11-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavez JA. Summers SA. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim Biophys Acta. 2010;1801:252–265. doi: 10.1016/j.bbalip.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriksen EJ. Exercise training and the antioxidant α-lipoic acid in the treatment of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2006;40:3–12. doi: 10.1016/j.freeradbiomed.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Kahn SE. Haffner SM. Heise MA. Herman WH. Holman RR. Jones NP. Kravitz BG. Lachin JM. O'Neill MC. Zinman B. Viberti G ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 11.Boden G. Zhang M. Recent findings concerning thiazolidinediones in the treatment of diabetes. Expert Opin Investig Drugs. 2006;15:243–250. doi: 10.1517/13543784.15.3.243. [DOI] [PubMed] [Google Scholar]
- 12.Vasudevan AR. Balasubramanyam A. Thiazolidinediones: a review of their mechanisms of insulin sensitization, therapeutic potential, clinical efficacy, and tolerability. Diabetes Technol Ther. 2004;6:850–863. doi: 10.1089/dia.2004.6.850. [DOI] [PubMed] [Google Scholar]
- 13.Lee BW. Kwon SJ. Chae HY. Kang JG. Kim CS. Lee SJ. Yoo HJ. Kim JH. Park KS. Ihm SH. Dose-related cytoprotective effect of alpha-lipoic acid on hydrogen peroxide-induced oxidative stress to pancreatic beta cells. Free Radic Res. 2009;43:68–77. doi: 10.1080/10715760802590400. [DOI] [PubMed] [Google Scholar]
- 14.Charbonnel BH. Matthews DR. Schernthaner G. Hanefeld M. Brunetti P QUARTET Study Group. A long term comparison of pioglitazone and gliclazide in patients with type 2 diabetes mellitus: a randomized, double-blind, parallel group comparison trial. Diabet Med. 2005;22:399–405. doi: 10.1111/j.1464-5491.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 15.Miller RA. Birnbaum MJ. An energetic tale of AMPK-independent effects of metformin. J Clin Invest. 2010;120:2267–2270. doi: 10.1172/JCI43661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Prato S. Marchetti P. Beta- and alpha-cell dysfunction in type 2 diabetes. Horm Metab Res. 2004;36:775–781. doi: 10.1055/s-2004-826163. [DOI] [PubMed] [Google Scholar]
- 17.Berrish TS. Hetherington CS. Alberti KG. Walker M. Peripheral and hepatic insulin sensitivity in subjects with impaired glucose tolerance. Diabetologia. 1995;38:699–704. doi: 10.1007/BF00401842. [DOI] [PubMed] [Google Scholar]
- 18.Evans JM. Ogston SA. Emslie-Smith MA. Morris A. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulphonylureas and metformin. Diabetologia. 2006;49:930–936. doi: 10.1007/s00125-006-0176-9. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JA. Majumdar SR. Simpson SH. Toth EL. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care. 2002;25:2244–2248. doi: 10.2337/diacare.25.12.2244. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E. Bolen S. Yeh HC. Wiley C. Wilson LM. Marinopoulos SS. Feldman L. Vassy J. Wilson R. Bass EB. Brancati FL. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008;168:2070–2080. doi: 10.1001/archinte.168.19.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anfossi G. Russo I. Bonomo K. Trovati M. The cardiovascular effects of metformin: further reasons to consider an old drug as a cornerstone in the therapy of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2010;8:327–337. doi: 10.2174/157016110791112359. [DOI] [PubMed] [Google Scholar]
- 22.Boyle PJ. King AB. Olansky L. Marchetti A. Lau H. Magar R. Martin J. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Clin Ther. 2002;24:378–396. doi: 10.1016/s0149-2918(02)85040-8. [DOI] [PubMed] [Google Scholar]
- 23.van Wijk JP. de Koning EJ. Martens EP. Rabelink TJ. Thiazolidinediones and blood lipids in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2003;23:1744–1749. doi: 10.1161/01.ATV.0000090521.25968.4D. [DOI] [PubMed] [Google Scholar]
- 24.Chiquette E. Ramirez G. Defronzo R. A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med. 2004;164:2097–2104. doi: 10.1001/archinte.164.19.2097. [DOI] [PubMed] [Google Scholar]
- 25.Tiikkainen M. Hakkinen A-M. Korsheninnikova E. Nyman T. Makimattila S. Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 26.van der Graaf M. Tack CJ. de Haan JH. Klomp DW. Heerschap A. Magnetic resonance spectroscopy shows an inverse correlation between intramyocellular lipid content in human calf muscle and local glycogen synthesis rate. NMR Biomed. 2010;23:133–141. doi: 10.1002/nbm.1433. [DOI] [PubMed] [Google Scholar]
- 27.Salgin B. Sleigh AJ. Williams RM. Jackson SJ. Bluck LJ. Murgatroyd PR. Humphreys SM. Harding S. Carpenter TA. Dunger DB. Intramyocellular lipid levels are associated with peripheral, but not hepatic, insulin sensitivity in normal healthy subjects. Clin Sci. 2009;117:111–118. doi: 10.1042/CS20080563. [DOI] [PubMed] [Google Scholar]
- 28.van Loon LJ. Koopman R. Manders R. van der Weegen W. van Kranenburg GP. Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab. 2004;287:E558–E665. doi: 10.1152/ajpendo.00464.2003. [DOI] [PubMed] [Google Scholar]
- 29.Boden G. Homko C. Mozzoli M. Showel LC. Nichols C. Cheung P. Thiazolidinediones up regulate fatty acid uptake and oxidation in adipose tissue in diabetic patients. Diabetes. 2005;54:880–885. doi: 10.2337/diabetes.54.3.880. [DOI] [PubMed] [Google Scholar]
- 30.Boden G. Cheung P. Mozzoli M. Fried SK. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism. 2003;52:753–759. doi: 10.1016/s0026-0495(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 31.Bourron O. Daval M. Hainault I. Hajduch E. Servant JM. Gautier JF. Ferré P. Foufelle F. Biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through activation of AMP-activated protein kinase. Diabetologia. 2010;53:768–778. doi: 10.1007/s00125-009-1639-6. [DOI] [PubMed] [Google Scholar]
- 32.Teranishi T. Ohara T. Maeda K. Zenibayashi M. Kouyama K. Hirota Y. Kawamitsu H. Fujii M. Sugimura K. Kasuga M. Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism. 2007;56:1418–1424. doi: 10.1016/j.metabol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Tamura Y. Watada H. Sato F. Kumashiro N. Sakurai Y. Hirose T. Tanaka Y. Kawamori R. Effects of metformin on peripheral insulin sensitivity and intracellular lipid contents in muscle and liver of overweight Japanese subjects. Diabetes Obes Metab. 2008;10:733–738. doi: 10.1111/j.1463-1326.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- 34.Malin SK. Gerber R. Chipkin SR. Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35:131–136. doi: 10.2337/dc11-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao CT. Giacca A. Lewis GF. Short-term oral α-lipoic acid does not prevent lipid-induced dysregulation of glucose homeostasis in obese and overweight nondiabetic men. Am J Physiol Endocrinol Metab. 2011;301:E736–E741. doi: 10.1152/ajpendo.00183.2011. [DOI] [PubMed] [Google Scholar]
- 36.Jacob S. Ruus P. Hermann R. Tritschler HJ. Maerker E. Renn W. Augustin HJ. Dietze GJ. Rett K. Oral administration of RAC-alpha lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Radic Biol Med. 1999;27:309–314. doi: 10.1016/s0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhang WJ. Wei H. Hagen T. Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]