Abstract
Background
Congestive heart failure (CHF) features disturbances in the interstitial environment that may affect the accuracy of subcutaneous continuous glucose monitoring (CGM).
Subjects and Methods
A pooled analysis of two studies of hospitalized patients with type 2 diabetes randomized to intravenous or subcutaneous insulin was conducted. One study enrolled patients with CHF exacerbation, whereas history of CHF was an exclusion criterion in the other. All patients wore a professional CGM device for at least 24 h. Intravenous insulin was administered according to the institution's nursing-run protocol (duration of 12 and 48 h in non-CHF and CHF protocols, respectively). Subcutaneous insulin was delivered similarly in both groups.
Results
Subjects with CHF (n=43) had higher admission glucose and hemoglobin A1c compared with non-CHF subjects (n=32), but the sensor glucose values were similar. Overall mean absolute relative difference (MARD) was similar between CHF and non-CHF subjects (0.11 vs. 0.08, respectively; P=0.12). MARD was higher in the 100–149 mg/dL (P=0.003) and >199 mg/dL (P=0.02) strata among CHF subjects. Static glucose and continuous glucose error grid analyses favored the non-CHF group. In multivariable analyses, only glucose coefficient of variation and log sensor time were independent predictors of elevated overall MARD >0.10. After adjustment for other factors, only increasing log sensor time was a significant predictor of elevated MARD in the 100–149 mg/dL strata.
Conclusions
Among hospitalized subjects with type 2 diabetes, CHF exacerbation is not associated with lower sensor accuracy after adjustment for other factors, but this requires confirmation over a wider glucose range.
Background
Subcutaneous (SQ) continuous glucose monitoring (CGM) is gaining increasing interest as a potential tool for use in addition to point-of-care glucose measurements in hospitalized patients due to the potential for increasing the safety and efficacy of insulin therapy.1–3 However, recent guidelines have recommended against the use of real-time interstitial CGM in the hospital because of lingering concerns regarding sensor accuracy, although improvements are being made with more recent technology.4,5
Many factors are known to affect the accuracy of point-of-care glucose monitoring devices, including fluid status, perfusion, pH, oxygenation, and medications.6 CGM accuracy may be affected in a similar manner. In the intensive care unit, CGM studies have demonstrated variable accuracy,6–12 although most were small, and some studies evaluated sensor accuracy over only a narrow glucose range.1 Outside of the intensive care unit, conditions may be more favorable for glucose monitoring. However, congestive heart failure (CHF) is one such condition that may be characterized by tissue edema and poor perfusion and thus may also affect CGM accuracy. Our previous research suggested that CHF may pose unique challenges to sensor accuracy in patients with type 2 diabetes, although glycemic control appeared to be a more important contributor.13 However, the previous study was not optimal for isolating the effects of CHF because comparator groups grossly differed by several other factors, such as type of diabetes. This study compares the accuracy of CGM by CHF status within insulin-requiring hospitalized non–intensive care unit patients with type 2 diabetes. Furthermore, we compare the effects of treatment modality (intravenous [IV] vs. SQ insulin).
Subjects and Methods
Patients
Study subjects consisted of two groups: CHF and non-CHF patients. Patients were enrolled as part of separate ongoing studies.14 Inclusion criteria for both studies included hospitalized adults with type 2 diabetes and significant insulin use (>20 units/day) or hyperglycemia (blood glucose >10 mmol/L on at least two occasions separated by at least 4 h apart). Exclusion criteria for both studies included type 1 diabetes, hyperglycemic emergency, critical illness (such as the need for mechanical ventilation and hypotension requiring vasopressors), corticosteroid use, end-stage renal or liver disease, hospital stay expected to be <48 h, inability to consent, imprisonment, and pregnancy. The CHF study specifically included patients who were admitted for CHF exacerbation as the primary diagnosis and excluded patients with acute myocardial infarction within the previous 3 months or predominantly right-sided heart failure. The non-CHF group excluded patients with known heart failure, arrhythmia, or autonomic neuropathy. All studies were approved by the Institutional Review Board at The Ohio State University, and all patients signed informed consent.
Intervention
Patients were randomly assigned to IV or SQ insulin, and all oral agents were discontinued. IV insulin was titrated using the study institution's universal nursing-run protocol. This protocol (target blood glucose level of 110–150 mg/dL) was adapted from a previously published algorithm.15 IV insulin was continued for 12 h in the non-CHF group and 48 h in the CHF group, a result of different study protocols. Patients who were eating received SQ prandial insulin with a rapid-acting insulin analog, using an algorithm that has been reported previously.14 SQ correction insulin was withheld during the IV infusion. In the SQ group, the total daily dose of insulin was calculated as 100% or 120% of the total home dose of insulin in patients with an enrollment glucose of <180 mg/dL or >180 mg/dL, respectively. Basal and prandial insulin were administered in approximately equal total daily doses with adjustments in the total daily dose of ±10–20% per day based on a published algorithm.16 The major exceptions were that prandial insulin was delivered according to carbohydrate intake and the target glucose range was 100–150 mg/dL.
A continuous glucose monitor (CGMS® iPro™; Medtronic, Northridge, CA) was used in accordance with the manufacturer's instructions. The sensor was inserted on the abdomen and downloaded after at least 24 h using CGMS Solutions software. Capillary glucose values (Accu-Chek® Inform®; Roche, Indianpolis, IN) were measured hourly during IV insulin and every 4–6 h in the SQ group. Calibrations were performed at four predetermined time points (closest to 7 a.m., 11 a.m., 4 p.m., and 9 p.m.) per day within the allowable glucose limits (40–400 mg/dL) of the software. Change in plasma volume (PV) was calculated as published previously, using the hemoglobin and hematocrit values from successive days.17 Patients with active bleeding or recent blood transfusions were excluded from PV calculations.
Analysis
The mean absolute difference (MAD) was calculated as the absolute value of the meter glucose level minus the sensor glucose level, and the mean absolute relative difference (MARD) was calculated as the absolute value of the meter minus the sensor value divided by the meter value: |meter BG–sensor BG|/meter BG. The MAD and MARD were compared, and MARD was stratified by glucose levels (<100 mg/dL, 100–149 mg/dL, 150–199 mg/dL, and >199 mg/dL). A strictly hypoglycemic stratum was not possible because of insufficient values in the hypoglycemic range. Thus, glucose strata were chosen to facilitate direct comparisons with previous studies.8,13 Overall and group-dependent Pearson's correlation coefficients were calculated. Bland–Altman plots were created. Clarke error grid analysis (EGA) and the continuous glucose EGA (CG-EGA) were performed.18,19
Non-normally distributed variables (diabetes duration, time in hypoglycemia, number of meter–sensor pairs, total sensor time, MAD, and MARD) were reported as median (interquartile range) and analyzed using Wilcoxon rank-sum tests. All other continuous variables had normal distributions and were reported as mean (SD) values and analyzed using unpaired t tests. Dichotomous variables were reported as number (percentage), and between-group comparisons were made using Fisher's exact test. Statistical significance was determined at a value of P<0.05.
Logistic regression analyses were performed for the dichotomous response variables “Overall MARD >0.10” or “MARD100–150>0.10” using backward stepwise methodology. The rationale for the cutoff of 0.10 was that it was the pooled median value for both groups in this study and is in agreement with other data.20 Variables were chosen for entry into the model based on clinical relevance (CHF status, insulin group) and univariable effect estimates (cutoff value of P<0.08). Non-normally distributed variables were log-transformed. Statistical analyses were performed using JMP version 9.0 software (SAS, Cary, NC). EGAs were conducted with software from The Epsilon Group® (www.tegvirginia.com).
Results
There were 43 subjects with CHF and 32 subjects without CHF. Characteristics are shown in Table 1. The CHF subjects were older (62 vs. 58 years, P=0.003) and more likely to be male (66% vs. 37%, P=0.02) than the non-CHF subjects; however, the hemoglobin A1c (8.0% vs. 9.5, P=0.01) and admission glucose (178 vs. 227 mg/dL, P=0.01) levels were lower in the CHF group. There was a nonsignificant trend for greater total time on the sensor and more sensor–meter pairs per subject in the CHF group than in the non-CHF group (P=0.09 for both). The first sensor glucose level, mean sensor glucose level, glucose coefficient of variation (CV), and time in hypoglycemia were similar between groups.
Table 1.
Baseline Characteristics and Glucose Values Among Patients Hospitalized with Type 2 Diabetes, by Congestive Heart Failure Status
| |
Non-CHF |
CHF |
|
||
|---|---|---|---|---|---|
| n | Value | n | Value | P value | |
| Patient characteristics | |||||
| Age (years) | 32 | 57.8±10.4 | 43 | 62.4±11.23 | 0.003 |
| Male | 32 | 21 (66%) | 43 | 16 (37%) | 0.02 |
| White | 32 | 29 (91%) | 43 | 35 (81%) | 0.34 |
| BMI (kg/m2) | 32 | 35.2±9.1 | 43 | 39.4±9.8 | 0.06 |
| Diabetes duration (years) | 31 | 12 (7–16) | 40 | 13 (10–20) | 0.17a |
| HbA1c (%) | 32 | 9.5±2.6 | 42 | 8.0±1.7 | 0.01 |
| Hypertension | 32 | 27 (84%) | 43 | 37 (86%) | >0.99 |
| Coronary artery disease | 32 | 12 (38%) | 43 | 25 (58%) | 0.10 |
| Retinopathy | 32 | 7 (22%) | 43 | 8 (19%) | 0.78 |
| Nephropathy | 32 | 10 (31%) | 43 | 6 (14%) | 0.09 |
| Neuropathy | 32 | 14 (44%) | 43 | 20 (47%) | >0.99 |
| Creatinine (mg/dL) | 32 | 1.38±0.78 | 43 | 1.67±0.67 | 0.11 |
| Change in plasma volume (%) | 21 | 2.7±8.6 | 41 | 0.10±7.5 | 0.25 |
| Insulin randomization group | |||||
| IV insulin | 16 | 16 (50%) | 17 | 17 (40%) | |
| SQ insulin | 16 | 16 (50%) | 26 | 26 (61%) | |
| Glucose data | |||||
| Number of meter–sensor pairs | 32 | 10.5 (7–18) | 43 | 11 (8–37) | 0.09a |
| Time on sensor (min) | 32 | 2,725 (2,038–3841) | 43 | 3,655 (3,585–3,810) | 0.09a |
| Admission glucose (mg/dL) | 32 | 227±83 | 43 | 178±54 | 0.01 |
| First sensor glucose (mg/dL) | 32 | 168±65 | 43 | 169±68 | 0.98 |
| Mean sensor glucose (mg/dL) | 31 | 172±43 | 43 | 164±37 | 0.37 |
| CV (%) | 31 | 27.9±11.2 | 43 | 23.5±8.2 | 0.07 |
| Time (%) in hypoglycemia | 0 (0–55) | 0 (0–0.17) | 0.16a | ||
| MAD (mg/dL) | 32 | 10 (3–20.8) | 43 | 13 (6–26.8) | <0.0001a |
| MARD (%) | 32 | 0.08 (0.07–0.12) | 43 | 0.11 (0.07–0.15) | 0.12a |
| Meter–sensor correlation (r) | 392 | 0.89 | 928 | 0.84 | <0.0001b |
Data are mean±SD values, median (interquartile range), or number (%), as indicated.
Differences between groups were tested using Wilcoxon's rank-sum test. The P value was determined using a t test for all other continuous variables. Fisher's exact test was used to find the P value for dichotomous variables.
Difference in correlations was determined using the Fisher's r-to-Z transformation.
BMI, body mass index; CHF, congestive heart failure; CV, coefficient of variation for glucose; HbA1c, hemoglobin A1c; MAD, mean absolute difference; MARD, mean absolute relative difference.
Overall accuracy
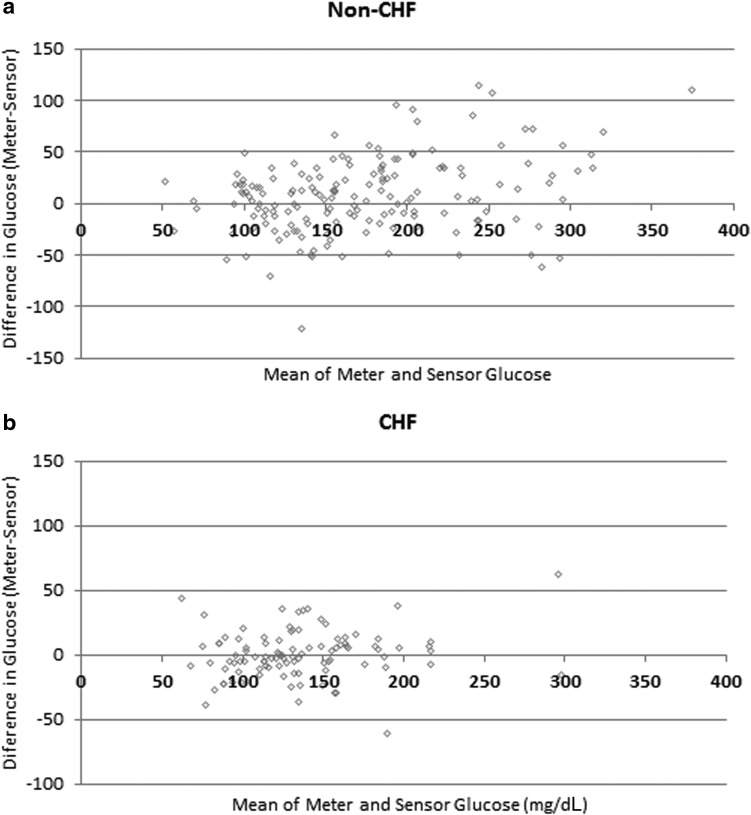
Bland–Altman plots are shown in Figure 1. The MAD and MARD were similar between CHF and non-CHF patients (Table 1). However, the meter–sensor correlation was higher in the non-CHF group (ρ=0.89 vs. 0.84 for non-CHF vs. CHF, respectively; P<0.0001) (Table 1). When stratified by glucose level, median MARD between 100 and 149 mg/dL (MARD100–149) was significantly lower in non-CHF patients compared with CHF patients (0. 07 vs. 0.10, P=0.003) (Table 2). MARD was significantly lower in the non-CHF group in the >199 mg/dL range (P=0.02), and there was a trend for lower MARD in the 150–199 mg/dL range (P=0.05), but values <100 mg/dL were not significantly different. There was no association between MARD and change in brain natriuretic peptide (ρ=0.06, P=0.69) in the CHF patients or between MARD and PV (ρ=−0.01, P=0.94) in all patients. Spearman's correlations between overall MARD and MARD100–150 and other study parameters are shown in Table 3. MARD was associated with IV insulin (ρ=0.21, P=0.046), CV of glucose (ρ=0.24, P=0.04), and total sensor time (ρ=0.42, P=0.002). MARD100–149 was correlated only with total sensor time (ρ=0.38, P=0.002).
FIG. 1.
Bland–Altman plots for (a) non-congestive heart failure (CHF) and (b) CHF subjects.
Table 2.
Mean Absolute Relative Difference by Glucose Strata in Hospitalized Patients With or Without Congestive Heart Failure
| |
Non-CHF |
CHF |
|
||
|---|---|---|---|---|---|
| Glucose strata (mg/dL) | n | Median (IQR) | n | Median (IQR) | P value |
| <100 | 38 | 0.08 (0.02–0.21) | 75 | 0.10 (0.06–0.26) | 0.11 |
| 100–149 | 146 | 0.07 (0.02–0.14) | 389 | 0.10 (0.04–0.18) | 0.003 |
| 150–199 | 117 | 0.07 (0.03–0.14) | 304 | 0.09 (0.04–0.16) | 0.05 |
| >200 | 91 | 0.05 (0.01–0.10) | 160 | 0.07 (0.03–0.15) | 0.02 |
Data are median (interquartile range [IQR]) values. Differences between groups were determined using Wilcoxon's rank-sum test.
CHF, congestive heart failure; MARD, mean absolute relative difference.
Table 3.
Correlations with Mean Absolute Relative Difference
| |
Overall MARD |
MARD 100–150 mg/dL |
||
|---|---|---|---|---|
| Variable | Spearman ρ | P value | Spearman ρ | P value |
| CHF | 0.19 | 0.11 | 0.13 | 0.30 |
| IV insulin | 0.21 | 0.046 | 0.24 | 0.05 |
| Age | 0.34 | 0.33 | 0.12 | 0.32 |
| Male | −0.12 | 0.31 | −0.03 | 0.80 |
| Diabetes duration | 0.01 | 0.94 | 0.06 | 0.644 |
| BMI | 0.158 | 0.21 | 0.22 | 0.07 |
| HbA1c | −0.04 | 0.72 | 0.18 | 0.15 |
| Sensor mean total | −0.16 | 0.18 | −0.03 | 0.82 |
| Sensor CV total | 0.24 | 0.04 | 0.14 | 0.28 |
| Hypoglycemia time | −0.002 | 0.99 | −0.01 | 0.92 |
| Admission glucose | −0.12 | 0.29 | −0.02 | 0.88 |
| First sensor glucose | −0.20 | 0.08 | −0.16 | 0.21 |
| Time on sensor | 0.42 | 0.0002 | 0.38 | 0.002 |
| Number of sensor–meter pairs | 0.03 | 0.74 | 0.03 | 0.81 |
| PV change | −0.03 | 0.83 | −0.11 | 0.43 |
BMI, body mass index; CHF, congestive heart failure; CV, coefficient of variation for glucose; HbA1c, hemoglobin A1c; IV, intravenous; MARD, mean absolute relative difference; PV, plasma volume.
Clinical accuracy
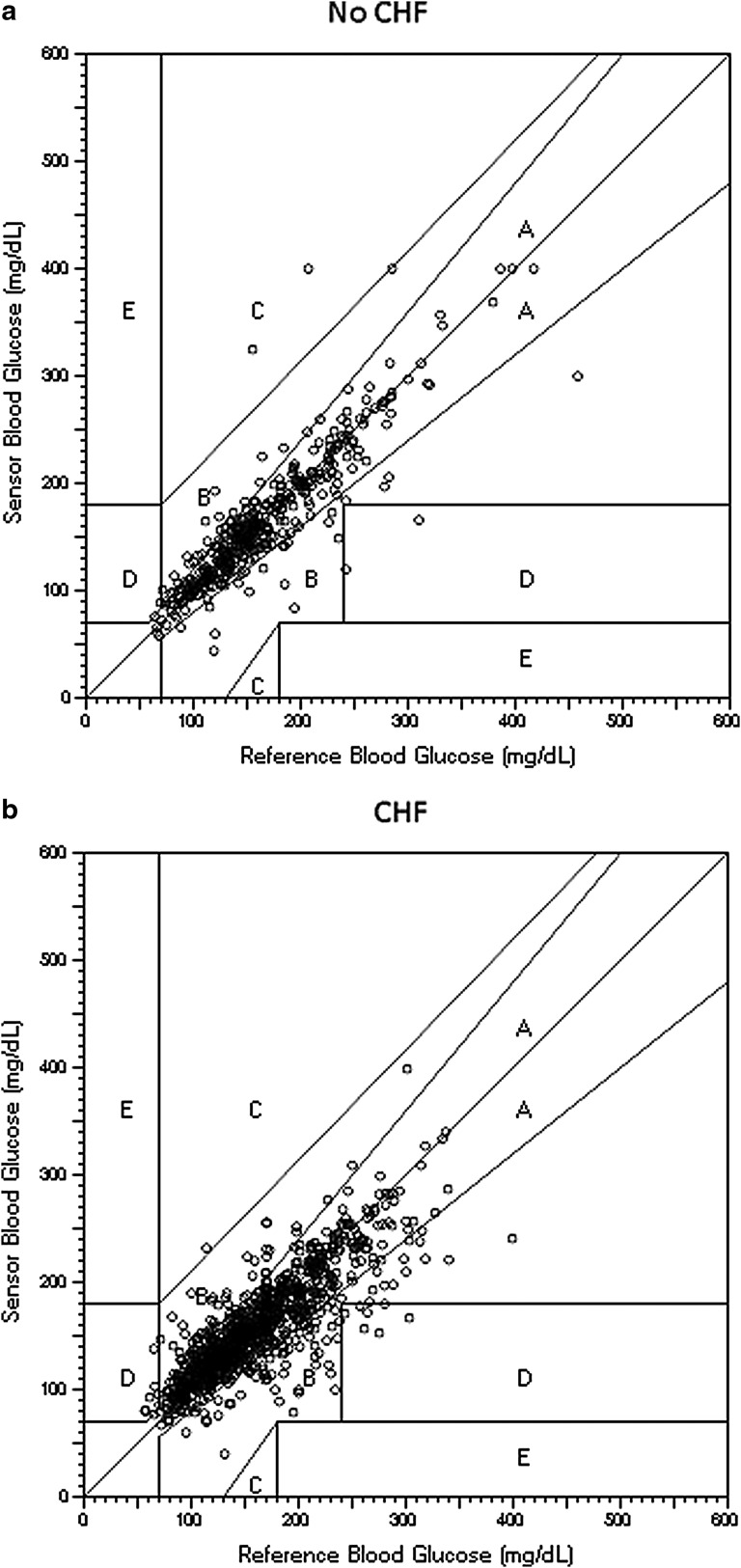
EGAs are shown in Table 4 and Figure 2. There were significantly more values in Zone A in the non-CHF group compared with the CHF group (86.7% vs. 79.3%, P=0.001) using the original EGA, but the number in Zones A+B did not differ (98.7% vs. 98.5%, P>0.99). CG-EGA found more values in Zone A in the non-CHF group in the hypoglycemic range (<70 mg/dL), but there were relatively few readings for comparison. CG-EGA percentages in the 71–180 mg/dL and >180 mg/dL range did not differ between the CHF and non-CHF groups.
Table 4.
Clinical Performance of Professional Continuous Glucose Monitoring by Congestive Heart Failure Status
| Non-CHF (%) | CHF (%) | P valuea | |
|---|---|---|---|
| Original EGA | |||
| Zone A | 86.7 | 79.3 | 0.001 |
| Zone A+B | 98.7 | 98.5 | >0.99 |
| CG-EGA | |||
| <70 mg/dL | |||
| Zone A | 100 | 12.5 | 0.01 |
| Zone A+B | 100 | 12.5 | 0.01 |
| Erroneous | 0 | 87.5 | 0.01 |
| 71–180 mg/dL | |||
| Zone A | 100 | 99.5 | >0.99 |
| Zone A+B | 100 | 99.5 | >0.99 |
| Erroneous | 0 | 0.5 | >0.99 |
| >180 mg/dL | |||
| Zone A | 97.7 | 97.8 | >0.99 |
| Zone A+B | 97.7 | 97.8 | >0.99 |
| Erroneous | 2.3 | 2.2 | >0.99 |
Differences between groups were determined using Fisher's exact test.
CG-EGA, continuous glucose error grid analysis; CHF, congestive heart failure; EGA, error grid analysis.
FIG. 2.
Original Clarke error grid analysis for (a) non-congestive heart failure (CHF) and (b) CHF subjects.
Models (Table 5)
Table 5.
Multivariable Models of Mean Absolute Relative Difference
| |
Overall MARD >0.10 |
MARD 100–150 mg/dL>0.10 |
||||
|---|---|---|---|---|---|---|
| Term | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value |
| Initial model | ||||||
| CHF | 2.40 | 0.80–7.74 | 0.13 | 1.83 | 0.61–5.72 | 0.29 |
| IV insulin | 0.67 | 0.17–2.81 | 0.57 | 0.57 | 0.15–2.32 | 0.42 |
| BMI (kg/m2) | — | — | — | 1.01 | 0.95–1.07 | 0.78 |
| Sensor CV total | 1.07 | 1.01–1.14 | 0.02 | — | — | — |
| Time on sensora | 2.70 | 1.01–9.08 | 0.07 | 2.33 | 0.80–7.87 | 0.14 |
| Final model | ||||||
| Sensor CV total | 1.06 | 1.01–1.24 | 0.03 | — | — | — |
| Time on sensora | 3.43 | 1.62–8.29 | 0.008 | 3.31 | 1.47–8.47 | 0.003 |
Log-transformed values.
BMI, body mass index; CHF, congestive heart failure; CI, confidence interval; CV, coefficient of variation for glucose; IV, intravenous.
In the initial model for overall MARD >0.10 adjusting for CHF status, IV insulin, sensor CV, and log-transformed sensor time, only sensor CV was a significant predictor of MARD >0.10 (odds ratio=1.07, 95% confidence interval 1.01–1.14, P=0.02). In the final model, sensor CV and log-transformed sensor time were significant predictors of higher MARD. In the initial model for MARD100–149>0.10, none of the variables was a significant predictor. After backward elimination, however, log-transformed sensor time emerged as the only significant predictor (P=0.003).
Discussion
CHF is commonly encountered among ambulatory and hospitalized non-critically ill patients with type 2 diabetes and may pose potential challenges to sensor accuracy. Overall, technical and clinical accuracy was acceptable in patients with and without CHF, with trends for decreasing accuracy in the lower glucose range (Tables 1 and 2). There were trends for higher MARD in CHF patients compared with non-CHF patients. After adjustment for other factors, CHF status did not remain significant in the overall MARD >0.10 model or in the model for normoglycemia (MARD100–149>0.10), again suggesting that residual differences in glycemic control (or the ability of the sensor to detect glucose excursions) between CHF and non-CHF groups are likely to contribute to the unadjusted analyses (Table 5).
The importance of glucose excursions on sensor accuracy is in agreement with our previous data, which showed that sensor accuracy is significantly degraded in hospitalized patients presenting with severe hyperglycemia.13 By comparison, a study of 174 medical intensive care unit patients requiring intensive insulin therapy reported an overall MARD of 7.3%, 99.1% of subjects in insulin titration EGA Zones A+B, and a correlation coefficient of 0.92 (2,045 sensor–meter pairs).10 This patient population, however, exhibited very tight glycemic control, with a mean glucose of 111 mg/dL, likely because of the inclusion of patients without preexisting diabetes.
Overall, MARD and values in Zone A of the EGA favored non-CHF patients. No direct measures of edema, tissue oxygenation, or perfusion were available. Both brain natriuretic peptide and estimation of PV were analyzed as readily available, although somewhat imprecise, indicators of CHF severity. Neither brain natriuretic peptide nor the indirect measure of change in PV was associated with MARD, challenging the role of CHF itself on sensor accuracy. However, within-patient changes in brain natriuretic peptide are reported to be a more reliable indicator of response to treatment than absolute values alone.21 After higher and lower glucose values were excluded, no effect of CHF was observed on MARD. Unfortunately, the effect of glucose excursions on sensor accuracy could not be vigorously analyzed, because of fewer sensor–meter pairs in the hypoglycemic and hyperglycemic ranges. In comparison, a small study of pediatric cardiac surgery patients did not demonstrate a significant association between sensor accuracy and edema, determined radiographically.9 Furthermore, other studies have not identified associations between sensor accuracy and vasopressor use.10,11 However, it is unclear how such studies apply to CHF, which may feature more complex disturbances in the sensor environment than that reported in studies of edema or hypotension alone.
Our previous data questioned whether glucose legacy may play a role in sensor accuracy. Such an effect might be mediated by local tissue changes during prolonged hyperglycemia (such as glycosylation or inflammation). In this study, there were significant differences in admission glucose and hemoglobin A1c among CHF and non-CHF patients, but the more extreme values occurred in the non-CHF patients (Table 1), indicating that glucose legacy was not responsible for the differences in sensor accuracy between CHF and non-CHF patients. Moreover, neither hemoglobin A1c nor admission glucose level was a significant univariate predictor of overall MARD or MARD100–149.
In this study, CV was a significant predictor of overall MARD, independent of other variables (Table 5); this is consistent with our previous data.13 Rapid glucose changes are known to affect the accuracy of CGM.17 Likewise, increasing log-transformed sensor time was a predictor of MARD >0.10, even after controlling for CV. The reasons for this finding are unclear, but the odds ratios were attenuated in the initial models, suggesting confounding by other variables, particularly route of insulin. For example, the odds ratio for log-transformed sensor time increased from 2.7 to 3.3 after removal of IV insulin and increased only slightly after removal of CHF. In a previous study, IV insulin was also independently associated with increasing overall MARD, even after adjusting for CV, raising the question of whether other factors, such as unmeasured fluid shifts, may be important.13 However, this observation was not confirmed in the current study. Further research is needed to determine whether the useful sensor life is reduced in subjects with CHF, but data in healthy subjects suggest that sensor accuracy does not degrade with up to 9 days of wear.22
The study is limited by the comparison of patients who, although differing by CHF status, may have had other characteristics, such as a difference in activity level, that could affect sensor accuracy. Thus, residual confounding is possible, even though we adjusted for multiple factors in the models. Formal assessments of cardiac function were not performed in patients without CHF, but none had clinical evidence of active heart failure exacerbation. In addition, capillary, rather than venous or arterial, glucose values were used for calibration. Although we specifically excluded patients who were hypotensive, edema may affect the accuracy of capillary blood glucose values, potentially influencing the calibration of CGM.23,24 However, calibration using capillary blood glucose is more likely to reflect typical use on the wards, where performing multiple venipunctures per day would be impractical. Regardless, calibration was performed in similar manner in both CHF and non-CHF patients and thus does not appear to explain all of the differences between groups in this study or in our previous data.13 Finally, it is important to note that accuracy determinations using professional CGM may not be directly comparable to that of real-time CGM, because of differences in calibration. The iPro software, in particular, has the benefit of using all calibration points in its recording period for calibration, resulting in greater accuracy. Thus, separate accuracy studies for real-time CGM are needed.
In conclusion, among hospitalized subjects with type 2 diabetes, CHF exacerbation is not associated with lower sensor accuracy after adjustment for other factors, but this requires confirmation over a wider glucose range.
Acknowledgments
The authors wish to thank Kelly Rogers for assistance with analysis of data. The project described was supported by the Ohio State University Clinical and Translational Research Center, award UL1RR025755 from the National Center for Research Resources, and by NIH grants R21DK081877 and K23DK080891 from the National Institutes of Health.
Author Disclosure Statement
K.D. reports research funding from Novo Nordisk and consulting fees from Eli Lilly and Diabetes Technology Management. K.G. and C.S. have no competing financial interests.
References
- 1.Klonoff DC. Buckingham B. Christiansen JS. Montori VM. Tamborlane WV. Vigersky RA. Wolpert H. Endocrine Society: Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:2968–2979. doi: 10.1210/jc.2010-2756. [DOI] [PubMed] [Google Scholar]
- 2.Moghissi ES. Korytkowski MT. DiNardo M. Einhorn D. Hellman R. Hirsch IB. Inzucchi SE. Ismail-Beigi F. Kirkman MS. Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 3.Blevins TC. Bode BW. Garg SK. Grunberger G. Hirsch IB. Jovanovic L. Nardacci E. Orzeck EA. Roberts VL. Tamborlane WV. Statement by the American Association of Clinical Endocrinologists Consensus Panel on continuous glucose monitoring. Endocr Pract. 2010;16:730–745. doi: 10.4158/EP.16.5.730. [DOI] [PubMed] [Google Scholar]
- 4.Keenan DB. Cartaya R. Mastrototaro JJ. Accuracy of a new real-time continuous glucose monitoring algorithm. J Diabetes Sci Technol. 2010;4:111–118. doi: 10.1177/193229681000400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Facchinetti A. Sparacino G. Guerra S. Luijf YM. Devries JH. Mader JK. Ellmerer M. Benesch C. Heinemann L. Bruttomesso D. Avogaro A. Cobelli C. the AP@home Consortium: Real-time improvement of continuous glucose-monitoring accuracy: the smart sensor concept. Diabetes Care. 2013;36:793–800. doi: 10.2337/dc12-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dungan K. Chapman J. Braithwaite SS. Buse J. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30:403–409. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 7.Corstjens AM. Ligtenberg JJ. van der Horst IC. Spanjersberg R. Lind JS. Tuleken JE. Meertens JH. Zijlstra JG. Accuracy and feasibility of point-of-care and continuous blood glucose analysis in critically ill ICU patients. Crit Care. 2006;10:R135. doi: 10.1186/cc5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg PA. Siegel MD. Russell RR. Sherwin RS. Halickman JI. Cooper DA. Dziura JD. Inzucchi SE. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technol Ther. 2004;6:339–347. doi: 10.1089/152091504774198034. [DOI] [PubMed] [Google Scholar]
- 9.Piper HG. Alexander JL. Shukla A. Pigula F. Costello JM. Laussen PC. Jaksic T. Agus MSD. Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics. 2006;118:1176–1184. doi: 10.1542/peds.2006-0347. [DOI] [PubMed] [Google Scholar]
- 10.Brunner R. Kitzberger R. Miehsler W. Herkner H. Madl C. Holzinger U. Accuracy and reliability of a subcutaneous continuous glucose-monitoring system in critically ill patients. Crit Care Med. 2011;39:659–664. doi: 10.1097/CCM.0b013e318206bf2e. [DOI] [PubMed] [Google Scholar]
- 11.Holzinger U. Warszawska J. Kitzberger R. Herkner H. Metnitz PG. Madl C. Impact of shock requiring norepinephrine on the accuracy and reliability of subcutaneous continuous glucose monitoring. Intensive Care Med. 2009;35:1383–1389. doi: 10.1007/s00134-009-1471-y. [DOI] [PubMed] [Google Scholar]
- 12.Logtenberg SJ. Kleefstra N. Snellen FT. Groenier KH. Slingerland RJ. Pre- and postoperative accuracy and safety of a real-time continuous glucose monitoring system in cardiac surgical patients: a randomized pilot study. Diabetes Technol Ther. 2009;11:31–37. doi: 10.1089/dia.2008.0028. [DOI] [PubMed] [Google Scholar]
- 13.Dungan KM. Han W. Miele A. Zeidan T. Weiland K. Determinants of the accuracy of continuous glucose monitoring in non-critically ill patients with heart failure or severe hyperglycemia. J Diabetes Sci Technol. 2012;6:884–891. doi: 10.1177/193229681200600420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dungan K. Osei K. Sagrilla C. Binkley P. Effect of the approach to insulin therapy on glycemic fluctuations and autonomic tone in hospitalized patients with diabetes. Diabetes Obes Metab. 2013;15:558–563. doi: 10.1111/dom.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg PA. Memoirs of a root canal salesman: the successful implementation of a hospital-wide intravenous insulin infusion protocol. Endocr Pract. 2006;12(Suppl 3):79–85. doi: 10.4158/EP.12.S3.79. [DOI] [PubMed] [Google Scholar]
- 16.Umpierrez GE. Smiley D. Jacobs S. Peng L. Temponi A. Mulligan P. Umpierrez D. Newton C. Olson D. Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34:256–261. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalra PR. Anagnostopoulos C. Bolger AP. Coats AJ. Anker SD. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol. 2002;39:1901–1908. doi: 10.1016/s0735-1097(02)01903-4. [DOI] [PubMed] [Google Scholar]
- 18.Clarke WL. The original Clarke Error Grid Analysis (EGA) Diabetes Technol Ther. 2005;7:776–779. doi: 10.1089/dia.2005.7.776. [DOI] [PubMed] [Google Scholar]
- 19.Kovatchev BP. Gonder-Frederick LA. Cox DJ. Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors: continuous glucose-error grid analysis illustrated by TheraSense Freestyle Navigator data. Diabetes Care. 2004;27:1922–1928. doi: 10.2337/diacare.27.8.1922. [DOI] [PubMed] [Google Scholar]
- 20.Welsh J. Kaufman FR. Lee SW. MARD data based on a recent Medtronic retrospective analysis of iPro2 algorithms. J Diabetes Sci Technol. 2012;6:475–476. doi: 10.1177/193229681200600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang WH. Girod JP. Lee MJ. Starling RC. Young JB. Van Lente F. Francis GS. Plasma B-type natriuretic peptide levels in ambulatory patients with established chronic symptomatic systolic heart failure. Circulation. 2003;108:2964–2966. doi: 10.1161/01.CIR.0000106903.98196.B6. [DOI] [PubMed] [Google Scholar]
- 22.Iscoe KE. Davey RJ. Fournier PA. Is the response of continuous glucose monitors to physiological changes in blood glucose levels affected by sensor life? Diabetes Technol Ther. 2012;14:135–142. doi: 10.1089/dia.2011.0194. [DOI] [PubMed] [Google Scholar]
- 23.Critchell CD. Savarese V. Callahan A. Aboud C. Jabbour S. Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33:2079–2084. doi: 10.1007/s00134-007-0835-4. [DOI] [PubMed] [Google Scholar]
- 24.Kanji S. Buffie J. Hutton B. Bunting PS. Singh A. McDonald K. Fergusson D. McIntyre LA. Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33:2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]