Abstract
Background
Continuous glucose monitoring (CGM) has been shown to be a valuable tool to improve glycemic control in patients with diabetes. The objective of this pilot study was to develop and implement CGM in an existing diabetes clinic for low-income patients on multiple daily injections.
Subjects and Methods
This was a single-center, prospective, randomized controlled, crossover pilot study. Initial focus groups were held to create low-literacy, Spanish and English guides to the use of carbohydrate counting and CGM. These tools were implemented to train participants on carbohydrate counting and insulin adjustments participants. Subjects were then randomized to start in Group A (CGM) or Group B (self-monitoring blood glucose and then switched after 28 weeks). Hemoglobin A1c (HbA1c) was obtained at baseline and at the end of both study phases.
Results
Twenty-five economically challenged, primarily Latino participants with minimal prior education on intensive diabetes management completed the study. No significant reduction in HbA1c or decrease in time spent in parameters of low and high blood glucose was shown. However, eighty percent of participants who completed the study wanted to continue to use CGM once the research study was over. The participants also felt that the CGM made adjusting insulin easier.
Conclusions
CGM can be implemented in patients from a low-income public clinic; however, HbA1c reduction was not achieved. Given the underlying lack of baseline self-management knowledge, a longer trial might be necessary to see benefit with CGM in this population.
Introduction
Maintaining near-normal blood glucose levels has been documented to reduce both acute and chronic complications of diabetes mellitus (DM).1 Over the past several years, continuous glucose monitoring (CGM) has been shown to be a valuable tool to improve glycemic control in patients with diabetes.2,3 However, there are no data on CGM use in underserved populations despite the fact that minority patients with DM often have higher hemoglobin A1c (HbA1c) levels and are at increased risk chronic complications.4,5
The aim of this study was to determine if providing CGM to patients seen in a type 1 diabetes clinic for low-income patients in East Los Angeles, CA, could be used to improve glycemic control and quality of life outcomes. Preliminary data of this study were presented in an abstract at the 70th Scientific Sessions of the American Diabetes Association in 2010.6
Research Design and Methods
This was a single-center, prospective, randomized controlled, crossover study. The study was conducted in the Endocrine Fellows Diabetes Clinic at the Roybal Comprehensive Health Center in East Los Angeles, a federally designated underserved health care region. Between August 2007 to December 2008, patients with a clinical diagnosis of type 1 diabetes were recruited to participate. Major inclusion criteria were as follows: diagnosis of DM for at least 6 months prior to study enrollment, subject self-report of self-monitoring blood glucose (SMBG) three or more times per day, on multiple daily insulin injections, and at least 18 years of age. Insulin pumps are not available for patients in the Los Angeles County Healthcare System. Patients did not have access to a dietitian knowledgeable in teaching carbohydrate (CHO) counting prior to the study. At baseline, no patient did advanced CHO counting, although some used sliding-scale premeal insulin. Patients were treated with basal insulin glargine and a rapid-acting premeal insulin. These patients were categorized as uninsured; however, state/federal funds did support their clinic visits and diabetes medications and supplies.
Study protocol
Before randomization, the first six patients participated in focus groups to create the educational manual for CGM and CHO counting in Spanish and English at or below a 6th grade reading level (the standard instruction manuals were estimated to be written at a 10th grade reading level, largely because of the technical terminology involved). Participants were randomized into two groups: CGM first (Group A) or SMBG first (Group B) in Period 1 and then switched to the other therapy, Period 2, for up to 28 weeks each period. HbA1c was obtained at baseline, the end of Period 1, and the end of Period 2. Before starting CGM use, all had 1 week of a CGM “blinded” period where participants were not able to see the glucose values recorded in the receiver. Participants were provided with education on CHO counting and insulin dose adjustments using developed educational materials. Patients were seen monthly to download CGM tracings and meter and to receive reinforcement of CHO counting. Diabetes management in the Endocrine Fellows Diabetes Clinic consisted of continuity care with an endocrine Fellow who was supervised by one of two endocrine Attendings. At each routine clinic visit, the participants brought in their meter for downloading in the clinic providing the Fellow with access to the patient CGM downloads and CHO counting logs. HbA1c was measured with a DCA Vantage 2000 analyzer (Bayer, Tarrytown, NY). The Dexcom® (San Diego, CA) SEVEN® sensor was used for the study. The CGM Satisfaction Scale, a 44-item 5-point Likert scale questionnaire, was administered at the end of the CGM use.7
Descriptive statistics were used to assess change in HbA1c and measures of CGM satisfaction. The Spearman correlation was used to evaluate percentage change in HbA1c with the extent of CGM use (days/week). For within-subject comparison before and after CGM, we conducted the Wilcoxon signed rank test on percentage of time spent above 180 mg/dL or below 70 mg/dL. All analyses were performed in SAS version 9.2 (SAS Institute, Cary, NC).
Results
Of the 39 patients enrolled, 25 completed both arms. The mean length of study for Group A was 21 weeks, and that for Group B was 22 weeks. In Group A, four patients dropped out prior to completing the CGM arm (two were lost to follow-up, one because of knee surgery, and one discontinued after the first week of CGM), and four dropped out during the SMBG arm (three were lost to follow-up after CGM was completed, and one stopped because of family health issues). In Group B, two dropped out prior to completing the SMBG arm (both were lost to follow-up after 1 week of SMBG), and four dropped during the CGM arm (one was lost to follow-up, one had difficulty with using the sensor, and two did not like the stress associated with the CGM and did not want to check more often so stopped after 1 week on CGM). Baseline demographics of the completed subjects were as follows: average age of 40 years; 13 years of diabetes duration; 48% female; 76% Latino; a high school average level of education, and 8.5%±1.7% baseline HbA1c (Table 1). Patients had limited to no prior training on CHO counting and limited formal diabetes education.
Table 1.
Baseline Factors by Completers and Noncompleters
| Completers (n=25) | Dropouts (n=14) | |
|---|---|---|
| Randomization group | ||
| Group A: CGM 1st/SMBG 2nd | 11 (44%) | 8 (57%) |
| Group B: SMBG 1st/CGM 2nd | 14 (56%) | 6 (43%) |
| Baseline HbA1c (%) | 8.5±1.7 | 8.2±2.2 |
| Age (years) | 40±13 | 42±11 |
| Range | 21–63 | 30–67 |
| 21–29 | 8 (32%) | 0 |
| 30–39 | 5 (20%) | 7 (50%) |
| 40–49 | 5 (20%) | 5 (36%) |
| 50–67 years | 7 (28%) | 2 (14%) |
| Gender female | 12 (48%) | 8 (57%) |
| Race/ethnicity | ||
| Hispanic/Latino | 19 (76%) | 7 (50%) |
| White | 4 (16%) | 3 (21%) |
| Black/African American | 2 (8%) | 2 (14%) |
| Asian/Pacific Islander | 0 | 2 (14%) |
| Diabetes duration (years) | 13 (10, 21) | 26 (14, 29) |
| Age 21–29 years | 11 (9, 16) | NA |
| Age 30–39 years | 11 (8, 13) | 15 (10, 24) |
| Age 40–49 years | 21 (11, 25) | 28 (28, 29) |
| Age 50–67 years | 21 (13, 31) | 36 (27, 45) |
| Number of SH events in 1 month before consenta | ||
| None | 20 (80%) [80%] | 4 (44%) [44%] |
| 1 | 2 (8%) [88%] | 1 (11%) [56%] |
| 2 | 1 (4%) [92%] | 3 (33%) [89%] |
| 3 | 1 (4%) [96%] | 1 (11%) [100%] |
| 4 | 1 (4%) [100%] | 0 [100%] |
Data are number (%), mean±SD values, or median (25th percentile, 75th percentile) as indicated.
Self-reported severe hypoglycemia (SH) event associated with loss of consciousness or the need of assistance from others to administer carbohydrate, glucagon, or other resuscitative actions. Cumulative percentage is given in brackets. Data from five subjects are missing (three in the continuous glucose monitoring [CGM] first group, 2 in the self-monitoring of blood glucose [SMBG] first group).
HbA1c, hemoglobin A1c; NA, not applicable.
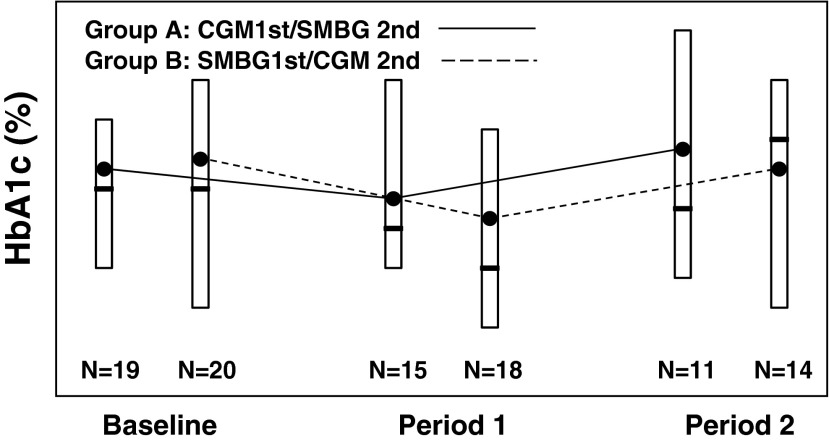
The change in HbA1c in Group A (CGM first/SMBG second) was from 8.3% to 8.0% and then up to 8.5% after the SMBG phase. In Group B (SMBG first/CGM second), the change in HbA1c was from 8.3% to 7.8% and then up to 8.3% after the CGM phase (Fig. 1). CGM usage was greater than 6 days/week in 67% of Group A and in 64% of Group B. For most subjects who dropped out of the study, it is not known if or how much CGM was used prior to leaving the study. There was a small trend (which did not reach statistical significance) toward a decrease in HbA1c with more consistent CGM use (data not shown). There was no decrease in time spent in the greater than 180 mg/dL or below 70 mg/dL range or statistical differences among measurements of glycemic variation such as SD or maximum amplitude of glycemic excursions compared with individual blinded and unblinded periods of CGM use.
FIG. 1.
Box plots of hemoglobin A1c (HbA1c) at three time points (baseline, Period 1, and Period 2) by treatment for all the subjects who completed both phases of the study. The open box denotes the 25th, 75th percentile, the solid line in the box denotes the median, and solid circle denotes the mean HbA1c level. CGM, continuous glucose monitoring; SMBG, self-monitoring of blood glucose.
Using a CGM satisfaction survey, out of a 5-point scale, the overall score for Group A was 3.9±0.4, and that for Group B was 3.8±0.5. Eighty percent of participants who completed the study wanted to continue to use CGM once the research study was over. The participants also thought that the CGM made adjusting insulin easier. Over 80% thought that CGM helped them prevent hypoglycemia and that it helped improve management of hypoglycemia.
Discussion
We have shown that low-income patients with DM who have had limited access to technology and advanced diabetes education can learn to use CGM. The strength of the study was that it was the first to evaluate the utility of CGM in a low-income, high-risk, primarily Latino population. Other studies have shown that CGM use improves glycemic control, but we were unable to show significant reductions in HbA1c.3,8–10 Although time was spent in adapting educational material used in previous studies, this proved to be less optimal for our population, perhaps because of their low health literacy, baseline lack of DM knowledge and experience with new technology for diabetes management, and difficulty in performing day-to-day self-adjustment in insulin doses. Although DM education was given at the beginning of the study, perhaps a longer period of education on DM management was needed given the low baseline DM knowledge. No home downloads were done, and no patients analyzed trend data over time, much less observed trends over time and adjusted accordingly.3,11,12 Other significant limitations include lack of power due to small sample size, poor retention, no run-in period, and no washout period to avoid carryover effect. Recruitment was limited because of the size and frequency of the clinic as well as a high no-show rate, as well as the transient nature of our population. Future studies need to address how to improve retention in this high-risk population. The limitations of the study made the duration of this study too brief to assess all the potential benefits with CGM in this primarily low-income Latino population.
In terms of individual preference, many of the patients liked wearing the device and would have continued with it if possible. Although it could be argued at this time that CGM is ineffective in an underserved population, additional studies are needed in this high-risk group to see how the use of newer technology can be implemented to improve outcomes.
Acknowledgments
This project was funded by JDRF Artificial Pancreas grant 22-2006-1119.
Author Disclosure Statement
P.S. has received funding from Sanofi. A.L.P. has received honoraria from Dexcom for giving lectures on CGM, has been a consultant/author for Abbott Diabetes Care, Amylin/Eli Lilly, Janssen Pharmaceuticals, Takeda Pharmaceuticals USA, Inc., Roche Pharmaceuticals, Sanofi, and BD, and a speaker/author for Amylin/Eli Lilly and Novo Nordisk Inc. L.M., V.R., D.X., V.C., and R.B. declare no competing financial interests exist.
References
- 1.Diabetes Control and Complication Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 3.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment. Diabetes Care. 2010;33:17–22. doi: 10.2337/dc09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris MI. Eastman RC. Cowie C. Flegal KM. Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22:403–408. doi: 10.2337/diacare.22.3.403. [DOI] [PubMed] [Google Scholar]
- 5.Delamater A. Shaw K. Applegate EB. Pratt IA. Eidson M. Lancelotta G. Gonzalez-Mendoza L. Ricthton S. Risk for metabolic control problems in minority youth with diabetes. Diabetes Care. 1999;22:700–705. doi: 10.2337/diacare.22.5.700. [DOI] [PubMed] [Google Scholar]
- 6.Sequeira PA. Ruelas V. Montoya L. Peters A. Continuous glucose monitoring (CGM) in underserved patients on multiple daily injections (MDI) [abstract] Diabetes. 2010;59:A542–A542. [Google Scholar]
- 7.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Validation of measures of satisfaction with and impact of continuous and conventional glucose monitoring. Diabetes Technol Ther. 2010;12:679–684. doi: 10.1089/dia.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deiss D. Bolinder J. Riveline JP. Battelino T. Bosi E. Tubiana-Rufi N. Kerr D. Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 9.Anderson J. Attvall S. Sternemalm L. Pivodic A. Fahlén M. Hanås R. Ekeroth G. Lind M. Effect on glycemic control by short- and long-term use of continuous glucose monitoring in clinical practice. J Diabetes Sci Technol. 2011;5:1472–1479. doi: 10.1177/193229681100500622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battelino T. Conget I. Olsen B. Schütz-Fuhrmann I. Hommel E. Hoogma R. Schierloh U. Sulli N. Bolinder J SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155–3162. doi: 10.1007/s00125-012-2708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayeaux EJ., Jr Murphy PW. Arnold C. Davis TC. Jackson RH. Sentell T. Improving patient education for patients with low literacy skills. Am Fam Physician. 1996;53:205–211. [PubMed] [Google Scholar]
- 12.Schillinger D. Grumbach K. Piette J. Wang F. Osmond D. Daher C. Palacios J. Diaz G. Sullivan G. Bindman A. Association of health literacy with diabetes outcomes. JAMA. 2002;288:475–482. doi: 10.1001/jama.288.4.475. [DOI] [PubMed] [Google Scholar]