Abstract
Background
Fatigue is one of the most frequently reported, distressing side effects reported by cancer survivors and often has significant long-term consequences. Research indicates that yoga can produce invigorating effects on physical and mental energy, and thereby may improve levels of fatigue. The objective of this systematic review was to examine the literature that reports the effects of randomized, controlled yoga interventions on self-reported fatigue in cancer patients and survivors. The online electronic databases, PubMed and PsycINFO, were used to search for peer-reviewed research articles studying the effects of yoga interventions on fatigue in cancer survivors. Combinations of yoga, cancer, and fatigue-related search terms were entered simultaneously to obtain articles that included all three elements. Studies were included if they met the following inclusion criteria: participants were male or female cancer patients or survivors participating in randomized, controlled yoga interventions. The main outcome of interest was change in fatigue from pre- to post-intervention. Interventions of any length were included in the analysis. Risk of bias using the format of the Cochrane Collaboration’s tool for assessing risk of bias was also examined across studies.
Results
Ten articles met inclusion criteria and involved a total of 583 participants who were predominantly female, breast cancer survivors. Four studies indicated that the yoga intervention resulted in significant reductions in self-reported fatigue from pre- to post-intervention. Three of the studies reported that there were significant reductions of fatigue among participants who attended a greater number of yoga classes. Risk of bias was high for areas of adequate selection, performance, detection, and patient-reported bias and mixed for attrition and reporting bias. Risk of bias was uniformly low for other forms of bias, including financial conflicts of interest.
Conclusions
Results of the studies included in this review suggest that yoga interventions may be beneficial for reducing cancer-related fatigue in women with breast cancer; however, conclusions should be interpreted with caution as a result of levels of bias and inconsistent methods used across studies. More well-constructed randomized controlled trials are needed to determine the impact of yoga interventions on fatigue in cancer patients and survivors.
Keywords: Yoga, cancer, fatigue
INTRODUCTION
Description of the Cancer-Related Fatigue (CRF)
Cancer and treatment for cancer often cause numerous physical and psychological problems that do not disappear with time, and can have negative effects on cancer survivors’ quality of life.1 Cancer patients commonly experience pain, depression, anxiety, and fatigue, which often continue even after the treatment is complete.2 Fatigue is one of the most frequently reported, distressing side effects reported by cancer survivors and often has significant long-term consequences.3–5 Cancer-related fatigue (CRF) has been defined by the National Comprehensive Cancer Network as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer-related treatment that is not proportional to recent activity and interferes with usual functioning.6 The occurrence of CRF in the literature is variable and has been found to range from 4% to 91%, depending on the population and assessment methods. Out of all side effects, CRF has been identified as the most distressing symptom, and the one that leads to the greatest amount of interference with daily life.7
Description of Yoga Interventions Tailored for Cancer Patients
Complementary and alternative medicine (CAM) techniques are frequently used in cancer patients to help manage their distressing side effects.8,9 Such techniques include a broad array of heterogeneous treatments, ranging from herbal medicine to yoga. Yoga has recently undergone empirical investigation for use as a complementary or alternative therapy for patients with cancer,10 and while the efficacy is still unclear, it is sometimes offered as a CAM for cancer patients.
Yoga is an ancient Eastern tradition that originated in India and consists of a combination of spiritual, moral, and physical practices aimed at attaining “self-awareness.” The traditional Indian form of yoga encompasses several domains, including ethical disciplines, physical postures, and spiritual practices, with the goal of uniting the mind, body, and spirit for health and self-awareness.10 There are a variety of types of yoga (Iyengar, Ashtanga, Hatha, Bikram, etc.), but the most common form adopted by Western society consists of three basic components, asanas (physicalposes), pranayama (breathcontrol), and dhyana (meditation). Specific postures are performed to help improve flexibility and strength, controlled breathing aims to increase focus and relaxation, and meditation aims to calm the mind.11,12 Yoga tailored for cancer patients aims to be a gentle practice that appropriately accommodates the needs of the patients. For patients, approval from an oncologist is typically needed before beginning the practice, and instructions are modified as needed to accommodate each individual patient’s needs.
The Oncology Nursing Society’s published guidelines recommend exercise as an evidence-based intervention for CRF.13 A systematic review of the occurrence, assessment, and treatment of fatigue in cancer patients indicated that exercise might help prevent or treat fatigue in some subsets of cancer patients.7 At least three recent systematic reviews have examined studies that examined physical and psychological effects of physical activity interventions in cancer populations. They all indicate that physical activity shows promising results for reducing cancer-related fatigue.14–16 Physical exercise during treatment for breast and prostate cancer seems to cause no health risks and may lead to reductions in fatigue.14 Research indicates that yoga can produce invigorating effects on physical and mental energy, which are similar to some of the effects of aerobic exercise, and thereby may improve levels of fatigue.17 The degree to which the meditative component versus the exercise component of yoga contributes to such outcomes is not clear. Few studies have directly compared the physical fitness benefits of yoga practice as compared to standard exercise regimens, although there are indications from the literature that the benefits of yoga, at least for older adults, may exceed those of conventional exercise interventions for self-rated health status and aerobic fitness.18
Gap in Existing Reviews
There are a number of systematic reviews assessing the effect of physical activity interventions on CRF.7,19–21 There are also several reviews examining complementary therapies for CRF21,22 and more general psychological and physical effects of yoga in cancer patients.10 There was one recent abbreviated systematic review in the format of a letter to the editor that addressed the topic of whether yoga can improve fatigue in breast cancer patients.23 They identified six randomized controlled trials on the topic that included a total of 362 patients. They concluded that the evidence suggests possible beneficial effects of yoga on fatigue in cancer patients, but that evidence is limited by risk of bias. To date, there is no detailed systematic review that we are aware of that specifically examines the effects of yoga interventions on fatigue in cancer survivors.
Objective
The objective of this review was to examine the literature that reports effects of yoga interventions on self-reported fatigue in cancer patients and cancer survivors.
METHODS
Eligibility Criteria
Studies were included in this review if they met the following inclusion criteria: participants were male or female cancer survivors participating in randomized, controlled yoga interventions. The main outcome of interest was change in fatigue from pre- to post-intervention. Interventions of any length were included in the analysis. Studies were excluded if they involved adjunctive interventions such as psychotherapy or nutritional consultation. Interventions based solely on meditation were not included. Case studies, conference abstracts, and non-experimental studies were also excluded.
Information Sources and Search Procedure
The online electronic databases, PubMed (1953–present) and PsycINFO (1806–present), were used to search for peer-reviewed research articles studying the effects of yoga interventions on self-reported fatigue in cancer survivors. The searches were last performed on May 31, 2012. Combinations of yoga, cancer, and fatigue-related search terms were entered simultaneously to obtain articles that included all three elements. Keywords entered as search terms included “(yoga OR yogic OR asana OR prana*) AND (fatigue OR exhaust* OR burnout OR letharg* OR weary OR weariness OR drows* OR tired*) AND (cancer OR metastatic OR leukemia OR lymphoma OR tumor OR oncology OR oncologist OR malignant OR malignancy OR chemotherapy OR radiation).” The reference lists of articles included in this review were also searched for relevant articles. The search was limited to English language articles that were accepted into publication into a peer review journal. All years available in the selected databases were included in the search.
Study Selection and Data Collection Process
Titles, abstracts, and keywords from the records retrieved from the searches were screened to determine whether they might meet inclusion and exclusion criteria. If a study appeared to meet the criteria, full text reports were read to determine eligibility and included in the review if they met all of the requirements. Studies were screened by the authors using a standard data extraction form consisting of the data items listed in the following section.
Data Items
The following information was gathered
General information: published/unpublished, title, authors, source, contact address, country, setting, language, year of publication, and source of funding.
Study characteristics: design, randomization (and method if stated), allocation concealment, and blinding of outcome assessors.
Participants: inclusion criteria, exclusion criteria, total number in intervention/control groups, sex, age, baseline characteristics, cancer diagnosis, and similarity of groups at baseline. Number of participants who refused or were excluded from entering the study as well as number of withdrawals/losses to end of intervention follow-up. Reasons for discontinuation of all participants allocated to the intervention.
Type of yoga intervention and comparator, frequency of yoga sessions, duration of study, assessment of adherence to intervention, training of yoga instructors, and use of a manual.
Measures of fatigue: methods for assessing fatigue, as well as baseline and post-intervention means with standard deviation (SD) for the intervention and control groups.
Risk of Bias in Individual Studies
Risk of bias was evaluated using the format of the Cochrane Collaboration’s tool for assessing risk of bias, which uses seven specific domains as listed below:
- Random sequence generation (selection bias)
- ○ Low risk, unclear risk, and high risk
- ○ Random sequence generation was rated as low risk if all the participants had the same possibility of being placed into treatment or wait-list, and if the investigator was unable to predict treatment allocation for each participant.
- Allocation concealment
- ○ Low risk: randomization was assigned using serially numbered, opaque, sealed envelopes, and there appears to be evidence of convincing concealment
- ○ Unclear risk: authors did not adequately report on method of concealment
- ○ High risk: allocation based on day of admission or case record number
- Blinding of participants and personnel (performance bias)
- ○ Low risk, unclear risk, and high risk
- ○ Not possible in yoga intervention studies unless a comparative design is used
- Blinding of outcome assessment (detection bias) (patient-reported bias)
- ○ Low risk: outcome assessors were blinded
- ○ Unclear risk: no adequate report on method of blinding
- ○ High risk: (a) outcome assessors were not blinded or (b) self-report measures were used and participants were not blinded
- Incomplete outcome data (attrition bias)
- ○ Low risk: fewer than 20% of participants were lost to follow-up, and reasons for loss were similar in both treatment and wait-list groups
- ○ Unclear risk: loss to follow-up was not reported
- ○ High risk: more than 20% of patients were lost to follow-up, or reasons for loss differed between treatment arms
- Selective reporting (reporting bias)
- ○ Low risk: free from suggestion of selective outcome reporting
- Unclear risk
- ○ High risk: suggestive of selective outcome reporting
- Other sources of bias
- ○ Primarily looked at possible financial conflicts of interest
- ○ Low risk: unlikely that other sources of bias influenced the results
- ○ Unclear risk: unclear if other sources of bias may have influenced the results
- ○ High risk: likely that other sources of bias influenced the results
Summary Measure
The principle summary measure was pre- to post-intervention difference in means of self-reported fatigue.
Risk of Bias Across Studies
Assessment of the risk of bias across studies was summarized using the Cochrane Collaboration’s tool for assessing risk of bias across studies.24
Low risk: most information is from studies at low risk of bias.
Unclear risk of bias: most information is from studies at low or unclear risk of bias.
High risk: the proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results.
RESULTS
Study Selection and Subject Characteristics
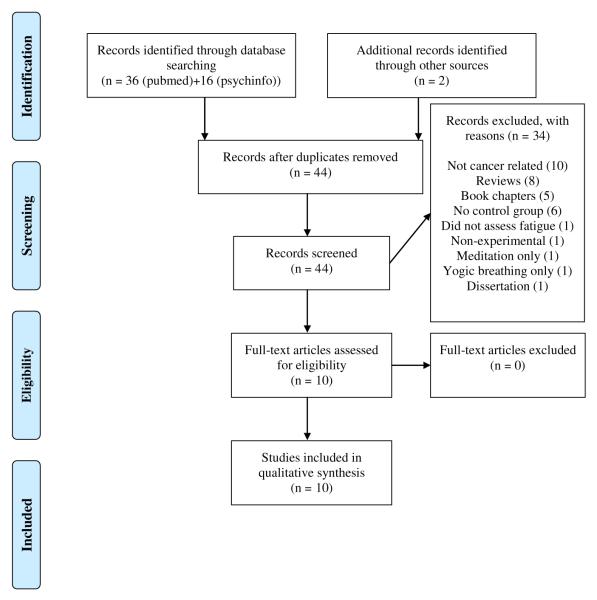
Forty-four non-redundant articles were identified, and 10 were potentially relevant after screening title and abstract (Figure 1). All 10 articles met inclusion criteria and involved a total of 583 participants (Table 1). Sample sizes across studies ranged from a low of 18 to a high of 164 (Table 2). Out of all 583 participants across the 10 included studies, only 17 (2.9%) were men. Eight of the studies included only women, one study25 included 15 (38%) men, and one study26 included only two (5%) men. The average age of participants was 54 years. Three studies did not report race or ethnicity. Of the other studies, 80–100% of the participants were Caucasian (see Table 2), with the exception of one study27 that was 42% African American, 31% Hispanic, 23% Caucasian, and 4% other. Trail sample size ranged from 18 to 164 participants.
Figure 1.
Flow diagram of records identified, included, and excluded.
Table 1.
Characteristics of Yoga Intervention
| References | Yoga Type | Length (minutes) |
Frequency (x/week) |
Duration (weeks) |
Training of Yoga Instructors | Manual |
|---|---|---|---|---|---|---|
| Banasik et al.30 | Iyengar yoga | 90 | 2 | 8 | Not reported | No |
| Bower et al.33 | Iyengar yoga | 90 | 2 | 12 | Certified Junior Intermediate Iyengar yoga instructor |
Yes |
| Carson et al.17 | Yoga of awareness | 120 | 1 | 8 | Experienced in teaching yoga to medical patients and underwent comprehensive training in traditional schools of yoga |
Yes |
| Chandwani et al.28 | Patanjali’s yoga tradition |
60 | 2 | 6 | VYASA-trained instructor | Yes |
| Cohen et al.25 | Tibetan yoga | Not reported | 1 | 7 | Experienced TY instructor (A.C.R.) | No |
| Culos-Reed et al.26 | Based on Hatha | 75 | 1 | 7 | BS in kinesiology, and certified as a yoga instructor |
No |
| Danhauer et al.32 | Based on Hatha | 75 | 1 | 10 | Cancer-specific yoga training who was registered by the National Yoga Alliance |
No |
| Littman et al.29 | Based on Hatha— viniyoga |
75 | 1 | 26 | Two certified yoga instructors with more than 10 years of experience in teaching yoga to cancer patients and survivors |
Yes |
| Moadel et al.27 | Based on Hatha | 90 | 1 | 12 | Certified yoga instructor, in consultation with experts in India and the US |
No |
| Vadiraja et al.34 | Integrated yoga program |
60 | 3 | 6 | Trained yoga therapist | No |
TY, tibetan yoga; BS, bachelor of science.
Table 2.
Sample Size and Study Completion Information
| Sample Size | Completion | |
|---|---|---|
| References | Yoga n; Control n |
Yoga n (%); Control n (%) |
| Banasik et al.30 | 9; 9 | 7 (78); 7 (78) |
| Bower et al.33 | 16; 15 | 13 (81); 13 (87) |
| Carson et al.17 | 17; 20 | 13 (76); 17 (85) |
| Chandwani et al.28 | 30; 31 | 27 (90); 31 (100) |
| Cohen et al.25 | 20; 19 | 16 (84); 14 (74) |
| Culos-Reed et al.26 | 20; 18 | 18 (90); 18 (100) |
| Danhauer et al.32 | 22; 22 | 13 (59); 14 (64) |
| Littman et al.29 | 32; 31 | 27 (84); 27 (87) |
| Moadel et al.27 | 108; 56 | 84 (78); 44 (79) |
| Vadiraja et al.34 | 44; 44 | 40 (91); 34 (77) |
Cancer diagnosis
Eight of the studies involved only breast cancer patients. One study included 85% breast cancer patients and did not report the diagnoses of other participants. One study25 included only participants with lymphoma. Studies included patients with various stages of cancer diagnosis, from stage I–IV, as well as patients with no active signs of cancer for at least two years (Table 3).
Table 3.
Patient Characteristics
| References | Age Yoga (SD); Control (SD) |
Gender % Women; % Men |
Race/Ethnicity | Cancer Type | Cancer Stage | Treatment Stage |
|---|---|---|---|---|---|---|
| Banasik et al.30 | 62.4 (7.3); 63.3 (6.9) | 100; 0 | 100% Caucasian | Breast cancer | Stage II–IV | At least two months post-treatment |
| Bower et al.33 | 54.4 (5.7); 53.3 (4.9) | 100; 0 | 87.1% Caucasian 6.5% Hispanic 3.2% Black 3.2% Other |
Breast cancer | Stage 0–II | At least six months post-treatment |
| Carson et al.17 | 53.9 (9.0); 54.9 (6.2) | 100; 0 | 81.1% Caucasian 18.9% African Am. |
Breast cancer | Stages IA–IIB ≥ two years before, no active cancer |
At least two years post-treatment |
| Chandwani et al.28 |
51.4 (8); 44.0 (10) | 100; 0 | 80% Caucasian 10% Latino 3% African Am. 3% Asian/Pacific Islander 3% Other |
Breast cancer | Stage 0–III | Undergoing radiotherapy |
| Cohen et al.25 | 51 (?); 51 (?) | 62; 38 | Not reported | Lymphoma | Stage I–IV | 85% receiving treatment (type not reported) |
| Culos-Reed et al.26 |
Total 51:2 (10.3) | 95; 5 | Not reported | 85% breast cancer; other 15% not reported |
Not reported | No current active treatment |
| Danhauer et al.32 |
54.3 (9.6); 57.2 (10.2) | 100; 0 | 88.6% Caucasian 6.8% African Am. 4.6% Asian/Pacific Islander |
Breast cancer | Stage 0–V | 34% currently receiving chemotherapy and/ or radiation treatment |
| Littman et al.29 | 60.6 (7.1); 58.2 (8.8) | 100; 0 | 93.6% Caucasian 4.8% African Am. 1.6% Other |
Breast cancer | Stage 0–III | At least three months post-treatment |
| Moadel et al.27 | 55.1 (10.1); 54.2 (9.8) | 100; 0 | 42% African Am. 31% Hispanic 23% Caucasian 4% Other |
Breast cancer | Not reported | 48% receiving medical treatment |
| Vadiraja et al.34 | Not reported (between 30 and 70) |
100; 0 | Not reported | Breast cancer | Stage II–III | Had undergone surgery as primary treatment and were receiving adjuvant radiotherapy |
Yoga intervention
There was no standard type of yoga intervention used throughout the studies; rather there were a variety of protocols with different components in each study (Table 4). The duration of the intervention and the frequency and length of yoga sessions were also variable across studies. Duration of the interventions ranged from six to 26 weeks, frequency ranged from one to five sessions per week, and length ranged from 60 to 120 min/session (one study did not report length).
Table 4.
Components of Yoga Interventions
| References | Stretch, Pose, and Breathe |
Deep Relaxation |
Meditation | Visualization | CD and Printed Materials for Home Practice |
Study of Pertinent Topics and Group Discussion |
|---|---|---|---|---|---|---|
| Banasik et al.30 | Yes | |||||
| Bower et al.33 | Yes | Yes | ||||
| Carson et al.17 | Yes | Yes | Yes | Yes | ||
| Chandwani et al.28 | Yes | Yes | Yes | Yes | ||
| Cohen et al.25 | Yes | Yes | Yes | Yes | ||
| Culos-reed et al.26 | Yes | Yes | ||||
| Danhauer et al.32 | Yes | Yes | ||||
| Littman et al.29 | Yes | Yes | Yes | |||
| Moadel et al.27 | Yes | Yes | Yes | |||
| Vadiraja et al.34 | Yes | Yes | Yes | Yes |
CD, compact disc.
Fatigue
There was little consistency in method of assessing fatigue across studies (Table 5). Fatigue was assessed with the Brief Fatigue Inventory in two studies,25,28 the Functional Assessment of Chronic Illness Therapy–Fatigue in two studies,27,29 Cella’s Functional Assessment of Cancer Therapy-Breast (FACT-B),30 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30,31 FACT-Fatigue,32 the Fatigue Symptom Inventory (FSI),33 the Rotterdam Symptom Checklist (RSCL),34 and a telephone reported 0–9 scale in which higher scores reflected greater fatigue.17
Table 5.
Assessment of Fatigue
| Reference | Time Points Assessed | Fatigue Assessment Method | Results |
|---|---|---|---|
| Banasik et al.30 | Baseline Post-treatment (8 weeks) |
Functional Assessment of Cancer Therapy-Breast (FACT- B): This is a self-report tool designed to measure perceived quality of life. Fatigue score is determined by averaging Likert scale responses to fatigue-related items. |
The yoga group reported less fatigue after yoga participation compared to baseline (P = .046), while the control group fatigue scores worsened over the course of the study period. In the control group, self-reported fatigue increased from a mean of 0.86 ± 0.90 at baseline to 1.57 ± 0.98 Analysis of variance with repeated measures comparing changes over time by group revealed a significant group- by-time interaction for fatigue score (P = .003). |
| Bower et al.33 | Baseline Post-treatment (12 weeks) Three-month follow-up |
Fatigue Symptom Inventory (FSI): This is a measure of fatigue that was designed for use with cancer patients |
Yoga led to statistically significant improvements in fatigue severity (P for group-by-time interaction = .032; effect size for predicted change from baseline to three-month; follow- up in the yoga versus health education group, d = 1.5). |
| Carson et al.17 | Baseline Post-treatment (eight weeks) Three-month follow-up |
Diary data was collected via an interactive telephone voice system using 0–9 scales in which higher scores reflected greater amounts of fatigue |
The yoga group showed significant post-treatment improvements relative to the controls in level of fatigue. These improvements were maintained at three-month follow-up. Greater practice was associated with less fatigue at post-treatment [r (27) = −0.413, P = .032] and at three-month follow-up [r (26) = −0.439, P = .025) |
| Chandwani et al.28 | Baseline One week post-treatment One month post-treatment Three months post-treatment |
Brief Fatigue Inventory (BFI): This is a nine-item questionnaire in which participants rate the severity of their fatigue at that moment and how much it interfered with their lives during the previous 24 hours. An average score is calculated, with higher scores representing worse fatigue; the range is 0–10 |
No significant difference |
| Cohen et al.25 | Baseline Post-treatment (seven weeks) Three-month follow-up |
BFI: see above | No significant difference |
| Culos-reed et al.26 | Baseline Post-treatment (seven weeks) |
EORTC QLQ-C30: This 30-item questionnaire includes five functional domains of quality of life: physical function (five items), emotional function (four items), cognitive function (two items), social function (two items), and role function (two items). There are also several symptom scales: Fatigue (three items), pain (two items), nausea and vomiting (two items), and one item each for dyspnea, sleep disturbance, appetite, constipation, diarrhea, and financial difficulties. |
No significant difference |
| Danhauer et al.32 | Baseline Post-treatment (10 weeks) |
Functional Assessment of Cancer Therapy–Fatigue (FACT-F): This is a 13-item instrument developed to assess fatigue in people with cancer. Responses are made on a five-point Likert scale ranging from 0 (not at all) to four (very much so) and summed to yield a total score. |
No significant difference |
| Littman et al.29 | Baseline Post-treatment (26 weeks) |
Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F): It was designed specifically for the cancer population and is used to assess limitations in daily activity and energy level. |
Six-month changes in fatigue did not differ between the groups. However, fatigue decreased more among women who attended a greater number of classes. Those who attended at least 24 classes had 4.2- and 3.5-point improvements (P < .05) compared with the control group. |
| Moadel et al.27 | Baseline Post-treatment (12 weeks) |
FACIT-F: see above | There was no significant main effect of the intervention on fatigue. Patients with high adherence (> six classes), low adherence (one to six classes), and no adherence (0 classes) were compared. Analysis of covariance indicated that post-treatment scores differed by adherence level on fatigue (F = 6.86; P = .002), controlling for baseline scores and covariates (chemotherapy, age, and race). Those who attended fewer classes tended to have increasing fatigue (t = 3.50; P < .001). |
| Vadiraja et al.34 | Baseline Post-treatment (six weeks) |
Rotterdam Symptom Checklist (RSCL): A questionnaire that measures symptoms that cause physical distress, psychological distress, and impairment in the activities of daily living. |
There was a significant group-by-time interaction effects for fatigue [F (1, 72) = 7.74, P = .007] and a significant between-subjects effects for fatigue [F (1, 72) = 8.26, P = .01]. |
| Post-hoc tests using Bonferroni’s correction showed significant differences between yoga and control groups in post-RT measures alone for fatigue (mean difference ± SE, −20.72 ± 5.36; P = .001) |
EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, SE, standard error, post-RT = postradiotherapy.
Risk of Bias Within Studies
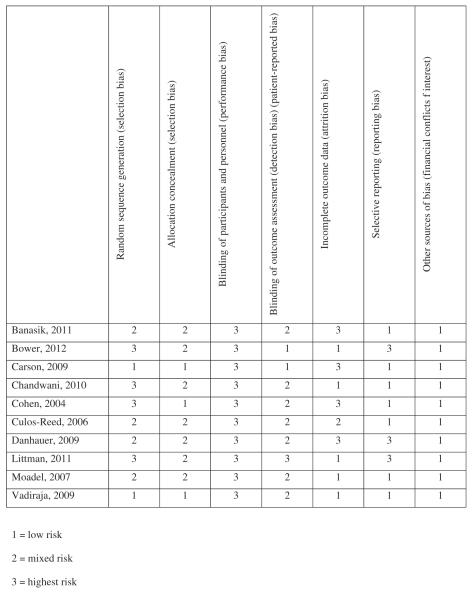
Random sequence generation
Selection bias
There appeared to be high risk of selection bias in the included studies. Only two studies17,34 included adequate description of the randomization process to suggest that all the participants had equal possibility of being placed into treatment or wait-list without the investigator being able to predict treatment allocation for each participant. Two studies used minimization, which is an adaptive assignment procedure intended to balance groups on selected patient characteristics. This type of assignment increases the chance that the investigator would be able to predict treatment allocation for each participant. Two studies used block randomization, which increases risk of bias. The remaining four studies made no mention of randomization procedures.
Allocation concealment
Selection bias
The risk of selection bias across studies was unclear, because only two of the studies mentioned using allocation concealment.17,25
Performance bias
There was a high risk of performance bias in the included studies. Due to the use of wait-list, health education, or brief supportive therapy as a control rather than comparative control groups, it was not possible for participants to be blinded to group assignment.
Detection bias and patient-reported bias
There was evidence of high risk of detection and patient-reported bias across most of the studies. Only two studies17,33 claimed blinding of outcome assessment, saying that the “research assistant collecting assessment data was kept blind with regard to patient condition assignments,” and “outcomes assessors for the performance tasks were blinded to group assignment.” One other study indicated blinding did not occur,29 and the other studies made no mention of blinding at all.
Attrition bias
There was evidence of mixed high and low risk of attrition bias across studies. Four of the studies showed evidence of high risk of attrition bias,17,25,30 five showed evidence of low risk,27–29,33,34 and one did not provide enough detail to make a judgment.31
Reporting bias
There was evidence of mixed high and low risk of reporting bias across studies. Three studies showed evidence of reporting bias by reported non-statistically significant trends.29,32,33 The remaining seven studies did not appear to show evidence of reporting bias.
Other bias
There appeared to be low risk of other bias in the included studies. None of the studies provided evidence of other sources of bias such as financial conflicts of interest, which could influence the results.
Summary risk of bias across studies
The risk of bias across studies was high. The proportion of information from studies at high risk of bias was sufficient to potentially affect the interpretation of results. Risk of bias was “high” for adequate selection, performance, detection, and patient-reported bias and “unclear” or mixed for attrition and reporting bias. Risk of bias was only uniformly “low” for other forms of bias including financial conflicts of interest (Figure 2).
Figure 2.
Risk of bias assessment.
Results of Individual Studies
The effects of yoga on fatigue are presented in Table 6. In four studies, the yoga group reported significant reductions in self-reported fatigue from pre- to post-intervention.17,30,33,34 Using the Functional Assessment of Cancer Therapy-Breast (FACT-B) and averaging responses to fatigue-related items, Banasik et al., found a significant group-by-time interaction for fatigue score (P = .003). The yoga group reported less fatigue after yoga participation compared to baseline, while the control group fatigue scores worsened over the course of the study period (P = .003). Bower et al., used the Brief Fatigue Inventory and reported that yoga led to statistically significant improvements in fatigue severity from baseline to three-month follow-up in the treatment group versus control group (P = .032). Carson et al., used diary data collected via an interactive telephone voice system using 0–9 scales in which higher scores reflected greater amounts of fatigue. Carson et al., reported that the yoga group showed significant post-treatment improvements (P = .032) relative to the controls in level of fatigue, and these improvements were maintained at three-month follow-up (P = .025). Vadiraja et al., reported a significant group-by-time interaction effect for fatigue [F(1, 72) = 7.74, P = .007] and a signi ficant between-subjects effects for fatigue [F(1, 72) = 8.26, P= .01] as measured by the Rotterdam Symptom Checklist.
Table 6.
Fatigue Measured Pre- and Post-Intervention
| Pre-Intervention |
Post-Intervention |
|||
|---|---|---|---|---|
| References | Yoga | Control | Yoga | Control |
| Banasik et al.30 | 1.9 (1.1) | 0.9 (0.9) | 1.0 (0.9) | 1.6 (1.0) |
| Bower et al.33 | 4.8 (1.1) | 5.1 (1.0) | 3.4 (1.8) | 4.9 (1.3) |
| Carson et al.17 | 3.1 (?) | 3.8 (?) | 2.9 (?) | 4.3 (?) |
| Chandwani et al.28 | 2.3 (0.3) | 2.3 (0.4) | 1.9 (0.7) | 2.5 (0.8) |
| Cohen et al.25 | 3.1 (2.4) | 2.8 (2.2) | 3.1 (1.5) | 3.1 (1.5) |
| Culos-reed et al.26 | Not reported | Not reported | Not reported | Not reported |
| Danhauer et al.32 | 30.1 (13.4) | 32.7 (11.8) | 39.8 (11.5) | 32.6 (15.5) |
| Littman et al.29 | 43.1 (5.8) | 43.2 (8.5) | 45.0 (5.3) | 43.1 (10.3) |
| Moadel et al.27 | 35.7 (11.7) | 34.9 (13.6) | 34.4 (11.3) | 33.8 (13.0) |
| Vadiraja et al.34 | Not reported | Not reported | Not reported | Not reported |
Three of the studies reported that there were significant reductions of fatigue among participants who attended a greater number of yoga classes. Carson, 2009 found that greater practice was associated with less fatigue at post-treatment [r(27) = −0.413, P = .032] and at three-month follow-up [r(26) = −0.439, P= .025]. Using the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), Littman et al., found that participants who attended at least 24 classes had 4.2- and 3.5-point improvements (P < .05) compared with the control group. Measuring with the FACIT-F, Moadel et al., indicated that post-intervention fatigue scores differed by adherence level (F = 6.86; P = .002), controlling for baseline fatigue and covariates (chemotherapy, age, and race).
Four studies reported no significant changes in self-reported fatigue and no association between the number of classes attended and fatigue.25,28,31,32 Two of these studies used the Brief Fatigue Inventory (BFI), one used the EORTC QLQ-C30, and one used the Functional Assessment of Cancer Therapy–Fatigue (FACT-F).
DISCUSSION
While aspects of this reviews’ findings suggest that yoga may have a beneficial effect on cancer-related fatigue, caution is warranted. One of the studies that reported positive findings only had 14 participants out of 18 completed the intervention, and a significant risk of bias was evident.30 Also, training of the yoga instructor was not mentioned, and treatment manuals were not utilized. Vadiraja et al., reported positive findings and had a relatively low risk of bias, but they did not report using a manual for treatment, did not provide information on training of yoga instructors, and did not provide demographic information such as age and ethnicity of participants. Another study that reported positive findings had relatively low risk of bias and did use a manual and did report information about training of yoga instructors.17 The study also included unique intervention components of study of relevant topics and group discussion that were not used in any of the other studies. A notable fact is that none of the studies reported an increase in fatigue in the yoga intervention group, which suggests that there is no evidence that yoga is detrimental for fatigue in this population.
Importantly, there was also evidence of significant reductions of fatigue among participants who attended a greater number of yoga classes. This observation was supported by a large sample size of 212 participants across three studies that completed the interventions.17,27,29 All three provided information about training of yoga instructors, and two out of three used a manual.
There are some study limitations that need to be acknowledged. The conclusions were drawn from a sample of 583 participants across 10 studies who were primarily Caucasian female breast cancer patients. The characteristics of the sample and the data provided make it difficult to interpret the generalizability of the findings to women and men with other types of cancer. Also, patients with all stages of cancer at various phases of treatment were generally grouped together in comparison of means analysis, making it difficult to determine whether improvements were equal in each subgroup. In addition, the clinical relevance of our findings is not clear because a wide variety of measurement tools were used for assessing fatigue. Estimates of clinically important differences were not provided in the included studies. Finally, while two major databases (PubMed and PsycINFO) were searched, and reference lists for all relevant articles were checked, it is possible that articles were missed. Since the search was limited to articles written in English, it is also possible that articles originating in other countries such as India were left out.
CONCLUSIONS
Results of the studies included in this review suggest that yoga interventions may be beneficial for reducing cancer-related fatigue in Caucasian women with breast cancer. Since intervention adherence appears to be a key factor in successful fatigue reduction, it is important that cancer survivors choose an alternative therapy method that is the right fit for their personal needs and interests. Special care should be taken to look for ways to increase adherence.
Clearly, more well-constructed randomized controlled trials are needed to better determine the impact of yoga interventions on fatigue in cancer patients and survivors. Studies should expand recruiting efforts to groups other than Caucasian women with breast cancer. There are a wide variety of cancers affecting men and women of every race and ethnicity that deserve to be studied in order to receive the best possible treatment options. Variability in the quality and nature of the yoga interventions and inconsistency in the method of assessing fatigue make it difficult to systematically evaluate the effect of yoga on fatigue in cancer patients and survivors. Given the apparent variability in components offered in yoga interventions, it is important to examine which specific components of yoga are most beneficial in order to ensure that the most effective components are offered in treatment. A unified effort should be made to ensure that manuals and instructor training requirements are comparable across studies. Since each posture can then be adapted for each individual, it is important to start from a point of consistency. In addition, each individual’s cancer stage and treatment phase should be included in analysis in order to determine whether improvements vary across subgroup. Also, since several studies reported greater decreases in fatigue in individuals who attended a greater number of classes, future studies should investigate factors associated with adherence to the intervention. Also, comparison studies should be conducted to determine whether yoga offers comparable fatigue reduction to that found in physical activity interventions and other CAM treatments. Healthcare providers should be clear about the evidence if suggesting yoga as a CAM for reducing fatigue in cancer patients or survivors.
REFERENCES
- 1.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(S11):2577–2592. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick D, Ferketich S, Frame P, et al. National institutes of health state-of-the-science conference statement: symptom management in cancer: pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst. 2003;95(15):1110. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 3.Carlson L, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90(12):2297–2304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knobel H, Loge JH, Nordøy T, et al. High level of fatigue in lymphoma patients treated with high dose therapy. J Pain Symptom Manage. 2000;19(6):446–456. doi: 10.1016/s0885-3924(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 5.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4) doi: 10.1200/JCO.2000.18.4.743. 743 2000;18(4) [DOI] [PubMed] [Google Scholar]
- 6.Mock V, Atkinson A, Barsevick A, et al. Oncology. 11A. Vol. 14. Williston Park, NY: 2000. NCCN practice guidelines for cancer-related fatigue; p. 151. [PubMed] [Google Scholar]
- 7.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;2004(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein BJ, Grasso T. Oncology. 10. Vol. 15. Williston Park, NY: 2001. Prevalence of complementary and alternative medicine use in cancer patients; p. 1267. [PubMed] [Google Scholar]
- 9.Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16(4):655. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 10.Smith KB, Pukall CF. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009;18(5):465–475. doi: 10.1002/pon.1411. [DOI] [PubMed] [Google Scholar]
- 11.Riley D. Hatha yoga and the treatment of illness. Altern Ther Health Med. 2004;10(2):20. [PubMed] [Google Scholar]
- 12.Pilkington K, Kirkwood G, Rampes H, Richardson J. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13–24. doi: 10.1016/j.jad.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SA, Beck SL, Hood LE, Moore K, Tanner ER. Putting evidence into practice: evidence-based interventions for fatigue during and following cancer and its treatment. Clin J Oncol Nurs. 2007;11(1):99–113. doi: 10.1188/07.CJON.99-113. [DOI] [PubMed] [Google Scholar]
- 14.Velthuis MJ, Agasi-Idenburg SC, van der Wall E, Aufdemkampe G, Wittink HM. Physical training to reduce fatigue levels during cancer treatment; a meta-analysis of clinical trials. Ned Tijdschr Geneeskd. 2011;155(45):A3679. [Google Scholar]
- 15.McMillan EM, Newhouse IJ. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: a metaanalysis. Appl Physiol Nutr Metab. 2011;36(6):2. doi: 10.1139/h11-082. [DOI] [PubMed] [Google Scholar]
- 16.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008:2. doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL. Yoga of awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17(10):1301–1309. doi: 10.1007/s00520-009-0587-5. [DOI] [PubMed] [Google Scholar]
- 18.Patel NK, Newstead AH, Ferrer RL. The effects of yoga on physical functioning and health related quality of life in older adults: a systematic review and meta-analysis. J Altern Complement Med. 2012;18(10):902–917. doi: 10.1089/acm.2011.0473. [DOI] [PubMed] [Google Scholar]
- 19.Velthuis M, Agasi-Idenburg S, Aufdemkampe G, Wittink H. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol. 2010;22(3):208–221. doi: 10.1016/j.clon.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 21.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of nonpharmacological therapies for cancer patients. Psychol Bull. 2008;134(5):700. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 22.Sood A, Barton DL, Bauer BA, Loprinzi CL. A critical review of complementary therapies for cancer-related fatigue. Integr Cancer Ther. 2007;6(1):8–13. doi: 10.1177/1534735406298143. [DOI] [PubMed] [Google Scholar]
- 23.Cramer H, Lange S, Klose P, Paul A, Dobos G. Can yoga improve fatigue in breast cancer patients? A systematic review Acta Oncol. 2012;51(4):559–560. doi: 10.3109/0284186X.2011.637960. [DOI] [PubMed] [Google Scholar]
- 24.Green S, Higgins JPT, Alderson P, Clarke M, Mulrow C, Oxman A. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration. John Wiley & Sons; Chichester (UK): 2008. [Google Scholar]
- 25.Cohen L, Warneke C, Fouladi RT, Rodriguez M, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100(10):2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 26.Nicole Culos-Reed S, Carlson LE, Daroux LM, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psychooncology. 2006;15(10):891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 27.Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387. doi: 10.1200/JCO.2006.06.6027. [DOI] [PubMed] [Google Scholar]
- 28.Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8(2):43–55. [PubMed] [Google Scholar]
- 29.Littman AJ, Bertram LC, Ceballos R, et al. Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: effects on quality of life and anthropometric measures. Support Care Cancer. 2011;20(2):267–277. doi: 10.1007/s00520-010-1066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23(3):135–142. doi: 10.1111/j.1745-7599.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- 31.Nicole CR. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psychooncology. 2006;15(10):891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 32.Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18(4):360–368. doi: 10.1002/pon.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors. Cancer. 2012;118(15):3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vadiraja SH, Rao MR, Nagendra RH, et al. Effects of yoga on symptom management in breast cancer patients: a randomized controlled trial. Int J Yoga. 2009;2(2):73. doi: 10.4103/0973-6131.60048. [DOI] [PMC free article] [PubMed] [Google Scholar]