Abstract
C-Reactive Protein (CRP) is related to adiposity, metabolic risk, and predicts events in adults.
Objective
To determine if relationships between adiposity and CRP have similar magnitudes in adolescents as adults.
Methods
Healthy African Americans (484 adults and 282 adolescents) were recruited from similar environments. In both cohorts measurements included anthropometrics, blood pressure (BP), metabolic risk factors and inflammatory markers. After stratification by high sensitivity CRP (hsCRP: ≤1, 1-≤3, >3 mg/dl), adults and adolescents were compared with regard to body mass index (kg/m2; BMI), waist circumference (cm; WC), BP, and other risk factors. hsCRP was regressed on BMI and WC with covariates including cohort, age, sex, BP, insulin resistance, smoking, alcohol, and other biomarkers. Interaction terms and a subset of the covariates were subject to a supervised variable selection procedure for a final model. Skewed variables were log-transformed and summarized by geometric means (GMs) with first and third quartiles [Q1, Q3].
Results
Among adolescents (16.3%) and adults (34.1%) having high hsCRP (> 3 mg/dl), BMI was distributed similarly (GM=36.4 [32.7, 43.1] and GM=34.7 [28.8, 40.8], respectively) as was WC (GM=104.2 [93.0, 119.0] and GM=104.9 [93.0, 117.2], respectively). In an adjusted regression model, for a given BMI, elevated WC was associated with elevated hsCRP (p=0.02). While elevated BMI was significantly associated with elevated hsCRP, the relationship was stronger among adolescents (interaction p=0.04).
Conclusion
These findings demonstrate that in African Americans obesity is associated with inflammation and adverse changes in metabolic parameters among both adolescents and young adults.
Keywords: Obesity, CRP, Inflammation, Adolescents, African Americans
Introduction
The associations between obesity, inflammation and cardiovascular risk are receiving increasing interest. There is now substantial evidence that inflammation contributes to onset and progression of atherosclerosis including plaque development, disruption and thrombosis.1 C-Reactive Protein (CRP) is a biomarker of inflammation that mediates multiple effects including up-regulation of adhesion molecules, complement binding, and decreasing vasodilation by reducing endothelial nitric oxide synthase.2 Among healthy adults there is a strong association of CRP with obesity.3 There is also a strong clinical association of CRP with cardiovascular disease. Among older adults, CRP has been linked to cardiovascular events and mortality.4 Although less is known about CRP in childhood, some reports from studies in children also describe an association of CRP with obesity and with insulin resistance.5–7 Investigators from the Cardiovascular Risk in Young Finns study reported that childhood CRP levels were predictive of adult CRP levels.8 The Pathobiological Determinants of Atherosclerosis in Youth study detected an association between serum CRP levels and raised lesions in the abdominal aorta and right coronary artery, suggesting that CRP could be a biomarker of an early phase of atherosclerosis.9 Less is known, however, about the strength of the associations of CRP with cardiovascular or metabolic risk factors in childhood, especially among African American youth. In particular, it is not known whether the CRP-obesity relationships among adolescents are similar to that of adults. The purpose of this study was to compare African American adolescents to young adult African Americans with regard to associations between plasma levels of CRP and adiposity. In addition, relationships of CRP to insulin resistance, other inflammatory markers, and adiponectin were examined.
Methods
Study Samples
Data obtained in two separate observational cohort studies were analyzed. A young adult cohort study enrolled African Americans 19–45 years of age between 2006 and 2009. African American ethnicity was determined by self-report. All participants were recruited from local communities and were without chronic health problems with the exception of high blood pressure (>130/85 mm Hg) in approximately half of the participants. Obesity, defined as BMI ≥30 kg/m2, was present in 50% of the cohort. Individuals with known diabetes or other chronic disease were excluded from enrollment. The study protocol was approved by the Institutional Review board of Thomas Jefferson University. Written informed consent was obtained from each participant at the time of enrollment. The design and method details of the young adult cohort study have been published10,11 An adolescent cohort enrolled African Americans age 13 to 18 years of age from 2009 to 2011. African American ethnicity was based on self-report by the adolescent participant and his/her parent. Using a similar design, the adolescent study enrolled participants with and without high blood pressure (≥120/80 mm Hg); and with and without obesity, defined as body mass index (BMI) ≥95th percentile. The adolescents were recruited from primary care clinics in Pediatrics and Family Medicine at Thomas Jefferson University and from community primary care practices. Exclusion criteria for adolescent participants were known diabetes, secondary hypertension, stage 2 hypertension, renal disease, and other chronic disease. The study and protocol were approved by the Institutional review Board of Thomas Jefferson University and the A.I. DuPont Hospital for Children. Written informed consent was obtained from 18 year old participants. For adolescents age <18 years, consent was obtained from the parent or guardian at enrollment and assent was obtained from the child.
Study Methods
Similar methods and procedures were applied in both cohort studies. Data on health status, medication use, and health related behaviors were obtained by self-report from each participant. Clinical assessment consisted of BP and anthropometric measurements (height, weight, and waist circumference). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). For the adolescent subjects, obesity was defined according to the CDC criteria (http://www.cdc.gov/obesity/childhood/defining.html), which are based on the child’s age, sex, and BMI. BP measurements were obtained on each subject, by trained research nurses, following a 10-minute rest period in a seated position with feet flat on the floor and back supported. BP was measured by auscultation using a cuff of appropriate size according to the circumference of the right arm. Four separate measurements of systolic BP (SBP) and diastolic BP (DBP) were obtained at each of two separate visits. For adolescents with suspected high BP (≥120/80 mm Hg) a third set of BP measurements were obtained. The average of all measures of SBP and DBP was used as the BP value for each participant.
An oral glucose tolerance test (OGTT) was conducted after a 12-hour overnight fast. A fasting blood sample was obtained for plasma glucose, insulin, and lipids. Samples of fasting plasma were also prepared and stored for later assay of cytokines including hsCRP, Interlukin-6 (IL-6), Plasminogen activator inhibitor (PAI-1), tumor necrosis factor alpha (TNF-α), tumor necrosis factor alpha receptor (TNF-αR) and adiponectin. Following the ingestion of 75 g of glucose solution (Glucola; Ames Diagnostics, Elkhart, IN), blood samples were then obtained at 30, 60 and 120 minutes post-ingestion and assayed for plasma glucose and insulin concentrations. Plasma glucose concentration was analyzed with the glucose oxidase technique (YS Model 27; Glucostat, Yellow Springs, OH). Plasma insulin concentration was determined with a solid phase radioimmunoassay (RIA) (Coat-a-Count; Diagnostic Products Corp, Los Angeles, CA). Coefficients of variation for intra- and inter-assay variability for glucose and insulin assays were <5%. Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA).12 Higher HOMA values indicate greater insulin resistance. In addition, all glucose and insulin values on each participant’s OGTT were used to compute a composite insulin sensitivity index (Composite ISI) according to the equations of Matsuda and DeFronzo.13 Lower Composite ISI values indicate greater insulin resistance. Fasting lipids including total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), and triglycerides (TG) were measured using the Hitachi 704 standard enzymatic method in the Lipid Laboratory of Thomas Jefferson University. All assays for the cytokines were performed by ELISA in duplicate using commercially available kits. Kits for Adiponectin, IL-6, TNF-α TNF-αR and hsCRP were obtained from R&D Systems (Minneapolis, MN). The kits for PAI-1 were obtained from Aniara (Mason, OH). The coefficient of variation for these assays was consistently <10% and most <6%.
Glucose tolerance status was determined using fasting and two hour OGTT glucose values: normal glucose tolerance was defined as fasting blood glucose <100 mg/dL and two hour post OGTT glucose < 140 mg/dL; impaired glucose tolerance was defined as fasting blood glucose 100–125 mg/dL or two hour glucose of 140–199 mg/dL; and diabetic was defined as fasting blood glucose >125 mg/dL or two hour post OGTT glucose >199 mg/dL. Metabolic syndrome for adults was defined according to NCEP/ATP III guidelines.14 These criteria were modified for adolescents by using ≥120/80 mm Hg for high BP and ≥ 110 mg/dl for elevated triglyceride.15
Statistical Methods
Subjects in each cohort were classified, according to hsCRP level, into three groups: low (hsCRP ≤ 1 mg/dl), middle (1 < hsCRP ≤ 3 mg/dl), and high (hsCRP > 3 mg/dl). Categorical and continuous variables in each hsCRP group were compared within and between the adolescent and adult cohorts. Categorical variables were summarized by frequency counts with percentages. Continuous variables were summarized by arithmetic means with standard deviations or, if skewed, were log transformed and summarized by geometric means (GM) with first and third quartiles. Study variables were tabled and compared across hsCRP and cohort groups. Student’s t-tests or ANOVA F-tests were used to evaluate differences in means and Fisher’s exact tests were used to evaluate differences in proportions. Adjustments were made to p-values to help control the overall false discovery rate.16 The significance level was set at α = 0.05.
A multivariable regression model for hsCRP was determined by application of a two-staged supervised selection process. In the first stage, log transformed hsCRP was regressed on BMI and waist circumference (WC) with covariates including cohort (i.e., an adolescent cohort indicator), age, sex, BP, hypertension medication, smoking, and alcohol consumption. In the next stage, linear terms for insulin resistance (log HOMA) and biomarkers (including adiponectin, log IL-6, PAI-1, log TNF-α, and log TNF-α receptor) as well as their respective two-way cohort interaction terms and respective two-way cohort interaction terms with BMI, WC, systolic BP, and gender were either removed or selected to remain in the first stage model by the hybrid Least Angle Regression (LAR) method.17 All continuous covariates were mean-centered prior to modeling. Anti-logged regression coefficients, representing geometric mean ratios (GMRs), with 95% confidence intervals and p-values from the selected model are presented.
All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
A total of 505 African American adults were enrolled in the study. Of these, complete data for this analysis were available on 484 subjects. For the adolescent cohort, a total of 301 African American adolescents were enrolled. Of these, complete data were available for this analysis on 282 adolescents. When comparing adolescents and adults, we found that on average hsCRP was 47% lower among adolescents (GM 0.78 vs. 1.66). However, when the cohorts were stratified by hsCRP level, more complicated relationships become apparent. Table 1 provides the plasma hsCRP GM in each adolescent and adult hsCRP group as well as the clinical and demographic characteristics, by categorical variable, of each group. GM hsCRP plasma level was similar for adolescents and adults in the middle (GM hsCRP 1.79 vs. 1.74) and high (GM hsCRP 5.19 vs. 5.17) hsCRP groups. Among the adolescents, 16.3% (N = 46) of the cohort had high hsCRP(≥ 3 mg/dl), compared to 34.1% (N = 165) having high hsCRP in the young adult cohort. Gender was similarly represented in the hsCRP groups among adolescents and adults, with female gender being more frequent among high vs. low hsCRP groups. Metabolic syndrome was also similarly represented in the hsCRP groups among adolescents and adults. Figure 1 depicts the log-transformed hsCRP values among individuals with and without metabolic syndrome in both cohorts. Among those without metabolic syndrome, hsCRP tended to be lower among adolescents compared to adults. Among those with metabolic syndrome, hsCRP was distributed similarly in both adolescents and adults, and was higher than those without metabolic syndrome. Accordingly, the distribution of metabolic syndrome was also similar in both adults and adolescents having high hsCRP (40.0% vs 32.6%, respectively: Table 1).
Table 1.
Study Variables by Cohort and hsCRP group.
| Categorical Variables: Frequencies (Percents) Continuous Variables: Mean (SD) or Geometric Mean [1st quartile, 3rd quartile] |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (hsCRP ≤ 1) | Middle (1 < hsCRP≤ 3) | High (hsCRP > 3) | |||||||
| Adult | Adolescent | p† | Adult | Adolescent | p† | Adult | Adolescent | p† | |
| Variable | (N = 159) | (N = 165) | (N = 160) | (N = 71) | (N = 165) | (N = 46) | |||
| hsCRP (mg/dl)‡ | 0.49 [0.30, 0.90] | 0.32 [0.20, 0.60] | <.001 | 1.74 [1.40, 2.20] | 1.79 [1.40, 2.30] | 0.680 | 5.17 [3.90, 6.50] | 5.19 [3.80, 6.90] | 0.934 |
| Age (yrs) | 37.08 (7.91) | 16.22 (1.75) | 38.16 (7.31) | 16.08 (1.61) | 37.94 (7.26) | 16.25 (1.58) | |||
| Gender Female | 55 (34.6%) | 69 (41.8%) | 0.282 | 76 (47.5%) | 38 (53.5%) | 0.563 | 111 (67.3%) | 29 (63.0%) | 0.680 |
| Smoking | 106 (66.7%) | 6 (3.7%) | <.001 | 95 (59.4%) | 3 (4.3%) | <.001 | 99 (60.0%) | 0 (0%) | <.001 |
| Alcohol Use | 83 (52.2%) | 8 (4.9%) | <.001 | 81 (50.6%) | 8 (11.6%) | <.001 | 68 (41.2%) | 1 (2.3%) | <.001 |
| Obesity | 43 (27.0%) | 51 (30.9%) | 0.561 | 86 (53.8%) | 53 (74.6%) | 0.007 | 114 (69.1%) | 41 (89.1%) | 0.014 |
| Hypertensive Meds | 47 (29.6%) | 58 (36.3%) | 64 (38.8%) | ||||||
| Blood Pressure Pre HTN | 37 (23.3%) | 34 (20.6%) | <.001 | 38 (23.8%) | 21 (29.6%) | <.001 | 42 (25.5%) | 14 (30.4%) | <.001 |
| HTN | 60 (37.7%) | 6 (3.6%) | 74 (46.3%) | 2 (2.8%) | 73 (44.2%) | 1 (2.2%) | |||
| Metabolic Syndrome | 18 (11.3%) | 7 (4.2%) | 0.037 | 42 (26.3%) | 17 (23.9%) | 0.787 | 66 (40.0%) | 15 (32.6%) | 0.499 |
| Glucose Tolerance: Impaired | 22 (13.8%) | 27 (16.5%) | 0.444 | 19 (11.9%) | 15 (21.1%) | 0.063 | 33 (20.0%) | 13 (28.3%) | 0.363 |
| Diabetic | 4 (2.5%) | 1 (0.6%) | 13 (8.1%) | 1 (1.4%) | 18 (10.9%) | 2 (4.3%) | |||
Fishers exact test (categorical) or Student's t-test (continuous); adjustments made for multiple testing
Data natural log transformed: geometric means with [first quartile, third quartile] presented
Obesity: ≥95th percentile (adolescents), ≥30 BMI (adults)
Metabolic Syndrome: 3 or more of following: Waist circumference ≥ 102 cm (males), ≥88 (females); SBP ≥120/80 mmHg (adolescents), ≥130/85 or HTN Rx (adults); HDL <40 mg/dl (males) or <50 (females); Triglycerides ≥110 mg/dl (adolescents) ≥150 (adults); Fasting glucose ≥110 mg/dl
HTN: BP ≥ 140/90 mmHg or HTN Rx (adults), BP percentile ≥ 95 (adolescents)
PreHTN: BP ≥ 120/80 mmHg. To convert hsCRP to SI units multiply by 9.524
Figure 1. hsCRP in Adolescents and Adults with Metabolic Syndrome.
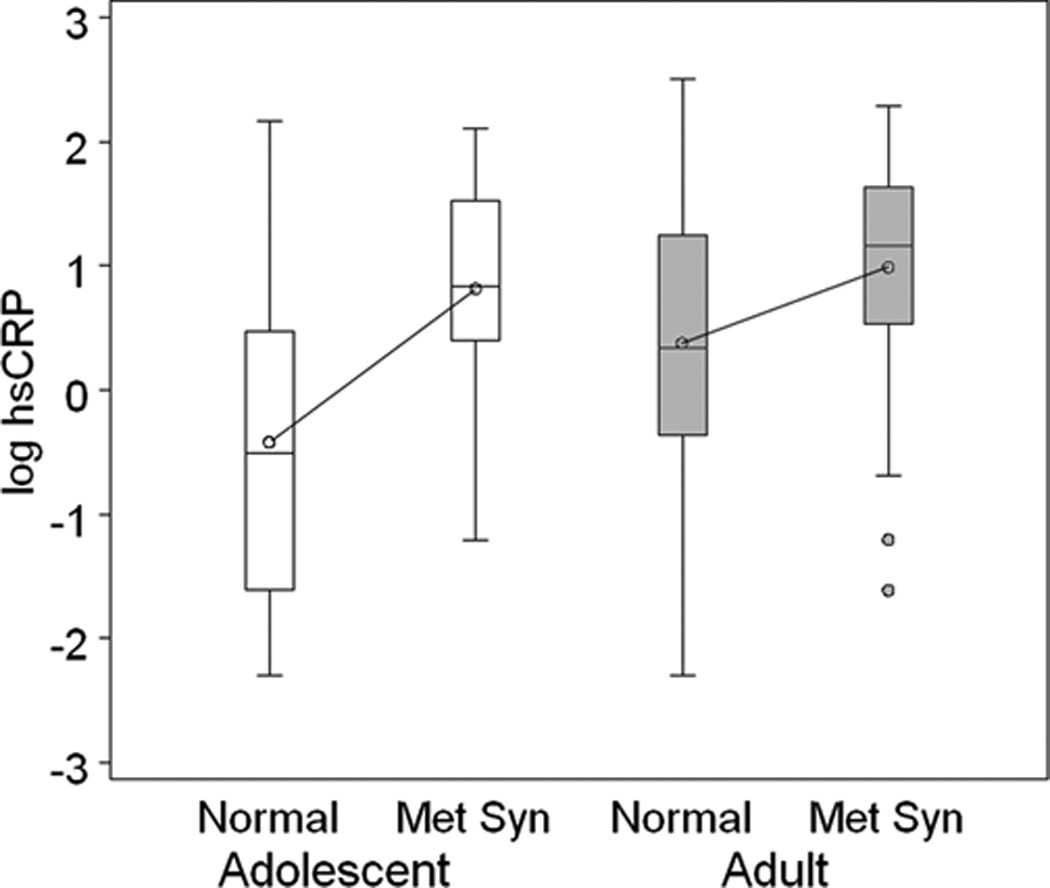
The box plots provide the distribution of log-transformed hsCRP among adolescents (open boxes) with and without metabolic syndrome (Met Syn) and adults (gray boxes) with and without metabolic syndrome. The median and distribution of hsCRP in adolescents with metabolic syndrome is similar to adults with metabolic syndrome.
Table 2 provides data on continuous variables for adolescent and adult participants according to hsCRP group. Within both adolescent and adult cohorts, there was a progressive and significant increase in BMI and waist circumference with increasing hsCRP group. In comparing adolescents in the high hsCRP group with adults in the high hsCRP group, BMI was comparable (GM =36.4 [32.7, 43.1] and GM =34.7 [28.8, 40.8], respectively) as was waist circumference (GM =104.2 [93.0, 119.0] and GM =104.9 [93.0, 117.2], respectively). There were no significant differences in systolic BP or diastolic BP across the adult hsCRP groups or across the adolescent hsCRP groups except for higher diastolic BP among the adult middle hsCRP group. Within both adolescent and adult cohorts, HDL-cholesterol was significantly lower in the high vs. low hsCRP groups, while triglycerides were significantly higher among the high compared to low hsCRP groups. Average insulin resistance, as estimated by the GM of HOMA, was significantly greater in the high hsCRP group compared to low hsCRP group within both adolescent (p<.001) and adult (p<.001) cohorts. A similar relationship was present for insulin sensitivity based on calculated Composite ISI. The Composite ISI was significantly lower in the high hsCRP group compared to low hsCRP group within both adolescent (p<0.001) and adult (p<0.001) cohorts. Within each cohort, adiponectin was inversely related to hsCRP group, and the inflammatory cytokines, IL6, PA1 and TNF a receptor, were positively associated with hsCRP group. Urine albumin excretion was not significantly associated with hsCRP.
Table 2.
Study variables by cohort and hsCRP group.
| Mean (SD) or Geometric Mean [1st quartile, 3rd quartile] |
||||||||
|---|---|---|---|---|---|---|---|---|
| Adult | Adolescent | |||||||
| Low (hsCRP ≤ 1) | Middle (1 < hsCRP ≤ 3) | High (hsCRP > 3) | p† | Low (hsCRP ≤ 1) | Middle (1 < hsCRP ≤ 3) | High (hsCRP > 3) | p† | |
| Variable | (N = 159) | (N = 160) | (N = 165) | (N = 165) | (N = 71) | (N = 46) | ||
| hsCRP (mg/dl)‡ | 0.49 [0.30, 0.90] | 1.74 [1.40, 2.20] | 5.17 [3.90, 6.50] | 0.32 [0.20, 0.60] | 1.79 [1.40, 2.30] | 5.19 [3.80, 6.90] | ||
| Waist Circumference (cm)‡ | 87.98 [80.00, 96.00] | 98.16 [89.00, 109.00] | 104.93 [92.99, 117.50] | <.001 | 79.58 [70.10, 87.50] | 94.39 [87.00, 107.50] | 104.15 [93.00, 119.40] | <.001 |
| BMI (kg/m2)‡ | 26.87 [23.06, 30.34] | 30.72 [26.78, 35.23] | 34.68 [28.80, 40.78] | <.001 | 25.24 [21.10, 29.17] | 31.84 [27.83, 36.28] | 36.37 [32.69, 43.06] | <.001 |
| SBP (mmHg)[‡ | 118.56 [109.0, 128.0] | 122.86 [112.00, 133.50] | 120.60 [110.0, 130.0] | 0.089 | 111.98 [105.0, 119.3] | 114.16 [106.33, 122.67] | 114.85 [106.7, 121.3] | 0.211 |
| DBP (mmHg)‡ | 73.30 [64.00, 82.00] | 77.17 [70.00, 84.00] | 73.21 [66.00, 80.00] | 0.005 | 62.25 [57.67, 68.00] | 62.45 [57.67, 67.00] | 63.20 [58.67, 67.00] | 0.794 |
| HDL (mg/dl) | 50.51 (14.59) | 48.68 (15.39) | 44.78 (13.75) | 0.003 | 55.16 (12.35) | 48.86 (12.59) | 49.37 (11.83) | 0.001 |
| LDL (mg/dl) | 104.17 (29.48) | 110.73 (26.89) | 110.52 (30.69) | 0.114 | 85.94 (23.80) | 91.15 (27.77) | 94.39 (33.44) | 0.218 |
| Total Cholesterol (mg/dl) | 169.43 (31.67) | 176.30 (29.55) | 172.56 (35.62) | 0.200 | 153.93 (27.67) | 155.23 (31.27) | 159.11 (35.31) | 0.713 |
| Triglycerides (mg/dl)‡ | 65.74 [48.00, 87.00] | 76.86 [52.00, 106.50] | 74.81 [54.00, 97.00] | 0.016 | 57.46 [44.00, 71.00] | 67.00 [49.00, 88.00] | 70.23 [54.00, 97.00] | 0.003 |
| Fasting Glucose (mg/dl) | 99.45 (11.81) | 103.34 (15.60) | 108.04 (25.29) | <.001 | 95.98 (8.69) | 96.54 (7.80) | 100.80 (17.14) | 0.257 |
| Composite ISI‡ | 7.61 [5.11, 11.98] | 6.30 [3.77, 11.32] | 4.98 [2.88, 8.86] | <.001 | 5.78 [3.91, 8.49] | 4.15 [2.64, 7.02] | 3.13 [2.10, 5.11] | <.001 |
| HOMA‡ | 1.22 [0.76, 1.70] | 1.50 [0.83, 2.45] | 2.01 [1.02, 3.67] | <.001 | 1.46 [0.94, 2.12] | 2.33 [1.32, 3.63] | 3.13 [1.97, 5.28] | <.001 |
| UAE (mg/gmcreat)‡ | 4.03 [1.49,11.11] | 5.45 [1.96,15.61] | 5.76 [2.25,14.28] | 0.102 | 2.61 [1.10, 6.49] | 2.22 [1.07, 3.51] | 2.59 [0.82, 7.19] | 0.680 |
| Adiponectin (μg/ml)‡ | 5.63 [3.50, 9.10] | 4.51 [3.05, 7.35] | 4.34 [2.80, 6.90] | 0.002 | 6.41 [4.30,10.10] | 4.75 [3.50, 7.10] | 4.18 [2.50, 6.00] | <.001 |
| IL6 (pg/ml)‡ | 1.57 [1.00, 2.40] | 2.12 [1.50, 3.10] | 3.92 [2.30, 6.70] | <.001 | 2.58 [1.90, 3.60] | 3.18 [2.10, 4.20] | 4.26 [3.30, 5.20] | <.001 |
| PAI1 (ng/ml) | 60.32 (29.54) | 71.62 (32.59) | 75.71 (31.72) | <.001 | 50.65 (23.93) | 69.61 (25.88) | 79.21 (25.91) | <.001 |
| TNFα (pg/ml)‡ | 6.23 [5.20, 9.40] | 6.73 [5.70, 9.40] | 7.50 [6.00, 9.50] | 0.043 | 8.49 [7.20, 9.60] | 8.26 [6.80, 9.90] | 8.15 [6.50,10.00] | 0.786 |
| TNFα Receptor (pg/ml)‡ | 0.80 [0.60, 1.00] | 0.82 [0.60, 1.00] | 0.94 [0.70, 1.20] | 0.004 | 0.88 [0.70, 1.10] | 0.96 [0.70, 1.20] | 1.07 [0.90, 1.30] | 0.003 |
Fishers exact test (categorical) or ANOVA F-test (continuous); adjustments made for multiple testing
Data natural log transformed: geometric means with [first quartile, third quartile] presented. The SI conversion factor for HDL, LDL, and Total Cholesterol is 0.0259. The SI conversion factor for glucose is 0.0555. The SI conversion factor for PAI1 is 19.231. The SI conversion factor (approximate) for Il-6 is 0.131. The SI conversion factor (approximate) for TNFα is 0.086
Figure 2 represents the relationship between log hsCRP and obesity according to BMI groups for adolescents and adults. Among adults, there was a progressive increase in the distribution of hsCRP with increasing BMI (Figure 2, panel A). This trend was similar for the adolescent cohort (Figure 2, panel B). Among adolescents with BMI <25 kg/m2, hsCRP tended to be lower as compared to adults. However, among the very obese (BMI >35 kg/m2), the distribution of hsCRP was nearly identical in adolescents and adults. As can be seen in the figure, the slope of the curve interpolating the group means is steeper in the adolescent cohort.
Figure 2. hsCRP and Body Mass Index (BMI) in Adults and Adolescents.
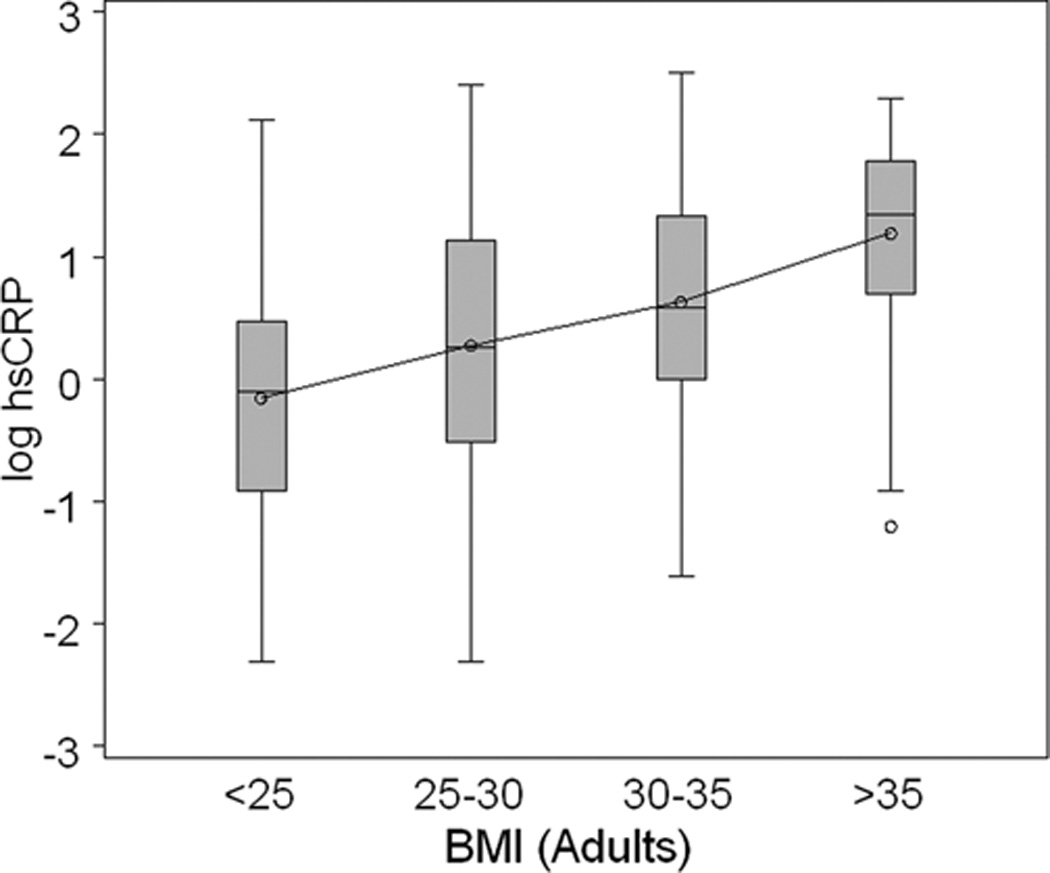
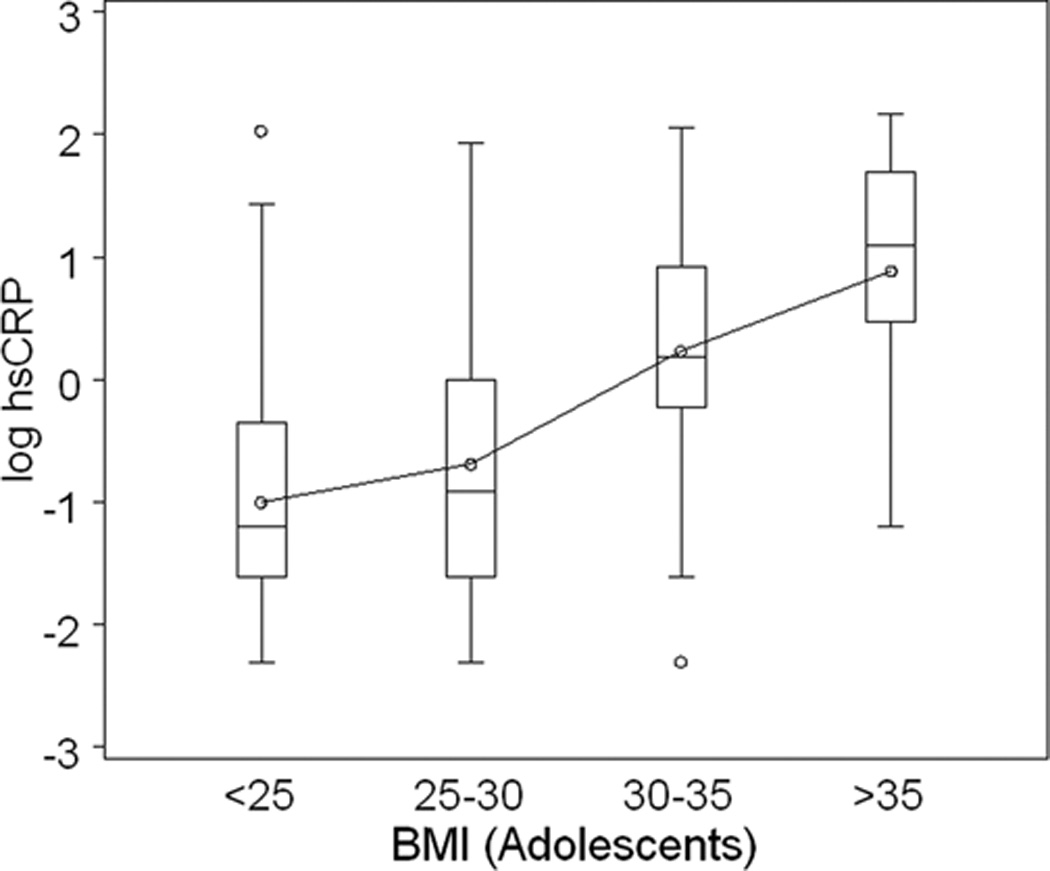
The box plots provide the distribution of log-transformed hsCRP in adults (A. upper panel depicted in gray boxes) according to BMI group; and in adolescents (B. lower panel depicted in open boxes) according to BMI group. The increase in hsCRP with increasing adiposity is present in adults and adolescents. As BMI exceeds 30 kg/m2, the log hsCRP distribution is similar in adolescents and adults.
An adjusted regression model was selected to evaluate the linear association between log hsCRP and clinical variables in all study participants. The results of this model are presented in Table 3. Age, smoking, alcohol consumption, hypertension medication, and BP generally had no association with hsCRP in the model after adjusting for other variables. Female gender was associated with a 27% higher hsCRP (GMR 1.27, p<0.01). WC showed a significant 1.3% increase in hsCRP per cm (GMR =1.013, p=0.01). Adiponectin, and log IL-6 were also significantly associated with hsCRP (GMR =0.87, p=0.014 and GMR =1.55, p<0.01, respectively).
Table 3.
Final model: hsCRP geometric mean ratios for adolescent and adult participants.
| Variable | GMR (95% CI) | p-value |
|---|---|---|
| Age | 0.998 (0.987, 1.009) | 0.719 |
| Gender female | 1.27 (1.10, 1.46) | <.001 |
| Smoke | 0.97 (0.82, 1.14) | 0.711 |
| Alcohol consumption | 1.05 (0.90, 1.23) | 0.555 |
| HTN Rx | 1.15 (0.96, 1.38) | 0.125 |
| Waist circumference (cm) | 1.013 (1.005, 1.021) | 0.002 |
| SBP | 0.994 (0.989, 1.000) | 0.064 |
| DBP | 1.006 (0.997, 1.014) | 0.182 |
| Adiponectin‡ | 0.87 (0.78, 0.97) | 0.014 |
| Log IL6‡ | 1.55 (1.40, 1.71) | <.001 |
| Adolescent (vs. adult) | 0.53 (0.40, 0.71) | <.001 |
| BMI | 1.01 (0.99, 1.03) | 0.303 |
| Adolescent * BMI Interaction | 1.03 (1.01, 1.05) | 0.002 |
| Log HOMA‡ | 1.06 (0.95, 1.18) | 0.317 |
| Adolescent * Log HOMA‡ Interaction | 1.25 (1.02, 1.52) | 0.028 |
| Log TNFᇠ| 1.09 (0.95, 1.24) | 0.213 |
| Adolescent * Log TNFᇠInteraction | 0.68 (0.50, 0.94) | 0.020 |
Continuous predictors were mean-centered. GMR: geometric mean ratio
log-transformed data
Significant two-way interactions were detected between cohorts (adolescents vs. adults) for three clinical variables (BMI, log HOMA, and log TNFα) as they related to log hsCRP. After adjusting for WC and other covariates, a unit increase in BMI was associated with a non-significant 1% increase (GMR =1.01, p=0.303) in hsCRP among adults. The significant BMI interaction indicated that BMI has a stronger hsCRP association among adolescents suggesting their hsCRP tended to increase by an additional 3% per unit of BMI (GMR =1.03, p=0.002). The model suggests that adolescents tend to have lower hsCRP on average (GMR =0.53, p<0.001), however, as BMI increases (per unit), the expected difference in hsCRP between an adult and a similar adolescent will be reduced by about 3% per unit difference in their BMI. These results reflect how adolescent hsCRP tends to “catch up” with adult hsCRP as BMI increases as shown in Figure 2. There was also a significant interaction detected between adolescents and insulin resistance. Specifically, log HOMA was not significantly associated with hsCRP among adults (GMR =1.06, p=0.317) However among adolescents, for each unit increase in log HOMA, there was an additional 25% greater average increase in hsCRP (GMR =1.25, p=0.028). A unit increase in log TNFα was associated with a non-significant 9% increase (GMR =1.09, p=0.213) in hsCRP among adults. However, the significant TNFα interaction was antagonistic (GMR =0.68, p=0.020), indicating that TNFα was associated with decreased hsCRP among adolescents and their hsCRP tended to decrease by a total of (1.09 × 0.68 × 100) = 74% per unit of TNFα. This finding with log TNFα is most likely of limited relevance because, as seen in Table 2, the TNFα levels are similar across hsCRP groups within the adolescent cohort; and within the adult cohort the TNFα levels show a slight increase with increasing hsCRP.
Discussion
Although the mean hsCRP levels were higher in the young adult African American cohort compared to the adolescent cohort (GM 1.66 vs 0.78), adolescents with BMI exceeding 30 kg/m2 had levels of hsCRP and other inflammatory biomarkers that were similar to obese adults. Chronic inflammation, as estimated by hsCRP and other cytokines, was associated with differences in metabolic risk factors in both adolescents and adults. The significant BMI interaction that was detected illustrates a significantly steeper relationship of BMI with hsCRP among adolescents that is in contrast to adults. The results of this study demonstrate that, as indicated by hsCRP, obese African American adolescents have levels of obesity-related inflammation exposure similar to adults.
Obesity-related inflammation has been previously described in adults. Among middle-aged and elderly African Americans enrolled in the Jackson Heart Study, there was a strong correlation between BMI and CRP.18 Studies have also documented associations between obesity and inflammatory cytokines, including TNFα and IL-6.19 Adiponectin generally has an inverse association with obesity.20 In our young adult cohort, we found similar associations of hsCRP, other inflammatory cytokines, and adiponectin with BMI. Associations between obesity in adults and other metabolic cardiovascular risk factors have also been described. Specifically, obesity has been linked to insulin resistance, dyslipidemia and metabolic syndrome.21 The obese adults in our study, similarly had evidence of insulin resistance, as evidenced by greater HOMA.
There is emerging data that demonstrate associations of obesity with biomarkers of inflammation among both children and adolescents. Data on over 8,500 children and adolescents ages 3–16 years from the US, National Health and Nutrition Examination Survey (NHANES) document a significant association of plasma CRP level with measures of BMI and skinfold thickness.22 Musso et al,22 demonstrated similar associations between BMI and CRP among adolescents aged 11–14 years in South America. Further, these authors found unfavorable changes in metabolic parameters among the overweight and obese adolescents, particularly increased triglyceride/HDL cholesterol ratio. Vikram et al,23 also reported significant associations between obesity measures (BMI, waist circumference and skinfold thickness) and CRP among Asian Indian adolescents. In a predominantly Caucasian adolescent cohort, Sinaiko et al24 quantified insulin sensitivity by insulin clamp and demonstrated that insulin resistance confers an effect on metabolic risk factors that is in addition to adiposity. Despite less severe obesity in their cohort these investigators reported associations of CRP and adiponectin with BMI that are similar to our findings in African American adolescents. Also similar is our finding that with increasing insulin resistance (HOMA) there is a greater increase in hsCRP in adolescents compared to adults.
The inflammatory and metabolic profiles of obese adults and adolescents were similar in our two cohorts. Obesity-related inflammation among adolescents has implications for subsequent cardiovascular and metabolic disease. Longitudinal data from the Cardiovascular Risk in Young Finns Study, which examined children and adolescents through young adulthood found that childhood BMI and CRP were predictive of adverse health consequences in adulthood.25 Adverse health consequences of elevated BMI and CRP in childhood may not be limited to cardiovascular or metabolic events, especially among females. In both our adolescent and young adult African American cohorts, a larger portion of the high CRP groups were female. This observation may be relevant to a recent meta-analysis reported by Rebelo et al.26 In their meta-analysis of 18 separate studies the investigators found that young women with elevated CRP levels were at increased risk for preeclampsia. Thus adolescent girls with high CRP may also be at greater risk for subsequent reproductive complications.
When the adolescent and young adult cohorts were compared on the parameters of insulin resistance (HOMA) and insulin sensitivity (Composite ISI), adolescents manifest relative insulin resistance compared to adults. As seen in Table 2, at each hsCRP category HOMA is higher and Composite ISI is lower in adolescent groups compared to adult groups. When we stratified the cohorts by BMI categories as normal weight (BMI <25), overweight (BMI 25-<30), and obese (BMI ≥30), and compared HOMA and Composite ISI values (data not shown), the same difference in insulin resistance were present in each BMI category. The data demonstrating greater insulin resistance in adolescents compared to adults that is present across all CRP categories and all weight categories is most likely due to the relative insulin resistance of adolescence. Previous clinical studies in healthy adolescents have demonstrated a transient increase in insulin resistance that occurs during normal pubertal development.27–29 The factors that contribute to the changes in insulin action during puberty have not been clearly defined. As in adults, insulin resistance in adolescents is strongly associated with BMI. However, the relative insulin resistance of puberty is not explained by differences in BMI or adiposity.29
Our study is limited by the cross-sectional design, which does not allow determination of causality. Because there was intentional over sampling of obese participants in both cohorts our data do not accurately represent the population prevalence of high CRP in an African American population. The results of our study may not be generalizable to other racial and ethnic groups because enrollment was limited to African Americans. Finally, subjects were enrolled from a single center which enhances the internal validity of comparing the young adults to the adolescents, but it also creates some limitations on generalizability.
Our data demonstrate that among African Americans obesity is associated with inflammation and adverse changes in metabolic parameters among both adolescents and young adults. We also detected evidence that the associations of inflammation with obesity and insulin resistance, estimated by HOMA, may be somewhat stronger in adolescents compared to adults. The most recent NHANES data indicate that the prevalence of obesity in children and adolescents is nearly 17%.30 The combined prevalence of overweight and obesity is 31.8% with higher rates among minority children. Our findings are consistent with emerging evidence that childhood onset obesity confers early and prolonged exposure to obesity associated inflammation.
Acknowledgments
This work was supported by a grant from the National Institutes of Health HL092030 and a grant from the Pennsylvania Department of Health. The Department of Health disclaims responsibility for any analyses, interpretations of conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Source of Funding:
Each author certifies that he/she has no conflicts of interest relative to the materials presented in this manuscript.
References
- 1.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devaraj S, Singh U, Jialal I. The evolving role of C-reactive protein in atherothrombosis. Clin Chem. 2009;55:229–238. doi: 10.1373/clinchem.2008.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? ArteriosclerThromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 4.Kistorp C, Raymond I, Pedersen F, et al. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES. C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2003;108:1053–1058. doi: 10.1161/01.CIR.0000080913.81393.B8. [DOI] [PubMed] [Google Scholar]
- 6.Moran A, Steffen LM, Jacobs DR, Jr, et al. Relation of C-reactive protein to insulin resistance and cardiovascular risk factors in youth. Diabetes Care. 2005;28:1763–1768. doi: 10.2337/diacare.28.7.1763. [DOI] [PubMed] [Google Scholar]
- 7.Cook DG, Mendall MA, Whincup PH, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 8.Juonala M, Viikari JS, Ronnemaa T, et al. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2006;26:1883–1888. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- 9.Zieske AW, Tracy RP, McMahan CA, et al. Elevated serum C-reactive protein levels and advanced atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 2005;25:1237–1243. doi: 10.1161/01.ATV.0000164625.93129.64. [DOI] [PubMed] [Google Scholar]
- 10.Huan YDS, Keith SW, Pequignot EC, et al. High blood pressure and obesity increase the risk of abnormal glucose tolerance in young adult African Americans. J Clin Hypertens (Greenwich, Conn) 2011;13:397–403. doi: 10.1111/j.1751-7176.2010.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLoach SHY, Keith SW, Martinez Cantarin MP, et al. Relationship of blood pressure and obesity with inflammatory cytokines among African Americans. Ther Adv Cardiovasc Dis. 2011;5:149–157. doi: 10.1177/1753944711408757. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 14.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini YH. Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 17.Efron BHT, Johnstone I, Tibshirani R. Least angle regression (with discussion) Ann Stat. 2004;32:407–499. [Google Scholar]
- 18.Fox ER, Benjamin EJ, Sarpong DF, et al. Epidemiology, heritability, and genetic linkage of C-reactive protein in African Americans (from the Jackson Heart Study) Amer J Cardiol. 2008;102:835–841. doi: 10.1016/j.amjcard.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–S73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kern PA, Di Gregorio GB, Lu T, et al. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 21.Palaniappan L, Carnethon MR, Wang Y, et al. Predictors of the incident metabolic syndrome in adults: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:788–793. doi: 10.2337/diacare.27.3.788. [DOI] [PubMed] [Google Scholar]
- 22.Musso C, Graffigna M, Soutelo J, et al. Cardiometabolic risk factors as apolipoprotein B, triglyceride/HDL-cholesterol ratio and C-reactive protein, in adolescents with and without obesity: cross-sectional study in middle class suburban children. Pediatr Diabetes. 12:229–234. doi: 10.1111/j.1399-5448.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- 23.Vikram NK, Misra A, Dwivedi M, et al. Correlations of C-reactive protein levels with anthropometric profile, percentage of body fat and lipids in healthy adolescents and young adults in urban North India. Atherosclerosis. 2003;168:305–313. doi: 10.1016/s0021-9150(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 24.Sinaiko AR, Steinberger J, Moran A, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111:1985–1991. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 25.Juonala M, Juhola J, Magnussen CG, et al. Childhood environmental and genetic predictors of adulthood obesity: the cardiovascular risk in young Finns study. J Clin Endocrinol Metab. 2011;96:E1542–E1549. doi: 10.1210/jc.2011-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebelo F, Schlussel MM, Vaz JS, et al. C-reactive protein and later preeclampsia: systemic review and meta-analysis taking into account the weight status. J Hypertens online publication. 2012 doi: 10.1097/HJH.0b013e32835b0556. [DOI] [PubMed] [Google Scholar]
- 27.Amiel SA, Sherwin RS, Simonson DC, et al. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 28.Caprio S, Plewe G, Diamond MP, et al. Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr. 1989;114:963–967. doi: 10.1016/s0022-3476(89)80438-x. [DOI] [PubMed] [Google Scholar]
- 29.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 30.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999–2010. JAMA. 2012;307 doi: 10.1001/jama.2012.40. published online January 17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


