Abstract
The effects of inhibition of the Raf/MEK/ERK and PI3K/Akt/mTOR signaling pathways and chemotherapeutic drugs on cell cycle progression and drug sensitivity were examined in cytokine-dependent FL5.12 hematopoietic cells. We examined their effects, as these cells resemble normal hematopoietic precursor cells as they do not exhibit “oncogene-addicted” growth, while they do display “cytokine-addicted” proliferation as cytokine removal resulted in apoptosis in greater than 80% of the cells within 48 h. When cytokine-dependent FL5.12 cells were cultured in the presence of IL-3, which stimulated multiple proliferation and anti-apoptotic cascades, MEK, PI3K and mTOR inhibitors transiently suppressed but did not totally inhibit cell cycle progression or induce apoptosis while chemotherapeutic drugs such as doxorubicin and paclitaxel were more effective in inducing cell cycle arrest and apoptosis. Doxorubicin induced a G1 block, while paclitaxel triggered a G2/M block. Doxorubicin was more effective in inducing cell death than paclitaxel. Furthermore the effects of doxorubicin could be enhanced by addition of MEK, PI3K or mTOR inhibitors. Cytokine-dependent cells which proliferate in vitro and are not “oncogene-addicted” may represent a pre-malignant stage, more refractory to treatment with targeted therapy. However, these cells are sensitive to chemotherapeutic drugs. It is important to develop methods to inhibit the growth of such cytokine-dependent cells as they may resemble the leukemia stem cell and other cancer initiating cells. These results demonstrate the enhanced effectiveness of targeting early hematopoietic progenitor cells with combinations of chemotherapeutic drugs and signal transduction inhibitors.
Keywords: cell cycle progression, chemotherapeutic drugs, drug resistance, leukemia stem cells, targeted therapy, Raf, Akt, PI3K
Introduction
Proliferation and suppression of apoptosis in many hematopoietic precursor cells is promoted by interleukin-3 (IL-3) and other cytokines/growth factors.1-13 Hematopoietic cell lines have been isolated which require IL-3 for cell proliferation and survival.1,3 The FL5.12 cell line is an IL-3-dependent cell line isolated from the fetal liver of BALB/c mice and is viewed as a model of early hematopoietic progenitor cells.1 Cytokine-deprivation of these cells results in rapid cessation of growth with subsequent death by apoptosis, (programmed cell death) within 48 h.2,9,10 In the presence of IL-3, these cells proliferate continuously, however, they are non-tumorigenic when injected into immunocompromised mice.6-8 Spontaneous factor-independent cells are rarely recovered from the FL5.12 cell line (< 10−7), making it an attractive model to analyze the effects various genes have on signal transduction and leukemogenesis, since abrogation of cytokine-dependence is an important factor in the development of leukemia.6-8,11 Furthermore, this cell line is a model for examining the effects of signal transduction inhibitors and chemotherapeutic drugs on the induction of death in early hematopoietic precursor cells and potentially leukemia stem cells (LSC) as these cells and their transformed derivative lines, share markers expressed on LSCs and other cancer initiating cells.5,11,14-21
IL-3 exerts its biological activity by binding to the IL-3 receptor (IL-3R) which activates the Ras/Raf/MEK/ERK, PI3K/Akt/mTOR and other signaling and anti-apoptotic cascades.3 Aberrant expression of the Ras/Raf/MEK/ERK and PI3K/Akt/mTOR pathways have been detected in many AML samples and their joint overexpression is usually associated with a poor prognosis.22 IL-3R is reported to be expressed on LSCs.5,23,24 Aberrant expression of PI3K/Akt/mTOR and other signaling pathways have been observed in LSCs and other CICs.5,16,25-30
Relatively little is known regarding the interactions between the Raf/MEK/ERK and PI3K/Akt/mTOR pathways in “non-oncogene” addicted, non-malignant cells and the sensitivity of such cells to signal transduction inhibitors and classical chemotherapy.31,32 Understanding the roles the Raf/MEK/ERK and PI3K/Akt/mTOR cascades play in the control of normal and malignant cell cycle progression will enhance our knowledge of how these pathways regulate the sensitivity of CICs and the remaining “bulk” cancer cells to various therapeutic approaches.4,5,31-48 It is important to learn how targeting these pathways may suppress the growth of CICs. These same pathways are also being considered for targeting in aging and decreasing their activities may suppress aging.49-57 Thus these are critical pathways implicated in various types of human diseases and aging.
In the following studies, we sought to determine the effects of Raf/MEK/ERK and PI3K/Akt/mTOR on cell cycle progression and drug resistance by inhibiting the Raf/MEK/ERK and PI3K/Akt/mTOR pathways in cytokine-dependent hematopoietic cells with MEK, PI3K or mTOR inhibitors in the presence and absence of chemotherapeutic drugs. While “non-oncogene” addicted cells were not as sensitive to signal transduction inhibitors as “oncogene-addicted” cells,58,59 the “non-oncogene addicted” cells were sensitive to chemotherapeutic drugs and the therapeutic efficacy can be enhanced by targeted therapy. Therefore, it may be efficacious to target “non-oncogene addicted” pre-leukemia cells before the development of leukemic cells with combinations of chemotherapy and signal transduction inhibitors.
Results
Effects of inhibition of the Raf/MEK/ERK and PI3K/Akt/mTOR pathways on cell cycle progression in cytokine-dependent FL5.12 cells
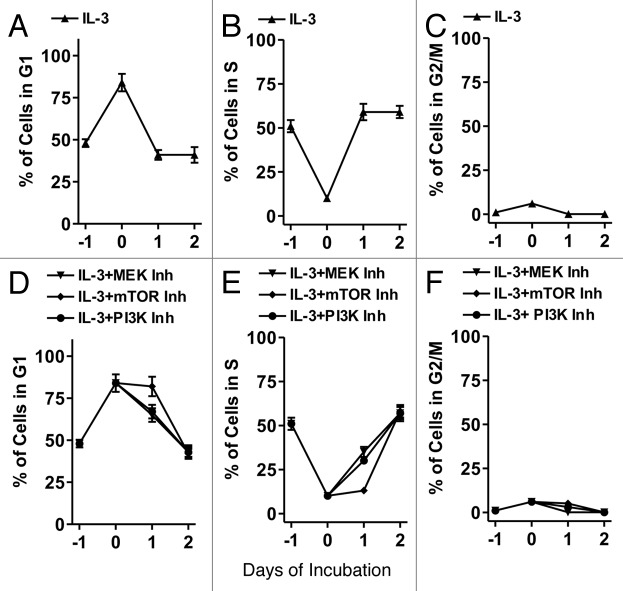
To elucidate the roles of the Raf/MEK/ERK and PI3K/Akt/mTOR pathways on cell cycle progression in cytokine-dependent hematopoietic cells, FL5.12 cells were deprived of IL-3 for 24 h and then stimulated with IL-3 in the presence and absence of inhibitors which target Raf/MEK/ERK or PI3K/Akt/mTOR pathways. When FL5.12 cells were deprived of IL-3 for 24 h, they accumulated in the G1 phase (Fig. 1A) and exited the S phase (B). Upon addition of IL-3 at day 0, the cells exited G1 (A) and entered S phase (B).
Figure 1. Cell cycle progression in FL5.12 cells in the presence of signal transduction inhibitors. FL5.12 cells were collected, washed with PBS twice and then plated in phenol-red free medium containing 5% CS FBS (Day -1). After 24 h (Day 0) of incubation, IL-3 and in some cases 10 μM U0126 (MEK inhibitor), 100 nM rapamycin (mTOR inhibitor) or 10 μM PI3K inhibitor (LY294002) were added. The percentage of cells in the different stages of the cell cycle determined after PI staining and FACS analysis with the Modfit computer program. These experiments were repeated three times and averaged together.
Similar experiments were performed with the FL5.12 cells that were treated with IL-3 and MEK1 (10 μM U0126), PI3K (10 μM LY294002) and mTOR (100 nM) inhibitors. These concentrations of MEK1, PI3K and mTOR inhibitors inhibit activation/phosphorylation of ERK1,2, Akt, p70S6K and S6 in FL5.12 cells and other cell lines.11,54,55 Addition of the MEK1 or PI3K inhibitors increased the percentage of cells in G1 (D) compared with untreated cells for the first 24 h of treatment (A). Furthermore these inhibitors suppressed the entry of FL5.12 cells into the S phase after the first 24 h of treatment (E). After treatment with the mTOR inhibitor for 24 h, there was a more profound block in G1 (D) and inhibition of entry into S phase (E). After two days of treatment, similar levels of cells treated with the MEK, PI3K and mTOR inhibitors were in S phase as compared with untreated cells. Thus after a single inhibitor treatment, a transient suppression in cell cycle progression was observed.
Effects of doxorubicin and paclitaxel on cell cycle progression in FL5.12 cells
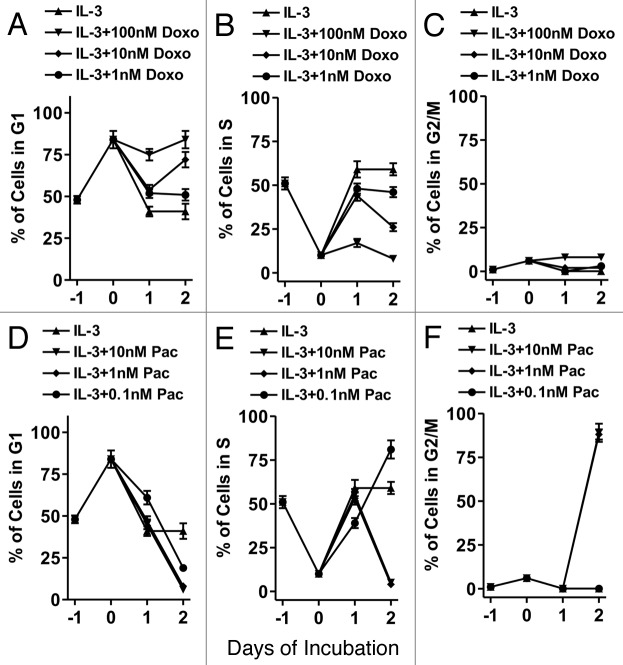
The effects of two chemotherapeutic drugs on cell cycle progression in FL5.12 cells were examined to determine the ability of these agents to induce cell cycle arrest and apoptosis in cytokine-dependent cells (Fig. 2). Addition of doxorubicin, which inhibited DNA synthesis, at concentrations between 10 and 100 nM, resulted in a blockage of the cells in the G1 phase of the cell cycle (A). This arrest in G1 was more readily observed at day 1 and day 2 when 100 nM doxorubicin was used. Doxorubicin prevented entry into S phase in a dose-dependent fashion (B).
Figure 2. Cell cycle progression in FL5.12 cells in the presence of chemotherapeutic drugs. In FL5.12 cells were collected, washed with PBS twice and then plated in phenol-red free medium containing 5% CS FBS (Day -1). After 24 h (Day 0) of incubation, IL-3 and the indicated concentrations of doxorubicin (A–C) or paclitaxel (D–F) were added. One ml aliquots (1 x 105 cells) were removed at the indicated time points and the percentage of cells in the different stages of the cell cycle determined after PI staining and FACS analysis with the Modfit computer program. These experiments were repeated three times and averaged together.
In contrast, treatment of FL5.12 cells with IL-3 and either 1 or 10 nM paclitaxel, which prevented microtubule disassembly, resulted in an exit of the cells from the G1 (D) and S (E) phases and a block in G2/M. The blockage in G2/M occurred after 2 d of paclitaxel addition (F). Thus doxorubicin and paclitaxel differed in how they affected cell cycle progression in cytokine-dependent FL5.12 cells.
Effects of MEK and mTOR inhibitors on prevention of cell cycle progression induced by doxorubicin and paclitaxel
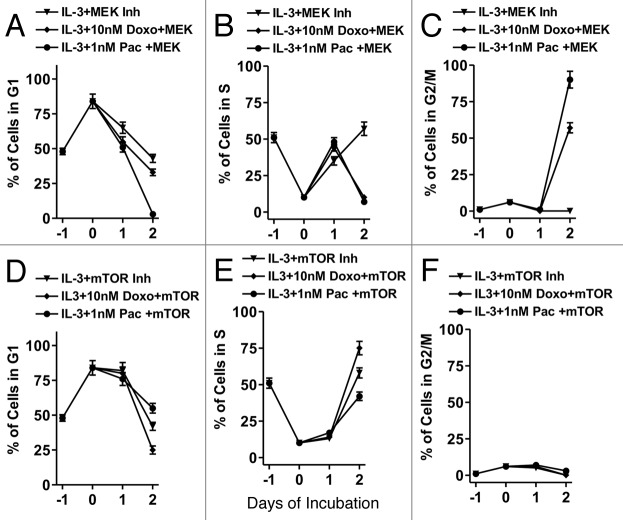
The effects of combining signal transduction inhibitors with chemotherapeutic drugs on cell cycle progression were determined (Fig. 3). When cells were treated with the MEK inhibitor and either doxorubicin or paclitaxel for 2 d, the cells had exited S phase (B) a dramatic arrest in G2/M occurred (C). In contrast, when they were treated with the mTOR inhibitor and either doxorubicin or paclitaxel, they were blocked from entering G2/M (F) and many of the cells remained in S phase (E).
Figure 3. Cell cycle progression in FL5.12 cells in the presence of signal transduction inhibitors and chemotherapeutic drugs. In FL5.12 cells were collected, washed with PBS twice and then plated in phenol-red free medium containing 5% CS FBS (Day -1). After 24 h (Day 0) of incubation, IL-3 and 10 μM MEK inhibitor and either doxorubicin or paclitaxel (A–C) or -3 and 100 nM mTOR inhibitor and either doxorubicin or paclitaxel (D–F) were added. One ml aliquots (1 x 105 cells) were removed at the indicated time points and the percentage of cells in the different stages of the cell cycle determined after PI staining and FACS analysis with the Modfit computer program. These experiments were repeated three times and averaged together.
Effects of MEK and mTOR inhibitors on the induction of apoptosis induced by doxorubicin
The ability of targeted therapy and classical chemotherapy to induce apoptosis in FL5.12 cells was determined by annexin V/PI binding (Table 1). As a control, some cells were deprived of IL-3 for 48 h which resulted in a 2.5-fold increase in apoptotic cells compared with cells continuously cultured in IL-3. When the cells were grown in medium containing IL-3, the MEK1 inhibitor by itself did not have a significant effect on the induction of apoptosis. In contrast, treatment with either the PI3K or mTOR inhibitors did have greater effects on the induction of apoptosis, consistent with the involvement of this pathway on the prevention of apoptosis.
Table 1. Fold increase in apoptosis due to chemotherapeutic drugs and signal transduction inhibitors in FL5.12 cells.
| |
No inhibitor |
+10 μM U0126 (MEK Inh) |
+10 μM LY294002 (PI3K Inh) |
+100 nM rapamycin (mTOR Inh) |
|---|---|---|---|---|
| Fold Induction of Apoptosis | ||||
| No IL-3 |
2.5 ± 0.23 |
|
|
|
| IL-3 |
1 ± 0.10 |
1.1 ± 0.14 |
1.4 ± 0.14 |
1.6 ± 0.18 |
| IL-3 + 1 nM Doxorubicin |
1.1 ± 0.14 |
1.2 ± 0.13 |
1.4 ± 0.18 |
1.6 ± 0.19 |
| IL-3 + 10 nM Doxorubicin |
1.3 ± 0.17 |
1.7 ± 0.17 |
1.4 ± 0.14 |
1.9 ± 0.08 |
| IL-3 + 100 nM Doxorubicin |
1.8 ± 0.22 |
2.0 ± 0.22 |
2.0 ± 0.03 |
2 ± 0.22 |
| IL-3 + 0.1 nM Paclitaxel |
1.4 ± 0.15 |
1.4 ± 0.14 |
1.5 ± 0.14 |
1.6 ± 0.16 |
| IL-3 + 1 nM Paclitaxel |
1.8 ± 0.23 |
1.8 ± 0.22 |
1.7 ± 0.19 |
1.9 ± 0.21 |
| IL-3 + 10 nM Paclitaxel | 1.8 ± 0.22 | 1.8 ± 0.20 | 1.9 ± 0.21 | 2.1 ± 0.25 |
The effects of doxorubicin on the induction of apoptosis were measured by the Annexin V/PI assay in the presence of and absence of different concentration of the MEK1 inhibitor U0126 or the mTOR inhibitor Rapamycin. These experiments were repeated 5 times and similar results were observed. Results were normalized to IL-3-treated cells.
Both doxorubicin and paclitaxel induced apoptosis in FL5.12 cells in a dose-dependent fashion. The effects of co-addition of signal transduction pathway inhibitors on the induction of apoptosis by doxorubicin and paclitaxel were determined. Co-addition of MEK and mTOR inhibitors, but not the PI3K inhibitor LY294002, increased the extent of apoptosis induced by 10 nM doxorubicin. In contrast, co-addition of MEK, mTOR or PI3K inhibitors with paclitaxel did not increase the extent of apoptosis as compared with the level of apoptosis induced by paclitaxel by itself. Thus the apoptosis inducing ability of certain chemotherapeutic drugs can be enhanced by MEK and mTOR inhibitors.
Effects of IL-3 on induction of MAPK pathway and cell cycle regulatory gene expression in FL5.12 cells
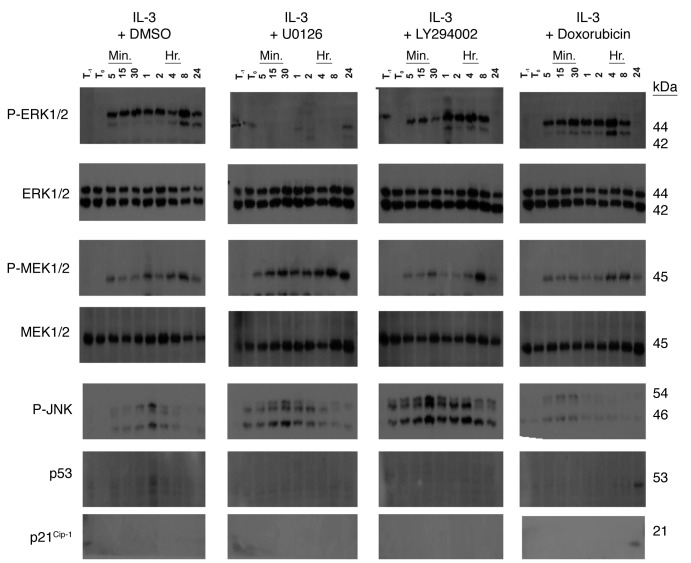
The effects of IL-3 activation on the MAPK pathways and cell cycle regulatory gene expression were examined in FL5.12 cells. In these experiments, FL5.12 cells were collected, washed with PBS twice to remove IL-3 and cultured in the absence of IL-3 in medium containing FBS before treatment with a signal transduction inhibitor or doxorubicin for one hour and then subsequently stimulated with IL-3 for the varying time points. In the absence of treatment with targeted therapy or chemotherapy, IL-3 induced the activation of MEK1 and the MAPKs, ERK1/2 and JNK (Fig. 4) after 5 min of IL-3 stimulation. Interestingly it appeared that after IL-3 stimulation of IL3-deprived cells, the predominant ERK that was being detected was ERK1 and not ERK2. The JNK MAPK has been associated with both proliferation and cell stress.60-63 Treatment with IL-3 resulted in activation of JNK. When the cells were stimulated with IL-3, essentially no p53 or p21Cip-1 were detected.
Figure 4. Effects of MEK1, PI3K inhibitors and doxorubicin on MAPK and cell cycle gene expression in FL5.12 cells. The effects of MEK1, PI3K inhibitors and doxorubicin on MAPK and cell cycle gene expression was examined in FL5.12 cells that had been deprived of IL-3 for 24 h (T-1) and then treated with the indicated inhibitors (10 μM U0126 or 10 μM LY294002) or 100 nM doxorubicin for 1 h (T0) and then stimulated with IL-3 for the indicated time periods. Western blot analysis was performed with antibodies which recognized activated ERK1/2, MEK1/2, JNK, p53 and p21Cip-1. These experiments were repeated three times and similar results were obtained.
The effects of the MEK inhibitor U0126, the PI3K inhibitor LY294002 and the chemotherapeutic drug doxorubicin on MAPK and cell cycle regulatory gene expression were examined. In these experiments, the IL3-deprived cells were treated with UO126, LY294002 or doxorubicin, 1 h prior to the addition of IL-3 and aliquots of the cells were removed at the indicated time intervals. As expected 10 μM U0126 suppressed ERK1/2 activation from the 5 min to 8 h after IL-3 treatment but did not inhibit the detection of activated MEK1/2. U0126 has been previously shown to inhibit MEK1/2 activity, but not MEK1/2 activation. There is a complicated negative feedback loop which is suppressed by the MEK1/2 inhibitors which actually results in higher levels of activated MEK1/2 as the negative feedback ERK1,2 loop on MEK1/2 is suppressed. Interesting and consistent with the cell cycle data presented earlier, activated ERK1/2 levels did rebound 24 h after IL-3 and U0126 treatment.
Treatment with IL-3 plus the MEK inhibitor UO126 or IL-3 plus the PI3K inhibitor LY294002 resulted in activation of JNK. Higher levels of activated JNK were detected after IL-3 plus PI3K inhibitor treatment. Slightly higher levels of activated JNK were detected after U0126 and IL-3 treatment than after stimulation with IL-3 by itself.
In contrast and as expected, the PI3K inhibitor did not suppress ERK1/2 activation from 5 min after IL-3 treatment until 8 h, however, no activated ERK1/2 was detected 24 h after treatment with the PI3K inhibitor and IL-3. Treatment with the PI3K inhibitor and IL-3 actually resulted in higher levels of activated JNK than detected in the other treatment conditions suggesting that either inhibition of the PI3K pathway could result in JNK activation. It has been reported that the PI3K pathway can negatively regulate JNK.66 Thus inhibition of PI3K may result in activation of JNK.
The effects of the chemotherapeutic drug doxorubicin on activation of ERK and JNK were also examined. After IL-3 and doxorubicin treatment and until 8 h, activated ERK1/2 was detected, however, no activated ERK1/2 was detected 24 h after treatment with doxorubicin and IL-3. After 24 h of doxorubicin treatment, p53 and p21Cip-1 were induced. Doxorubicin treatment did not result in increased induction of JNK compared with cells just treated with IL-3. Likewise it did not appear to suppress ERK or MEK induction.
Discussion
Cytokine-dependent FL5.12 cells are highly sensitive to cytokine deprivation. Cytokine deprivation resulted in the rapid exit from the cell cycle and accumulation of cells in the G1 phase. After two days of cytokine-deprivation, the cells underwent apoptosis. Furthermore the cytokine-dependent cells are not particularly sensitive to treatment with MEK1, PI3K or mTOR inhibitors, by themselves, indicating that suppression of a single pathway will not prevent cell cycle progression or induce apoptosis in cells which remain cytokine-dependent and their growth does not appear to be “addicted” to a particular oncogene.
In our study, the effects of classical chemotherapy were compared with signal transduction inhibitors which target the Raf/MEK/ERK or PI3K/Akt/mTOR pathways. Classical chemotherapeutic drugs such as doxorubicin or paclitaxel are very effective in inducing cell cycle arrest and apoptosis in cytokine-dependent cells. Doxorubicin normally induces a block in G1 while paclitaxel induces a block in G2/M. Thus agents which inhibit DNA synthesis such as doxorubicin inhibit entry into S phase while agents such as paclitaxel, which affect the disassembly of the mitotic spindle apparatus, induce a block in G2/M. The effects of these chemotherapeutic drugs on inhibition of cell cycle progression can be enhanced with inhibitors which target MEK and mTOR.
Both doxorubicin (Fig. 4) and paclitaxel (unpublished observations) induce the activation of ERK1/2. Activation of ERK1/2 can have pro-proliferative, anti-apoptotic effects on cells and contribute to drug resistance.11 Hence, it is rational to combine certain chemotherapeutic treatments with agents which will suppress ERK1/2 activity.
Inhibition of MEK, PI3K or mTOR by themselves only partially suppressed cell cycle progression and weakly induced apoptosis in cytokine-dependent cells. Single inhibitor treatment may be ineffective in cytokine-dependent cells as the cytokines induce multiple signaling pathways which have overlapping anti-apoptotic effects.
Clearly signal transduction pathways induced by cytokines and chemotherapeutic drugs interact to result in the regulation of cell cycle progression and apoptosis. Devising mechanisms to inhibit these interactions may result in more effective anti-cancer therapies. Some of these signaling pathways are associated with chemotherapeutic drug resistance.11,20,21,31,32,37-43,58,59,67,68 Furthermore, the p53 tumor suppressor is a common target of many chemotherapeutic drugs and can interact with many signaling pathways.69-72 Approaches that combine prolongation of wild-type p53 activity may enhance the effects of chemotherapeutic drugs, either in the presence of absence of signal transduction inhibitors.73-78 Elucidating the mechanisms of interaction of chemotherapy, p53 activators and signal transduction inhibitors and how they enhance cell death by various mechanisms may yield more effective therapy.
This therapeutic strategy is relevant in those solid and hematological neoplasias which show upfront resistance to classical cytostatic drugs e.g., acute myeloid leukemia (AML) of the elderly, or which become resistant during ongoing treatment. In addition targeted therapy alone has only been a breakthrough in a few entities such as Gleevec (Imatinib) in chronic myeloid leukemia (CML) and gastro-intestinal stroma tumor (GIST) in which neoplastic transformation relies on a singular activating hit (BCR-ABL in CML, c-Kit in GIST).3-5,79,80 As in many other cancers pathway activation is more complex and interdependent, it can be anticipated, that combined therapeutic strategies will be of increasing importance to enhance the efficiency of classical cytostatic drugs, to limit their side effects and to overcome resistance.
Cells which proliferate continuously like cytokine-dependent FL5.12 cells represent a very appropriate model cell system to evaluate novel signal transduction inhibitors and chemotherapeutic drugs as they are not addicted on a particular oncogene for growth and they do not normally form tumors upon injection into immunocompromised mice. Targeting these cytokine-dependent, non-malignant cells, which can readily be transformed to cytokine-independent, tumorigenic cells, by various activated oncogenes (BCR-ABL, JAK, Flt-3) could result in the discovery of therapeutic approaches which are effective against LSCs or CICs.
Materials and Methods
Cell lines and growth factors
Cells were maintained in a humidified 5% CO2 incubator with RPMI-1640 [(RPMI) Invitrogen, Carlsbad, CA, USA] supplemented with 5% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA, USA). The IL-3 dependent FL5.12 murine cell line was cultured in this medium supplemented with 10% WEHI-3B(D-) conditioned medium (WCM) as a source of IL-3.1,2,11
In some cases, cells were treated with the MEK1 inhibitor U0126 (Promega, Madison, WI), the PI3K inhibitor LY294002 (Calbiochem, Los Angeles, CA) or the mTOR inhibitor rapamycin (Sigma-Aldrich, Saint Louis, MO) as described.11 These inhibitors were dissolved in dimethyl-sulfoxide (DMSO, Sigma-Aldrich). Some cells were treated with different concentrations of doxorubicin, or paclitaxel (Sigma-Aldrich).
Analysis of cell cycle distribution
Cells were incubated at approximately 2 x 105 cells/ml in 3 mls of phenol-red free RPMI containing FCS but lacking IL-3. This initial time point is designated (Day-1). Twenty-four hours later at the T0 point, IL-3 (10% WEHI clear conditioned medium), and various concentrations of doxorubicin, paclitaxel, U0126, LY294002 or rapamycin were added to the cultures. Aliquots of approximately 1 x 105 cells (0.5 mls) were subsequently removed at the indicated time points. Analysis of cell cycle distribution was performed as described previously.81
Annexin V apoptotic assays
Annexin V/PI binding assays were performed as previously described with kits purchased from Roche (Indianapolis, IN).9,11
Western blot analysis
Cells were washed twice with PBS and then cultured in the presence of phenol red free RPMI 1640 containing 5% charcoal stripped (CS) FBS for 24 h. An aliquot of cells was removed (T-1 Hr). Cells were treated with the vehicle (DMSO) or the indicated signal transduction inhibitors or doxorubicin for 1 h and an aliquot was removed (T0). Cells were then incubated with IL-3 in the presence and absence of the chemotherapeutic drugs and signal transduction inhibitors for the indicated time intervals and aliquots removed. Western blots were performed with antibodies specific for phospho and total MEK, ERK, JNK, p53 and p21Cip-1 as we have previously described.9-11 All antibodies used in this study were purchased from Cell Signaling (Beverly, MA).
Acknowledgments
This work was supported in part by grants from the US Public Health Service, National Institutes of Health, National Cancer Institute (NCI R01CA098195) and a Brody Brothers Endowment Fund (MT7826) to J.A.M. A.M.M. was supported by grants from Progetti Strategici Unibo EF 2006 and MIUR PRIN 2008.
02/10/10
02/15/10
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/11544
References
- 1.McKearn JP, McCubrey JA, Fagg B. Enrichment of hematopoietic precursor cells and cloning of multipotential B-lymphocyte precursors. Proc Natl Acad Sci U S A. 1985;82:7414–8. doi: 10.1073/pnas.82.21.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCubrey JA, Holland G, McKearn J, Risser R. Abrogation of factor-dependence in two IL-3-dependent cell lines can occur by two distinct mechanisms. Oncogene Res. 1989;4:97–109. [PubMed] [Google Scholar]
- 3.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 4.McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22:708–22. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 5.Misaghian N, Ligresti G, Steelman LS, Bertrand FE, Bäsecke J, Libra M, Nicoletti F, Stivala F, Milella M, Tafuri A, et al. Targeting the leukemic stem cell: the Holy Grail of leukemia therapy. Leukemia. 2009;23:25–42. doi: 10.1038/leu.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayo MW, Wang X-Y, Algate PA, Arana GF, Hoyle PE, Steelman LS, McCubrey JA. Synergy between AUUUA motif disruption and enhancer insertion results in autocrine transformation of interleukin-3-dependent hematopoietic cells. Blood. 1995;86:3139–50. [PubMed] [Google Scholar]
- 7.Wang X-Y, McCubrey JA. Malignant transformation induced by cytokine genes: a comparison of the abilities of germline and mutated interleukin 3 genes to transform hematopoietic cells by transcriptional and posttranscriptional mechanisms. Cell Growth Differ. 1996;7:487–500. [PubMed] [Google Scholar]
- 8.Wang XY, McCubrey JA. Differential effects of retroviral long terminal repeats on interleukin-3 gene expression and autocrine transformation. Leukemia. 1997;11:1711–25. doi: 10.1038/sj.leu.2400793. [DOI] [PubMed] [Google Scholar]
- 9.Shelton JG, Steelman LS, Lee JT, Knapp SL, Blalock WL, Moye PW, Franklin RA, Pohnert SC, Mirza AM, McMahon M, et al. Effects of the RAF/MEK/ERK and PI3K/AKT signal transduction pathways on the abrogation of cytokine-dependence and prevention of apoptosis in hematopoietic cells. Oncogene. 2003;22:2478–92. doi: 10.1038/sj.onc.1206321. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand FE, Steelman LS, Chappell WH, Abrams SL, Shelton JG, White ER, Ludwig DL, McCubrey JA. Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia. 2006;20:1254–60. doi: 10.1038/sj.leu.2404217. [DOI] [PubMed] [Google Scholar]
- 11.McCubrey JA, Abrams SL, Ligresti G, Misaghian N, Wong EW, Steelman LS, Bäsecke J, Troppmair J, Libra M, Nicoletti F, et al. Involvement of p53 and Raf/MEK/ERK pathways in hematopoietic drug resistance. Leukemia. 2008;22:2080–90. doi: 10.1038/leu.2008.207. [DOI] [PubMed] [Google Scholar]
- 12.Gaundar SS, Bradstock KF, Bendall LJ. p38MAPK inhibitors attenuate cytokine production by bone marrow stromal cells and reduce stroma-mediated proliferation of acute lymphoblastic leukemia cells. Cell Cycle. 2009;8:2975–83. doi: 10.4161/cc.8.18.9545. [DOI] [PubMed] [Google Scholar]
- 13.Brasier AR. Expanding role of cyclin dependent kinases in cytokine inducible gene expression. Cell Cycle. 2008;7:2661–6. doi: 10.4161/cc.7.17.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roper S, Hemberger M. Defining pathways that enforce cell lineage specification in early development and stem cells. Cell Cycle. 2009;8:1515–25. doi: 10.4161/cc.8.10.8381. [DOI] [PubMed] [Google Scholar]
- 15.Gonda TA, Tu S, Wang TC. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8:2005–13. doi: 10.4161/cc.8.13.8985. [DOI] [PubMed] [Google Scholar]
- 16.Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–8. doi: 10.4161/cc.7.10.5954. [DOI] [PubMed] [Google Scholar]
- 17.Blagosklonny MV. Cancer stem cell and cancer stemloids: from biology to therapy. Cancer Biol Ther. 2007;6:1684–90. doi: 10.4161/cbt.6.11.5167. [DOI] [PubMed] [Google Scholar]
- 18.Stuart SA, Minami Y, Wang JYJ. The CML stem cell: evolution of the progenitor. Cell Cycle. 2009;8:1338–43. doi: 10.4161/cc.8.9.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Borodyansky L, Yang Y. Genomic instability en route to and from cancer stem cells. Cell Cycle. 2009;8:1000–2. doi: 10.4161/cc.8.7.8041. [DOI] [PubMed] [Google Scholar]
- 20.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–66. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller-Sieburg C, Sieburg HB. Stem cell aging: survival of the laziest? Cell Cycle. 2008;7:3798–804. doi: 10.4161/cc.7.24.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, Estey EH, Andreeff M. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–65. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gosliga D, Schepers H, Rizo A, van der Kolk D, Vellenga E, Schuringa JJ. Establishing long-term cultures with self-renewing acute myeloid leukemia stem/progenitor cells. Exp Hematol. 2007;35:1538–49. doi: 10.1016/j.exphem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–84. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 25.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–80. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 26.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–90. doi: 10.1016/S0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–82. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–22. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Zhang Y. Cancer stem cells: Models, mechanisms and implications for improved treatment. Cell Cycle. 2008;7:1360–70. doi: 10.4161/cc.7.10.5953. [DOI] [PubMed] [Google Scholar]
- 30.Sabisz M, Skladanowski A. Cancer stem cells and escape from drug-induced premature senescence in human lung tumor cells: implications for drug resistance and in vitro drug screening models. Cell Cycle. 2009;8:3208–17. doi: 10.4161/cc.8.19.9758. [DOI] [PubMed] [Google Scholar]
- 31.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCubrey JA, Abrams SL, Stadelman K, Chappell WH, Lahair M, Ferland RA, Steelman LS. Targeting signal transduction pathways to eliminate chemotherapeutic drug resistance and cancer stem cells. Adv Enzyme Regul. 2010;50:285–307. doi: 10.1016/j.advenzreg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ligresti G, Militello L, Steelman LS, Cavallaro A, Basile F, Nicoletti F, Stivala F, McCubrey JA, Libra M. PIK3CA mutations in human solid tumors: role in sensitivity to various therapeutic approaches. Cell Cycle. 2009;8:1352–8. doi: 10.4161/cc.8.9.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carracedo A, Baselga J, Pandolfi PP. Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle. 2008;7:3805–9. doi: 10.4161/cc.7.24.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krymskaya VP, Goncharova EA. PI3K/mTORC1 activation in hamartoma syndromes: therapeutic prospects. Cell Cycle. 2009;8:403–13. doi: 10.4161/cc.8.3.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster DA, Toschi A. Targeting mTOR with rapamycin: one dose does not fit all. Cell Cycle. 2009;8:1026–9. doi: 10.4161/cc.8.7.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JT, Lehmann BD, Terrian DM, Chappell WH, Stivala F, Libra M, Martelli AM, Steelman LS, McCubrey JA. Targeting prostate cancer based on signal transduction and cell cycle pathways. Cell Cycle. 2008;7:1745–62. doi: 10.4161/cc.7.12.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steelman LS, Navolanic PM, Sokolosky ML, Taylor JR, Lehmann BD, Chappell WH, Abrams SL, Wong EW, Stadelman KM, Terrian DM, et al. Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors. Oncogene. 2008;27:4086–95. doi: 10.1038/onc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagadinou ED, Ziros PG, Tsopra OA, Dimas K, Kokkinou D, Thanopoulou E, Karakantza M, Pantazis P, Spyridonidis A, Zoumbos NC. c-Jun N-terminal kinase activation failure is a new mechanism of anthracycline resistance in acute myeloid leukemia. Leukemia. 2008;22:1899–908. doi: 10.1038/leu.2008.192. [DOI] [PubMed] [Google Scholar]
- 40.Gomes AR, Brosens JJ, Lam EWF. Resist or die: FOXO transcription factors determine the cellular response to chemotherapy. Cell Cycle. 2008;7:3133–6. doi: 10.4161/cc.7.20.6920. [DOI] [PubMed] [Google Scholar]
- 41.Nimbalkar D, Quelle FW. Phosphoinositide 3-kinase signaling overrides a G2 phase arrest checkpoint and promotes aberrant cell cycling and death of hematopoietic cells after DNA damage. Cell Cycle. 2008;7:2877–85. doi: 10.4161/cc.7.18.6675. [DOI] [PubMed] [Google Scholar]
- 42.Palomero T, Dominguez M, Ferrando AA. The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell Cycle. 2008;7:965–70. doi: 10.4161/cc.7.8.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papa V, Tazzari PL, Chiarini F, Cappellini A, Ricci F, Billi AM, Evangelisti C, Ottaviani E, Martinelli G, Testoni N, et al. Proapoptotic activity and chemosensitizing effect of the novel Akt inhibitor perifosine in acute myelogenous leukemia cells. Leukemia. 2008;22:147–60. doi: 10.1038/sj.leu.2404980. [DOI] [PubMed] [Google Scholar]
- 44.Martelli AM, Nyåkern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, Cocco L. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–28. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 45.Tazzari PL, Tabellini G, Bortul R, Papa V, Evangelisti C, Grafone T, Martinelli G, McCubrey JA, Martelli AM. The insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 induces apoptosis in acute myeloid leukemia cells exhibiting autocrine insulin-like growth factor-I secretion. Leukemia. 2007;21:886–96. doi: 10.1038/sj.leu.2404643. [DOI] [PubMed] [Google Scholar]
- 46.Skladanowski A, Bozko P, Sabisz M, Larsen AK. Dual inhibition of PI3K/Akt signaling and the DNA damage checkpoint in p53-deficient cells with strong survival signaling: implications for cancer therapy. Cell Cycle. 2007;6:2268–75. doi: 10.4161/cc.6.18.4705. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Vidal A, Mazars A, Gautier EF, Prévost G, Payrastre B, Manenti S. Upregulation of the CDC25A phosphatase down-stream of the NPM/ALK oncogene participates to anaplastic large cell lymphoma enhanced proliferation. Cell Cycle. 2009;8:1373–9. doi: 10.4161/cc.8.9.8302. [DOI] [PubMed] [Google Scholar]
- 48.Chiarini F, Fala F, Tazzari PL, Ricci F, Astolfi A, Pession A, et al. Dual inhibition of class IA phosphatidylionsitol 3-kinase and mTOR as a new therapeutic option for T-cell acute lymphoblastic leukemia. Cancer Res. 2009;69:3520–8. doi: 10.1158/0008-5472.CAN-08-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blagosklonny MV. Aging, stem cells and mammalian target of rapamycin: a prospect of pharmalogical rejuvenation of aging stem cells. Rejuv Res. 2008;11:801–8. doi: 10.1089/rej.2008.0722. [DOI] [PubMed] [Google Scholar]
- 50.Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–54. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- 51.Blagosklonny MV, Campisi J. Cancer and aging: more puzzles, more promises? Cell Cycle. 2008;7:2615–8. doi: 10.4161/cc.7.17.6626. [DOI] [PubMed] [Google Scholar]
- 52.Blagosklonny MV. Program-like aging and mitochondria: instead of random damage by free radicals. J Cell Biochem. 2007;102:1389–99. doi: 10.1002/jcb.21602. [DOI] [PubMed] [Google Scholar]
- 53.Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–61. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- 54.Demidenko ZN, Shtutman M, Blagosklonny MV. Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle. 2009;8:1896–900. doi: 10.4161/cc.8.12.8809. [DOI] [PubMed] [Google Scholar]
- 55.Demidenko ZN, Blagosklonny MV. At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle. 2009;8:1901–4. doi: 10.4161/cc.8.12.8810. [DOI] [PubMed] [Google Scholar]
- 56.Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–95. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- 57.Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle. 2009;8:1883–7. doi: 10.4161/cc.8.12.8815. [DOI] [PubMed] [Google Scholar]
- 58.Steelman LS, Abrams SL, Shelton JG, Chappell WH, Bäsecke J, Stivala F, Donia M, Nicoletti F, Libra M, Martelli AM, et al. Dominant roles of the Raf/MEK/ERK pathway in cell cycle progression, prevention of apoptosis and sensitivity to chemotherapeutic drugs. Cell Cycle. 2010;9:1629–38. doi: 10.4161/cc.9.8.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abrams SL, Steelman LS, Shelton JG, Wong EW, Chappell WH, Bäsecke J, Stivala F, Donia M, Nicoletti F, Libra M, et al. The Raf/MEK/ERK pathway can govern drug resistance, apoptosis and sensitivity to targeted therapy. Cell Cycle. 2010;9:1781–91. doi: 10.4161/cc.9.9.11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiarini F, Del Sole M, Mongiorgi S, Gaboardi GC, Cappellini A, Mantovani I, Follo MY, McCubrey JA, Martelli AM. The novel Akt inhibitor, perifosine, induces caspase-dependent apoptosis and downregulates P-glycoprotein expression in multidrug-resistant human T-acute leukemia cells by a JNK-dependent mechanism. Leukemia. 2008;22:1106–16. doi: 10.1038/leu.2008.79. [DOI] [PubMed] [Google Scholar]
- 61.Oktay K, Buyuk E, Oktem O, Oktay M, Giancotti FG. The c-Jun N-terminal kinase JNK functions upstream of Aurora B to promote entry into mitosis. Cell Cycle. 2008;7:533–41. doi: 10.4161/cc.7.4.5660. [DOI] [PubMed] [Google Scholar]
- 62.Nica AF, Tsao CC, Watt JC, Jiffar T, Kurinna S, Jurasz P, Konopleva M, Andreeff M, Radomski MW, Ruvolo PP. Ceramide promotes apoptosis in chronic myelogenous leukemia-derived K562 cells by a mechanism involving caspase-8 and JNK. Cell Cycle. 2008;7:3362–70. doi: 10.4161/cc.7.21.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee K, Song K. Basal c-Jun N-terminal kinases promote mitotic progression through histone H3 phosphorylation. Cell Cycle. 2008;7:216–21. doi: 10.4161/cc.7.2.5155. [DOI] [PubMed] [Google Scholar]
- 64.Catalanotti F, Reyes G, Jesenberger V, Galabova-Kovacs G, de Matos Simoes R, Carugo O, Baccarini M. A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nat Struct Mol Biol. 2009;16:294–303. doi: 10.1038/nsmb.1564. [DOI] [PubMed] [Google Scholar]
- 65.McCubrey JA, Steelman LS, Abrams SL, Chappell WH, Russo S, Ove R, Milella M, Tafuri A, Lunghi P, Bonati A, et al. Emerging MEK inhibitors. Expert Opin Emerg Drugs. 2010;15:203–23. doi: 10.1517/14728210903282760. [DOI] [PubMed] [Google Scholar]
- 66.Autret A, Martin-Latil S, Brisac C, Mousson L, Colbère-Garapin F, Blondel B. Early phosphatidylinositol 3-kinase/Akt pathway activation limits poliovirus-induced JNK-mediated cell death. J Virol. 2008;82:3796–802. doi: 10.1128/JVI.02020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tazzari PL, Cappellini A, Ricci F, Evangelisti C, Papa V, Grafone T, Martinelli G, Conte R, Cocco L, McCubrey JA, et al. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia. 2007;21:427–38. doi: 10.1038/sj.leu.2404523. [DOI] [PubMed] [Google Scholar]
- 68.Follo MY, Finelli C, Bosi C, Martinelli G, Mongiorgi S, Baccarani M, Manzoli L, Blalock WL, Martelli AM, Cocco L. PI-PLCbeta-1 and activated Akt levels are linked to azacitidine responsiveness in high-risk myelodysplastic syndromes. Leukemia. 2008;22:198–200. doi: 10.1038/sj.leu.2404855. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, Elf SE, Asai T, Miyata Y, Liu Y, Sashida G, Huang G, Di Giandomenico S, Koff A, Nimer SD. The p53 tumor suppressor protein is a critical regulator of hematopoietic stem cell behavior. Cell Cycle. 2009;8:3120–4. doi: 10.4161/cc.8.19.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choong ML, Yang H, Lee MA, Lane DP. Specific activation of the p53 pathway by low dose actinomycin D: a new route to p53 based cyclotherapy. Cell Cycle. 2009;8:2810–8. doi: 10.4161/cc.8.17.9503. [DOI] [PubMed] [Google Scholar]
- 71.Sohr S, Engeland K. RHAMM is differentially expressed in the cell cycle and downregulated by the tumor suppressor p53. Cell Cycle. 2008;7:3448–60. doi: 10.4161/cc.7.21.7014. [DOI] [PubMed] [Google Scholar]
- 72.París R, Henry RE, Stephens SJ, McBryde M, Espinosa JM. Multiple p53-independent gene silencing mechanisms define the cellular response to p53 activation. Cell Cycle. 2008;7:2427–33. doi: 10.4161/cc.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wade M, Rodewald LW, Espinosa JM, Wahl GM. BH3 activation blocks Hdmx suppression of apoptosis and cooperates with Nutlin to induce cell death. Cell Cycle. 2008;7:1973–82. doi: 10.4161/cc.7.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaseva AV, Marchenko ND, Moll UM. The transcription-independent mitochondrial p53 program is a major contributor to nutlin-induced apoptosis in tumor cells. Cell Cycle. 2009;8:1711–9. doi: 10.4161/cc.8.11.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia M, Knezevic D, Tovar C, Huang B, Heimbrook DC, Vassilev LT. Elevated MDM2 boosts the apoptotic activity of p53-MDM2 binding inhibitors by facilitating MDMX degradation. Cell Cycle. 2008;7:1604–12. doi: 10.4161/cc.7.11.5929. [DOI] [PubMed] [Google Scholar]
- 76.Bykov VJ, Lambert JM, Hainaut P, Wiman KG. Mutant p53 rescue and modulation of p53 redox state. Cell Cycle. 2009;8:2509–17. doi: 10.4161/cc.8.16.9382. [DOI] [PubMed] [Google Scholar]
- 77.Varmark H, Sparks CA, Nordberg JJ, Koppetsch BS, Theurkauf WE. DNA damage-induced cell death is enhanced by progression through mitosis. Cell Cycle. 2009;8:2951–63. doi: 10.4161/cc.8.18.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhuang Z, Lu J, Lonser R, Kovach JS. Enhancement of cancer chemotherapy by simultaneously altering cell cycle progression and DNA-damage defenses through global modification of the serine/threonine phospho-proteome. Cell Cycle. 2009;8:3303–6. doi: 10.4161/cc.8.20.9689. [DOI] [PubMed] [Google Scholar]
- 79.Vicente-Dueñas C, Pérez-Caro M, Abollo-Jiménez F, Cobaleda C, Sánchez-García I. Stem-cell driven cancer: “hands-off” regulation of cancer development. Cell Cycle. 2009;8:1314–8. doi: 10.4161/cc.8.9.8217. [DOI] [PubMed] [Google Scholar]
- 80.Belinsky MG, Rink L, Cai KQ, Ochs MF, Eisenberg B, Huang M, von Mehren M, Godwin AK. The insulin-like growth factor system as a potential therapeutic target in gastrointestinal stromal tumors. Cell Cycle. 2008;7:2949–55. doi: 10.4161/cc.7.19.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang F, McCubrey JA. p21Cip1 induced by Raf is associated with increased Cdk4 activity in hematopoietic cells. Oncogene. 2001;20:4354–64;. doi: 10.1038/sj.onc.1204564. [DOI] [PubMed] [Google Scholar]