Abstract
Excess vitamins, especially folate, are consumed during pregnancy but later-life effects on the offspring are unknown. High multivitamin (10-fold AIN-93G, HV) gestational diets increase characteristics of metabolic syndrome in Wistar rat offspring. We hypothesized that folate, the vitamin active in DNA methylation, accounts for these effects through epigenetic modification of food intake regulatory genes. Male offspring of dams fed 10-fold folate (HFol) diet during pregnancy and weaned to recommended vitamin (RV) or HFol diets were compared with those born to RV dams and weaned to RV diet for 29 weeks. Food intake and body weight were highest in offspring of HFol dams fed the RV diet. In contrast, the HFol pup diet in offspring of HFol dams reduced food intake (7%, p = 0.02), body weight (9%, p = 0.03) and glucose response to a glucose load (21%, p = 0.02), and improved glucose response to an insulin load (20%, p = 0.009). HFol alone in either gestational or pup diet modified gene expression of feeding-related neuropeptides. Hypomethylation of the pro-opiomelanocortin (POMC) promoter occurred with the HFol pup diet. POMC-specific methylation was positively associated with glucose response to a glucose load (r = 0.7, p = 0.03). In conclusion, the obesogenic phenotype of offspring from dams fed the HFol gestational diet can be corrected by feeding them a HFol diet. Our work is novel in showing post-weaning epigenetic plasticity of the hypothalamus and that in utero programming by vitamin gestational diets can be modified by vitamin content of the pup diet.
Keywords: DNA methylation, folate, gene expression, gestation, glucose response, hypothalamus, obesity, post-weaning
Introduction
Vitamin intake during pregnancy has increased due to wide availability of multivitamin supplements, liberalized fortification policies and dietary advice encouraging pregnant women to consume adequate nutrients. In particular, folate is recommended to reduce the risk of neural tube defects in newborns.1,2 Although vitamins are critical in fetal development, the effects of high vitamin intake during pregnancy and the mechanisms by which vitamins modify the offspring phenotype have received limited attention.
We have previously shown that high multivitamin (HV, 10-fold the recommended multivitamin content) diets fed during pregnancy to Wistar rats produce offspring with increased food intake, obesity and characteristics of the metabolic syndrome.3,4 These offspring were programmed to overeat, which is a primary cause of metabolic syndrome, suggesting that some component(s) of the multivitamin preparation is impacting development of the feeding pathways in the brain. A possible explanation for altered development of intake regulatory systems resides in epigenetic mechanisms involving methyltransfer reactions. Because our recent data have demonstrated that the obesogenic phenotype of offspring of dams fed a HV diet is prevented by a post-weaning diet in either multivitamins or folate,5 it can be suggested that folate alone within the multivitamin preparation is impacting the feeding neurocircuitry. Folate is a methyl metabolism co-factor in processes of DNA methylation,6 which in turn can modify gene expression and function in regulatory systems. However, it is unknown whether folate alone in the HV gestational diet is responsible for the obesogenic phenotype in mature offspring, and whether folate in the pup diet can provide protection against these effects.
We report here the role of folate in the gestational and post-weaning diets in food intake regulatory systems in the offspring. According to the predictive adaptive response (PAR) hypothesis,7,8 a mismatch between the maternal and postnatal nutrition increases susceptibility of the offspring to chronic disease in later life. If folate in the HV gestational diet is the vitamin that is responsible for the obesogenic phenotype in the offspring, it can be predicted that providing folate in the post-weaning diet, thereby matching the vitamin content between the gestational and post-weaning diets, would prevent the obesogenic phenotype later in life. DNA methylation provides a plausible mechanism by which high folate (HFol; 10-fold folate) diet fed during gestation alters food intake regulation favoring obesity in the offspring. Moreover, the correction of the obesogenic phenotype by feeding them a HFol diet is expected to also be due to methylation events occurring postnatally.9 We measured similar metabolic outcomes such as food intake, body weight and glucose response to a glucose or insulin load as our previous studies3,4 to determine whether folate in the gestational and pup diets account for the effects of the HV diets. Glucose response to a glucose or insulin load was measured after week 7 post-weaning and 2–3 alternating weeks apart because performing these challenges early on when food intake patterns have not differentiated among the diet groups may disrupt the offspring feeding behavior. We also focused on gene expression of previously identified feeding-related neuropeptides altered by the HV gestational diet.5
Therefore, we hypothesized that folate alone during gestation contributes to the obesogenic phenotype in the offspring due to epigenetic effects on hypothalamic mechanisms regulating food intake, and these effects are prevented by increasing the folate content in the pup diet. Our objective was to determine the effect of HFol diets consumed during pregnancy and post-weaning on food intake, body weight, glucose control, hypothalamic gene expression of feeding-related neuropeptides and DNA methylation status in the offspring compared with those born to dams fed the recommended vitamin (RV) diet and weaned to RV diet.
Results
Food intake and body weight
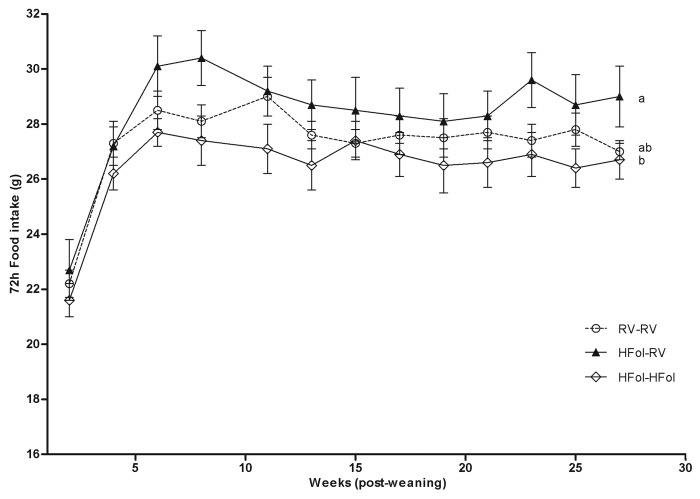
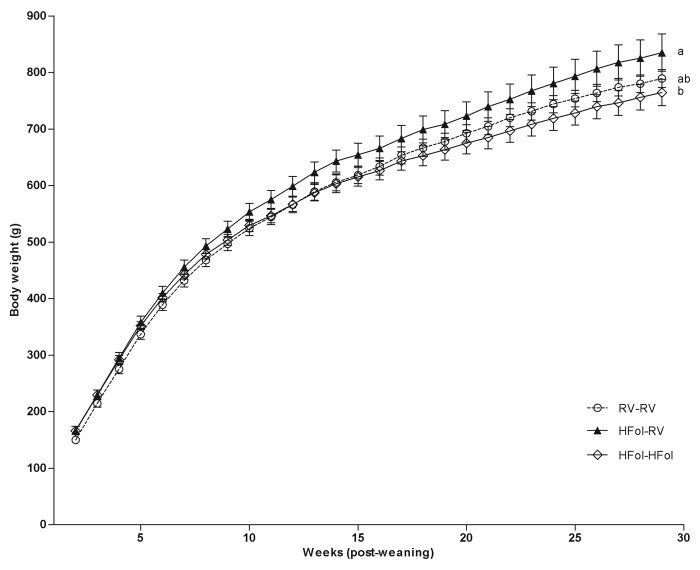
Seventy-two-h food intake and body weight were highest in male offspring of HFol dams fed the RV diet compared with those fed the HFol diet and the control offspring. In contrast, the HFol pup diet in offspring of HFol dams reduced 7% food intake (Diet: p = 0.02, Time: p < 0.0001, Diet*Time Interaction: p = 1.0) (Fig. 1) and 9% body weight (Diet: p = 0.03, Time: p < 0.0001, Diet*Time Interaction: p = 0.7) (Fig. 2) compared with the RV pup diet at 29 weeks post-weaning. Body weight at birth and weaning was similar among the diet groups.
Figure 1. Food intake over 72 h of male offspring from 0–29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HFol, RV+10-fold the folate content. Gestational and pup diets denoted before and after the dash line, respectively. Diet (p = 0.02), Time (p < 0.0001), Diet*Time (p = 1.0). ab Significantly different by PROC MIXED model repeated measures. Values are mean ± SEM, n = 10–12/group.
Figure 2. Body weight of male offspring from 0–29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HFol, RV+10-fold the folate content. Gestational and pup diets denoted before and after the dash line, respectively. Weight Gain: Diet (p = 0.03), Time (p < 0.0001), Diet*Time (p = 0.7). ab Significantly different by PROC MIXED model repeated measures. Values are mean ± SEM, n = 11–12/group.
Glucose response
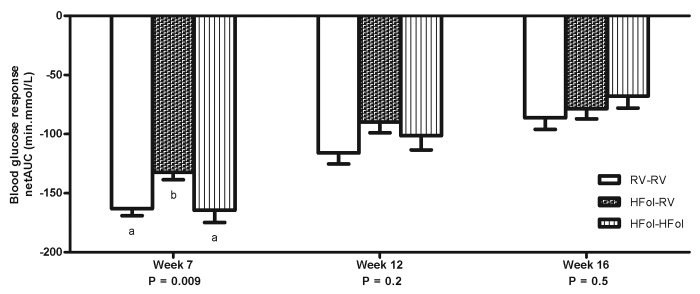
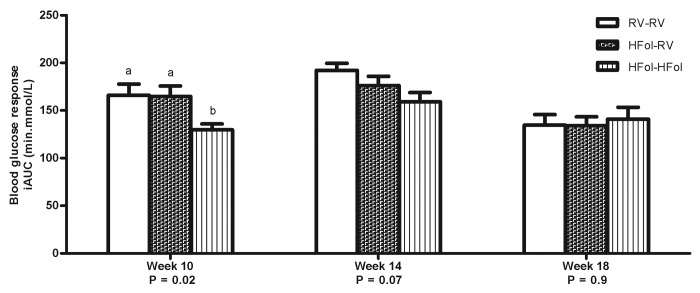
Male offspring from the HFol dams weaned to a RV diet had 20% reduced glucose response to an insulin load at 7 weeks post-weaning (p = 0.009) compared with those from the RV dams (Fig. 3). However, feeding a HFol pup diet improved the glucose response in the offspring of HFol dams (Fig. 3). At 10 weeks post-weaning, male offspring from the HFol dams weaned to HFol diet had 21% lower glucose response to a glucose load (p = 0.02) compared with those from the RV dams and HFol dams weaned to the RV pup diet (Fig. 4). No other differences were observed in all other weeks.
Figure 3. Blood glucose response to an insulin load (0.75 U of insulin per kg of body weight) as net area under the curve (netAUC) of male offspring at 7, 12 and 16 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HFol, RV+10-fold the folate content. Gestational and pup diets denoted before and after the dash line, respectively. ab Significantly different by one-way ANOVA followed by Tukey’s post-hoc test. Values are mean ± SEM, n = 9–12/group.
Figure 4. Blood glucose response to a glucose load (5 g of glucose per kg of body weight) as incremental area under the curve (iAUC) of male offspring at 10, 14 and 18 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HFol, RV+10-fold the folate content. Gestational and pup diets denoted before and after the dash line, respectively. ab Significantly different by one-way ANOVA followed by Tukey’s post-hoc test. Values are mean ± SEM, n = 9–12/group.
Hypothalamic gene expression
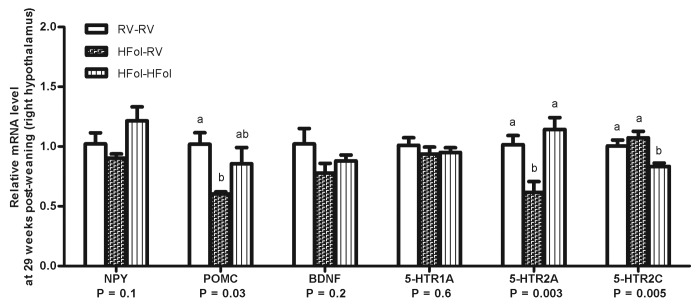
HFol alone in the gestational diet reduced POMC (40%, p = 0.03) and 5-HTR2A (40%, p = 0.003) in the right hypothalamus compared with the male offspring from dams fed the RV diet. The HFol pup diet brought 5-HTR2A expression to control levels and attenuated the decrease in POMC expression caused by the HFol gestational diet (Fig. 5). 5-HTR2C expression was unaffected by the gestational diet but was lowered by the HFol pup diet in the offspring born to dams fed the HFol diet (20%, p = 0.005) (Fig. 5). Expression of other genes was similar among the diet groups.
Figure 5. Hypothalamic gene expression of neuropeptide Y (NPY), pro-opiomelanocortin (POMC), brain-derived neurotrophic factor (BNDF) and serotonin receptors (5-HTR1A/2A/2C) of right hypothalamus in male offspring at 29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HFol, RV+10-fold the folate content. Gestational and pup diets denoted before and after the dash line, respectively. ab Significantly different by one-way ANOVA followed by Tukey’s post-hoc test. Values are mean ± SEM, n = 4–7/group.
DNA methylation
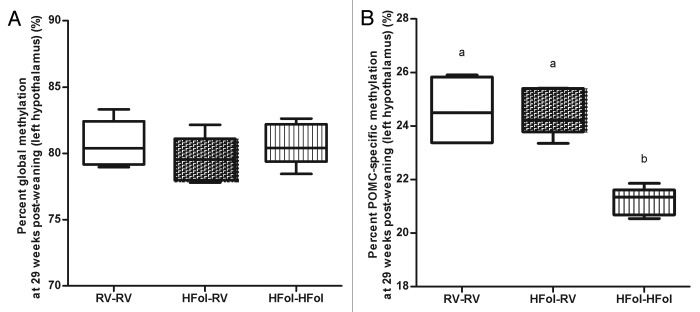
Neither the gestational nor post-weaning diet had any effect on the global methylation status in the left hypothalamus of the male offspring at 29 weeks post-weaning (Fig. 6A). However, the HFol pup diet in offspring of HFol dams lowered POMC DNA methylation (p = 0.0001) compared with the RV pup diet and those born to the RV dams (Fig. 6B). POMC-specific methylation was positively associated with glucose response to a glucose load at 10 weeks post-weaning (r = 0.7, p = 0.03) (Fig. 7).
Figure 6. DNA methylation at (A) global and (B) pro-opiomelanocortin (POMC)-specific of left hypothalamus in male offspring at 29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HFol, RV+10-fold the folate content. Gestational and pup diets denoted before and after the dash line, respectively. Global DNA methylation not significant, n = 5/group. ab POMC-specific methylation p = 0.0001 by one-way ANOVA followed by Tukey’s post-hoc test, n = 4–6/group. Values are mean ± SEM.
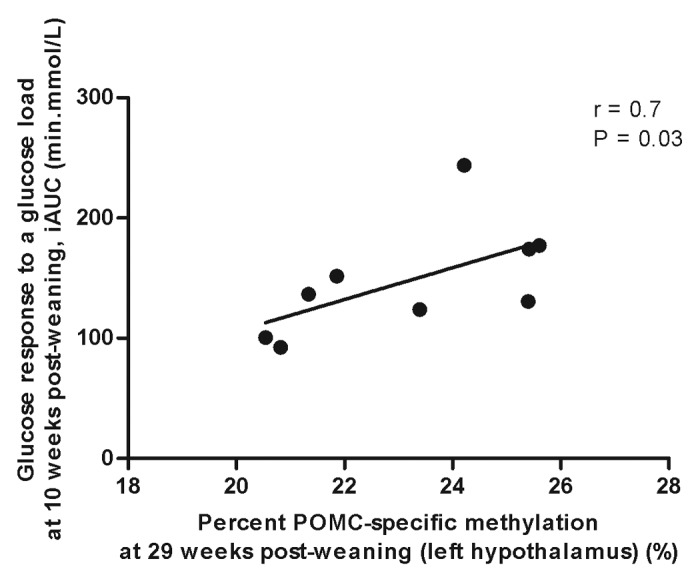
Figure 7. Correlation between pro-opiomelanocortin (POMC)-specific methylation and glucose response to a glucose load at 10 weeks post-weaning by Spearman’s correlation coefficient, n = 9.
Folate concentration
The HFol pup diet increased folate levels in the brain (p = 0.002) (Fig. 8A) and plasma (p = 0.004) (Fig. 8B) without the effect of gestational diets.
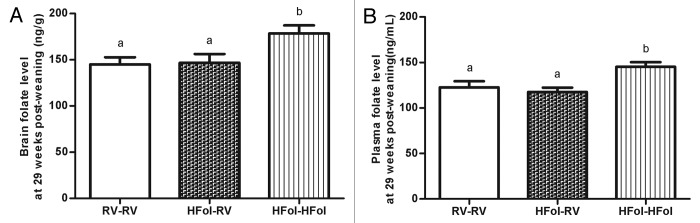
Figure 8. Folate concentrations in the (A) brain and (B) plasma of male offspring at 29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HFol, RV+10-fold the folate content. Gestational and pup diets denoted before and after the dash line, respectively. ab Brain folate: p = 0.002, n = 9–11 and plasma folate: p = 0.004, n = 13–15 by one-way ANOVA followed by Tukey’s post-hoc test. Values are mean ± SEM.
Discussion
Our findings support the hypothesis that folate alone accounts for some expression of the obesogenic phenotype in the offspring of dams fed HV diets but can be prevented by increasing the folate content of the pup diet possibly due to epigenetic alterations involving DNA methylation. We demonstrate for the first time that folate in either the gestational or post-weaning diets modifies hypothalamic feeding pathways in mature offspring. These results are novel in showing epigenetic plasticity of the hypothalamus that responds to folate consumption not only in utero but also in later life.
Folate and obesogenic phenotype
Folate alone in the gestational diet resulted in increased body weight and food intake in the offspring, suggesting that folate alone accounts for the effects of the HV diet. Because previous studies have shown that nutrients involved in methyl metabolism (folate, vitamins B6 and B12 and choline together) powerfully influence methylation,10,11 interaction of folate with other methyl vitamins may be required to exacerbate obesity. Surprisingly, the HFol pup diet lowered body weight and food intake in the offspring of the HFol dams, indicating that the post-weaning diet has a strong effect on overall phenotypic changes.
Consistent with higher body weight and food intake, the HFol gestational diet impaired glucose response to an insulin load in the offspring. However, feeding the offspring the HFol diet normalized the glucose response, thus supporting the PAR hypothesis, that matching the folate content in the gestational and pup diets protected against glucose dysregulation. The HFol pup diet has a potential to ameliorate in utero programming of the offspring for insulin resistance caused by the HFol gestational diet. Similarly after the glucose load, only the HFol pup diet reduced the glucose response preventing the gestational diet effect. The difference in glucose response detected at earlier weeks in contrast to later suggests that there may be a critical window of development at which the brain responds to vitamins to alter glucose metabolism. Importantly, it reflects overall disturbance in the feeding neurocircuitry, which may have translated into sustained food intake and body weight differences in mature offspring.
Role of folate in the hypothalamic food intake regulatory system
Overall, lower expression of appetite-suppressing neuropeptides support our hypothesis that the obesogenic phenotype in the offspring of dams fed the HV diet may be due to folate altering the hypothalamic feeding mechanisms. The HFol gestational diet lowered the expression of POMC and 5-HTR2A, both of which are known to partake in appetite suppression. These changes are consistent with our previous study reporting decreased POMC expression by the HV gestational diet.5 In contrast, unlike the HV diet, we did not find a reduction in BDNF, which is also an anorexigen, adding evidence that possibly vitamins other than folate regulate its expression.
Because we found that folate in the pup diet also modified gene expression, our data show plasticity in feeding pathways in response to vitamin content of the post-weaning diet. The HFol pup diet increased expression of POMC and 5-HTR2A, which also corresponded to similar reversal effects observed in the glucose response to an insulin load in the offspring of the HFol dams. Furthermore, we found that the folate levels in the brain and plasma were increased and maintained by the HFol pup diet, suggesting minimal metabolic adaptation occurs in catabolism of the excess folate, and that vitamins fed during post-weaning may have a more sustained impact on the offspring compared with that of the gestation. Moreover, the HFol pup diet resulted in lower 5-HTR2C expression, which was not affected by the gestational diet, perhaps because specific receptor subtypes respond to folate differently during maturation.
Folate and DNA methylation
The reduction in POMC-specific methylation in the HFol offspring weaned to the HFol diet strengthens the concept of epigenetic remodeling that responds to environmental factors to establish phenotype throughout life. Hypomethylation at the POMC promoter was driven primarily by the HFol post-weaning diet, which may explain the higher POMC expression.12 Although a different model, feeding a high-fat diet for 32 weeks post-weaning also decreased methylation of the cytosine-phosphate-guanine (CpG) near the transcription start site of the brain melanocortin-4 receptor, which is a downstream effector of POMC, accompanied by increased gene expression in the obese Berlin fat mouse inbred line.13 These results are consistent with our data suggesting that POMC and downstream effectors are epigenetically altered involving DNA methylation by nutrients possibly contributing to gene expression changes and subsequent alterations in phenotype. Our findings also add to the studies investigating the effect of folate supplementation on epigenetic changes in different tissues such as the liver and mammary tissue. Both the folate-supplemented maternal and post-weaning diets alter methylation at the promoter of liver phosphoenolpyruvate carboxykinase, which is an enzyme at the rate-limiting step in gluconeogenesis, in rat offspring at post-weaning day 54–60.14 Similarly, maternal but not post-weaning folic acid supplementation in rats reduced global DNA methylation in non-neoplastic mammary tissue at 28 weeks post-weaning.15 However, we provide the first evidence of folate effects on feeding pathways in the brain.
Our data show that DNA methylation offers a link between the vitamin diet and phenotypic alterations in the mature offspring. Higher methylation at POMC implies lower gene expression,12 thus less appetite suppression, which manifests as obesogenic phenotype indicated by higher glycemia, as it would be expected with insulin resistance. Consistent with this, we observed a positive correlation between POMC-specific methylation and glucose response to a glucose load (Fig. 7) and previous studies have associated gene-specific DNA methylation status with childhood adiposity at 9 y16 and 2-h post-oral glucose tolerance test blood glucose concentrations.17 Neonatal over-feeding resulting from reducing the number of pups per litter also resulted in hyper-methylation of the POMC promoter in the mouse hypothalamus at postnatal day 21 and the offspring had higher insulin concentrations suggesting insulin resistance.18 In our study, the HFol diet during pregnancy decreased glucose response to an insulin load in the offspring but feeding the HFol post-weaning diet reversed these effects. Because small changes in methylation together are known to determine the risk of chronic diseases,19 the association between POMC-specific methylation and glucose response to a glucose load at 10 weeks post-weaning demonstrates important implications at the phenotypic level.
Relevance to humans
Our findings may have relevance to humans as increasing amounts of vitamins, especially folate, are consumed during pregnancy. A recent Canadian Health Measures Survey (2007–2009) has shown that the red blood cell folate concentrations of 40% of female participants were above the high-concentration cut-off, 1,360 nmol/L.20 The consequences of these high levels are uncertain but may be of concern because folic acid use after 12 weeks of gestation influences human offspring repeat element and imprinted gene methylation in cord blood.21 Another study has shown that periconceptional maternal folic acid use of 400 μg per day is positively related to methylation of the insulin-like growth factor 2 gene in children between 12–18 mo of age.22 These studies highlight the need of further examination on the consequences and mechanisms by which folate programs the offspring for obesity and chronic diseases in later life.
Limitations
There are some limitations in interpreting the data. One weakness of our study is that gene expression does not necessarily indicate function. Protein expression downstream of the genes that were modified due to folate gestational and pup diets would inform functional consequences. In addition, examining the role of vitamins involved in DNA methylation, not only folate but also methyl group vitamins together, in regulating feeding mechanisms would provide better understanding of their interaction in contributing to epigenetic changes in the brain. Furthermore, we are uncertain at which point in the critical windows of development that folate induced its effect on the offspring. Although the critical windows of development are generally not well-defined, they include not only the periconception, mid- and late-gestation and early postnatal life, but also extend to post-weaning and puberty.23
Conclusion
In conclusion, the obesogenic phenotype of offspring from dams fed the HFol gestational diet can be corrected by feeding them a HFol diet perhaps due to epigenetic changes involving methylation.
Materials and Methods
Animals and diets
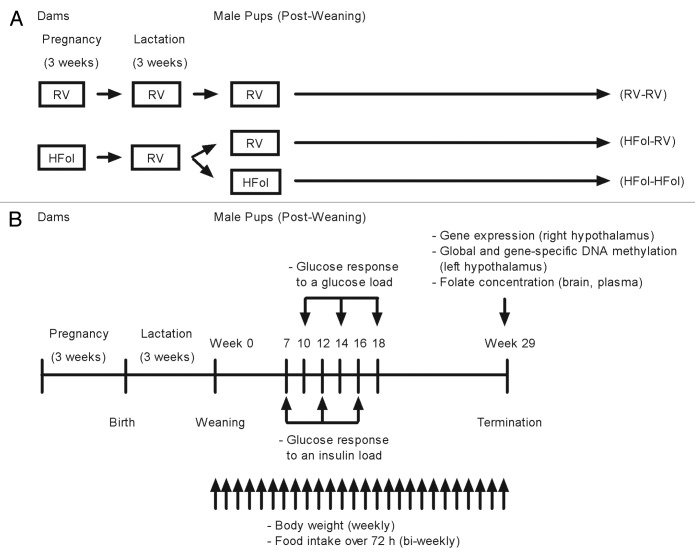
First-time, 2- to 3-d pregnant Wistar rats (Charles River) were housed individually in ventilated plastic transparent cages with bedding in a 12:12 h light-dark cycle (lights on at 0700 at 22 ± 1 °C). Food and water were provided ad libitum throughout the study period. The experimental procedure was approved by the University of Toronto Animal Care Committee. From third day of pregnancy to term, dams (n = 10 per gestational diet group) were fed the AIN-93G diet24 containing either the recommended vitamin (RV) content or RV+10-fold the folate content (HFol, high folate), both of which have the same energy density (3.8 kcal/g). The composition (in g/kg) of the RV AIN-93G diet is 529.5 cornstarch, 200 casein, 100 sucrose, 70 soybean oil, 50 cellulose, 10 vitamin mixture, 35 mineral mixture, 3 L-cystine, 2.5 choline bitartarate and 0.014 tert-butyl hydroquinone. The RV diet has 2 mg/kg of folate. In order to formulate the 10-fold HFol diet, we added 18 mg of folate to make a total of 20 mg/kg. Figure 9A shows the study design. Within 24 h of delivery, each litter was culled to 10 unsexed pups to minimize the difference in milk availability. All dams were fed the RV diet during lactation. At weaning, one male offspring from each litter was terminated whereas another male was followed for 29 weeks. The offspring from dams fed the HFol diet were weaned to RV or HFol diets. A control group was the offspring from dams fed a RV diet weaned to a RV diet.
Figure 9. Schematic sketch of the study (A) design and (B) protocol for male offspring from 0–29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HFol, RV+10-fold the folate content. Gestational and pup diets denoted before and after the dash line, respectively.
Food intake and body weight
Figure 9B illustrates the study protocol. Seventy-two-hour food intake was measured every 2 weeks from 0–29 weeks post-weaning. Body weight was measured at birth and weaning, and weight gain was calculated weekly from 0–29 weeks post-weaning.
Glucose response to an insulin or glucose load
After 10 h overnight fasting, a blood sample was withdrawn from the tail vein and baseline glucose was immediately assayed using a commercial glucometer (MediSense Precision Xtra, Abbott Laboratories). Glucose response was measured after an intraperitoneal injection of insulin (0.75 U of insulin per kg body weight) at 7, 12 and 16 weeks post-weaning or after a glucose gavage (0.375 g glucose mL−1, 5 g of glucose per kg body weight) at 10, 14 and 18 weeks post-weaning. Upon the insulin injection or glucose gavage, blood glucose concentrations were determined at 15, 30, 45 and 60 min later. The net area under the curve (netAUC) for glucose response to an insulin load and incremental area under the curve (iAUC) for glucose response to a glucose load were calculated as previously described.25
Hypothalamic gene expression
Whole brains of offspring at 29 weeks post-weaning were removed rapidly after decapitation and immediately frozen on top of dry ice, and then stored at −80 °C. The right and left sides of the hypothalamus were dissected separately on ice using the previously reported method.26 The right side of the hypothalamus was homogenized using a tissue ruptor homogenizer (Qiagen Tech). The RNA from the homogenized hypothalamus was isolated using Trizol and chloroform extraction by the manufacturer’s protocol (Invitrogen) and quantified by Nanodrop 1000. The cDNA from the extracted right hypothalamus was synthesized using the High Capacity cDNA Archive Kit (Applied Biosystems Inc.; ABI) Gene Amp PCR System 2700.
Real-time RT-PCR was performed on the ABI PRISM 7000 Sequence Detection System (SDS) using Taqman assays for the following genes (ABI): neuropeptide Y (NPY; Rn01410146_m1); pro-opiomelanocortin (POMC; Rn00595020_m1); brain-derived neurotrophic factor (BDNF; Rn02531967_s1); serotonin receptor 1A (5-HTR1A; Rn00561409_s1); serotonin receptor 2A (5-HTR2A; Rn01468302_m1); serotonin receptor 2C (5-HTR2C; Rn00562748_m1). The cycle conditions were 50 °C for 2 min, 95 °C for 10 min, 40 cycles for 95 °C for 15 sec and 60 °C for 1 min. The relative quantification method was performed using glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Rn99999916_s1), which was the endogenous housekeeping gene selected for its lowest variation from a preliminary screen. Results were expressed as fold-change by the 2−ΔΔCT method27 and analyzed using ABI DataAssist software version 3.0.
Hypothalamic global and gene-specific DNA methylation analysis
The left side of the hypothalamus was used for DNA methylation status. DNA was isolated using the standard protocol outlined by the DNeasy Blood and Tissue Kit (Qiagen Tech). Two spectrophotometric readings of the DNA samples were averaged to determine the concentration for a ratio of A260 to A280 between 1.8 and 2.0 and free of RNA and protein contamination. The global methylation status of hypothalamic genomic DNA and gene-specific methylation of the hypothalamic POMC promoter were assessed by a pyrosequencing assay of sodium bisulfate converted DNA as previously described.28 For each sample, the methylation levels of 5 CpG sites were verified and averaged. Sequencing was performed by PyroMark Q24 (Qiagen, 9001514) and long interspersed nuclear element-1 repeat element methylation was used as an indicator of global methylation levels as previously described.29Figure 10 shows the primer sequence of POMC promoter and location of 5 CpG sites.

Figure 10. Location of 5 cytosine-phosphate-guanine (CpG) sites in the pro-opiomelanocortin (POMC) gene promoter shown by the shadowed areas.
Folate concentration analysis
The left side of the remaining brain was used for folate concentration measurement. Trunk blood was collected and centrifuged to obtain plasma. Brain and plasma folate concentrations were determined by a standard microbiological microtiter 96-well plate assay using Lactobacillus casei, which demonstrates a growth response to folate.30,31
Statistical analyses
The PROC MIXED model procedure in SAS (Version 9.2, SAS Institute Inc.) was used to determine the treatment effects on 72 h food intake and weight gain with gestational diets, pup diets and time as the main factors. Variables with non-normal distribution were normalized. The means at each time point and for glucose response to a glucose or insulin load, hypothalamic gene expression, global and POMC-specific methylation status and folate concentrations were compared by one-way analysis of variance using the PROC GLM followed by a Tukey’s post-hoc test. Correlation analyses between DNA methylation status and outcome measures were performed by using Spearman’s correlation coefficient. Significant differences were reported at p < 0.05. All data are expressed as mean ± standard error of the mean (SEM).
Acknowledgments
We thank Sanaa Choufani from Rosanna Weksberg’s laboratory for technical assistance with methylation assay. This research is supported by the Canadian Institute of Health Research, Institute of Nutrition, Metabolism and Diabetes (CIHR-INMD), Reference MOP-93624. Clara E. Cho is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Postgraduate Scholarship (PGS). Diana Sanchez Hernandez and Sandra A. Reza- López are supported by the Consejo Nacional de Ciencia y Tecnologia (Mexico).
Glossary
Abbreviations:
- BDNF
brain-derived neurotrophic factor
- CpG
cytosine-phosphate-guanine
- HFol
high folate
- HV
high multivitamin
- iAUC
Incremental Area Under the Curve
- netAUC
Net Area Under the Curve
- NPY
neuropeptide Y
- PAR
Predictive Adaptive Response
- POMC
pro-opiomelanocortin
- RV
recommended vitamin
- SEM
standard error of the mean
- 5-HTR1A
serotonin receptor 1A
- 5-HTR2A
serotonin receptor 2A
- 5-HTR2C
serotonin receptor 2C
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/24948
References
- 1.Wilson RD, Johnson JA, Wyatt P, Allen V, Gagnon A, Langlois S, et al. Genetics Committee of the Society of Obstetricians and Gynaecologists of Canada and The Motherrisk Program Pre-conceptional vitamin/folic acid supplementation 2007: the use of folic acid in combination with a multivitamin supplement for the prevention of neural tube defects and other congenital anomalies. J Obstet Gynaecol Can. 2007;29:1003–26. doi: 10.1016/S1701-2163(16)32685-8. [DOI] [PubMed] [Google Scholar]
- 2.De Wals P, Tairou F, Van Allen MI, Uh SH, Lowry RB, Sibbald B, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med. 2007;357:135–42. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 3.Szeto IM, Aziz A, Das PJ, Taha AY, Okubo N, Reza-Lopez S, et al. High multivitamin intake by Wistar rats during pregnancy results in increased food intake and components of the metabolic syndrome in male offspring. Am J Physiol Regul Integr Comp Physiol. 2008;295:R575–82. doi: 10.1152/ajpregu.90354.2008. [DOI] [PubMed] [Google Scholar]
- 4.Szeto IM, Das PJ, Aziz A, Anderson GH. Multivitamin supplementation of Wistar rats during pregnancy accelerates the development of obesity in offspring fed an obesogenic diet. Int J Obes (Lond) 2009;33:364–72. doi: 10.1038/ijo.2008.281. [DOI] [PubMed] [Google Scholar]
- 5.Cho CE, Sanchez-Hernandez D, Reza-Lopez SA, Huot PS, Kim YI, Anderson GH. Obesogenic phenotype of offspring of dams fed a high multivitamin diet is prevented by a post-weaning high multivitamin or high folate diet. Int J Obes. 2013 doi: 10.1038/ijo.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135:2703–9. doi: 10.1093/jn/135.11.2703. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527–33. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 10.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–57. [PubMed] [Google Scholar]
- 11.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroda A, Rauch TA, Todorov I, Ku HT, Al-Abdullah IH, Kandeel F, et al. Insulin gene expression is regulated by DNA methylation. PLoS One. 2009;4:e6953. doi: 10.1371/journal.pone.0006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widiker S, Karst S, Wagener A, Brockmann GA. High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet. 2010;51:193–7. doi: 10.1007/BF03195727. [DOI] [PubMed] [Google Scholar]
- 14.Hoile SP, Lillycrop KA, Grenfell LR, Hanson MA, Burdge GC. Increasing the folic acid content of maternal or post-weaning diets induces differential changes in phosphoenolpyruvate carboxykinase mRNA expression and promoter methylation in rats. Br J Nutr. 2012;108:852–7. doi: 10.1017/S0007114511006155. [DOI] [PubMed] [Google Scholar]
- 15.Ly A, Lee H, Chen J, Sie KK, Renlund R, Medline A, et al. Effect of maternal and postweaning folic acid supplementation on mammary tumor risk in the offspring. Cancer Res. 2011;71:988–97. doi: 10.1158/0008-5472.CAN-10-2379. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C, et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes. 2011;60:1528–34. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houde A, Hivert M, Bouchard L. Fetal epigenetic programming of adipokines. Adipocyte. 2013;2:41–6. doi: 10.4161/adip.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plagemann A, Harder T, Brunn M, Harder A, Roepke K, Wittrock-Staar M, et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587:4963–76. doi: 10.1113/jphysiol.2009.176156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heijmans BT, Tobi EW, Lumey LH, Slagboom PE. The epigenome: archive of the prenatal environment. Epigenetics. 2009;4:526–31. doi: 10.4161/epi.4.8.10265. [DOI] [PubMed] [Google Scholar]
- 20.Colapinto CK, O’Connor DL, Tremblay MS. Folate status of the population in the Canadian Health Measures Survey. CMAJ. 2011;183:E100–6. doi: 10.1503/cmaj.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haggarty P, Hoad G, Campbell DM, Horgan GW, Piyathilake C, McNeill G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am J Clin Nutr. 2013;97:94–9. doi: 10.3945/ajcn.112.042572. [DOI] [PubMed] [Google Scholar]
- 22.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, et al. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4:e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955–9. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 24.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127(Suppl):838S–41S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 25.Wolever TM. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br J Nutr. 2004;91:295–301. doi: 10.1079/BJN20031054. [DOI] [PubMed] [Google Scholar]
- 26.Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–69. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Byun HM, Wong HL, Birnstein EA, Wolff EM, Liang G, Yang AS. Examination of IGF2 and H19 loss of imprinting in bladder cancer. Cancer Res. 2007;67:10753–8. doi: 10.1158/0008-5472.CAN-07-0329. [DOI] [PubMed] [Google Scholar]
- 29.Kiss NB, Kogner P, Johnsen JI, Martinsson T, Larsson C, Geli J. Quantitative global and gene-specific promoter methylation in relation to biological properties of neuroblastomas. BMC Med Genet. 2012;13:83. doi: 10.1186/1471-2350-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. doi: 10.1016/S0076-6879(97)81007-5. [DOI] [PubMed] [Google Scholar]
- 31.Sie KK, Chen J, Sohn KJ, Croxford R, Thompson LU, Kim YI. Folic acid supplementation provided in utero and during lactation reduces the number of terminal end buds of the developing mammary glands in the offspring. Cancer Lett. 2009;280:72–7. doi: 10.1016/j.canlet.2009.02.004. [DOI] [PubMed] [Google Scholar]