Summary
Acinetobacter baumannii is an opportunistic pathogen that has emerged as a prevalent source of nosocomial infections, most frequently causing ventilator-associated pneumonia. The emergence of pan-drug resistant strains magnifies the problem by reducing viable treatment options and effectively increasing the mortality rate associated with Acinetobacter infections. In light of this rising threat, research on A. baumannii epidemiology, antibiotic resistance, and pathogenesis is accelerating. The recent development of both in vitro and in vivo models has enabled studies probing the host-Acinetobacter interface. Bacterial genetic screens and comparative genomic studies have led to the identification of several A. baumannii virulence factors. Additionally, investigations into host defense mechanisms using animal models or cell culture have provided insight into the innate immune response to infection. This review highlights some of the key attributes of A. baumannii virulence with an emphasis on bacterial interactions with the innate immune system.
Introduction
Acinetobacter baumannii is a Gram-negative, aerobic coccobacillus that belongs to a genus comprised of both environmental species and opportunistic pathogens. Interestingly, A. baumannii is an opportunistic pathogen that unlike others in the genus is not ubiquitous in nature and exhibits a low human carrier rate. This resilient organismis notorious for its ability to withstand desiccation and disinfection and to persist in the hospital environment. Contributing to the fortitude of A. baumannii is its propensity to form biofilms on a number of abiotic surfaces, including catheters, ventilators, and other medical devices, enhancing bacterial transmission (Vidal et al., 1996; Tomaras et al., 2003; Pour et al., 2011). This organism is a particular challenge in intensive care units where it is responsible for a diverse range of infections including ventilator-associated pneumonia, skin and wound infections, urinary tract infections, and bacteremia (Doyle et al., 2011). In addition to ventilator-associated pneumonia, there have also been reports of severe community-acquired pneumonia caused by A. baumannii (Falagas et al., 2007). The escalating number of infections involving multi-drug and pan-drug resistant strains necessitates the development of new treatment options against this emerging threat. Considerable progress has been made towards understanding the epidemiology, mechanisms of antibiotic resistance, and persistence of A. baumannii in the hospital environment, and there are several recent reviews on these topics (Peleg et al., 2008a; Garnacho-Montero et al., 2010; Gordon et al., 2010; Durante-Mangoni et al., 2011). In contrast, much less is known regarding the pathogenesis of A. baumannii disease. In fact, we are only beginning to uncover the immune pathways that are critical to host defense towards A. baumannii, and a small number of virulence factors have been identified in this organism. This review summarizes the current understanding of the pathogenesis of A. baumannii infections with an emphasis on the innate immune response to this pathogen.
A. baumannii elicits a pro-inflammatory immune response
Due to vigilant monitoring by immune cells, penetration of A. baumannii into the vertebrate host does not go undetected, and recognition by sentinel receptors triggers a rapid immune response. Bacterial pathogen-associated molecular patterns (PAMPs) are detected by innate immune receptors on host cells. As a result, intracellular signaling pathways promote transcription, processing, and secretion of inflammatory mediators. Although there are multiple inflammatory signaling cascades leading to cytokine and chemokine secretion, A. baumannii PAMPs are known to activate the NF-κB and MAPK pathways (March et al., 2010). This induces release of numerous chemokines, including macrophage inflammatory protein 2 (MIP-2), monocyte chemoattractant protein (MCP-1), and keratinocyte-derived chemokine (KC)/IL-8, and pro-inflammatory cytokines such as TNF-β, IL-1β, and IL-6 (Knapp et al., 2006; van Faassen et al., 2007). Bearing in mind the complexity that comprises the immune response to a given pathogen, the range of pro-inflammatory mediators and pathways activated during A. baumannii infection certainly extends beyond those listed here.
Like many Gram-negative bacteria, the LPS of A. baumannii is highly immunostimulatory (Garcia et al., 1999). TLR4 and its co-receptor CD14 recognize A. baumannii LPS, leading to NF-κB activation, secretion of MIP-2 and KC/IL-8, and subsequent neutrophil recruitment (Knapp et al., 2006; Erridge et al., 2007). Of the three components of LPS (lipid A, core polysaccharide, and O-antigen), lipid A is the main immune-activating portion of the molecule. Remarkably, several colistin-resistant mutants of A. baumannii have been described with mutations in lipid A biosynthetic genes that eliminate LPS expression and increase membrane permeability (Moffatt et al., 2010). The ability of A. baumannii to permit loss of LPS without a concomitant loss of viability is a surprising feature of this organism. Finally, the loss of the glycosyltransferase lpsB truncates LPS at the core polysaccharide and reveals that full-length LPS is necessary for serum resistance and survival in vivo, though immunostimulatory properties of this truncated LPS have not been explored (Luke et al., 2010).
The inflammatory response to A. baumannii does not appear to be exclusively dependent on TLR4. TLR2 is also involved, although its relative contribution is less understood. TLR2-deficient mice exhibit accelerated neutrophil influx, improved clearance of A. baumannii, and reduced pro-inflammatory responses (Knapp et al., 2006). This suggests that TLR2 signaling is detrimental to the host in the context of A. baumannii infection and may participate in anti-inflammatory activity. Others have shown in similar models that TLR2 activation is pro-inflammatory, up-regulating NF-κB and secretion of IL-8 in response to A. baumannii infection (Erridge et al., 2007; March et al., 2010). Clearly, further research is needed to elucidate the contribution of TLR2 to the pathogenesis of A. baumannii infection.
The armamentarium of recruited neutrophils controls A. baumannii infection.
A. baumannii-induced activation of the innate immune response stimulates chemokine secretion and subsequent recruitment of immune cells to the site of infection in both pneumonia and septicemia models. Neutrophils are the largest population of recruited cells and are required for controlling infection; however, macrophages and natural killer (NK) cells have also been detected following A. baumannii challenge (van Faassen et al., 2007; Breslow et al., 2011; Tsuchiya et al., 2011). NK cells participate in neutrophil recruitment through increased expression of KC/IL-8 (Tsuchiya et al., 2011). Lymphocytes and granulocytes produce a number of antimicrobial factors in response to infection, including defensins, cathelicidins, reactive oxygen species (ROS), and reactive nitrogen species (RNS). ROS and the related myeloperoxidase as well as β-defensin-2 have been assigned roles in A. baumannii killing, with a minor contribution by RNS (Knapp et al., 2006; Qiu et al., 2009; March et al., 2010). Undoubtedly, there are additional immune cell effectors and antimicrobial peptides in the neutrophil arsenal that are relevant to A. baumannii infection.
Host-Acinetobacter interactions shape the composition of the immune response
The immune response is principally dependent on how the host interacts with a given pathogen. More specifically, the particular immune receptors that detect antigens or PAMPs are dictated by the host cell type and the subcellular location of these sentinel proteins. A. baumannii is a versatile pathogen that can adhere to and invade numerous cell types, yet different cell types display varying degrees of susceptibility to invasion (Choi et al., 2008c). Diverse strains of A. baumannii also have distinct capacities for cell adherence and invasion (Lee et al., 2006; de Breij et al., 2010; Eijkelkamp et al., 2011b). A. baumannii attaches to bronchial epithelial cells by means of short, fimbrial-like protrusions on the bacterial cell surface (Lee et al., 2008). Following attachment, A. baumannii can invade epithelial cells in a microfilament- and microtubule-dependent, zipper-like mechanism (Choi et al., 2008c). This interaction leads to host cell cytotoxicity. Specifically, during infection host epithelial cells up-regulate caspase-3, -8, -9, and poly[ADP-ribose] polymerase (PARP) that correlate with secretion of cytochrome c and apoptosis inducing factor (AIF) from the mitochondria (Choi et al., 2005). The stimuli and signaling pathways implicated in cell death are not established; however, they involve imbalanced calcium homeostasis, pro-inflammatory cytokines, and oxidative stress (Smani et al., 2011). Several significant questions regarding the lifecycle of A. baumannii remain to be answered. For instance, understanding the functional relevance of A. baumannii invasion and the intracellular trafficking patterns is crucial, as this can profoundly shape the host cell responses. In addition, the mechanisms whereby A. baumannii modulates virulence factor expression to adapt to the host environment are not clear.
The balance between anti- and pro-inflammatory responses affects pathogenesis
Although A. baumannii stimulates the pro-inflammatory immune response, it induces a weaker response than the less-pathogenic A. junii (de Breij et al., 2010). This phenomenon is extended to individual strains and clinical isolates of A. baumannii that differ in virulence. More specifically, highly virulent strains induce more severe lung pathology and lower levels of anti-inflammatory cytokines without exhibiting increased bacterial organ burdens or dissemination (de Breij et al., 2012). Therefore, there is a direct correlation between virulence of a given strain and the strength of the pro-inflammatory response. This observation also highlights the need to balance the pro- and anti-inflammatory responses to a pathogen such that there is sufficient inflammation to eradicate the invader while not severely injuring the host.
Relatedly, most patients with A. baumannii infection are immune-compromised, often in a heightened inflammatory state. Postsurgical and trauma patients have a pre-existent acute phase response characterized by the presence of proteins such as C-reactive protein, serum amyloid A (SAA), and serum amyloid P and concurrent changes to the inflammatory response. In an acute phase response model, A. baumannii infection results in decreased pro-inflammatory cytokine secretion and decreased neutrophil recruitment to the lungs due at least in part to SAA (Renckens et al., 2006). Additionally, in an allergic asthma model, A. baumannii infection suppresses allergic-like responses, most notably a decrease in eosinophil influx into the lung and reduction of Th2 cytokines (Qiu et al., 2011). These studies indicate that the pre-existing immunological environment can impact the host response to A. baumannii and ultimately the outcome of infection.
Complement defenses in the serum are circumvented by A. baumannii
Complement is a major bactericidal component in serum that limits microbial dissemination. Typically, one of three pathways is activated leading to deposition of complement factors on the surface of bacteria and consequent bacterial lysis or opsonin-mediated phagocytosis. The alternative complement pathway is responsible for A. baumannii killing in human serum; however, clinically relevant strains can be resistant to complement activity. There is considerable debate over the mechanism behind the serum resistance of A. baumannii. Proposed models include bacterial-mediated inactivation of the alternative complement pathway inhibitor Factor H (Kim et al., 2009), A. baumannii release of LPS (Garcia et al., 2000), and the modification of peptidoglycan by the penicillin-binding protein PBP-7/8 (Russo et al., 2009). Furthermore, the presence of surface polysaccharides or capsule protects Gram-negative bacteria from host antimicrobials in serum, and several strains of A. baumannii produce a polysaccharide capsule (Russo et al., 2010; Fregolino et al., 2011). To date, two genes have been associated with capsule production, ptk and epsA, and both are required for serum resistance (Russo et al., 2010).
Nutritional immunity is countered by A. baumannii metal acquisition systems
An archetypal example of the host-microbe interface during infection is the struggle for essential nutrient metals. The sequestration of these vital nutrients from invading pathogens is a principal component of host defense against all microbial invaders. Bacteria can tolerate the nutrient-limiting environment by altering metabolic pathways; however, they also directly counter this affront by utilizing diverse nutrient and metal acquisition systems. Bacterial acquisition of non-iron metals is a burgeoning field, but for A. baumannii, the little that is known about metal acquisition focuses on iron. In iron-limiting conditions, the canonical iron-sensing repressor Fur releases transcription of iron uptake genes in A. baumannii (Daniel et al., 1999). Iron-limiting conditions lead to expression changes not only for iron acquisition genes, but also for genes involved in various processes such as respiration and motility (Eijkelkamp et al., 2011a; Nwugo et al., 2011). The best characterized iron scavenging molecule in A. baumannii is the siderophore acinetobactin, which steals iron from transferrin and lactoferrin and is essential for replication in the host (Yamamoto et al., 1994; Gaddy et al., 2012). The systems involved in acinetobactin synthesis, secretion, and import have all been described (Dorsey et al., 2004; Mihara et al., 2004; Zimbler et al., 2009). In addition to siderophore-mediated iron acquisition, putative heme-uptake and ferrous iron uptake systems have been identified in A. baumannii (Zimbler et al., 2009; Antunes et al., 2011). Interestingly, distinct clinical strains of A. baumannii vary in their expression of many iron acquisition systems including the genes involved in acinetobactin production (Echenique et al., 1992; Actis et al., 1993; Yamamoto et al., 1994). While securing iron via systems described above is critical for A. baumannii virulence, A. baumannii likely exploits additional strategies to acquire non-iron metals and further circumvent nutritional immunity.
OmpA contributes to multiple aspects of pathogenesis
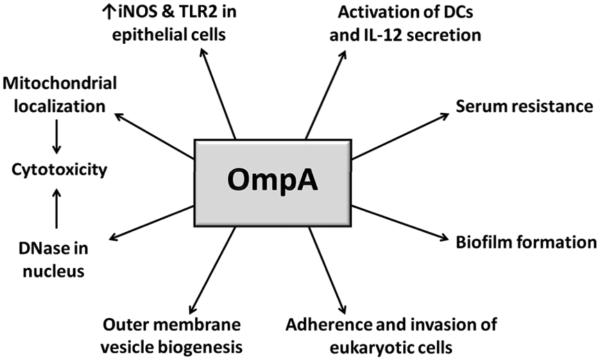
OmpA or Omp38 is a trimeric outer membrane porin involved in solute transport; however, it is also associated with several aspects of A. baumannii virulence. OmpA can be secreted via outer membrane vesicles (OMVs) and contributes to the biogenesis of such vesicles (Jin et al., 2011; Moon et al., 2012). Exposure to these OMVs or purified OmpA induces host cell death by apoptosis. OmpA-mediated apoptosis is attributed to its localization and presumed activity in the mitochondria or DNase activity in the nucleus (Choi et al., 2005; Choi et al., 2008a; Choi et al., 2008b; Lee et al., 2010), although no surface receptors or host protein targets in the nucleus or mitochondria have been identified. Finally, there is evidence that other bacterial proteins may independently contribute to induction of apoptosis in host cells (Gaddy et al., 2009).
OmpA is also immunomodulatory. Although no changes in pro-inflammatory cytokine or chemokine secretion by OmpA-treated cells have been observed, human laryngeal cells up-regulate nitric oxide synthase (iNOS) as well as TLR2 in response to OmpA (Kim et al., 2008). The cytotoxic effects of oxidative stress also provide another connection of OmpA to cell death. At sublethal concentrations, OmpA activates dendritic cells through TLR2 and both MAPK and NF-κB pathways, which results in stimulation of CD4+ T cells towards a Th1 response (Lee et al., 2007). In addition to its role in cell death and immune stimulation, OmpA is required for eukaryotic cell adherence and invasion, and partially contributes to biofilm formation and serum resistance (Choi et al., 2008c; Gaddy et al., 2009; Kim et al., 2009). Figure 1 summarizes the numerous roles attributed to OmpA as an A. baumannii virulence factor. While the possibility exists that OmpA is multifunctional, the number of distinct processes in which OmpA is involved suggests that some of these ascribed effects are indirect.
Figure 1.
Outline of the multiple contributions of A. baumannii OmpA to pathogenesis.
Multiple virulence determinants permit A. baumannii to flourish in the host
In addition to numerous proteins involved in antimicrobial resistance, other A. baumannii proteins have been described that enable A. baumannii to persist in the host. Sequencing and bioinformatic analyses of the genomes of several A. baumannii strains have led to the identification of other putative virulence factors (Fournier et al., 2006; Smith et al., 2007; Adams et al., 2008; Iacono et al., 2008; Vallenet et al., 2008). In several cases, functional expression of these potential virulence determinants has not been confirmed or expression is not universal among strains and clinical isolates. This latter aspect underscores the genomic plasticity observed among Acinetobacter species and strains of A. baumannii. Comparison of A. baumannii ATCC 17978 and A. baylyi ADP1 has revealed at least 28 putative pathogenicity islands that encode antibiotic resistance determinants and potential virulence genes (Smith et al., 2007). The field has since acquired the genome sequences for numerous strains of A. baumannii. Comparative studies of these genomes have identified frequent genetic differences among strains that highlight the pronounced capacity for horizontal gene acquisition and genome rearrangement that likely account for the variation in pathogenicity (Imperi et al., 2011). In this regard, genomic analyses suggest that A. baumannii encodes groups of proteins with homology to type IV and type VI secretion systems, which are often associated with virulence in other bacterial pathogens (Smith et al., 2007; Henry et al., 2011). Furthermore, in response to DNA damage and oxidative stress experienced by A. baumannii in the host, the DNA repair protein RecA is required for bacterial survival (Aranda et al., 2011). Finally, a phospholipase D protein, a phospholipase C protein, and a sensor kinase GacS are necessary for full virulence in animal models (Peleg et al., 2008b; Camarena et al., 2010; Jacobs et al., 2010).
An important aspect of pathogenesis is transmission to a host, which is enhanced through bacterial biofilm formation on abiotic surfaces such as catheters and ventilators. Several factors involved in the different phases of biofilm formation have been identified in A. baumannii. First, Type IV pili have been implicated in motility, which would allow for spread to new surfaces (Eijkelkamp et al., 2011b). The CsuA/BABCDE chaperone-usher pili assembly system is required for adherence to abiotic surfaces, though not for adherence to eukaryotic cells (Tomaras et al., 2003; de Breij et al., 2009). Following adherence, the production of the biofilm extracellular matrix is dependent on synthesis of the polysaccharide poly-β-(1,6)-N-acetylglucosamine (PNAG) (Choi et al., 2009). The outer membrane protein Bap is necessary for biofilm maturation and also for adherence to eukaryotic cells (Loehfelm et al., 2008; Brossard et al., 2011). Regulation of biofilm formation and motility involves the regulatory elements BfmSR and an N-acyl-homo-serine lactone (AHL) signaling molecule (Niu et al., 2008; Tomaras et al., 2008; Clemmer et al., 2011; Eijkelkamp et al., 2011b). Although all of these proteins may not be essential within the host, biofilm formation by A. baumannii alters expression of proteins involved in numerous activities, including those involved in other aspects of A. baumannii virulence (Shin et al., 2009; Cabral et al., 2011; Marti et al., 2011). This latter point emphasizes the importance of motility and biofilm formation to virulence.
Conclusions and future directions
The increasing threat of A. baumannii infections in hospitals combined with the decreasing capacity to effectively treat antibiotic resistant strains has fueled A. baumannii research. Significant strides have been made towards understanding its antibiotic resistance and its ability to survive in the hospital environment. On the other hand, there is a paucity of information about host-Acinetobacter interactions and the mechanisms by which they impact pathogenesis. Research in this area should be facilitated through the employment of the established pneumonia and sepsis models as well as the recently developed rat soft tissue infection model (Russo et al., 2008). Several other models that have been useful in the identification of A. baumannii virulence determinants include the amoebae Dictyostelium discoideum (Smith et al., 2007), the nematode Caenorhabditis elegans (Smith et al., 2004), and larvae of the insect Galleria mellonella (Peleg et al., 2009). These models have been useful to investigate the general host response; however, studies aiming to delineate innate immune signaling pathways have been limited. Furthermore, the environment of the immune-compromised host and the contribution of the anti-inflammatory response to controlling damaging inflammation have important implications to pathogenesis that we are only beginning to appreciate. Immunomodulatory therapeutics in patients with compromised immune status may be a beneficial strategy for treatment of Acinetobacter infections. However, the development of such therapies is dependent on a more complete understanding of the host immune response to A. baumannii. Antigen presentation to the host immune system depends on the intracellular or extracellular localization of A. baumannii within the infected tissue. Beyond the ability to adhere to and enter cells, virtually nothing is known about the intracellular lifecycle and trafficking of A. baumannii or the bacterial and host factors mediating these processes. For many bacterial pathogens, the virulence factor repertoire includes proteins that directly modulate the host immune response. Other than OmpA, bacterial proteins that directly manipulate the host have yet to be identified. Nevertheless, the heterogeneity of the survival strategies and virulence factors exhibited by A. baumannii strains highlights the adaptability of the organism to a variety of host assaults. Figure 2 illustrates both the host and bacterial defense tactics employed at the frontline of the host-pathogen battlefield existing during an A. baumannii infection. The recent development of bacterial genetic tools and animal infection models should help address the deficiencies in our understanding of Acinetobacter virulence. Knowledge gained from such research will facilitate the identification of drug targets and guide the design of effective therapeutics to target this emerging threat.
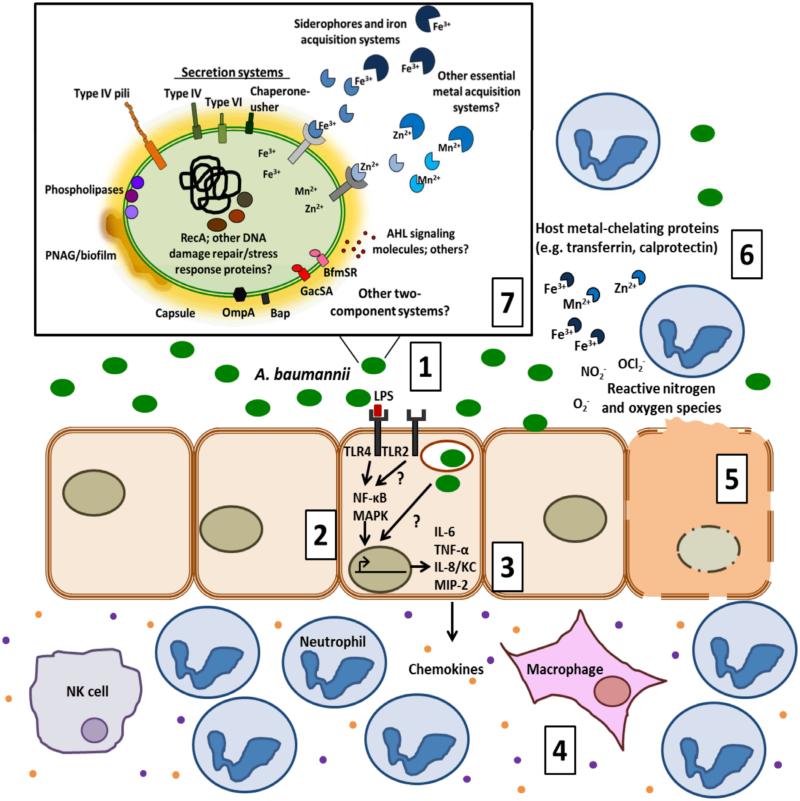
Figure 2. Summary of the dynamic interplay between A. baumannii and the host.
(1) A. baumannii can adhere and invade host cells, leading to stimulation of the pro-inflammatory immune response. (2) The inflammatory response is initiated by TLR4 recognition of LPS which then activates MAPK and NF-κB pathways. TLR2 is also reported to detect A. baumannii. (3) Activation of these receptor proteins leads to subsequent transcription and secretion of pro-inflammatory mediators such as cytokines IL-6 and TNF-β and chemokines KC/IL-8 and MIP-2. (4) These chemokines recruit granulocytes and lymphocytes that are required for controlling infection. (5) Following A. baumannii infection host cells also undergo apoptosis. (6) Other host defenses include nutritional immunity, ROS/RNS production, and antimicrobial peptides. (7) In response to the host environment, A. baumannii expresses several virulence factors implicated in pathogenesis, which are displayed in the inset of the figure. The illustration depicts those proteins and molecules that are functionally characterized and those that are predicted to be expressed. The question marks designate areas in which there are significant gaps in our knowledge.
Acknowledgements
The authors would like to thank the members of the Skaar lab for their thoughtful editorial comments on the manuscript. Work in the Skaar lab is supported by AI091771, AI069233, and AF073843. EPS is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases.
References
- Actis LA, Tolmasky ME, Crosa LM, Crosa JH. Effect of iron-limiting conditions on growth of clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 1993;31:2812–2815. doi: 10.1128/jcm.31.10.2812-2815.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MD, Goglin K, Molyneaux N, Hujer KM, Lavender H, Jamison JJ, et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol. 2008;190:8053–8064. doi: 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes LC, Imperi F, Towner KJ, Visca P. Genome-assisted identification of putative iron-utilization genes in Acinetobacter baumannii and their distribution among a genotypically diverse collection of clinical isolates. Res Microbiol. 2011;162:279–284. doi: 10.1016/j.resmic.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Aranda J, Bardina C, Beceiro A, Rumbo S, Cabral MP, Barbe J, Bou G. Acinetobacter baumannii RecA protein in repair of DNA damage, antimicrobial resistance, general stress response, and virulence. J Bacteriol. 2011;193:3740–3747. doi: 10.1128/JB.00389-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow JM, Meissler JJ, Jr., Hartzell RR, Spence PB, Truant A, Gaughan J, Eisenstein TK. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance. Infect Immun. 2011;79:3317–3327. doi: 10.1128/IAI.00069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossard KA, Campagnari AA. The Acinetobacter baumannii biofilm associated protein (Bap) plays a role in adherence to human epithelial cells. Infect Immun. 2012;80:228–233. doi: 10.1128/IAI.05913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral MP, Soares NC, Aranda J, Parreira JR, Rumbo C, Poza M, et al. Proteomic and functional analyses reveal a unique lifestyle for Acinetobacter baumannii biofilms and a key role for histidine metabolism. J Proteome Res. 2011;10:3399–3417. doi: 10.1021/pr101299j. [DOI] [PubMed] [Google Scholar]
- Camarena L, Bruno V, Euskirchen G, Poggio S, Snyder M. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog. 2010;6:e1000834. doi: 10.1371/journal.ppat.1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AH, Slamti L, Avci FY, Pier GB, Maira-Litran T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-β-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191:5953–5963. doi: 10.1128/JB.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Hyun SH, Kim J, Lee YC, Seol SY, Cho DT, Lee JC. Nuclear translocation and DNAse I-like enzymatic activity of Acinetobacter baumannii outer membrane protein A. FEMS Microbiol Lett. 2008a;288:62–67. doi: 10.1111/j.1574-6968.2008.01323.x. [DOI] [PubMed] [Google Scholar]
- Choi CH, Hyun SH, Lee JY, Lee JS, Lee YS, Kim SA, et al. Acinetobacter baumannii outer membrane protein A targets the nucleus and induces cytotoxicity. Cell Microbiol. 2008b;10:309–319. doi: 10.1111/j.1462-5822.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- Choi CH, Lee EY, Lee YC, Park TI, Kim HJ, Hyun SH, et al. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7:1127–1138. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- Choi CH, Lee JS, Lee YC, Park TI, Lee JC. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol. 2008c;8:216. doi: 10.1186/1471-2180-8-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmer KM, Bonomo RA, Rather PN. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology. 2011;157:2534–2544. doi: 10.1099/mic.0.049791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C, Haentjens S, Bissinger MC, Courcol RJ. Characterization of the Acinetobacter baumannii Fur regulator: cloning and sequencing of the fur homolog gene. FEMS Microbiol Lett. 1999;170:199–209. doi: 10.1111/j.1574-6968.1999.tb13375.x. [DOI] [PubMed] [Google Scholar]
- de Breij A, Dijkshoorn L, Lagendijk E, van der Meer J, Koster A, Bloemberg G, et al. Do biofilm formation and interactions with human cells explain the clinical success of Acinetobacter baumannii? PLoS One. 2010;5:e10732. doi: 10.1371/journal.pone.0010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Breij A, Eveillard M, Dijkshoorn L, van den Broek PJ, Nibbering PH, Joly-Guillou ML. Differences in Acinetobacter baumannii strains and host innate immune response determine morbidity and mortality in experimental pneumonia. PLoS One. 2012;7:e30673. doi: 10.1371/journal.pone.0030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Breij A, Gaddy J, van der Meer J, Koning R, Koster A, van den Broek P, et al. CsuA/BABCDE-dependent pili are not involved in the adherence of Acinetobacter baumannii ATCC19606(T) to human airway epithelial cells and their inflammatory response. Res Microbiol. 2009;160:213–218. doi: 10.1016/j.resmic.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Dorsey CW, Tomaras AP, Connerly PL, Tolmasky ME, Crosa JH, Actis LA. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology. 2004;150:3657–3667. doi: 10.1099/mic.0.27371-0. [DOI] [PubMed] [Google Scholar]
- Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ. Epidemiology of infections acquired in intensive care units. Semin Respir Crit Care Med. 2011;32:115–138. doi: 10.1055/s-0031-1275525. [DOI] [PubMed] [Google Scholar]
- Durante-Mangoni E, Zarrilli R. Global spread of drug-resistant Acinetobacter baumannii: molecular epidemiology and management of antimicrobial resistance. Future Microbiol. 2011;6:407–422. doi: 10.2217/fmb.11.23. [DOI] [PubMed] [Google Scholar]
- Echenique JR, Arienti H, Tolmasky ME, Read RR, Staneloni RJ, Crosa JH, Actis LA. Characterization of a high-affinity iron transport system in Acinetobacter baumannii. J Bacteriol. 1992;174:7670–7679. doi: 10.1128/jb.174.23.7670-7679.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp BA, Hassan KA, Paulsen IT, Brown MH. Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genomics. 2011a;12:126. doi: 10.1186/1471-2164-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp BA, Stroeher UH, Hassan KA, Papadimitrious MS, Paulsen IT, Brown MH, Lo R. Adherence and motility characteristics of clinical Acinetobacter baumannii isolates. FEMS Microbiol Lett. 2011b;323:44–51. doi: 10.1111/j.1574-6968.2011.02362.x. [DOI] [PubMed] [Google Scholar]
- Erridge C, Moncayo-Nieto OL, Morgan R, Young M, Poxton IR. Acinetobacter baumannii lipopolysaccharides are potent stimulators of human monocyte activation via Toll-like receptor 4 signalling. J Med Microbiol. 2007;56:165–171. doi: 10.1099/jmm.0.46823-0. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Karveli EA, Kelesidis I, Kelesidis T. Community-acquired Acinetobacter infections. Eur J Clin Microbiol Infect Dis. 2007;26:857–868. doi: 10.1007/s10096-007-0365-6. [DOI] [PubMed] [Google Scholar]
- Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregolino E, Gargiulo V, Lanzetta R, Parrilli M, Holst O, Castro CD. Identification and structural determination of the capsular polysaccharides from two Acinetobacter baumannii clinical isolates, MG1 and SMAL. Carbohydr Res. 2011;346:973–977. doi: 10.1016/j.carres.2011.03.024. [DOI] [PubMed] [Google Scholar]
- Gaddy JA, Arivett BA, McConnell MJ, Lopez-Rojas R, Pachon J, Actis LA. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun. 2012;80:1015–1024. doi: 10.1128/IAI.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy JA, Tomaras AP, Actis LA. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun. 2009;77:3150–3160. doi: 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A, Salgado F, Solar H, Gonzalez CL, Zemelman R, Onate A. Some immunological properties of lipopolysaccharide from Acinetobacter baumannii. J Med Microbiol. 1999;48:479–483. doi: 10.1099/00222615-48-5-479. [DOI] [PubMed] [Google Scholar]
- Garcia A, Solar H, Gonzalez C, Zemelman R. Effect of EDTA on the resistance of clinical isolates of Acinetobacter baumannii to the bactericidal activity of normal human serum. J Med Microbiol. 2000;49:1047–1050. doi: 10.1099/0022-1317-49-11-1047. [DOI] [PubMed] [Google Scholar]
- Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis. 2010;23:332–339. doi: 10.1097/QCO.0b013e32833ae38b. [DOI] [PubMed] [Google Scholar]
- Gordon NC, Wareham DW. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int J Antimicrob Agents. 2010;35:219–226. doi: 10.1016/j.ijantimicag.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH, et al. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids and poly-β-1,6-N-acetylglucosamine. Antimicrob Agents Chemother. 2012;56:59–69. doi: 10.1128/AAC.05191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono M, Villa L, Fortini D, Bordoni R, Imperi F, Bonnal RJ, et al. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob Agents Chemother. 2008;52:2616–2625. doi: 10.1128/AAC.01643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperi F, Antunes LC, Blom J, Villa L, Iacono M, Visca P, Carattoli A. The genomics of Acinetobacter baumannii: insights into genome plasticity, antimicrobial resistance and pathogenicity. IUBMB Life. 2011;63:1068–1074. doi: 10.1002/iub.531. [DOI] [PubMed] [Google Scholar]
- Jacobs AC, Hood I, Boyd KL, Olson PD, Morrison JM, Carson S, et al. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect Immun. 2010;78:1952–1962. doi: 10.1128/IAI.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JS, Kwon SO, Moon DC, Gurung M, Lee JH, Kim SI, Lee JC. Acinetobacter baumannii secretes cytotoxic outer membrane protein A via outer membrane vesicles. PLoS One. 2011;6:e17027. doi: 10.1371/journal.pone.0017027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SA, Yoo SM, Hyun SH, Choi CH, Yang SY, Kim HJ, et al. Global gene expression patterns and induction of innate immune response in human laryngeal epithelial cells in response to Acinetobacter baumannii outer membrane protein A. FEMS Immunol Med Microbiol. 2008;54:45–52. doi: 10.1111/j.1574-695X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- Kim SW, Choi CH, Moon DC, Jin JS, Lee JH, Shin JH, et al. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol Lett. 2009;301:224–231. doi: 10.1111/j.1574-6968.2009.01820.x. [DOI] [PubMed] [Google Scholar]
- Knapp S, Wieland CW, Florquin S, Pantophlet R, Dijkshoorn L, Tshimbalanga N, et al. Differential roles of CD14 and toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am J Respir Crit Care Med. 2006;173:122–129. doi: 10.1164/rccm.200505-730OC. [DOI] [PubMed] [Google Scholar]
- Lee HW, Koh YM, Kim J, Lee JC, Lee YC, Seol SY, Cho DT. Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin Microbiol Infect. 2008;14:49–54. doi: 10.1111/j.1469-0691.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- Lee JC, Koerten H, van den Broek P, Beekhuizen H, Wolterbeek R, van den Barselaar M, et al. Adherence of Acinetobacter baumannii strains to human bronchial epithelial cells. Res Microbiol. 2006;157:360–366. doi: 10.1016/j.resmic.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Lee JS, Choi CH, Kim JW, Lee JC. Acinetobacter baumannii outer membrane protein A induces dendritic cell death through mitochondrial targeting. J Microbiol. 2010;48:387–392. doi: 10.1007/s12275-010-0155-1. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lee JC, Lee CM, Jung ID, Jeong YI, Seong EY, et al. Outer membrane protein A of Acinetobacter baumannii induces differentiation of CD4+ T cells toward a Th1 polarizing phenotype through the activation of dendritic cells. Biochem Pharmacol. 2007;74:86–97. doi: 10.1016/j.bcp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Loehfelm TW, Luke NR, Campagnari AA. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol. 2008;190:1036–1044. doi: 10.1128/JB.01416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke NR, Sauberan SL, Russo TA, Beanan JM, Olson R, Loehfelm TW, et al. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect Immun. 2010;78:2017–2023. doi: 10.1128/IAI.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March C, Regueiro V, Llobet E, Moranta D, Morey P, Garmendia J, Bengoechea JA. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS One. 2010;5:e10033. doi: 10.1371/journal.pone.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti S, Nait Chabane Y, Alexandre S, Coquet L, Vila J, Jouenne T, De E. Growth of Acinetobacter baumannii in pellicle enhanced the expression of potential virulence factors. PLoS One. 2011;6:e26030. doi: 10.1371/journal.pone.0026030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K, Tanabe T, Yamakawa Y, Funahashi T, Nakao H, Narimatsu S, Yamamoto S. Identification and transcriptional organization of a gene cluster involved in biosynthesis and transport of acinetobactin, a siderophore produced by Acinetobacter baumannii ATCC 19606T. Microbiology. 2004;150:2587–2597. doi: 10.1099/mic.0.27141-0. [DOI] [PubMed] [Google Scholar]
- Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DC, Choi CH, Lee JH, Choi CW, Kim HY, Park JS, et al. Acinetobacter baumannii outer membrane protein a modulates the biogenesis of outer membrane vesicles. J Microbiol. 2012;50:155–160. doi: 10.1007/s12275-012-1589-4. [DOI] [PubMed] [Google Scholar]
- Niu C, Clemmer KM, Bonomo RA, Rather PN. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol. 2008;190:3386–3392. doi: 10.1128/JB.01929-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwugo CC, Gaddy JA, Zimbler DL, Actis LA. Deciphering the iron response in Acinetobacter baumannii: A proteomics approach. J Proteomics. 2011;74:44–58. doi: 10.1016/j.jprot.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC, Jr., Mylonakis E. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother. 2009;53:2605–2609. doi: 10.1128/AAC.01533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008a;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Tampakakis E, Fuchs BB, Eliopoulos GM, Moellering RC, Jr., Mylonakis E. Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008b;105:14585–14590. doi: 10.1073/pnas.0805048105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour NK, Dusane DH, Dhakephalkar PK, Zamin FR, Zinjarde SS, Chopade BA. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol Med Microbiol. 2011;62:328–338. doi: 10.1111/j.1574-695X.2011.00818.x. [DOI] [PubMed] [Google Scholar]
- Qiu H, Kuolee R, Harris G, Chen W. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect Immun. 2009;77:1015–1021. doi: 10.1128/IAI.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Kuolee R, Harris G, Zhou H, Miller H, Patel GB, Chen W. Acinetobacter baumannii infection inhibits airway eosinophilia and lung pathology in a mouse model of allergic asthma. PLoS One. 2011;6:e22004. doi: 10.1371/journal.pone.0022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renckens R, Roelofs JJ, Knapp S, de Vos AF, Florquin S, van der Poll T. The acute-phase response and serum amyloid A inhibit the inflammatory response to Acinetobacter baumannii pneumonia. J Infect Dis. 2006;193:187–195. doi: 10.1086/498876. [DOI] [PubMed] [Google Scholar]
- Russo TA, Beanan JM, Olson R, MacDonald U, Luke NR, Gill SR, Campagnari AA. Rat pneumonia and soft-tissue infection models for the study of Acinetobacter baumannii biology. Infect Immun. 2008;76:3577–3586. doi: 10.1128/IAI.00269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, Luke NR, Beanan JM, Olson R, Sauberan SL, MacDonald U, et al. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun. 2010;78:3993–4000. doi: 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, MacDonald U, Beanan JM, Olson R, MacDonald IJ, Sauberan SL, et al. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J Infect Dis. 2009;199:513–521. doi: 10.1086/596317. [DOI] [PubMed] [Google Scholar]
- Shin JH, Lee HW, Kim SM, Kim J. Proteomic analysis of Acinetobacter baumannii in biofilm and planktonic growth mode. J Microbiol. 2009;47:728–735. doi: 10.1007/s12275-009-0158-y. [DOI] [PubMed] [Google Scholar]
- Smani Y, Docobo-Perez F, McConnell MJ, Pachon J. Acinetobacter baumannii-induced lung cell death: role of inflammation, oxidative stress and cytosolic calcium. Microb Pathog. 2011;50:224–232. doi: 10.1016/j.micpath.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Smith MG, Des Etages SG, Snyder M. Microbial synergy via an ethanol-triggered pathway. Mol Cell Biol. 2004;24:3874–3884. doi: 10.1128/MCB.24.9.3874-3884.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, Snyder M. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 2007;21:601–614. doi: 10.1101/gad.1510307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology. 2003;149:3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- Tomaras AP, Flagler MJ, Dorsey CW, Gaddy JA, Actis LA. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology. 2008;154:3398–3409. doi: 10.1099/mic.0.2008/019471-0. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Nakao N, Yamamoto S, Hirai Y, Miyamoto K, Tsujibo H. NK1.1(+) cells regulate neutrophil migration in mice with Acinetobacter baumannii pneumonia. Microbiol Immunol. 2012;56:107–116. doi: 10.1111/j.1348-0421.2011.00402.x. [DOI] [PubMed] [Google Scholar]
- Vallenet D, Nordmann P, Barbe V, Poirel L, Mangenot S, Bataille E, et al. Comparative analysis of Acinetobacters: three genomes for three lifestyles. PLoS One. 2008;3:e1805. doi: 10.1371/journal.pone.0001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun. 2007;75:5597–5608. doi: 10.1128/IAI.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal R, Dominguez M, Urrutia H, Bello H, Gonzalez G, Garcia A, Zemelman R. Biofilm formation by Acinetobacter baumannii. Microbios. 1996;86:49–58. [PubMed] [Google Scholar]
- Yamamoto S, Okujo N, Sakakibara Y. Isolation and structure elucidation of acinetobactin, a novel siderophore from Acinetobacter baumannii. Arch Microbiol. 1994;162:249–254. doi: 10.1007/BF00301846. [DOI] [PubMed] [Google Scholar]
- Zimbler DL, Penwell WF, Gaddy JA, Menke SM, Tomaras AP, Connerly PL, Actis LA. Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii. Biometals. 2009;22:23–32. doi: 10.1007/s10534-008-9202-3. [DOI] [PubMed] [Google Scholar]