Abstract
Purpose of the review
We aim to review the most recent advances in the field of epilepsy genetics with particular focus on the progress in gene discovery in monogenic epilepsies, identification of risk genes in complex genetic epilepsies and recent findings in the field of epilepsy pharmacogenomics.
Recent findings
During the last 12 months, the use of massive parallel sequencing technologies has allowed for the discovery of several genes for monogenic epilepsies. Most importantly, PRRT2 was identified as the long-sought gene for Benign Familial Infantile Seizures (BFIS). Mutations in KCNT1 were found in two seemingly unrelated monogenic epilepsies including Malignant Migrating Partial Seizures of Infancy (MMPSI) and severe Autosomal Dominant Nocturnal Frontal Lobe Epilepsy (ADNFLE). A genome-wide association study in Idiopathic Generalized Epilepsy (IGE) revealed the first common risk variants for human seizure disorders including variants in VRK2, PNPO and SCN1A. Furthermore, a landmark study provided evidence that screening for the HLAB*1502 variant may prevent carbamazepine-induced side effects in the Taiwanese population. Also, HLA-A*3101 variants were identified as a risk factor for carbamazepine side effects in Europeans.
Summary
Novel technologies and an unprecedented level of international collaboration has resulted in novel genes for monogenic and complex genetic epilepsies as well as risk factors for side effects of antiepileptic drugs. This review provides an overview of the most relevant studies in the last year and highlights the future direction of the field.
Keywords: Epilepsy, genetics, pharmacogenomics, epileptic encephalopathies
Introduction
A genetic contribution to the epilepsies has been recognized since antiquity and is well established through twin studies and family studies. However, despite intense research over the last decades, the majority of the genetic burden in the epilepsies remains unsolved. While this “missing heritability problem” [1] is also observed in many other disorders, the gap appears to be particularly pronounced in the epilepsies. Compared to fields such as autism and schizophrenia research, genetic research in the epilepsies is lagging behind in terms of funding and publications. The main reason for this discrepancy is the phenotypic and genetic complexity of the epilepsies, which poses a particular problem for modern large-scale genetic studies relying on sample sizes in the range of thousands. However, present and emerging international research collaborations are likely to overcome some of these obstacles [2]. With several large-scale genomic studies on the way, the epilepsies are virtually on the eve of their genetic revolution.
In some areas, the effect of this approaching revolution can already be felt, including research on (1) monogenic epilepsies comprising familial epilepsies and epileptic encephalopathies, (2) complex genetic epilepsies, and (3) pharmacogenomics. All three fields use slightly different paradigms and will therefore be discussed separately. The scope of this review is to discuss the recent advances and highlight the future directions.
Deciphering the genetic architecture of the epilepsies: paradigms and technologies
Genetic technology and knowledge about the normal variation in the human genome has significantly advanced over the last 12 months. Whole genome sequencing has become affordable, and exome sequencing, i.e. the sequencing of large parts of the protein-coding region of the human genome, is well-established as a standard technology.
The main result from population studies including the 1000 Genomes Project is the unexpected amount of rare genetic variation in the human genome. This flood of rare variants does not only include a significant amount of private genetic variation limited to single individuals, but also a unforeseen number of de novo mutations in each genome. This variation provides the backdrop for all genetic studies, which aim to identify genetic risk factors on a genome-wide level.
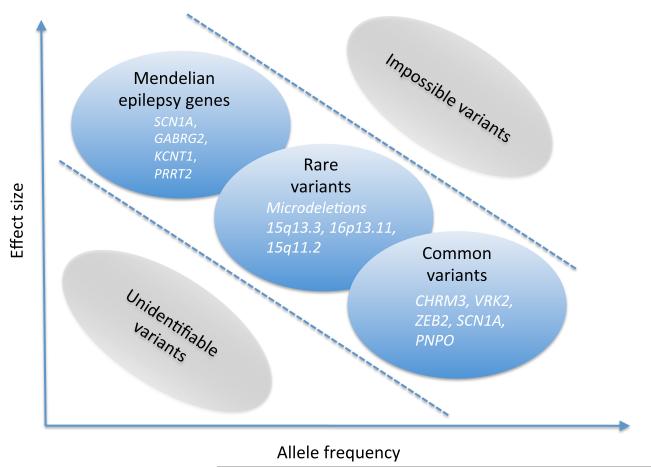
Genetic risk factors for seizure disorders can be classified according to several criteria, including effect size and allele frequency (Figure 1). In addition, genetic epilepsies can present with various degrees of genetic heterogeneity. Dravet Syndrome, for example, is caused by mutations in SCN1A in >80% of cases and represents a relatively genetically homogeneous epilepsy [3, 4], while other epilepsies such as the Idiopathic Generalized Epilepsies are more heterogeneous. As some epilepsies such as Malignant Migrating Partial Seizures of Infancy (MMPSI) appear to be unexpectedly homogeneous (see below), there is good reason to believe that further research may uncover additional, surprising findings in distinct epilepsy syndromes.
Figure 1.
The dimensions of the genetic architecture of the epilepsies
Effect size refers to the increase in risk carried by a given genetic variant. While the effect size is very high for monogenic variants, common genetic variants such as Single Nucleotide Polymorphism (SNPs) usually confer a very small risk. Rare genetic variants located between both extremes currently pose a major problem for genetic research, as established frameworks to interpret this variant are still lacking. Variants can be frequent or rare in the general and patient population, and there is usually an inverse correlation between effect size and frequency. A variant with a strong effect is usually rare. This inverse correlation, however, only applies to disease risk variants, as the low frequency of the disease in the population only permits such a correlation. In genetic risk factors for medication side effects, for example, both the frequency and effect size can be high, which allows for these studies to be performed with a modest sample size.
Progress in monogenic epilepsies
Monogenic epilepsies include both familial epilepsies and severe epilepsies caused by de novo mutations in particular genes. In both groups, massive parallel sequencing technologies have contributed immensely to gene discovery.
Familial Epilepsies
The year 2012 witnessed the answer to one of epilepsy genetics most prominent mysteries: the genetics of Benign Familial Infantile Seizures (BFIS). BFIS represents a usually benign epilepsy syndrome with self-limiting seizures in the first year of life. BFIS is genetically and phenotypically different from Benign Familial Neonatal Seizures (BFNS, KCNQ2, KCNQ3) and Benign Familial Neonatal-Infantile Seizures (BFNIS, SCN2A). Even though a linkage to chromosome 16 was already described in 1997 [5], the gene had escaped detection until 2011.
Somewhat ironically, the initial gene finding through Next Generation Sequencing (NGS) was not achieved in BFIS, but in paroxysmal kinesogenic dyskinesia (PKD), a movement disorder that is observed in some BFIS families [6]. PRRT2 was subsequently found to be the main cause of BFIS, explaining up to 70% of families [7, 8]. Furthermore PRRT2 mutations were also identified in several other benign infantile epilepsies [9-11]. During the course of 2012, more than 20 publications on PRRT2 in infantile epilepsies were published, highlighting the importance of this gene in infantile epilepsies.
The identification of KCNT1 mutations in Malignant Migrating Partial Seizures of Infancy (MMPSI) and severe Autosomal Dominant Nocturnal Frontal Lobe Epilepsy (ADNFLE) was another surprising finding in the last 12 months [12, 13]. MMPSI represents a severe infantile epilepsy syndrome with a peculiar electroclinical pattern. ADNFLE, in contrast, has an onset in adolescence or adulthood with prominent frontal seizures. Mutation in CHRNA4, CHRNB2 and CHRNA2 are known to cause ADNFLE [14]. KCNT1 mutations were found through exome sequencing in 6/12 patients with MMPSI including three patients with the same de novo mutation [13]. Functional studies suggest that these mutations results in an activation of the KCNT1 channel, a potassium channel expressed in the Central Nervous System. In four families with severe ADNFLE and accompanying psychiatric features and intellectual disability, mutations in KCNT1 were identified [12]. MMPSI and severe ADNFLE might therefore represent the extremes of a novel, unpredicted spectrum of epilepsy phenotypes due mutations in a single gene.
Epileptic encephalopathies
Gene discovery in severe early onset epilepsies, the so-called “epileptic encephalopathies”, aims to identify de novo variants or recessive variants in small families or patient-parent trios using genome-wide sequencing technology. When parents are included in the sequencing, de novo variants can be assessed on a genome-wide level, a method that has been shown to be effective in several other neurodevelopmental disorders [15-19]. Using whole genome sequencing of a family quartet, SCN8A mutations were identified in a family with infantile epileptic encephalopathy and Sudden Unexplained Death in Epilepsy (SUDEP, [20]). In addition, one study identified mutations in KCNQ2 in Ohtahara Syndrome, an early onset severe epilepsy [21]. Large-scale family sequencing or trio sequencing have not yet been published, but data on comparable studies in autism, schizophrenia or intellectual disability already provide a first insight into this method [15-19]. While de novo mutations can be identified in almost every individual sequenced, double hits in a single gene are rare and require modest to large sample sizes for detection. Interestingly, up to half of the patients reported in studies on autism and ID also had epilepsy. Hence, many of the genes recurrently affected by de novo mutations including CHD8 and DYRK1A also represent interesting candidates for seizure disorders. While many of the rare variants may not be pathogenic, finding mutations in a known epilepsy gene or finding double hits are established paradigms to approach family exome sequencing data. In December 2012, the Epi4K consortium [2] presented their first analysis on large-scale family exome sequencing in patients with Infantile Spasms and Lennox-Gastaut-Syndrome who were identified through the Epilepsy Phenome/Genome Project. The Epi4K researchers analyzed 165 patient-parent trios for de novo mutations on an exome-wide level and found causative variants in 15% of patients [22, 23]. The genes identified included known genes for epileptic encephalopathies including CDKL5, ARX and STXBP1, but also several novel genes, which require further follow-up. In summary, this first data shows that the methods used for autism, schizophrenia and intellectual disability can be translated to identifying genes in epilepsies and that de novo mutations significantly contribute to the genetic morbidity of epileptic encephalopathies.
Progress in complex genetic epilepsies
The vast majority of epilepsies are complex genetic, i.e. a strong genetic contribution is known from family and twin studies [24, 25], but the epilepsies are not inherited in a monogenic fashion. The identification of genetic risk factors in complex genetic epilepsies occurs through association studies, and the recruitment of sufficiently large patient cohorts for powerful association studies has been a long-standing weakness of the field.
Rare genetic variants
Insight into the genetic architecture of sporadic epilepsies has largely occurred through the study of copy number variants, submicroscopic chromosomal deletions or duplications. A surprising finding of the last five years has been the identification of recurrent microdeletions predisposing to a wide range of neurodevelopmental disorders including autism, schizophrenia and intellectual disability [26]. In addition, many of these variants were found to be inherited from unaffected parents, a puzzling finding in the light of the strong genetic risk conferred by some of these variants [27]. In the last 12 months, two papers have been published that describe analyses of the genetic architecture of microdeletions. A paper by Girirarjan and collaborators assayed the role of double hits, i.e. two independent copy number variants in patients with intellectual disability, showing that several microdeletions associated with disease preferentially co-occur with other microdeletions in patients [28]. This paper, building up on previous work postulating a double hit model for intellectual disability [28], is a first step in explaining the genetics of neurodevelopmental disorders through oligogenic inheritance. However, these studies have not yet been extended to patients with epilepsy. A further paper investigated the apparent contradiction of association and segregation for epilepsy-related microdeletions and developed models to translate odds ratio of rare variants into segregation patterns [29]. The main message of this model is the conclusion that rare variants with an intermediate risk usually are not expected to show a clear segregation pattern in families. Rare genetic variants in Idiopathic Generalized Epilepsy were also investigated in a study by Heinzen and collaborators, who aimed to identify rare genetic sequence variants through exome sequencing [30]. The authors identified 3900 rare genetic variants through exome sequencing of 118 IGE patients and 242 controls and followed these variants up in a large validation cohort. No variant could be identified with genome-wide significance, suggesting that rare variants of intermediate frequency (so-called “goldilocks variants”) are not easily identified in complex genetic epilepsies. The authors suggested that the genetic architecture of IGE might be more heterogeneous and that a gene-based approach as opposed to a variant-based approach might be more advantageous.
The analysis of common genetic variants
Common genetic variants or Single Nucleotide Polymorphisms require large case-control studies to achieve significant results. Previous attempts to identify common genetic variants in common epilepsies did not result in variants significant at a genome-wide level [31]. Finally, in October 2012, the first common genetic variants associated with IGE were published by the European EPICURE consortium [32]. The authors found common variants in CHRM3, VRK2, ZEB2, SCN1A and PNPO. Except for SCN1A and CHRM3, all other genes are not intuitive candidates for IGE susceptibility genes, hinting at as yet unknown pathways besides the involvement of ion channels. Either way, with the publication of the first “successful” GWAS, the curse of failing association studies in epilepsy research appears to be broken.
Progress in epilepsy pharmacogenomics
Epilepsy pharmacogenomics aims to investigate genetic markers associated with response to antiepileptic drugs (AED) or side effects. While promising genetic variants predicting AED response are still lacking, important advances have been made in identifying genetic markers associated with AED side effects. Simultaneously, two studies were published investigating the role of HLA markers in association with side effects for carbamazepine (CBZ). Based on previous knowledge regarding the high risk of CBZ side effects in patients with the HLA-B*1502 variant in Taiwanese patients, Chen and colleagues reported an intervention study, in which the HLA-B*1502 genotype was determined prior to AED treatment [33]. Patients with the risk variant were given another AED rather than CBZ, and a dramatic decrease in cutaneous side effects caused by AED treatment was observed, also in comparison to historical data. In a second study, McCormack and colleagues identified an HLA risk variant for CBZ-related side effects in European populations [34]. Taken together, both studies suggest that cutaneous side effects of CBZ are HLA-related and may at least in part be prevented through prior genetic screening.
Future directions of the field
With the revolution of next generation sequencing technologies only about to start, we expect a large group of monogenic epilepsies to be resolved in the next five years. Familial epilepsies with monogenic inheritance are ideally suited to be unraveled through this technology. As in the case of MMPSI/ADNFLE and PRRT2, some surprises can be expected along the way, i.e. genes that unexpectedly explain a larger phenotypic fraction than assumed. However, the fraction of the expected proportion of “epilepsies explained” by high-throughput sequencing studies may be overestimated. Recent studies in autism, intellectual disability and schizophrenia suggest that no more than 10% of cases are caused by de novo mutations [15-17]. Distinguishing causative from non-causative variation will become increasingly difficult with the increasing amount of data generated through massive parallel sequencing studies. In addition, rare genetic variants, which are expected to contribute significantly to genetic morbidity of human disease, are still difficult to identify and require large sample sizes.
Areas with research needs
There are several areas in the field of epilepsy genetics where either the progress has been stagnating or where the future direction is difficult to estimate, including research on (1) Febrile Seizures, (2) endophenotypes, (3) psychosocial effects of genetic testing, and (4) somatic mutations.
Febrile Seizures
Even though Febrile Seizures (FS) are the most common epilepsy syndrome with a strong genetic basis, there has been little progress in this area over the last decade. In contrast to common FS, the genetics of febrile-seizure related epilepsy syndromes such as Genetic Epilepsy with Febrile Seizures Plus (FS+) is well understood [35-37]. In families with pure FS, however, gene findings are rare. We are currently not aware of any large-scale research effort investigating the genetics of FS.
Endophenotypes
Even though much work had been done on the genetics of epilepsy-related endophenotypes in the 80s and 90s, few advances have been made in this field over the last ten years. Endophenotypes are subclinical phenotypes that are thought to be closer to the genetic basis of the disease than the disease itself [38]. Therefore, a less complicated genetic architecture is expected. EEG patterns have been investigated as possible epilepsy endophenotypes and some evidence for linkage exists for the centrotemporal spikes associated with Benign Roland Epilepsy [39] and for photosensitivity [40]. However, the initial assumptions that these endophenotypes were transmitted in monogenic fashion could not be validated. Novel impetus for epilepsy-related endophenotypes might come from advanced imaging studies including functional MRI and connectivity studies.
Psychosocial effects of genetic testing
While most genetic testing in patients with epilepsy does not affect treatment decisions, discovering the causative gene for a patient’s epilepsy may have a profound psychosocial impact. In the last decade, support groups such as the Dravet Foundation have been initiated, highlighting the psychosocial aspects of epilepsy genetics to create shared sense of identity for patients and caregivers. These psychological and social aspects, however, are largely unexplored to date. A pioneer study found that patients with epilepsy tend to dramatically overestimate the genetic burden of their disease [41], possibly a reflection on the widespread media coverage of genetic research. Many current large-scale research grants implement work packages on patient outreach and investigations of psychosocial effects of genetic testing to study the impact on a patient level.
Somatic mutations
Some initial reports have investigated the role of somatic mutations in patients with hemispheric brain malformations such as hemimegalencephaly [42]. Using exome sequencing, mutations in genes affecting the mTOR pathway have been identified, including PIK3CA, AKT3 and MTOR genes, suggesting that modern technologies are capable of identifying disease-related somatic mutations for brain malformations. The role of somatic mutations is a largely unexplored field [43], and future studies assessing their role in patients undergoing epilepsy surgery may help define the role of these mutations in common epilepsies including Temporal Lobe Epilepsy.
Conclusion
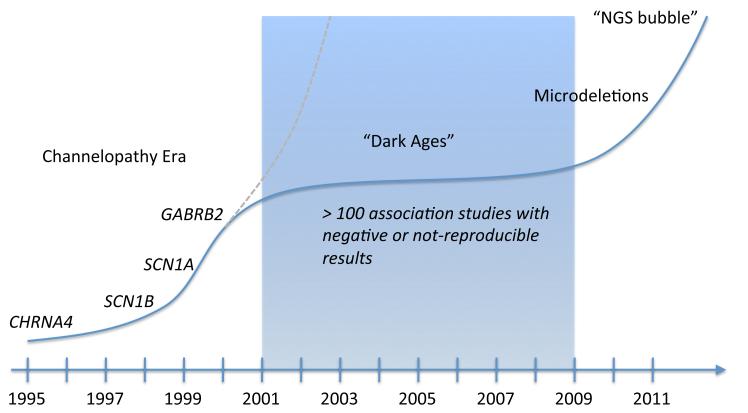
The genetic burden of the epilepsies will continue to be unraveled, and we expect novel gene findings will deepen our understanding of the pathophysiology of seizure disorders (Figure 2). Novel technologies will undoubtedly contribute significantly to gene discovery, but also pose novel challenges including a “flood of rare variants”, which will make it difficult to distinguish causative from random generation. The sharing of research data, central repositories for genetic data and collaboration will generate the resources that the field needs to interpret this data and to identify the genetic risk variants for the seizure disorders we see in our patients.
Figure 2.
The history of gene identifications in epilepsy research
The history of gene discovery in epilepsy began in 1995 with the discovery of CHRNA4 as the causative gene in Autosomal Dominant Nocturnal Frontal Lobe Epilepsy (ADNFLE). After a series of gene discoveries in monogenic epilepsies, it was found that most causative genes were ion channel genes, postulating the Channelopathy concept of human epilepsy. However, subsequent association studies mainly on ion channel genes have failed, and the initial hope for rapid detection of further risk variants (gray) has diminished. The field entered a period of association studies, which largely were not replicable. Finally, with the discovery of epilepsy-related microdeletions, the first risk genes for common epilepsies were identified. With the advent of Next-Generation Sequencing technologies (NGS), more gene discovery is likely. The expectations of this technology, however, parallel a typical “bubble phenomenon” and first indications from other neurodevelopmental disorders suggest that only a small subset of genes can be identified.
Key points.
Several new genes responsible for monogenic epilepsy syndromes (including Benign Familial Infantile Seizures, Malignant Migrating Partial Seizures of Infancy and severe Autosomal Dominant Nocturnal Frontal Lobe Epilepsy) have been identified in the past year.
De novo mutations in a wide variety of genes appear to be the basis of a significant proportion of the epileptic encephalopathies.
A recent GWAS study of over 3,000 individuals with idiopathic generalized epilepsy provided the first evidence for common genetic variants as the basis for epilepsy.
Specific HLA variants have been shown to be associated with side effects of carbamazepine, and a recent study has demonstrated that prospective screening for the variants can be used to guide treatment and reduce side effects.
The emergence of large-scale, international collaborative efforts combined with the power of next generation sequencing technologies hold the promise of major advances in the identification of the genetic risk variants across a wide spectrum of the epilepsies in the coming five years.
Acknowledgements
This work was supported by funding from the German Research Foundation (HE 5415/3-1) within the Eurocores program EuroEPINOMICS of the European Science Foundation, from the Federal Ministry of Education and Research (MAR 10/012) and from the University of Kiel, Germany to I.H., and grants from the National Institute of Neurological Disorders and Stroke (NS053998, NS077274 and NS077276) to D.H.L.
Literature
- 1.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epi4K: gene discovery in 4,000 genomes. Epilepsia. 2012;53:1457–67. doi: 10.1111/j.1528-1167.2012.03511.x. * This review provides an overview of the Epi4K consortium and outlines the workflow of gene discovery in the era of high-throughput genomics.
- 3.Claes L, Ceulemans B, Audenaert D, et al. De novo SCN1A mutations are a major cause of severe myoclonic epilepsy of infancy. Hum Mutat. 2003;21:615–21. doi: 10.1002/humu.10217. [DOI] [PubMed] [Google Scholar]
- 4.Harkin LA, McMahon JM, Iona X, et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007;130:843–52. doi: 10.1093/brain/awm002. [DOI] [PubMed] [Google Scholar]
- 5.Szepetowski P, Rochette J, Berquin P, et al. Familial infantile convulsions and paroxysmal choreoathetosis: a new neurological syndrome linked to the pericentromeric region of human chromosome 16. American Journal of Human Genetics. 1997;61:889–898. doi: 10.1086/514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen WJ, Lin Y, Xiong ZQ, et al. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;43:1252–5. doi: 10.1038/ng.1008. [DOI] [PubMed] [Google Scholar]
- 7.Heron SE, Grinton BE, Kivity S, et al. PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am J Hum Genet. 2012;90:152–60. doi: 10.1016/j.ajhg.2011.12.003. ** This study identified PRRT2 as the long-sought gene for Benign Familial Infantile Seizures, ending an almost decade-long gene hunt for the missing gene for this disease.
- 8.Schubert J, Paravidino R, Becker F, et al. PRRT2 mutations are the major cause of benign familial infantile seizures. Hum Mutat. 2012;33:1439–43. doi: 10.1002/humu.22126. [DOI] [PubMed] [Google Scholar]
- 9.Scheffer IE, Grinton BE, Heron SE, et al. PRRT2 phenotypic spectrum includes sporadic and fever-related infantile seizures. Neurology. 2012 doi: 10.1212/WNL.0b013e3182752c6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardiner AR, Bhatia KP, Stamelou M, et al. PRRT2 gene mutations: From paroxysmal dyskinesia to episodic ataxia and hemiplegic migraine. Neurology. 2012 doi: 10.1212/WNL.0b013e3182752c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marini C, Conti V, Mei D, et al. PRRT2 mutations in familial infantile seizures, paroxysmal dyskinesia, and hemiplegic migraine. Neurology. 2012 doi: 10.1212/WNL.0b013e3182752ca2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heron SE, Smith KR, Bahlo M, et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 2012;44:1188–90. doi: 10.1038/ng.2440. ** This study is one of two publications linking KCNT1 with genetic epilepsies. In this study, KCNT1 was identified as a gene for several autosomal dominant frontal lobe epilepsy, a familial epilepsy that has previously only been linked to mutations in genes for nicotinergic acetylcholine receptors.
- 13.Barcia G, Fleming MR, Deligniere A, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44:1255–9. doi: 10.1038/ng.2441. ** A parallel study describing KCNT1 in Malignant Migrating Partial Seizures of Infancy (MMPSI), a rare catastrophic epilepsy of previously unknown origin. A significant proportion of patients with MMPSI carry mutations in KCNT1.
- 14.Marini C, Guerrini R. The role of the nicotinic acetylcholine receptors in sleep-related epilepsy. Biochem Pharmacol. 2007;74:1308–14. doi: 10.1016/j.bcp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Neale BM, Kou Y, Liu L, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–5. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders SJ, Murtha MT, Gupta AR, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–41. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Roak BJ, Deriziotis P, Lee C, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–9. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B, Roos JL, Dexheimer P, et al. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat Genet. 2011;43:864–8. doi: 10.1038/ng.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Ligt J, Willemsen MH, van Bon BW, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367:1921–9. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 20.Veeramah KR, O’Brien JE, Meisler MH, et al. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am J Hum Genet. 2012;90:502–10. doi: 10.1016/j.ajhg.2012.01.006. * This study describes the discovery of a SCN8A as a novel epilepsy gene using exome sequencing in a quartett, demonstrating the power of this approach in families with a single affected individual.
- 21.Saitsu H, Kato M, Koide A, et al. Whole exome sequencing identifies KCNQ2 mutations in Ohtahara syndrome. Ann Neurol. 2012;72:298–300. doi: 10.1002/ana.23620. [DOI] [PubMed] [Google Scholar]
- 22.Sherr E. Massive Parallel Sequencing in Epilepsy. In: Berkovic SF, De Jonghe P, editors. 66th Annual Meeting of the American Epilepsy Society; San Diego, USA. AES; 2012. [Google Scholar]
- 23.Helbig I. De novo mutations in Infantile Spasms and Lennox-Gastaut Syndrome. In: Helbig I, Krause R, editors. Beyond the Ion Channel - The Channelopathist. EuroEPINOMICS consortium; Kiel, Germany: 2012. EuroEPINOMICS blog. [Google Scholar]
- 24.Berkovic SF, Howell RA, Hay DA, et al. Epilepsies in twins: genetics of the major epilepsy syndromes. Ann Neurol. 1998;43:435–45. doi: 10.1002/ana.410430405. [DOI] [PubMed] [Google Scholar]
- 25.Helbig I, Scheffer IE, Mulley JC, et al. Navigating the channels and beyond: unravelling the genetics of the epilepsies. Lancet Neurol. 2008;7:231–45. doi: 10.1016/S1474-4422(08)70039-5. [DOI] [PubMed] [Google Scholar]
- 26.Mefford HC, Eichler EE. Duplication hotspots, rare genomic disorders, and common disease. Curr Opin Genet Dev. 2009;19:196–204. doi: 10.1016/j.gde.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girirajan S, Rosenfeld JA, Cooper GM, et al. A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet. 2010;42:203–9. doi: 10.1038/ng.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girirajan S, Rosenfeld JA, Coe BP, et al. Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N Engl J Med. 2012;367:1321–31. doi: 10.1056/NEJMoa1200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helbig I, Hodge SE, Ottman R. Familial cosegregation of rare genetic variants with disease in complex disorders. Eur J Hum Genet. 2012 doi: 10.1038/ejhg.2012.194. * This study aims to translate odds ratios derived from case-control studies to sibling risk in patients with microdeletions, aiming to provide a framework for the apparent lack of familial segregation in some microdeletions with highly significant association findings.
- 30.Heinzen EL, Depondt C, Cavalleri GL, et al. Exome sequencing followed by large-scale genotyping fails to identify single rare variants of large effect in idiopathic generalized epilepsy. Am J Hum Genet. 2012;91:293–302. doi: 10.1016/j.ajhg.2012.06.016. * This study aimed to identify recurrent rare variants in Genetic Generalised Epilepsy/Idiopathic Generalised Epilepsy (GGE/IGE) in a sufficiently powered cohort. The negative results of this study suggest that recurrent single rare variants might not be main contributors to the pathogenesis of GGE/IGE.
- 31.Kasperaviciute D, Catarino CB, Heinzen EL, et al. Common genetic variation and susceptibility to partial epilepsies: a genome-wide association study. Brain. 2010;133:2136–47. doi: 10.1093/brain/awq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.EPICURE Consortium et al. Genome-wide association analysis of genetic generalized epilepsies implicates susceptibility loci at 1q43, 2p16.1, 2q22.3 and 17q21.32. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds373. ** This landmark study is the first genome-wide association study on Genetic Generalised Epilepsy/Idiopathic Generalised Epilepsy and the first association study in epilepsy to yield robust association results in a large cohort, identifying several novel candidate genes for a disease that was traditionally considered a channelopathy.
- 33.Chen P, Lin JJ, Lu CS, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364:1126–33. doi: 10.1056/NEJMoa1009717. [DOI] [PubMed] [Google Scholar]
- 34.McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–43. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace RH, Marini C, Petrou S, et al. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 36.Wallace RH, Scheffer IE, Barnett S, et al. Neuronal sodium-channel alpha1-subunit mutations in generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2001;68:859–65. doi: 10.1086/319516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace RH, Wang DW, Singh R, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–70. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 38.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 39.Neubauer BA, Fiedler B, Himmelein B, et al. Centrotemporal spikes in families with rolandic epilepsy: linkage to chromosome 15q14. Neurology. 1998;51:1608–12. doi: 10.1212/wnl.51.6.1608. [DOI] [PubMed] [Google Scholar]
- 40.de Kovel CG, Pinto D, Tauer U, et al. Whole-genome linkage scan for epilepsy-related photosensitivity: a mega-analysis. Epilepsy Res. 2010;89:286–94. doi: 10.1016/j.eplepsyres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Helbig KL, Bernhardt BA, Conway LJ, et al. Genetic risk perception and reproductive decision making among people with epilepsy. Epilepsia. 2010 doi: 10.1111/j.1528-1167.2009.02507.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Huynh M, Silhavy JL, et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet. 2012;44:941–5. doi: 10.1038/ng.2329. ** This study identified mutations in the mTOR-related pathway in patients with hemispheric brain malformations, highlighting the huge potential of next-generation sequencing technologies in identifying causative somatic mutations in epilepsies due to malformations.
- 43.Lindhout D. Somatic mosaicism as a basic epileptogenic mechanism? Brain. 2008;131:900–1. doi: 10.1093/brain/awn056. [DOI] [PubMed] [Google Scholar]