Abstract
Background
Slow waves modulate the pattern of small intestine contractions. However, the large-scale spatial organization of intestinal slow wave pacesetting remains uncertain because most previous studies have had limited resolution. This study applied high-resolution (HR) mapping to evaluate intestinal pacesetting mechanisms and propagation patterns in-vivo.
Methods
HR serosal mapping was performed in anesthetized pigs using flexible arrays (256 electrodes; 32×8; 4 mm spacing), applied along the jejunum. Slow wave propagation patterns, frequencies, and velocities were calculated. Slow wave initiation sources were identified and analyzed by animation and isochronal activation mapping.
Key Results
Analysis comprised 32 recordings from nine pigs (mean duration 5.1±3.9 min). Slow wave propagation was analyzed, and a total of 26 sources of slow wave initiation were observed and classified as focal pacemakers (31%), sites of functional re-entry (23%) and circumferential re-entry (35%), or indeterminate sources (11%). The mean frequencies of circumferential and functional re-entry were similar (17.0±0.3 vs 17.2±0.4 cycle min−1; p=0.5), and greater than that of focal pacemakers (12.7±0.8 cycle min−1; p<0.001). Velocity was anisotropic (12.9±0.7 mm s−1 circumferential vs 9.0±0.7 mm s−1 longitudinal; p<0.05), contributing to the onset and maintenance of re-entry.
Conclusions & Inferences
This study has shown multiple patterns of slow wave initiation in the jejunum of anesthetized pigs. These results constitute the first description and analysis of circumferential re-entry in the gastrointestinal tract and functional re-entry in the in-vivo small intestine. Re-entry can control the direction, pattern, and frequency of slow wave propagation, and its occurrence and functional significance merit further investigation.
Keywords: Pacemaker, High-resolution mapping, Electrophysiology, Frequency gradient, Interstitial cells of Cajal
Introduction
Slow waves generated by the interstitial cells of Cajal modulate the pattern of gastrointestinal contractions (1). The initiation and organization of normal gastric slow waves is well documented and understood (2),(3),(4). However, slow wave organization in the small intestine remains much less certain, and particularly, the organization of small intestine slow wave pacesetting remains a matter of some debate.
Previous studies have revealed that an initial pacemaker site exists in the proximal duodenum, followed by a declining frequency gradient along the intestine in the form of a series of frequency plateaus (5),(6),(7). Investigations into the mechanism of these frequency plateaus have presented differing outcomes. In-vivo studies have suggested that the frequency gradient and plateaus can result from a hierarchy of peripheral pacemakers ‘competing’ along the intestinal tract (8),(9),(10), whereas a feline in-vitro study instead showed that they could result from successive propagation blocks to the antegrade wavefront (11). Recently, another in-vitro study found that functional re-entrant circuits can occur in the healthy and diabetic rat small intestine (12), which might also control slow wave frequency. However, re-entry has not yet been examined in the in-vivo small intestine.
Historically, the technical complexity of recording and analyzing slow wave activity over large areas of the intestine proved a barrier to defining slow wave propagation in spatial detail (8). Classic studies primarily focused on analyzing slow wave frequency using small numbers of electrodes in a linear configuration spaced along the intestine (6),(7),(9), preventing a spatial analysis of propagation dynamics, pacemaker behaviors, and wavefront interactions. However, spatial analysis is necessary to accurately resolve, quantify, and classify pacemaking mechanisms (13),(14). More recently, high-resolution (HR) platforms for mapping GI slow waves have been introduced, whereby simultaneous recordings are taken from dense arrays of electrodes, to define activation sequences in fine spatiotemporal detail (15). New techniques have recently been developed to map curved anatomical surfaces in HR and to efficiently analyze the results, now presenting the opportunity to study intestinal slow wave pacesetting dynamics in more comprehensive spatial detail (16),(17),(18).
The aim of this study was to apply these new HR mapping techniques to record slow wave activity around the intestinal circumference, in a large animal model, and to use these data to better define the mechanisms governing the organization and patterns of intestinal slow wave pacesetting in-vivo. We hypothesized that spatiotemporal analysis of slow wave activation over the intestinal circumference could reveal a combination of mechanisms to influence small intestine slow wave frequency in-vivo, such as focal pacemakers, conduction blocks, and possibly re-entry.
Materials and Methods
Animal preparation
Ethical approval was obtained from the University of Auckland Animal Ethics Committee (R698). All experiments were performed in-vivo on white cross-breed weaner pigs (n=9; 33.1 ± 2.6 kg). These animals and their preparation methods were similar to those used in a previous study defining porcine gastric slow wave activity (4). In summary, the animals were fasted overnight and then subjected to general anesthesia, induced with Zoletil (Tiletamine HCl 50 mg mL−1 and Zolazepam HCl 50 mg mL−1) and maintained with isoflurane (2.5–5% with an oxygen flow of 400 mL within a closed circuit anesthetic system). Vital signs were continually monitored and temperature was maintained in the normal physiological range (38.5–39.5 °C) by external heating. A midline laparotomy was performed to expose the small intestine. At the conclusion of the experiments, the animals were euthanized with a bolus injection of 50 mL of a saturated solution of magnesium sulfate while they were still under anesthesia.
Recording Methods
Serosal HR slow wave mapping was performed using validated flexible printed-circuit-board (PCB) multi-electrode arrays (16),(18). Arrays of 256 electrodes were arranged in a 32 × 8 configuration with an inter-electrode spacing of 4 mm, covering approximately 35 cm2. The PCB arrays were contained in gauze-padded silicone cradles that were sized to match the circumference of the porcine jejunum, with the cradled arrays placed such that the electrodes conformed around most of the intestinal circumference, while exerting only gentle pressure on the serosa (18).
Recordings were conducted at periodic intervals along the proximal jejunum, with sequential recordings then obtained across a segment of the jejunum that displayed a frequency change. All of these intestinal manipulations were accomplished with the minimum necessary visceral handling. During recordings, the intestine was replaced into the abdomen, the wound edges were approximated, and the residual opening packed with warm, saline-soaked gauze to prevent tissue cooling and drying.
Unipolar recordings were acquired at a sampling frequency of 512 Hz using the ActiveTwo System (Biosemi, Amsterdam, Netherlands), with reference electrodes placed on the lower left abdomen and the left hindquarter thigh.
Signal Processing and Analysis
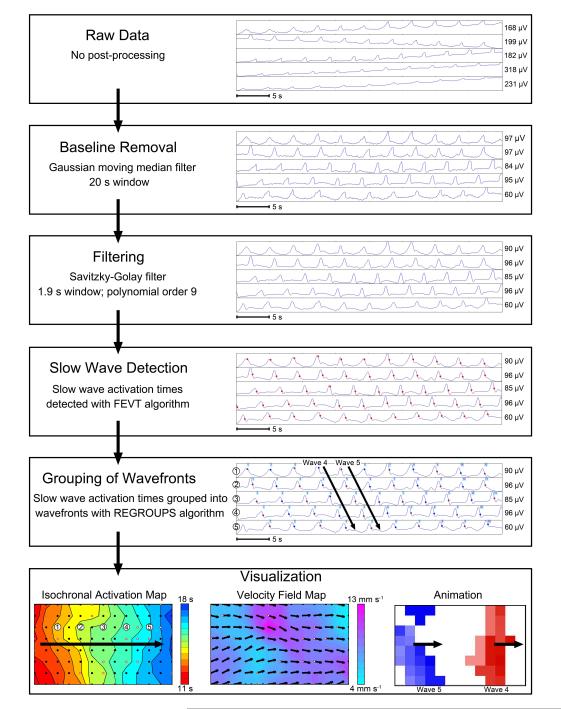
The data analysis pipeline was performed as summarized in Figure 1. These methods were implemented in the Gastrointestinal Electrical Mapping Suite (GEMS) software v1.5 (19). All data were first filtered with a Gaussian moving median filter (20 second moving window) to remove baseline drift and a Savitzky-Golay filter (‘low-pass’, window 1.7 s, polynomial order 9) to remove high frequency noise (20). Slow wave activation times were then detected using the falling-edge variable-threshold (FEVT) algorithm (17), with parameters tuned for porcine small intestine slow wave detection (18). Automatically-detected slow wave events were manually reviewed to correct false-positive and false-negative marks.
Figure 1.
Pipeline of the data analysis process from raw data through visualization. Sample electrograms are shown for each step, and each step is described in the ‘Signal Processing and Analysis’ section of the methods. Identified slow wave activation times are marked by red dots. Three forms of visualization are shown. The isochronal activation map shows the propagation of Wave 4 from the electrogram above (see Figure 2 for a complete description of the activation maps). The velocity field map displays the magnitude as a color gradient and the direction at each electrode as an arrow. A single frame of the propagation animation shows multiple waves in the mapped area at one time, represented in different colors and corresponding to Wave 4 and Wave 5 in the electrogram above.
Animations of slow wave activation were generated for all recorded data sequences (19) and propagation patterns were quantified and classified. Based on this classification, recordings that showed sites of wavefront initiation or re-entrant activities were focused on for comprehensive analysis. An automated grouping algorithm (‘REGROUPS’), initially designed for gastric recordings, was applied to cluster individual slow waves into groups representing the same cycle of activity (21), although subsequent manual correction was often necessary, particularly around pacemaker and re-entrant sites. Isochronal mapping of activation times was then performed to visualize and quantify the propagation of successive wavefronts, using automated methods (21). Areas of conduction block were manually indicated as thick black lines, and inaccuracies in the automated maps were manually corrected (22).
Slow wave frequencies were determined by calculating the cycle-to-cycle intervals of successive events across an array of electrodes over the duration of interest. Velocities were calculated using a finite difference approach with a Gaussian smoothing filter (23), and circumferential and longitudinal propagation patterns were analyzed and compared.
Statistical Methods
Slow wave frequencies and velocities were averaged and compared using Student’s t-test or one-way ANOVA with Tukey’s post-test, as appropriate. Means ± SEMs are reported unless otherwise specified.
Results
Analysis comprised a total of 32 recordings from nine pigs, of mean duration 5.1 ± 3.9 min (total duration 163.1 min; approximately 450,000 individual slow wave events).
Propagation Direction, Velocity, and Initiation
Slow wave activity was observed to propagate in the antegrade, retrograde, and circumferential directions, with wavefront collisions and multiple propagation patterns sometimes being observed in the duration of a single recording. Antegrade propagation was the most prevalent pattern, present in 30/32 recordings, with 11 of those recordings characterized exclusively by antegrade propagation (Figure 2A). Retrograde propagation was observed in 21/32 recordings, with only two recordings characterized exclusively by retrograde propagation (Figure 2B). Circumferential propagation was observed in 15/32 recordings, always accompanying a source of wavefront initiation and, thus, accompanied by antegrade and retrograde propagation. The velocity of propagation in the circumferential direction (12.9 ± 0.7 mm s−1) was higher than that of propagation in the longitudinal direction (9.0 ± 0.7 mm s−1); (p<0.05).
Figure 2.
Antegrade and retrograde slow wave propagation. An 8 × 13 electrode section of the array is shown in these examples. The electrode array was wrapped around the circumference of the intestine such that the top and bottom of each map correspond to nearly-adjacent tissue at the mesenteric border. Electrograms are shown on the left, and isochronal activation maps for three successive slow wave cycles (a, b, and c) are shown on the right. The displayed electrograms correspond to the channels labeled on maps A(a) and B(a). Each activation map shows a single wavefront, with each color band indicating the area of slow wave propagation per 0.5 s in A and 1.0 s in B, progressing from red (early) to blue (late). Each black dot represents an electrode, and white dots outlined in red represent electrodes where activity was interpolated. A. Consistent antegrade propagation. Slow wave activity originated oral to the array and propagated antegrade across the array. B. Consistent retrograde propagation. Slow wave activity originated aboral to the array and propagated retrograde across the array. (See also: Supplemental Animation 2A and Supplemental Animation 2B of the same data).
Spatiotemporal HR analyses revealed a total of 26 incidences of wavefront initiation occurring in 15 recordings from 6 animals. These were classified into three distinct mechanisms of wavefront initiation (refer to Table 1 for a glossary of terms): focal pacemakers (n = 8/26; illustrated in Figure 3), functional re-entry (n = 6/26; illustrated in Figure 4), and circumferential re-entry (n = 9/26; illustrated in Figure 5). In three instances, the source of wavefront initiation could not be definitively classified because of insufficient data coverage at those sites. Wavefronts emanated from all of these sources, and due to the more rapid circumferential velocity, these wavefronts ultimately developed into complete rings of activation propagating longitudinally along the organ in the antegrade and retrograde directions. Collisions between antegrade and retrograde propagating wavefronts were frequently observed.
Table 1.
Glossary of terms used to describe intestinal slow wave propagation and re-entry. Several cardiac electrophysiology terms (25),(28) have been adapted to describe similar phenomena in the intestine.
| Terminology | Description |
|---|---|
| Leading Edge | Depolarized / excited tissue at the front of a
propagating slow wave wavefront. |
| Refractory Tail | Depolarized tissue behind the leading edge of
a slow wave that is unable to be excited. |
| Wavefront Spacing | The distance between two successive wavefronts. |
| Anisotropic Conduction | A propagation profile that is directionally
non-uniform. In the case of gastric and intestinal slow waves, anisotropic conduction manifests as circumferential propagation being faster than longitudinal propagation (36). |
| Conduction Block | Abnormal termination of a propagating
wavefront, for example when refractory tissue or a structural defect is encountered. |
| Functional Block | A specific type of conduction block resulting
from dynamic interactions between the leading edge and refractory tissue rather than by anatomical means. |
| Focal Pacemaker | Slow wave/s arising from a point-source and
propagating in all directions, colliding in the opposite aspect of the intestine circumferentially, and forming rings of activation propagating longitudinally. |
| Re-entry | A slow wave propagation pattern where slow
wave activity propagates in a circuit around a defined obstacle, forming a loop pattern of activation and re-activating that circuit over successive cycles. |
| Excitable Gap | A section of excitable tissue between the
refractory tail of one cycle of re-entry and the leading edge of the following cycle. The presence of an excitable gap is necessary to maintain re- entry, as re-entry will terminate if there is a collision between the leading edge and refractory tail. The size of the excitable gap is governed by the size of the re-entrant circuit, the slow wave velocity, and the refractory period. |
| Anatomical Re-entry | A type of re-entry where a structural feature
forms the obstacle around which slow wave activity establishes a re- entrant loop (27). |
| Circumferential Re-entry | A specific type of anatomical re-entry where
the intestinal lumen forms the anatomical obstacle around which slow wave activation establishes a re-entrant loop. |
| Functional Re-entry | A type of re-entry where a functional
conduction block forms the obstacle around which slow wave activation establishes a re-entrant loop of propagation (24)(25). |
| Figure-of-Eight Re-entry | A variant of functional re-entry where a
common wavefront divides into clockwise and anticlockwise wavelets that propagate around separate functional blocks before rejoining, forming a re-entrant circuit in a figure-of-eight pattern (26). |
Figure 3.

Focal pacemaker. The figure panel is arranged as described in Figure 2. Each map depicts a single wavefront and each color band represents 0.25 s of propagation time. An 8 × 11 electrode section of the array is shown. Slow wave activity was observed to originate at a point near the mesentery, and spread in all directions. The circumferentially-directed propagation traveled around the intestinal circumference (from top and bottom of map), colliding at a point approximately opposite the site of activation (near the middle of the map; also observed in converging waveforms in the electrograms). Focal pacemakers generated wavefronts traveling longitudinally along the intestine in both the antegrade and retrograde directions (see also: Supplemental Animation 3 of the same data).
Figure 4.
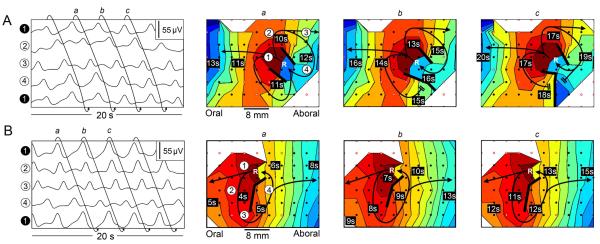
Functional re-entry. The figure panels are arranged as described in Figure 2, with each map depicting a single re-entrant cycle and each color band representing the area of propagation per 0.5 s. Blank areas represent locations where data was not reliably recorded. An 8 × 10 electrode section of the array is shown in these examples. The top electrode in the electrogram (‘electrode 1’) is repeated at the bottom of the electrogram to demonstrate the continuous propagation. A. In this example, slow wave activity formed a ‘figure-of-eight’ re-entrant circuit where a single wavefront broke into two wavelets (wave fragments that separate from the main wavefront) that propagated in clockwise and anti-clockwise directions around two distinct lines of functional block. These wavelets repeatedly re-excited the same loops of tissue, reactivating after subsequent cycles (at point marked ‘R’ in maps a, b, and c) and forming a re-entrant circuit. In this example, the anti-clockwise propagation loop was slightly out-paced by the clockwise loop, causing the clockwise loop to become dominant and the anti-clockwise loop to collide and terminate at a functional block in maps b and c. B. An episode of functional re-entry from a second animal, occurring in an anti-clockwise direction. The loop of activation again traveled around a line of functional activation block and re-excited the same tissue circuit. In both A and B, wavefronts were seen to propagate antegrade and retrograde from the sites of functional re-entry, thereafter assuming ‘rings’ of activation moving longitudinally. (see also: Supplemental Animation 4A and Supplemental Animation 4B).
Figure 5.
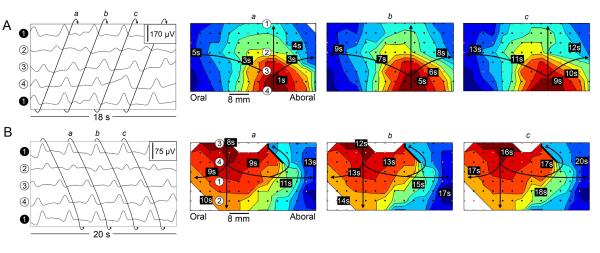
Circumferential re-entry. The figure panels are arranged as described in Figure 2, with each map depicting a single wavefront and each color band representing the area of propagation per 0.5 s. An 8 × 14 electrode section of the array is shown. A. Slow wave activity was observed to propagate from the bottom to top of the maps and re-enter at the bottom, illustrating a continuous loop wavefront that propagated circumferentially around the intestine (i.e., the wavefront at the top of map a propagated continuously onto bottom of map b, and so on). B. A further example from a second animal again illustrates a continuous loop of slow wave activity that propagated around the intestinal circumference, in this case in the opposite direction as in A. An additional conduction block was present in this case at the top of the map, represented by the thick black line. In A and B, ring wavefronts were observed to travel longitudinally antegrade and retrograde along the intestine from the site of circumferential re-entry (see also: Supplemental Animation 5A and Supplemental Animation 5B).
Animations corresponding to Figures 2-5 are also provided as supplementary material.
Focal Pacemakers
In 8/26 sources of wavefront initiation, slow wave activity was observed to originate from a focal pacemaker and to propagate in all directions from that location (observed in 4 animals). Antegrade and retrograde wavefronts were established, together with circumferential propagation that traveled around the intestine in opposite directions, colliding and terminating at a site opposite to the focal pacemaker. An example is shown in Figure 3 (and Supplementary Animation 3). The location of focal pacemakers occasionally migrated by a few centimeters over the duration of the pacemaker before other competing slow wave activity overtook and entrained the tissue where the focal pacemaker had been located.
Re-entrant Activities
The majority of sources of wavefront initiation were re-entrant foci (15/26), where slow wave activity propagated over a ‘loop’ of tissue, re-activating (i.e., ‘re-entering’) that loop over multiple successive cycles. Two distinct types of slow wave re-entry were observed, as described below.
Functional Re-entry
Functional re-entry was identified by repeated slow wave activation occurring in either a clockwise or anticlockwise loop around a line of functional block (24)(25), and was seen to be the source of wavefront initiation in six instances (6/15 re-entries; 6/26 sources of wavefront initiation; 2 animals). In all of these instances, wavefronts were observed to propagate through the area where re-entry had occurred, either prior to, or after, the actual re-entrant activity, which indicated that these re-entrant circuits were best explained by functional mechanisms rather than by structural defects or anatomical mechanisms (24)(25). In one instance, ‘figure-of-eight’ re-entry (26), as defined in Table 1, was observed (Figure 4A and Supplementary Animation 4A). An additional example of functional re-entry from a different animal is shown in Figure 4B (and Supplementary Animation 4B).
Circumferential Re-entry
Circumferential re-entry was characterized by a single wavefront establishing a continuous and repeated loop around the intestinal circumference, and was observed in nine instances (9/15 re-entries; 9/26 sources of wavefront initiation; 4 animals). Two examples of circumferential re-entry from different animals are shown in Figure 5 (and Supplemental Animation 5A and Supplemental Animation 5B). In some instances, functional blocks were also present adjacent to the area of circumferential re-entry, with subsequent propagation activating the tissue behind the block in a further non re-entrant loop, as shown in Figure 5B.
Re-entry frequency and duration
The mean frequencies of functional and circumferential re-entry were similar (17.2 ± 0.4 vs 17.0 ± 0.3 cycle min−1; p=0.5), and both were higher than the frequency of the focal pacemakers (12.7 ± 0.8 cycle min−1) (p<0.001). Wavefronts arising from re-entrant circuits propagated antegrade and retrograde from the site of re-entry at the same frequency as that re-entry, thereby establishing a high-frequency ‘plateau’ along adjacent sections of intestinal tissue (Figures 4 and 5, and corresponding animations, supplementary material).
The duration of the three observed slow wave initiation mechanisms was analyzed. There was no significant difference in the average recorded duration of focal pacemakers (median 41 s; range 20-200 s), functional re-entry (median 41.5 s; range 25-150 s), or circumferential re-entry (median 80 s; range 37-200 s) (p=0.14). In some cases, focal pacemakers and circumferential re-entry were present at the beginning of the recording (2 focal; 3 circumferential) or persisted to the termination of the recording period (1 focal; 3 circumferential), meaning that the duration values were an underestimate for those recording periods.
Physical Principles of Re-entry
Re-entry has physical implications for the frequency, velocity, and wavefront spacing (refer to glossary in Table 1) of the resultant slow wave entrainment (27). The physical principles of the observed re-entrant events were formulated in terms of mathematical equations in Figure 6, which mirror those previously established in cardiac re-entry (27). In order to maintain re-entry, the period of re-entry must be greater than the combined activation and refractory periods, such that there is an excitable gap of tissue between cycles of re-entry (28), as described in Table 1. The frequency, and thereby also the period, of circumferential re-entry is governed by the relationship between intestinal circumference and circumferential velocity. For any pacemaker site, the spacing of resultant longitudinally-propagating wavefronts is governed by the slow wave frequency and longitudinal velocity. For circumferential re-entry, this wavefront spacing can be further formulated in terms of longitudinal velocity, circumferential velocity, and intestinal circumference (Figure 6D).
Figure 6.
Mathematical properties of re-entry. Slow wave propagation is represented with arrows, where red represents the wavefront and white represents the excitable gap (see Table 1 for definition of terminology). A. Schematic of a focal pacemaker, shown on a cross-section of the intestine. Activity originates from a point represented with the asterisk, travels in both directions around the circumference of the intestine, and collides on the opposite side. B. Schematic of circumferential re-entry, shown on a cross-section of the intestine. A wavefront propagates continuously in a loop around the circumference of the intestine, re-exciting the same circuit of tissue over successive cycles. C. Schematic of functional re-entry, shown on the serosa of the intestine. Activity propagates in a loop around a functional block, represented by a thick black line. The functional block is created by the refractory period such that the leading edge of one cycle follows the refractory tail of the previous cycle. In both circumferential and functional re-entry, an excitable gap must be present between the leading edge of one cycle and the refractory period of the previous cycle to avoid a collision between the two, resulting in termination of the re-entry. D. Equations outlining the physical properties governing re-entrant slow wave activity.
To further validate that the data presented above was indicative of circumferential re-entry, the above principles were applied to the experimental data recorded in this study by inserting the average frequency (f = 17.0 cycles min−1), circumferential velocity (VC = 12.9 mm s−1), and longitudinal velocity (VL = 9.0 mm s−1) into the formulae presented in Figure 6. The result was a predicted average intestinal circumference of φ = 46 mm and an average longitudinal wavefront spacing resulting from a site of circumferential re-entry of λ = 32 mm (as illustrated in Supplemental Animation 5A and Supplemental Animation 5B). This estimate agrees closely with intestinal circumference data from porcine controls in another study (median: 43 mm; range: 33-55 mm) (29).
Discussion
In this study, HR electrical mapping was performed around the circumference of the porcine jejunum to examine the range of slow wave propagation patterns and wavefront initiation mechanisms occurring in the in-vivo small intestine. In addition to observing focal pacemakers, re-entrant slow wave propagation was observed and quantified, including both functional re-entry and a novel form of circumferential re-entry. These re-entrant propagation patterns determine the direction, frequency, and period of slow wave propagation along the jejunum, and operated at a higher frequency than focal pacemakers. This study serves as the first description of circumferential slow wave re-entry in the gastrointestinal tract, and the discovery of functional re-entry in the in-vivo small intestine complements the recent descriptions of functional re-entry in the gastric antrum and corpus (13),(30) and isolated in-vitro small intestine (12).
This study demonstrated how organ-level mechanisms may influence the pattern of intestinal slow wave propagation. Previously, it has generally been assumed that in-vivo intestinal slow wave frequencies and propagation patterns are governed solely by the intrinsic frequencies of ICC, influenced by the variable coupling between adjacent segments (5). The enteric nervous system and intrinsic agents, such as prostaglandins, are also known to influence slow wave frequencies (31),(32). Our study showed that in-vivo intestinal slow wave frequency can also be governed by re-entrant foci, in which frequencies of ICC are entrained according to relationships of velocity, wavelength, and organ geometry, as seen in Figure 6. It is likely that re-entry, particularly circumferential re-entry, has been missed in previous studies due to insufficient spatial resolution, limited flexibility or coverage of HR mapping arrays, or preparation methods of in-vitro tissue (e.g., opening the sample along the longitudinal axis, which eliminates the circumferential re-entrant pathway). The flexible PCB electrode arrays that wrapped around the intestinal circumference in this study were vital to our ability to record circumferential re-entry. Circumferential coverage was not complete in experimental cases, due to the necessity for a small gap at the location of mesenteric attachment (approximately 5-30%, depending on subject variability). However, the slow wave activation times, distances, velocities, and lack of any circumferential wavefront collisions (Figure 5 and Supplemental Animation 5A and Supplemental Animation 5B) were indicative that circumferential re-entry was the most favorable explanation for these sources of wavefront initiation, as quantified above.
‘Plateaus’ of stable slow wave frequency have been shown to occur along the intestine (6),(7), caused by either intermittent conduction blocks in-vitro (11), or a hierarchy of ‘peripheral’ pacemakers in-vivo (9),(10). Our study further supports the concept of peripheral pacemakers as the dominant in-vivo pattern, albeit with the added complexity of re-entry also causing frequency plateaus. Discrepant in-vivo and in-vitro findings may be due to the fact that preparation of tissues for in-vitro studies may elevate ICC frequencies and / or impair frequency gradients, for example, as a consequence of prostaglandin release (32). Suzuki et al. previously demonstrated that ‘waxing and waning’ (or ‘spindling’), where the amplitude of slow waves cyclically increases and decreases, thought to be a consequence of adjacent frequencies operating slightly out of phase, was greatly diminished in the isolated in-vitro intestine, compared to in-vivo preparations (8), suggesting a loss of competing peripheral pacemakers in-vitro.
The anisotropy of small intestine conduction reported in this study (velocity 40% faster circumferentially than longitudinally) accords with a previous study of anisotropy in the feline small intestine (velocity 30% faster circumferentially) (33). This velocity anisotropy is important for the initiation and maintenance of circumferential re-entry because it enhances the likelihood of circumferentially-directed activation to re-enter a circuit of tissue before a competing longitudinal wavefront arrives at the excitable gap at that location. The cause of this velocity anisotropy requires further investigation. Conduction through ICC-MP (myenteric plexus ICC) is considered to be isotropic based on small animal studies (34),(35), and the anisotropy demonstrated here could be a result of the coupling and differing conductivities between ICC-MP and other classes of ICC and / or smooth muscle layers (36).
Lammers et al. recently demonstrated the occurrence of functional re-entry in the rat small intestine in-vitro (12). In that study, re-entry was observed in 15% (10/66) of preparations studied, in both control and streptozocin-induced diabetic rats. Unlike in our porcine study, however, it was found that in rats the re-entrant frequency operated within a similar frequency range to the intrinsic intestinal frequency, likely reflecting differences in conduction velocity and refractory periods between these species. Our study serves as the first description of the figure-of-eight re-entry pattern in the intestine, which is well known in the heart, and was recently observed in the canine antrum (13),(26).
In cardiology, re-entry has been widely studied and occurs exclusively as a dysrhythmic pattern, including in ventricular fibrillation (25). Re-entry in the gastrointestinal tract is a relatively new avenue of research, with its implications only beginning to be investigated. Gastric re-entry is considered dysrhythmic and is associated with tachygastria (13),(30), though it has not yet been demonstrated in disease states or to be associated with significant organ dysfunction. Lammers et al. also considered intestinal functional re-entry to be dysrhythmic in their in-vitro rat study because although the slow wave frequency was unchanged, re-entry was accompanied by increased retrograde propagation (12). In this study, re-entry was observed to occur in the healthy porcine small intestine. However, the experimental manipulations in this study, although limited, may have played a role in promoting re-entrant slow wave activity as a dysrhythmic pattern (30). It is also possible that re-entry will occur in the normal small intestine in health, given the inherent anisotropy of the tissue. To help resolve this question further, it would be useful to perform circumferential mapping in the healthy human small intestine. Importantly, the methods used in this study are suitable for human research (18). Furthermore, a recovery study with chronic recordings may help to determine whether re-entry is associated only with acute surgery, or also occurs in the recovered fed state and disease models.
As was detailed in Figure 6, and previously in the cardiac field (27),(28),(37), the physical properties of re-entry are characterized by a complex relationship between the size of the re-entrant circuit, refractory period, and slow wave velocity, which is further affected by anisotropic conductivity (38). In functional re-entry, the leading edge directly follows the refractory tail of the previous cycle, and the circuit size is thereby kept to a minimum, defined by the velocity and refractory period. By contrast, the size of the circuit in circumferential re-entry is anatomically determined by the intestinal circumference, and is larger than that in functional re-entry. It was therefore an interesting result that the frequencies of functional and circumferential re-entry were found to be similar, despite their differing mechanisms. This result could be explained by the anisotropic conduction. Although circumferential re-entry has a larger circuit, the entire circuit is in the direction of maximum velocity, whereas functional re-entry has a smaller circuit but also a slower velocity because a portion of the circuit will always be in the longitudinal direction.
The anisotropic conductivity should also serve to stabilize circumferential re-entry because rapid circumferential activation reduces the size of the excitable gap, reducing the likelihood for competing wavefronts to invade this gap and terminate reentry. A similar stabilizing effect of anisotropic conductivity on anatomical re-entrant excitation has been described in cardiac arrhythmias (28),(38).
This study investigated re-entry in the jejunum, but functional re-entry has previously been shown to also occur in the duodenum and ileum of the in-vitro rat (12). It is currently unknown whether circumferential re-entry could also occur in the in-vivo duodenum and ileum of large animals. However, the slow wave velocity has been shown to be high in the duodenum of dogs (in the order of 10 cm s-1) (10), such that the very long resultant wavelength makes duodenal reentry unlikely. The decreased velocity of slow waves (in the order of 1 cm s-1 in dogs) in the ileum means that re-entry may be more likely in that part of the tract (10),(12). Further experimental studies are now needed to confirm or refute the above theoretical considerations.
The effects on intestinal motility of re-entry and its accompanying high-frequency retrograde propagation require further research, but useful comparisons can be drawn with studies where pacing above intrinsic frequencies was applied to the distal limb of an isolated jejunal loop to similarly induce retrograde slow wave activity (39),(40). This pacing approach was explored as a potential therapy for short gut syndrome (39). These, and other, studies have shown that the intestinal transit, particularly of liquids, is substantially slowed with retrograde slow wave propagation, increasing proximal volume and absorption (39),(40). Similar effects could perhaps be expected from the relatively high-frequency retrograde propagation resulting from re-entry. If, as discussed above, re-entry is a dysrhythmic pattern in the intestine, then it could potentially be relevant to the pathophysiology of post-operative ileus and other motility disorders, such as intestinal ischemia, where dynamic conduction blocks and tachyarrhythmias have been shown to occur, but with uncertain mechanisms (41),(42). It is also possible that this type of re-entry may be associated with dysrhythmias observed in other parts of the gut, such as the stomach, where the high degree of anisotropy (circumferential velocity 2.5 times faster than longitudinal in human) promotes circumferentially-directed propagation during dysrhythmia (14).
Recent debate has arisen as to whether extracellular electrical recordings truly reflect slow wave potentials or movement artifact (43). Based on the morphological and biophysical characteristics of the recordings reported here, which are similar to those in other intestinal extracellular studies (6),(10), it is these authors’ view that the recorded events were strongly indicative of true bioelectrical potentials rather than contractions (44).
In summary, this study serves as the first description of circumferential re-entry in the GI tract, and the first example of functional re-entry in the in-vivo intestine. The occurrence of slow wave re-entry and its effect on intestinal function merits further investigation.
Supplementary Material
Supplemental Animation 2A and 2B These animations correspond directly with Figure 2 and show longitudinal slow wave propagation. Supplemental Animation 2A shows consistent antegrade propagation where slow wave activity traveled from orad (left) to aborad (right). Supplemental Animation 2B shows consistent retrograde propagation where slow wave activity traveled from aborad (right) to orad (left). Each square represents an electrode and lights up when the slow wave passes that electrode. Successive cycles of slow wave activity are colored differently for ease of visualization.
Supplemental Animation 3 This animation corresponds directly with Figure 3 and shows a focal pacemaker. Slow wave activity originated at a focal source and propagated in all directions, colliding circumferentially and establishing wavefronts that propagated both antegrade (aborad, right) and retrograde (orad, left).
Supplemental Animation 4A and 4B These animations correspond directly with Figure 3 and show functional re-entry of slow wave activity. In Supplemental Animation 4A, slow wave activity propagated as two wavelets in stable, repetitive clockwise and anticlockwise circuits around two distinct linear activation blocks, thus forming a figure-of-eight re-entrant circuit. Slow waves formed as ring wavefronts that propagated from the re-entrant circuit in both the antegrade (aborad, right) and retrograde (orad, left) directions. In Supplemental Animation 4B, slow wave activity propagated in a stable, repetitive anticlockwise circuit around a linear activation block, forming a continuous loop of activation and, thus, a re-entrant circuit. As above, slow waves formed ring wavefronts that propagated from the re-entrant circuit in both the antegrade (aborad, right) and retrograde (orad, left) directions.
Supplemental Animation 5A and 5B These animations correspond directly with Figure 4 and show circumferential re-entry of slow wave activity. In Supplemental Animation 5A, slow wave activity propagated in a stable, repeated circuit from the top (mesentery) to bottom (mesentery), and then reactivated the same circuit of tissue from the top of the animation. Since the electrode array was wrapped around the circumference of the intestine such that the top and bottom were nearly adjacent at the mesentery, this is best explained as a re-entrant slow wave circuit around the circumference of the intestine. As with functional re-entry (Figure 4, Supplemental Animation 4), slow waves formed ring wavefronts that propagated from the re-entrant circuit in both the antegrade (aborad, right) and retrograde (orad, left) directions. In Supplemental Animation 5B, another example of circumferential re-entry is shown, where slow wave activity propagated from the bottom (mesentery) to the top (mesentery), and then reactivated the same circuit of tissue at the bottom of the animation.
Acknowledgements
With great sadness, AJP passed away during the course of this project. We thank Linley Nisbet for her expert technical assistance.
TRA, GOG, AJP, & LKC designed the study; TRA, GOG, PD, & NP collected the experimental data; TRA, GOG, PD, & NP analyzed the data; TRA & GOG wrote the draft; AJP, IPB, and LKC supervised the research; all authors except AJP revised and approved the manuscript.
Funding This work and authors were supported in part by funding from the New Zealand Health Research Council, the National Institutes of Health (R01 DK64775), the Riddet Institute, the Earle Food Research Fund, and the Royal Society of New Zealand RHT Bates Postgraduate Scholarship.
Footnotes
Disclosures None of the authors have any professional, financial, or personal conflicts of interest in relation to this work.
References
- 1.Huizinga JD, Lammers WJEP. Gut peristalsis is coordinated by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:1–8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 2.O’Grady G, Du P, Cheng LK, et al. The origin and propagation of human gastric slow wave activity defined by high-resolution mapping. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):585–92. doi: 10.1152/ajpgi.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1200–10. doi: 10.1152/ajpgi.90581.2008. [DOI] [PubMed] [Google Scholar]
- 4.Egbuji JU, O’Grady G, Du P, et al. Origin, propagation and regional characteristics of porcine gastric slow wave activity determined by high-resolution mapping. Neurogastroenterol Motil. 2010;22:e292–300. doi: 10.1111/j.1365-2982.2010.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasler WL. Small intestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Elsevier Academic Press; USA: 2006. pp. 935–64. [Google Scholar]
- 6.Szurszewski JH, Elveback LR, Code CF. Configuration and frequency gradient of electrical slow wave over canine small bowel. Am J Physiol. 1970;218(5):1468–73. doi: 10.1152/ajplegacy.1970.218.5.1468. [DOI] [PubMed] [Google Scholar]
- 7.Diamant NE, Bortoff A. Nature of the intestinal slow-wave frequency gradient. Am J Physiol. 1969;216(2):301–307. doi: 10.1152/ajplegacy.1969.216.2.301. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki N, Prosser CL, DeVos W. Waxing and waning of slow waves in intestinal musculature. Am J Physiol. 1986;250:G28–34. doi: 10.1152/ajpgi.1986.250.1.G28. [DOI] [PubMed] [Google Scholar]
- 9.Diamant NE, Bortoff A. Effects of transection on the intestinal slow-wave frequency gradient. Am J Physiol. 1969;216(2):301–7. doi: 10.1152/ajplegacy.1969.216.2.301. [DOI] [PubMed] [Google Scholar]
- 10.Lammers WJ, Ver Donck L, Schuurkes JA, Stephen B. Peripheral pacemakers and patterns of slow wave propagation in the canine small intestine in vivo. Can J Physiol Pharmacol. 2005;83(11):1031–43. doi: 10.1139/y05-084. [DOI] [PubMed] [Google Scholar]
- 11.Lammers WJ, Stephen B. Origin and propagation of individual slow waves along the intact feline small intestine. Exp Physiol. 2008;93(3):334–46. doi: 10.1113/expphysiol.2007.039180. [DOI] [PubMed] [Google Scholar]
- 12.Lammers WJEP, Stephen B, Karam SM, et al. Functional reentry and circus movement arrhythmias in the small intestine of normal and diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2012;302(7):G684–9. doi: 10.1152/ajpgi.00332.2011. [DOI] [PubMed] [Google Scholar]
- 13.Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology. 2008;135(5):1601–11. doi: 10.1053/j.gastro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 14.O’Grady G, Angeli TR, Du P, et al. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143(3):589–98. doi: 10.1053/j.gastro.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lammers WJ, al-Kais A, Singh S, Arafat K, el-Sharkawy TY. Multielectrode mapping of slow-wave activity in the isolated rabbit duodenum. J Appl Physiol. 1993;74(3):1454–61. doi: 10.1152/jappl.1993.74.3.1454. [DOI] [PubMed] [Google Scholar]
- 16.Du P, O’Grady G, Egbuji JU, et al. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: methodology and validation. Ann Biomed Eng. 2009;37(4):839–46. doi: 10.1007/s10439-009-9654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson JC, O’Grady G, Du P, et al. Falling-edge, variable threshold (FEVT) method for the automated detection of gastric slow wave events in serosal high-resolution electrical recordings. Ann Biomed Eng. 2010;38(4):1511–29. doi: 10.1007/s10439-009-9870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angeli TR, O’Grady G, Erickson JC, et al. Mapping small intestine bioelectrical activity using high-resolution printed-circuit-board electrodes. Conf Proc IEEE Eng Med Biol Soc. 2011;1:4951–54. doi: 10.1109/IEMBS.2011.6091227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yassi R, O’Grady G, Paskaranandavadivel N, et al. The gastrointestinal electrical mapping suite (GEMS): software for analyzing and visualizing high-resolution (multi-electrode) recordings in spatiotemporal detail. BMC Gastroenterol. 2012;12:60. doi: 10.1186/1471-230X-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paskaranandavadivel N, O’Grady G, Du P, Cheng LK. Comparison of filtering methods for extracellular gastric slow wave recordings. Neurogastroenterol Motil. 2012 doi: 10.1111/nmo.12012. In press [doi: 10.1111/nmo.12012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson JC, O’Grady G, Du P, Egbuji JU, Pullan AJ, Cheng LK. Automated cycle partitioning and visualization of high-resolution activation time maps of gastric slow wave recordings: the Regional Growing Using Polynomial Surface-estimate stabilization (REGROUPS) algorithm. Ann Biomed Eng. 2011;39(1):469–83. doi: 10.1007/s10439-010-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potse M, Linnenbank AC, Grimbergen CA. Automated generation of isochronal maps in the presence of activation block. Int J Bioelectromagnetism. 2002;4(2):115–6. [Google Scholar]
- 23.Paskaranandavadivel N, O’Grady G, Du P, Pullan AJ, Cheng LK. An improved method for the estimation and visualization of velocity fields from gastric high-resolution electrical mapping. IEEE Trans Biomed Eng. 2012;59:882–9. doi: 10.1109/TBME.2011.2181845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41(1):9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- 25.Weiss JN, Qu Z, Chen P, et al. The dynamics of cardiac fibrillation. Circulation. 2005;112:1232–40. doi: 10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- 26.El-Sherif N, Mehra R, Gough WB, Zeiler RH. Reentrant ventricular arrhythmias in the late myocardial infarction period. Circulation. 1983;68(3):644–56. doi: 10.1161/01.cir.68.3.644. [DOI] [PubMed] [Google Scholar]
- 27.Mines GR. On dynamic equilibrium in the heart. J Physiol. 1913;46:349–82. doi: 10.1113/jphysiol.1913.sp001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 29.Ruurda JP, van Dongen KW, Dries J, Borel Rinkes IHM, Broeders IAMJ. Robot-assisted laparoscopic choledochojejunostomy. Surg Endosc. 2003;17(12):1937–42. doi: 10.1007/s00464-003-9008-x. [DOI] [PubMed] [Google Scholar]
- 30.O’Grady G, Egbuji JU, Du P, et al. High-resolution spatial analysis of slow wave initiation and conduction in porcine gastric dysrhythmia. Neurogastroenterol Motil. 2011;23:e345–55. doi: 10.1111/j.1365-2982.2011.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue S, Valdez DT, Tremblay L, Collman PI, Diamant NE. Electrical slow wave activity of the cat stomach: its frequency, gradient, and the effect of indomethacin. Neurogastroenterol Motil. 1995;7:157–167. doi: 10.1111/j.1365-2982.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 32.Forrest AS, Ordog T, Sanders KM. Neural regulation of slow-wave frequency in the murine gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2006;290:G486–95. doi: 10.1152/ajpgi.00349.2005. [DOI] [PubMed] [Google Scholar]
- 33.Lammers WJ, Stephen B, Slack JR, Dhanasekaran S. Anisotropic propagation in the small intestine. Neurogastroenterol Motil. 2002;14(4):357–64. doi: 10.1046/j.1365-2982.2002.00340.x. [DOI] [PubMed] [Google Scholar]
- 34.Hennig GW, Hirst GD, Park KJ, et al. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 2004;556(2):585–99. doi: 10.1113/jphysiol.2003.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park KJ, Hennig GW, Lee HT, et al. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006;290(5):C1411–27. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- 36.O’Grady G, Du P, Paskaranandavadivel N, et al. Rapid high-amplitude circumferential slow wave propagation during normal gastric pacemaking and dysrhythmias. Neurogastroenterol Motil. 2012;24(7):e299–312. doi: 10.1111/j.1365-2982.2012.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rensma PL, Allessie MA, Lammers WJEP, Bonke FIM, Schalij MJ. The length of the excitation wave and the susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 38.Spach MS, Josephson ME. Initiating re-entry: the role of nonuniform anisotropy in small circuits. J Cardiovasc Electr. 1994;5(2):182–209. doi: 10.1111/j.1540-8167.1994.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 39.Collin J, Kelly KA, Phillips SF. Absorption from the jejunum is increased by forward and backward pacing. Brit J Surg. 1979;66(7):489–92. doi: 10.1002/bjs.1800660712. [DOI] [PubMed] [Google Scholar]
- 40.Soper NJ, Geisler KL, Sarr MG, Kelly KA, Zinsmeister AR. Regulation of canine jejunal transit. Am J Physiol. 1990;259(6):G928–33. doi: 10.1152/ajpgi.1990.259.6.G928. [DOI] [PubMed] [Google Scholar]
- 41.Seidel SA, Hedge SS, Bradshaw LA, Ladipo JK, Richards WO. Intestinal tachyarrhythmias during small bowel ischemia. Am J Physiol. 1999;277(5):G993–9. doi: 10.1152/ajpgi.1999.277.5.G993. [DOI] [PubMed] [Google Scholar]
- 42.Lammers WJ, el-Kays A, Manefield GW, Arafat K, el-Sharkawy TY. Disturbances in the propagation of the slow wave during acute local ischaemia in the feline small intestine. Eur J Gastroenterol Hepatol. 1997;9(4):381–88. doi: 10.1097/00042737-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Bayguinov O, Hennig GW, Sanders KM. Movement artifacts may contaminate extracellular electrical recordings from GI muscles. Neurogastroenterol Motil. 2011;23:1029–42. doi: 10.1111/j.1365-2982.2011.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Grady G. Gastrointestinal extracellular electrical recordings: fact or artifact? Neurogastroenterol Motil. 2012;24:1–6. doi: 10.1111/j.1365-2982.2011.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Animation 2A and 2B These animations correspond directly with Figure 2 and show longitudinal slow wave propagation. Supplemental Animation 2A shows consistent antegrade propagation where slow wave activity traveled from orad (left) to aborad (right). Supplemental Animation 2B shows consistent retrograde propagation where slow wave activity traveled from aborad (right) to orad (left). Each square represents an electrode and lights up when the slow wave passes that electrode. Successive cycles of slow wave activity are colored differently for ease of visualization.
Supplemental Animation 3 This animation corresponds directly with Figure 3 and shows a focal pacemaker. Slow wave activity originated at a focal source and propagated in all directions, colliding circumferentially and establishing wavefronts that propagated both antegrade (aborad, right) and retrograde (orad, left).
Supplemental Animation 4A and 4B These animations correspond directly with Figure 3 and show functional re-entry of slow wave activity. In Supplemental Animation 4A, slow wave activity propagated as two wavelets in stable, repetitive clockwise and anticlockwise circuits around two distinct linear activation blocks, thus forming a figure-of-eight re-entrant circuit. Slow waves formed as ring wavefronts that propagated from the re-entrant circuit in both the antegrade (aborad, right) and retrograde (orad, left) directions. In Supplemental Animation 4B, slow wave activity propagated in a stable, repetitive anticlockwise circuit around a linear activation block, forming a continuous loop of activation and, thus, a re-entrant circuit. As above, slow waves formed ring wavefronts that propagated from the re-entrant circuit in both the antegrade (aborad, right) and retrograde (orad, left) directions.
Supplemental Animation 5A and 5B These animations correspond directly with Figure 4 and show circumferential re-entry of slow wave activity. In Supplemental Animation 5A, slow wave activity propagated in a stable, repeated circuit from the top (mesentery) to bottom (mesentery), and then reactivated the same circuit of tissue from the top of the animation. Since the electrode array was wrapped around the circumference of the intestine such that the top and bottom were nearly adjacent at the mesentery, this is best explained as a re-entrant slow wave circuit around the circumference of the intestine. As with functional re-entry (Figure 4, Supplemental Animation 4), slow waves formed ring wavefronts that propagated from the re-entrant circuit in both the antegrade (aborad, right) and retrograde (orad, left) directions. In Supplemental Animation 5B, another example of circumferential re-entry is shown, where slow wave activity propagated from the bottom (mesentery) to the top (mesentery), and then reactivated the same circuit of tissue at the bottom of the animation.