Abstract
The extracellular β-glucosidase from microorganisms is generally produced in low levels. Therefore, in this study, a β-glucosidase hyperproducing mutant was developed by multiple exposures of ethyl methyl sulfonate (EMS) and ultraviolet (UV) radiation (both individually and jointly) to Bacillus subtilis strain (PS). The developed mutants were screened, selected and characterized. The mutant, PS-UM1 developed after UV exposure alone, indicated a small increase in β-glucosidase production (718 U/l) in comparison to the wild-type strain, PS (675 U/l). The mutant, PS-CM5 developed after EMS exposure alone, displayed a slightly better production (762 U/l) than both the above strains. However, after exposure of the wild-type strain to both UV and EMS mutagens jointly, a better mutant (PS-CM5-UM3) was developed with 1.2-fold increase in production (806 U/l). Further, optimization of culture conditions by classical “one-variable-at-a-time” approach was done to determine the optimum, pH, temperature and nitrogen sources. The selected mutant (PS-CM5-UM3) produced up to 1,797 U/l enzyme and was found to be stable for ten generations. The β-glucosidase from the selected mutant (PS-CM5-UM3) was concentrated and purified using ammonium sulfate, dialysis and size-exclusion chromatography. The enzyme displayed maximal activity at 60 °C and it was found to be fairly stable at temperatures up to 70 °C for 30 min. Its molecular weight was determined to be around 60 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Keywords: β-Glucosidase, Bacillus subtilis, EMS mutagenesis, UV mutagenesis, SDS-PAGE, Size-exclusion chromatography
Introduction
β-Glucosidase (EC 3.2.1.21) together with endoglucanase (EC 3.2.1.4) and exoglucanase (EC 3.2.1.74) form a cellulase complex. All three enzymes work in a synergistic manner and hydrolyze the pretreated ligno-cellulosic biomass into glucose. β-Glucosidase catalyzes the final step in the cellulose hydrolysis, i.e., the breakdown of cellobiose into two glucose molecules. Thus, it plays an important role in improving the overall rate of cellulose degradation by removing the end product inhibition by cellobiose. β-Glucosidase catalyzes the hydrolysis of alkyl and aryl-β-glucosides, as well as diglucosides and oligosaccharides (Hoh et al. 1992; Mathew et al. 2008; Chen et al. 2012). β-Glucosidase is widely being used in biofuels, food, textile, leather, pulp and paper industries. It prevents the discoloration of fruit juices (Martino et al. 1994) and helps in the enzymatic release of aromatic compounds from glucosidic precursors present in fruits and fermenting products (Shoseyov et al. 1990; Guegen et al. 1996). Many natural flavor compounds such as monoterpenols, C-13 norisoprenoids and shikimate-derived compounds accumulate in fruits as flavorless precursors linked to mono or di-glycosides and require enzymatic or acidic hydrolysis for the liberation of their fragrances (Vasserot et al. 1995; Winterhalter and Skouroumounis 1997).
β-Glucosidase can be isolated from different sources such as plants, animals and microorganisms. Much research has been carried out on β-glucosidase of fungal origin (Mendels et al. 1971; Bailey and Nevalainene 1981; Sheir-Neiss and Montenecourt 1984; Gokhale et al. 1988; Durand et al. 1988; Rajoka et al. 1998, 2003, 2004; Chand et al. 2005; Dillon et al. 2006; Adsul et al. 2007; Chandra et al. 2009; Jun et al. 2009), but very less attention has been paid to bacterial sources (Zhou et al. 2008; Rashid and Siddiqui 1998). The production level of β-glucosidase in both fungal and bacterial culture filtrates is usually very low to be of any practical use (Lynd et al. 2002). Therefore, hyperproduction of β-glucosidase with better thermotolerant properties is required for industrial applications (Maki et al. 2009).
In this study, a selected Bacillus subtilis strain (PS) was improved with both physical (ultraviolet radiation) and chemical (ethyl methane sulfonate) mutagenesis. The developed mutants were screened and evaluated for enzyme production level. The β-glucosidase produced from a selected mutant was concentrated, purified and charecterized.
Materials and methods
Chemicals
Ethyl methyl sulfonate (EMS), p-nitrophenol β-d-glucopyranoside (pNPG), Luria–Bertani (LB) broth, Nutrient agar and other chemicals were procured from HiMedia Lab. Pvt. Ltd., Mumbai, India.
Microorganism and culture conditions
The B.subtilis strain PS, used in this study, was originally isolated from sugarcane bagasse (Rawat 2009). The LB broth was used to grow the bacterial strain. It was maintained as 2 ml glycerol stocks in cryovials and kept at −20 °C for long-term storage.
Mutagenesis and screening of mutants
A fresh active culture of B. subtilis strain PS was grown in LB media after overnight incubation at 37 °C from a glycerol stock. This strain was further cultured in 20 ml minimal media (containing K2HPO4 (5.8 g), KH2PO4 (4.5 g), (NH4)2SO4 (2.0 g), MgSO4 (0.16 g), Na2MoO4 (0.002 g), CaCl2 (0.02 g), FeSO4 (0.001 g), MnCl2 (0.001 g) and glucose (20 g) per liter of distilled water) with pH of 6.8 (Kumar et al. 2008). The bacterial cells were harvested at log phase (after 48 h of incubation at 37 °C) by centrifugation at 10,000 g for 10 min. The cells were further resuspended in 20 ml of minimal buffer (containing K2HPO4 (10.5 g), KH2PO4 (4.5 g), (NH4)2SO4 (1 g) and sodium citrate·2H2O (0.5 g) per liter). For EMS mutagenesis, 2 ml of diluted suspension of B. subtilis was treated with different concentrations of EMS (0.01, 0.02, 0.03, 0.04 EMS in ml) and incubated at 37 °C in an incubator shaker, each for 0, 10, 20, 30 and 40 min. On the other hand, ultraviolet fluorescence analysis (Macros Scientific Works Pvt. Ltd., India) was employed for UV mutagenesis. The UV lamp was about 20 cm above the surface of the cell suspension. During 30 min of the treatment process, 1 ml aliquots were withdrawn and placed on an ice bath for 5 min and then kept in the dark for 30 min. In both cases, all the collected aliquots were then serially diluted up to 10−6 and inoculated onto minimal media plates containing 200 μg of 5-bromo-4-chloro-3-indolyl-β-d-glucopyranoside (X-glucoside) with 0.2 % (w/v) cellobiose (minimal media-X-glucoside-cellobiose plates). The plates were incubated at 37 °C for 72 h and LD50 was determined. The potential EMS-generated mutant (PS-CM5) was further exposed to UV radiation. Screening and selection of potential mutants were done based on their β-glucosidase production. The positive strains producing β-glucosidase were identified as blue-colored colonies among the negative strains that were white colonies. Colonies were picked from minimal media plate on the basis of their color intensity and size. The selected colonies were further screened for higher enzyme production and genetic stability.
β-Glucosidase production in parent and mutant strains
The parent strain (PS) and mutants were batch cultured in minimal medium supplemented with 0.1 % glucose and incubated for 72 h at 37 °C in a 120 rpm orbital shaker. β-Glucosidase activity was assayed by incubating the reaction mixture containing 10 mM p-nitrophenyl-β-d-glucopyranoside (pNPG) and culture filtrate in 1:1 ratio for 1 h at 37 °C. The reaction was stopped by adding 2 ml of 1 M Na2CO3. The p-nitrophenol release was monitored at λ405 nm in UV–Vis spectrophotometer (Jäger et al. 2001). Protein content was determined by the method of Bradford (Bradford 1976). One unit (U) of β-glucosidase was defined as the amount of the enzyme to produce one μmole p-nitrophenol per minute under the assay conditions and the specific activity was defined as the number of units per mg of protein. β-Glucosidase production was calculated as enzyme units produced per liter of media (U/l) (Rajoka et al. 2004). In this step, only the best performing mutants (one each from the three mutagenesis strategies) were selected for further studies.
Genetic stability of selected mutant strains
The genetic stability of the selected mutants was determined by measuring the levels of β-glucosidase production for successive generations. The interval for each generation was 15 days (Jun et al. 2009).
Effect of incubation time on β-glucosidase production in parent and mutant strains
The effect of incubation period on β-glucosidase production by parent (PS) and selected mutant strain (PS-CM5-UM3) was studied. The growth and β-glucosidase production of both strains were determined at every interval of 2 h for 36 h at 37 °C.
Optimization of culture conditions
The culture conditions were optimized by the classical “one-variable-at-a-time approach”. Minimal broth with different pH (3–9) and incubation temperatures (20–70 °C) were studied for 30 h. The bacterial growth and β-glucosidase production were studied up to 30 h of incubation period. The effect of various nitrogen sources on β-glucosidase production was also studied. Organic nitrogen sources (peptone, yeast extract, methionine and glycine) at 1 % concentration and inorganic sources (urea, potassium nitrate and ammonium nitrate) at 0.1 % concentration were added to the minimal media. Culture growth was measured at 600 nm, while β-glucosidase production was measured as enzyme units produced per liter (U/l).
Partial purification of β-glucosidase
Ammonium sulfate was added to the cell-free culture supernatant up to 75 % saturation and the solutions were kept at 4 °C overnight. The precipitated protein was collected by centrifugation at 10,000 rpm for 20 min at 4 °C. The supernatant was decanted and the pellet was dissolved in minimum amount of acetate buffer (0.1 M, pH 5.0). The protein content and β-glucosidase activity were measured in each fraction. The ammonium sulfate fraction was carefully transferred into the dialysis tubes (10 cm) activated by boiling in solution containing 2 % (w/v) NaHCO3 and 1 mM EDTA. This was followed by washing repeatedly with lukewarm water. The ammonium sulfate fractions were dialyzed against 0.1 M acetate buffer (pH 5.0) on magnetic stirrer at 4 °C for 10–12 h with repeated changes of buffer. The partially purified fraction was concentrated against crystals of sucrose. All steps were performed at 4 °C. The fraction obtained was then assayed for protein content and β-glucosidase activity.
Size-exclusion chromatography with Sephadex-100
The partially purified β-glucosidase was further purified using Sephadex G-100 (size-exclusion limit 4–150 kDa) gel filtration columns (0.5 × 20 cm). The standard molecular weight marker proteins used for the determination of molecular weight were catalase (240 kDa), bovine serum albumin (66 kDa), ovalbumin (43 kDa) and lysozyme (14.3 kDa). 3 ml of the isolated concentrated protein sample (2.8 mg/ml) was applied on the column and eluted with a flow rate of 1 ml per minute. Fractions (1.5 ml) were collected. The protein content and the β-glucosidase activity in each of these fractions were determined by taking the absorbance at λ280 nm.
Analysis of protein samples by SDS (sodium dodecyl sulfate) polyacrylamide gel electrophoresis
The SDS-PAGE of partially purified protein samples was done using the method described by Laemmli (1970) with 10 % separating gel and 5 % stacking gel. After the preparation of the gel, the enzyme obtained after gel-filtration chromatography containing 40 μg of protein was loaded on the gel. The gel was run under a constant voltage of 80 volt till the sample was in stacking gel and after that the voltage was raised to 100 volts. The gel was allowed to run till the dye front reached 0.5 cm from the lower edge of the gel.
Determination of thermostability
The optimum temperature for the purified β-glucosidase was determined by incubating the reaction mixture at different temperatures; ranging from 30 to 70 °C for 30 min. The β-glucosidase activity was determined as described above and OD was taken at λ405 nm. The graph was plotted for temperature versus β-glucosidase activity (% of maximum).
Statistical analysis
Graphs were plotted either using Origin 6.0 or Microsoft Excel 2003. Each value in graphs and tables represents the mean of triplicate measurement and expressed as mean ± standard error at p < 0.05.
Results and discussion
Mutagenesis and screening/selection of mutants
Random mutagenesis using chemical agents and UV irradiation is an easy tool to achieve genetic and functional modifications of an organism. For mutagenesis experiments, 50 % lethality dose (LD50) for both EMS mutagenesis and UV mutagenesis was determined. It was found to be 0.03 ml EMS exposure for 20 min and 25 min exposure to far UV source from a distance of 20 cm. Chandra et al. (2009) have found that 99 % killing of Trichoderma reesei spores was achieved using N-methyl-N′-nitro-N-nitrosoguanidine (NG) for 6 h, followed by UV irradiation for 15 min. Santiago et al. (2006) reported that 50 % lethality was achieved after 3 min UV irradiation of Aspergillus niger spore suspension.
On the basis of color intensity and the size of the colonies on X-glucoside-containing plates, eight mutants after EMS mutagenesis, three after UV mutagenesis and nine after EMS plus UV mutagenesis were recovered and screened on the basis of their β-glucosidase production level (Table 1). Three mutants (PS-UM1, PS-CM5 and PS-CM5-UM3) were selected after primary screening for further studies. β-Glucosidase production in mutant PS-CM5-UM3 (806.10 U/l) was found to be better in comparison to other mutants (762.53 U/l for PS-UM1 and 806.10 U/l for PS-CM5, respectively). Therefore, under the same conditions, the mutant PS-CM5-UM3 showed 1.2-fold increase in β-glucosidase production over the parent strain PS. In a similar study, the mutant strain of B. amyloliquefaciens gave around 1.4-fold higher alpha amylase activity than the parental strain after mutation with EMS and UV radiations (Haq et al. 2010). Dillon et al. 2006 also reported 1.5-fold more cellulase productivity in Penicillium echinulatum by three repeated mutagenic treatment steps of UV, EMS and H2O2 (Durand et al. 1988). Chandra et al. (2009) have suggested that the enhancement of enzyme activities might be due to increased level of modulator proteins or co-factors promoting the enzyme activities.
Table 1.
β-glucosidase production in parent strain and screened mutants
| Strain/mutant | β-glucosidase production (U/l) |
|---|---|
| Without mutagenesis | |
| PS (parent) | 675.38 ±28.43 |
| EMS mutagenesis | |
| PS-CM1 | 108.93 ± 4.56 |
| PS-CM2 | 196.08 ± 8.96 |
| PS-CM3 | 544.66 ± 26.67 |
| PS-CM4 | 65.36 ± 2.88 |
| PS-CM5 | 762.53 ± 36.54 |
| PS-CM6 | 283.22 ± 13.21 |
| PS-CM7 | 675.38 ± 29.75 |
| PS-CM8 | 718.95 ± 34.43 |
| UV-mutagenesis | |
| PS-UM1 | 718.95 ± 34.08 |
| PS-UM2 | 65.36 ± 2.98 |
| PS-UM3 | 457.52 ± 20.45 |
| EMS plus UV-mutagenesis | |
| PS-CM5-UM1 | 762.53 ± 38.12 |
| PS-CM5-UM2 | 283.22 ± 15.02 |
| PS-CM5-UM3 | 806.10 ± 38.98 |
| PS-CM5-UM4 | 108.93 ± 5.34 |
| PS-CM5-UM5 | 631.81 ± 28.60 |
| PS-CM5-UM6 | 675.38 ± 32.04 |
| PS-CM5-UM7 | 21.79 ± 1.08 |
| PS-CM5-UM8 | 65.36 ± 3.42 |
| PS-CM5-UM9 | 326.80 ±12.74 |
Genetic stability of mutants
The genetic stability of the selected mutants (PS-UM1, PS-CM5 and PS-CM5-UM3) was determined by measuring the β-glucosidase production for ten successive generations. The PS-CM5 mutant strain was unstable in comparison to other selected mutants (PS-UM1 and PS-CM5-UM3). PS-UM1 was stable up to the eighth generation, while PS-CM5-UM3 was stable up to the tenth generation with 78 % of initial β-glucosidase production (Fig. 1). Hence, PS-CM5-UM3 was finally selected and characterized. In a similar report, fungal T. reesei mutant was found to be quite stable for cellulases and xylanase production up to seven generations (Jun et al. 2009).
Fig. 1.
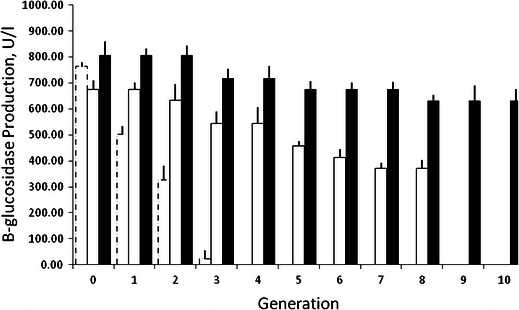
Genetic stability evaluation for the selected mutants. The genetic stability of the desired mutants was determined by measuring the levels of β-glucosidase production up to ten successive generations; the interval for each generation was 15 days. Mutant PS-CM5 (empty bar with dashed line); mutant PS-UM1 (empty bar with solid line); and mutant, PS-CM5-UM3 (black)
Growth and β-glucosidase production levels of parent and mutant strains
The selected mutant (PS-CM5-UM3) secreted two times higher β-glucosidase (1,302 U/l) after 20 h of incubation (Fig. 2) in comparison to the parent B. subtilis strain PS (680 U/l after 30 h). A similar finding was reported in the reduction of α-amylase production time in B. amyloliquefaciens from 72 to 48 h after mutation (Haq et al. 2010).
Fig. 2.
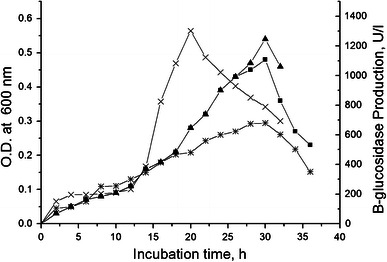
Growth and β-glucosidase production in parent and mutant strain. Both parent and mutant grow in minimal media up to 36 h. Growth was measured at OD600nm and β-glucosidase production was measured by standard method after 2 h interval. Growth of parent strain PS (filled squares); growth of mutant PS-CM5-UM3 (filled triangles); enzyme production of parent strain, PS (asterisks); and enzyme production of mutant PS-CM5-UM3 (times symbols)
Effect of pH and temperature on bacterial growth and β-glucosidase production
The maximum β-glucosidase production in parent (PS) and mutant (PS-CM5-UM3) was found at pH 7.0, i.e., 718 U/l and 1,209 U/l, respectively (Fig. 3a, b). Both at lower and higher pH than 7.0, poor growth was observed in both the parent and mutant strains with only meager amounts of β-glucosidase production. Similarly, maximum β-glucosidase production in parent (PS) and mutant (PS-CM5-UM3) was found with incubation at a temperature of 37 °C, i.e., 675 and 1,144 U/l, respectively. Temperatures lower or higher than 37 °C suppressed the growth and β-glucosidase production in both the parent and mutant strains.
Fig. 3.
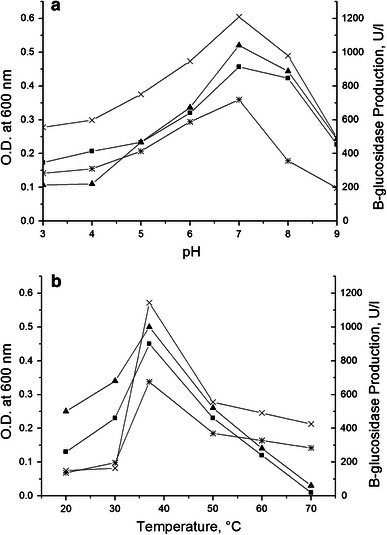
Growth and β-glucosidase production in parent and mutant strain at different pH (a) and temperatures (b). Growth of parent strain, PS (filled squares); growth of mutant strain, PS-CM5-UM3 (filled triangles); enzyme production of parent strain, PS (asterisks); and enzyme production of mutant strain, PS-CM5-UM3 (times symbols) strain of B. Subtilis
Effect of nitrogen sources on β-glucosidase production
Different nitrogen sources play a critical role in enzyme production. Therefore, the effect of various nitrogen sources on bacterial growth and β-glucosidase production was assessed. In parent strain PS, the maximum β-glucosidase production (1,198 U/l) was achieved when (NH4)2SO4 was used as nitrogen source, while the mutant PS-CM5-UM3 produced maximally (1,797 U/l) when peptone was used as the nitrogen source in minimal media. The β-glucosidase production level of mutant was 1.5 times higher than the parent strain in the presence of peptone (Table 2). PS-CM5-UM3 also showed increased β-glucosidase production (1,536 U/l) with (NH4)2SO4. Recently, Aparna et al. (2012) have reported that the choice of nitrogen source affects the biosurfactant production from Bacillus clausii strain. Demirkan (2011) has reported that tryptone was found to be better for amylase production by the parental and mutant strains in comparison to other nitrogen sources. Daroit et al. (2007); Barbosa et al. (2010) and Joo et al. (2010) have indicated that the production level of β-glucosidase was decreased when potassium nitrate was used as the nitrogen source. Aiyer (2004) and Felix et al. (1996) have reported that (NH4)2HPO4 was found to be the best nitrogen source for amylase production. Thippeswamy et al. (2006) have found that among the different nitrogen sources, peptone and yeast extract produced maximum amylase. Hence, the above reports suggest that diverse nitrogen sources may affect the production level of substrates differently. As no clear-cut explanation can be found in the above reports, it is imperative to study the effect of different nitrogen sources.
Table 2.
Effect of nitrogen source on β-glucosidase production in parent and mutant
| Nitrogen source | β-glucosidase production (U/l) | |
|---|---|---|
| Parent strain (PS) | Mutant strain (PS-CM5-UM3) | |
| Control | 675.38 ± 26.80 | 1,339.87 ± 52.48 |
| 1 % Peptone | 762.53 ± 30.45 | 1,797.39 ± 53.54 |
| 1 % Yeast extract | 936.82 ± 39.00 | 1,666.67 ± 75.08 |
| 0.1 % Urea | 239.65 ± 9.21 | 816.99 ± 34.00 |
| 0.1 % Potassium nitrate | 21.79 ± 0.83 | 32.68 ± 0.93 |
| 0.1 % Ammonium sulfate | 1,198.26 ± 42.33 | 1,535.95 ± 59.32 |
| 1 % Methionine | 457.52 ± 16.74 | 359.48 ± 62.54 |
| 1 % Glycine | 283.22 ± 11.34 | 163.40 ± 6.76 |
Partial purification of β-glucosidase and its characterization
β-Glucosidase from a mutant of Bacillus subtilis was successfully purified through ammonium sulfate precipitation, Sephadex G-100 and column chromatography (Table 3). β-Glucosidase was purified fivefold with 40 % retention of total extracellular activity. The specific activity of the purified enzyme was 391 U/mg of protein. These results coincide with those obtained by Han and Chen 2008, who obtained a 5.3-fold purification with 44 % recovery after purification through CM Sepharose and 16-fold purification with 21.5 % recovery after purification through Sephadex-75. SDS-PAGE analysis of the purified β-glucosidase indicated the presence of a single band when stained with Coomassie Blue R-250 (Fig. 4). Its apparent molecular mass was about 60 kDa. Han and Chen (2008) reported that β-glucosidase is represented by a single band of Mw 62.4 kDa. Karnchanatat et al. (2007) also reported that, SDS-PAGE analysis of the purified β-glucosidase showed the presence of a single band when stained with Coomassie BlueR-250 and its apparent molecular mass was about 64.2 kDa.
Table 3.
Partial purification of β-glucosidase
| Total activity (U) | Total protein (mg) | Specific activity (U/mg) | Purification (fold) | Yield (%) | |
|---|---|---|---|---|---|
| Culture filtrate | 1,264.05 | 28.33 | 44.61 | 1 | 100 |
| Ammonium sulfate precipitation | 850.19 | 10.93 | 77.761 | 1.74 | 67.25 |
| Dialysis | 611.92 | 7.08 | 86.38 | 1.93 | 48.41 |
| Sephadex G-100 (gel filteration) | 339.21 | 0.86 | 391.08 | 5.02 | 39.89 |
Fig. 4.
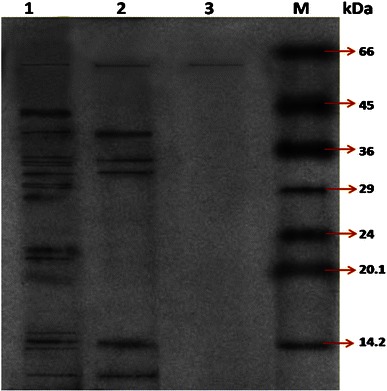
SDS-PAGE of purified β-glucosidase from mutant PS-5CM-UM3: lane 1, crude protein; lane 2, ammonium sulfate precipitate; lane 3, gel filtration chromatography, M molecular weight marker of protein standard
Thermostability of partially purified β-glucosidase
Thermostability of the partially purified β-glucosidase enzyme at different temperatures was monitored. The enzyme displayed maximal activity at 60 °C (Fig. 5) and it was found to be fairly stable at temperatures up to 70 °C for 30 min (Fig. 6). This is in contrast to the β-glucosidases isolated from corn stover which showed maximal activity around 37 °C, and showed depressed activity below 30 °C and above 40 °C. A total loss in activity was observed at 60 °C for 100 min and 70 °C for 1 min (Han and Chen 2008). Temperature stability of β-glucosidase from other sources has been reported to range from 50 to 65 °C (Christakopoulos et al. 1994; Yan and Lin 1997; Yazdi et al. 2003; Karnchanatat et al. 2007).
Fig. 5.
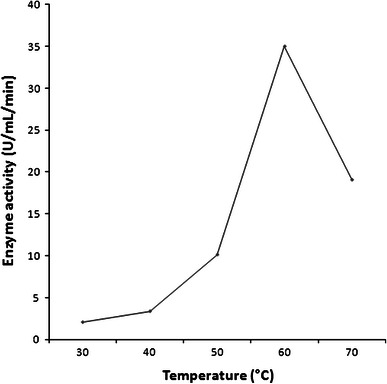
Optimization of incubation temperature for purified β-glucosidase
Fig. 6.
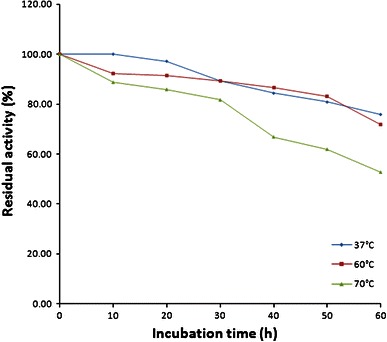
Thermostability of purified β-glucosidase at 37 °C (filled diamonds), 60 °C (filled squares) and 70 °C (filled triangles)
Acknowledgments
We acknowledge the support from the Department of Science and Technology (DST) (Govt. of India), New Delhi, for the award of fellowship and facilities under DST-FIST programme.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Ruchi Agrawal, Email: drruchiagrawal010@gmail.com.
Ashok Kumar Verma, Phone: +91-9412981859, FAX: +91-5944233473, Email: akv72@rediffmail.com.
References
- Adsul MG, Bastawde KB, Varma AJ, Gokhale DV. Strain improvement of Penicillium janthinellum NCIM 1171 for increased cellulase production. Biores Technol. 2007;98:1467–1473. doi: 10.1016/j.biortech.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Aiyer PVD. Effect of C:N ratio on alpha amylase production by Bacillus licheniformis SPT 27. Afr J Biotechnol. 2004;3:519–522. doi: 10.5897/AJB2004.000-2103. [DOI] [Google Scholar]
- Aparna DG, Reddy DSR (2012) Production of Lovastatin by Aspergillus parasiticus NCIM 696 using rice bran under solid state fermentation. J Chem Biol Phys Sci 2:284–291
- Bailey MJ, Nevalainene KMH. Induction, isolation and testing of stable Trichoderma reesei mutants with improved production of solubilizing cellulose. Enz Microb Technol. 1981;3:153–157. doi: 10.1016/0141-0229(81)90076-4. [DOI] [Google Scholar]
- Barbosa AM, Giese EC, Dekker RFH, Borsato D, Pérez AIB, Iranzo JFU. Extracellular b-glucosidase production by the yeast Debaryomyces pseudopolymorphus UCLM-NS7A: optimization using response surface methodology. N Biotechnol. 2010;27:374–381. doi: 10.1016/j.nbt.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Ann Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chand P, Aruna A, Maqsood AM, Rao LV. Novel mutation method for increased cellulase production. J Appl Microbiol. 2005;98:318–323. doi: 10.1111/j.1365-2672.2004.02453.x. [DOI] [PubMed] [Google Scholar]
- Chandra M, Kalra A, Sangwan NS, Gaurav SS, Darokar MP, Sangwan RS. Development of a mutant of Trichoderma citrinoviride for enhanced production of cellulases. Biores Technol. 2009;100:1659–1662. doi: 10.1016/j.biortech.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Chen HL, Chen YC, Lu MYJ, Chang JJ, Wang HTC, Wang TY, Ruan SK, Wang TY, Hung KY, Cho HY, Ke HM, Lin WT, Shih MC, Li WH. A highly efficient beta-glucosidase from a buffalo rumen fungus Neocallimastix patriciarum W5. Biotechnol Biofuels. 2012;5:24. doi: 10.1186/1754-6834-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakopoulos P, Goodenough PW, Kekos D, Macris BJ, Claeyssens M, Bhat MK. Purification and characterisation of an extracellular beta-glucosidase with transglycosylation and exo-glucosidase activities from Fusarium oxysporum. Eur J Biochem. 1994;224:379–385. doi: 10.1111/j.1432-1033.1994.00379.x. [DOI] [PubMed] [Google Scholar]
- Daroit DJ, Silveira ST, Hertz PF, Brandelli A. Production of extracellular β-glucosidase by Monascus purpureus on different growth substrates. Process Biochem. 2007;42:904–908. doi: 10.1016/j.procbio.2007.01.012. [DOI] [Google Scholar]
- Demirkan E. Production, purification, and characterization of α-amylase by Bacillus subtilis and its mutant derivates. Turk J Biol. 2011;35:705–712. [Google Scholar]
- Dillon AJP, Zorgi C, Camassola M, Henriques JA. Use of 2-deoxyglucose in liquid media for the selection of mutant strains of Penicillium echinulatum production increased cellulase increased cellulase and β-glucosidase activities. Appl Microbiol Biotechnol. 2006;70:740–746. doi: 10.1007/s00253-005-0122-7. [DOI] [PubMed] [Google Scholar]
- Durand H, Clanet M, Tiraby G. Genetic improvement of T. Reesei for large scale cellulase production. Enz Microb Technol. 1988;10:341–345. doi: 10.1016/0141-0229(88)90012-9. [DOI] [Google Scholar]
- Felix CR, Astolfi SF, Ulhoa CJ. Purification and characterization of truncated Bacillus subtilis alpha-amylase produced by Escherichia coli. Appl Microbiol Biotechnol. 1996;44:746–752. doi: 10.1007/BF00178613. [DOI] [PubMed] [Google Scholar]
- Gokhale DV, Puntambekar US, Deobagkar DN, Peberdy JF. Production of cellulolytic enzymes by mutant of Aspergillus niger NCIM 1207. Enz Microb Technol. 1988;10:442–445. doi: 10.1016/0141-0229(88)90040-3. [DOI] [Google Scholar]
- Guegen Y, Chemardin P, Janbon G, Arnaud A, Galzy P. A very efficient β-glucosidase catalyst for the hydrolysis of flavor precursors of wines and fruit juices. J Agri Food Chem. 1996;44:2336–2340. doi: 10.1021/jf950360j. [DOI] [Google Scholar]
- Han Y, Chen H. Characterization of β-glucosidase from corn stover and its application in simultaneous saccharification and fermentation. Biores Technol. 2008;99:6081–6087. doi: 10.1016/j.biortech.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Haq I, Ali S, Javed MM, Hameed U, Saleem A, Adnan F, Qadeer MA. Production of alpha amylase from a randomly induced mutant strain of Bacillus amyloliquefaciens and its application as a desizer in textile industry. Pak J Bot. 2010;42:473–484. [Google Scholar]
- Hoh YK, Yeoh HH, Tan TK. Properties of β-glucosidase purified from Aspergillus niger mutants USDB 0827 and USDB 0828. Appl Microbiol Biotechnol. 1992;37:590–593. [Google Scholar]
- Jäger S, Brumbauer A, Fehér E, Réczey K, Kiss L. Production and characterization of β-glucosidases from different Aspergillus strains. World J Microbiol Biotechnol. 2001;17:455–461. doi: 10.1023/A:1011948405581. [DOI] [Google Scholar]
- Joo AR, Jeya M, Lee KM, Moon HJ, Kimd YS, Lee JK. Production and characterization of β-1,4-glucosidase from a strain of Penicillium pinophilum. Process Biochem. 2010;45:851–858. doi: 10.1016/j.procbio.2010.02.005. [DOI] [Google Scholar]
- Jun H, Bing Y, Keying Z, Xuemei D, Daiwen C. Strain improvement of Trichoderma reesei Rut C-30 for increased cellulase production. Ind J Microbiol. 2009;49:188–195. doi: 10.1007/s12088-009-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnchanatat A, Petsom A, Sangvanich P, Piaphukiew J, Whalley AJS, Reynolds CD, Sihanonth P. Purification and biochemical characterization of an extracellular β-glucosidase from the wood-decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.)Rehm. FEMS Microbiol Lett. 2007;270:162–170. doi: 10.1111/j.1574-6968.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- Kumar R, Singh S, Singh OV. Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol. 2008;35:377–391. doi: 10.1007/s10295-008-0327-8. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lynd LR, Weimer PJ, Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66:506–577. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki M, Leung KT, Qin W. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci. 2009;5(5):500–516. doi: 10.7150/ijbs.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino A, Pifferi PG, Spagna G. Production of β-glucosidase by Aspergillus niger using carbon sources derived from agricultural wastes. J Chem Technol Biotechnol. 1994;60:247–252. doi: 10.1002/jctb.280600304. [DOI] [Google Scholar]
- Mathew GM, Sukumaram RK, Singhania RR, Pandey A. Progress in research on fungal cellulases for lignocellulose degradation. J Sci Ind Res. 2008;67:898–907. [Google Scholar]
- Mendels M, Weber J, Parizek R. Enhanced cellulase production by a mutant of Trichoderma viride. Appl Microbiol. 1971;21:152–154. doi: 10.1128/am.21.1.152-154.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajoka MI, Bashir A, Hussain MRA, Malik KA. Mutagenesis of Cellulomonas biazotea for improved production of cellulases. Folia Microbiol. 1998;43:15–22. doi: 10.1007/BF02815534. [DOI] [Google Scholar]
- Rajoka MI, Ashraf Y, Rashid H, Khalid AM. Kinetics and thermodynamics of the native and mutated extracellular endoglucanases from Cellulomonas biazotea. Protein Pept Lett. 2003;10:1–8. doi: 10.2174/0929866033478609. [DOI] [PubMed] [Google Scholar]
- Rajoka MI, Durrani IS, Khalid AM. Kinetics of improved production and thermostability of an intracellular β-glucosidase from a mutant derivative of Cellulomonas biazotea. Biotechnol Lett. 2004;26:281–285. doi: 10.1023/B:BILE.0000015426.74418.07. [DOI] [PubMed] [Google Scholar]
- Rashid MH, Siddiqui KS. Thermodynamic and kinetic study of stability of the native and chemically modified β-glucosidase from Aspergillus niger. Process Biochem. 1998;33:109–115. doi: 10.1016/S0032-9592(97)00036-8. [DOI] [Google Scholar]
- Rawat P (2009) Determination of physico-chemical and kinetic parameters of partially purified β-glucosidase of bacterial isolate from bagasse. M Sc Thesis, G B P U A and T, Pantnagar, Uttarakhand, India
- Santiago SDN, González CR, Almendárez BG, Fernández FJ, Jurado AT, Ochoa SH. Physiological, morphological, and mannanase production studies on Aspergillus niger uam-gs1 mutants. Electron J Biotechnol. 2006;9:50–60. doi: 10.2225/vol9-issue1-fulltext-2. [DOI] [Google Scholar]
- Sheir-Neiss G, Montenecourt BS. Characterization of the secreted cellulases of Trichoderma reesei parent type and mutants during controlled fermentations. Appl Microbiol Biotechnol. 1984;20:46–53. [Google Scholar]
- Shoseyov O, Bravdo BA, Ikan R, Chet I. Immobilized endo-β-glucosidase enriches flavor of wine and passion fruit juice. J Agri Food Chem. 1990;27:1973–1976. [Google Scholar]
- Thippeswamy S, Girigowda K, Mulimani VH. Isolation and identification of α-amylase producing Bacillus sp. from dhal industry waste. Indian J Biochem Biophys. 2006;43:295–298. [PubMed] [Google Scholar]
- Vasserot Y, Arnaud A, Galzy P. Monoterpenyl glycosides in plants and their biotechnological transformation. Acta Biotechnol. 1995;15:77–95. doi: 10.1002/abio.370150110. [DOI] [Google Scholar]
- Winterhalter P, Skouroumounis GK. Glycoconjugated aroma compounds: occurrence, role and biotechnological transformation. Adv Biochem Eng Biotechnol. 1997;55:73–105. doi: 10.1007/BFb0102063. [DOI] [PubMed] [Google Scholar]
- Yan TR, Lin YH. Purification and characterization of a glucose-tolerant β-glucosidase from Aspergillus niger CCRC 31494. Biosci Biotechnol Biochem. 1997;61:965–970. doi: 10.1271/bbb.61.965. [DOI] [PubMed] [Google Scholar]
- Yazdi MT, Khosravi AA, Nemati M, Davoodi N. Purification and characterization of two intracellular beta-glucosidases from the Neurospora crassa mutant cell-1. World J Microbiol Biotechnol. 2003;19:79–84. doi: 10.1023/A:1022531706036. [DOI] [Google Scholar]
- Zhou J, Wang YH, Chu J, Zhuang YP, Zhang L, Yin P. Identification and purification of the main components of cellulases from a mutant strain of Trichoderma viride T 100-14. Biores Technol. 2008;99:6826–6833. doi: 10.1016/j.biortech.2008.01.077. [DOI] [PubMed] [Google Scholar]


