Abstract
Plant proteomics has made tremendous contributions in understanding the complex processes of plant biology. Here, its current status in India and Nepal is discussed. Gel-based proteomics is predominantly utilized on crops and non-crops to analyze majorly abiotic (49 %) and biotic (18 %) stress, development (11 %) and post-translational modifications (7 %). Rice is the most explored system (36 %) with major focus on abiotic mainly dehydration (36 %) stress. In spite of expensive proteomics setup and scarcity of trained workforce, output in form of publications is encouraging. To boost plant proteomics in India and Nepal, researchers have discussed ground level issues among themselves and with the International Plant Proteomics Organization (INPPO) to act in priority on concerns like food security. Active collaboration may help in translating this knowledge to fruitful applications.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-013-0198-y) contains supplementary material, which is available to authorized users.
Keywords: Proteomics, Plants, Agriculture, Food security, Abiotic stress, Biotic stress
Ground-level issues in agriculture, biodiversity and sustainability
The Green revolution of the sixties led agricultural practices in the Indian subcontinent to improve, and fulfil the ever-increasing demand for plant-based foods. This was concomitant with the consumption of large doses of inorganic fertilizers and chemical pesticides to sustain crop productivity. Despite the transformation of India into a “bread basket”, a negative aspect of the then generally beneficial model, was the erosion of crop biodiversity through increasing industrialization of agriculture. This also gave rise to other ecological problems, such as decreasing soil fertility, chemical pollution of land and water resources, pesticide poisoning, and pest infestation due to growing pest resistance to pesticides. Agricultural crops or domesticated plants went through cycles of selections depending on needs and important traits. The traits kept changing as per the demands of particular eco-geography and/or demography, while the cycle of selection continued with limited number of genotypes in particular crop species. This practice resulted in a narrow primary or secondary gene pool of crop plants and their related species causing breeding depression, low polymorphism, and allied problems. Therefore, harnessing the available diversity only within crossable genotypes and/or species now limits conventional breeding. In fact, agriculture on the Indian subcontinent is once again at the crossroads. Further development of sustainable solutions will require deeper understanding of traits at molecular level that are critical in exploring and exploiting the existing molecular diversity in native plants. Molecular understanding of fertility barriers would be equally critical to utilize the well characterized, wider germplasm collections readily available for cereal crops.
Managing biotic and abiotic stresses in crop production remains a huge challenge. This is compounded with the perpetually rising numbers of pests and pathogens due to changing climate and pest/pathogen adaptation to chemicals. Harnessing the existing biodiversity in crop plants using their proximate species appears to be a sensible and straightforward approach. However, in reality it is highly complex and needs a deeper understanding of molecules at the transcript, protein and metabolite level. Genomics and transcriptomics approaches are becoming more feasible even in non-model plant systems due to tremendous advancements with invention of cheaper technologies in nucleic acid sequencing. Nonetheless, functional validation at the proteome level (enzymes, structural and regulatory proteins) remains elusive. Functional characterization of proteins with minor variations, in addition to the sophisticated and intricate mechanisms of their regulation and species/genome-wide variation, adds to the complexity and thus to the difficulty of understanding their specific roles.
Addressing the issues and where we are
Over the next 50 years, estimates project the world population to reach 9–12 billion, demanding 34–70 % increase in food production (FAO 2009, 2010). Green Revolution solved the problem of food insecurity during the last critical phase. However, because the principles of environmental sustainability were compromised, the modus of the first Green Revolution is impractical and cannot deliver any further increase in food production.
In order to avoid scarcity and maintain sustainability in agriculture, a combination of traditional and modern approaches, are being implemented. These include expansion of the cultivated land area, increase in cropping intensity (multiple cropping and shorter fallow periods, often through irrigation), zero-tilling, pollution control, and protection of the environment. Organic agriculture is a rapidly growing sector that espouses socio-economically and eco-geographically healthy agriculture. To feed the world sustainably into the future, and keeping in mind the environmental issues, local and global, along with policy and frame-work overhaul, a deeper understanding is required of the fundamental molecular principles that manifest into a healthy plant. According to a recent carbon footprint analysis, the entire food production chain contributes to 20 % of global greenhouse gas emissions. Reducing these greenhouse gas emissions and increasing the long-term storage of carbon in the soil are therefore essential measures to prevent a climate catastrophe.
Genetic engineering is one of the approaches to combat hunger and poverty without disrupting environmental and/or atmospheric factors because it is a powerful approach that allows the use of relevant and beneficial inter-species and even inter-kingdom genes in plants. At the same time it can provide new plant resources that are able to withstand the changing and adverse environmental conditions affecting yield. However, its use can be recommended only after rigorous deregulation and under strict biosafety conditions to avoid interbreeding of genetically modified organisms with natural organisms.
Different approaches and strategies used to date, including the first Green Revolution, have brought fruitful results towards tackling the sequentially increasing demands for food. Given the impending changes in demography and rise in related problems for food, fodder and fuel under the climate change scenarios, it is time to invent and apply new and faster technologies towards crop improvement. Hence, modern, high-throughput platform technologies are increasingly being applied to obtain information at different molecular levels e.g., RNA transcript, protein and metabolite at the plant systems level (Ehrhardt and Frommer 2012). Of these technologies, we will discuss here the proteomics technology and its utilization in India and Nepal which is contributing substantially to better understand the plant systems.
Proteomics technology and its applications in plants
Proteomics, as a technology, was conceptualized and defined in terms of studying all proteins by an Australian scientist Marc Wilkins (Wilkins et al. 1995). Explosive growth of proteomics and its applications in biological sciences triggered discussion on its original definition to have a more inclusive definition of proteomics (Agrawal and Rakwal 2008). Despite all discussions, no comprehensive definition is yet available to bring together all expanding areas of proteomics studies under the proteomics discipline.
Proteomics technology is by and large based on its two major complementary approaches, gel-based and gel-free. There are books (Agrawal and Rakwal 2008; Thiellement et al. 2007) and reviews (Lambert et al. 2005) detailing such approaches, and readers are referred to those for details. Briefly, in gel-based approach, one- or two-dimensional gel electrophoresis (1-D or 2-DGE) is used as a reliable and efficient method for protein separation in combination with MS for protein identification. The gel-based proteomics is difficult to automate – the ultimate objective for any high-throughput method. This was the basic reason behind the development of gel-free approaches. Most gel-free approaches use a bottom-up strategy, where proteins are first digested with a proteolytic enzyme (generally, trypsin) followed by analysis of the complex peptide mixture by usually reversed-phase chromatography coupled to an MS/MS instrument. Multidimensional protein identification technology (MudPIT) is one good example of a gel-free approach (Washburn et al. 2001; McDonald and Friedman 2010). MudPIT is also known as shotgun proteomics approach. Today, various gel-free strategies have been developed to increase proteome coverage. Both approaches have been now optimized for quantitative proteomics (Thelen and Peck 2007).
Proteomics technology and its approaches have been applied to study most of the plant tissues, organs, and organelles. Its applications are increasing day-by-day. Given the scope of this manuscript, it is not feasible to discuss all applications of proteomics reported already in plants. Nonetheless, the proteomics-based discoveries are now being translated to applications. Definition and some recent examples of translational proteomics research has been elegantly discussed in a recent review (Agrawal et al. 2012a).
Proteomics status in India and Nepal
Proteomics being a technology-driven/equipment-intensive research area has extensive financial demands towards purchasing and maintaining high-end equipments in good working condition. In addition, highly trained and specialized man-power is also required to handle proteomics equipments, as per the need of research and biological questions. These reasons are among the few supporting the fact that the state-of-the-art proteomics facilities are confined to well-funded and established laboratories only, mostly in developed countries like USA, Germany, France, Luxembourg, Japan, South Korea, Italy, Switzerland, and Sweden. Financial support for resource and capacity building to cater to the needs of researchers specifically in India is summarized in the following section. Unfortunately, such support is scarce in Nepal.
Funding organizations supporting proteomics research
There are many funding agencies that fund research projects and provide financial support to promote proteomics research and development of necessary infrastructure. In India, such agencies are Department of Biotechnology (DBT), Council of Scientific and Industrial Research (CSIR), Department of Science and Technology (DST), and University Grants Commission (UGC).
DBT has funded the development of proteomics facilities like National Institute of Plant Genome Research (NIPGR), New Delhi; Centre for DNA Fingerprinting and Diagnostics (CDFD), Hyderabad; and Institute of Bioinformatics (IOB), Bangalore. CSIR funded proteomics facilities include Centre for Cellular and Molecular Biology (CCMB) Hyderabad; Institute of Genomics & Integrative Biology (IGIB) Delhi; Central Drug Research Institute (CDRI) Lucknow; National Chemical Laboratory (NCL) Pune; National Botanical Research Institute (NBRI) Lucknow; Institute of Himalayan Bioresource and Technology (IHBT), Palampur, and Central Institute of Medicinal and Aromatic Plants (CIMAP) Lucknow. DST, through its Science and Engineering Research Council (SERC) division, promotes research and development programs in newly emerging and challenging areas of science including proteomics. In addition to funding research projects, DST also grants a Fund for Improvement of Science and Technology infrastructure in Universities and Higher Educational Institutions (FIST) for the establishment of proteomics research facilities. UGC also funds proteomics research, although in limited way.
In Nepal, national level funding is extremely limited. National Academy of Science and Technology (NAST) is the only top research organization which funds small-scale research projects. However, the grant money is so meager that it is impossible to conduct proteomics research. International funding is the main source for funding basic and applied research, developing infrastructure and training man-power in Nepal. However, the proximity of Nepal to India and a relative lack of language and cultural barriers portend strong links between the two countries to advance the knowledge of proteomics in Nepal through training of researchers and analysis of samples in India.
In addition to the proteomics facilities listed in Supplementary Table 1, some private companies also offer paid proteomic services. Most of the research groups use one or more of these services for proteomics-based studies. As far as Nepal is concerned, there is no government or private institutions, universities, or colleges that are equipped with proteomics facilities. The only possibility to conduct proteomics-based research is to collaborate with universities and institutes abroad or to utilize the paid services in other countries. Research Laboratory for Biotechnology and Biochemistry (RLABB) in Nepal is a model laboratory, which collaborates abroad to conduct proteomics research.
Research publications on proteomics
Result of the financial boost to plant proteomics has borne fruit, which is reflected in terms of the publications. Several impressive proteome-wide researches to better understand plant biology from accomplished and ongoing research works are summarized in Table 1 as per the PubMed search engine (http://www.ncbi.nlm.nih.gov/pubmed/) at the time of writing (as of April 2013). Based on the data in Supplementary Table 2, many research groups (~20 laboratories) in India are actively working in plant proteomics (Fig. 1, Supplementary Table 2). Unlike India, situation in Nepal is dramatically different mainly due to lack of infrastructure, trained human resource, awareness, and funding. RLABB is the only research organization, which is actively involved in plant proteomics research since 1999 (Rakwal et al. 1999). RLABB is a non-profit private research laboratory with main objective to address issues related to food security, safety, and human health in Nepal. Plant proteomics research led by Dr. Ganesh Kumar Agrawal (Associate Director of RLABB) has been very fruitful in understanding the biology of crop plants. Dr. Agrawal’s efforts in the plant proteomics have resulted in several publications (Table 1) in association with foreign research groups highlighting the importance of active collaboration.
Table 1.
Summary of plant proteomics research publications from India and Nepal
| Country | Plant | Separation method | Identification method | Software used | Proteins identified | Reference |
|---|---|---|---|---|---|---|
| INDIA | Mentha arvensis L. | 2-DGE | MALDI-TOF-TOF MS/MS | PD Quest software version 8.0.1, MASCOT & NCBI, KEGG | 60 | Sinha et al. 2013 |
| Phaseolus vulgaris L. | 2-DGE | LC-MS/MS | ImageMaster 2D Platinum software ver 6.0, MASCOT & MSDB | - | Kumar et al. 2013 | |
| Vigna mungo L. | 2-DGE | MALDI TOF/TOF | PD Quest software version 8.0.1, MASCOT & NCBI, KEGG | 109 | Kundu et al. 2013 | |
| Podophyllum hexandrum Royale. | 2-DGE | MALDI-TOF/TOF-MS | ImageMaster 2D Platinum software ver 6.0, MASCOT, NCBI, SwissProt | 60 | Dogra et al. 2013 | |
| Aconitum heterophyllum | 2-DGE | MALDI-TOF/TOF-MS | ImageMaster 2D Platinum software ver 6.0, MASCOT, NCBI, SwissProt | 40 | Rana and Sreenivasulu 2013 | |
| Seabuckthorn (Hippophae rhamnoides L.) | 2-DGE | nESI-LC − MS/MS | ImageMaster 2D Platinum software ver 6.0, MASCOT & NCBI, SignalP, SecretomeP 2.0 | 34 | Gupta and Deswal 2012 | |
| Kalanchoe pinnata L. | 2-DGE | MALDI-TOF/TOF | ImageMaster 2D Platinum software ver 6.0, MASCOT & NCBI | 10 | Abat and Deswal, 2012 | |
| Podophyllum hexandrum L. | 2-DGE | MALDI-TOF/TOF | PDQuest version 8.0.1, MASCOT & NCBI | 105 | Bhattacharayya et al. 2012 | |
| Gossypium herbaceum L. | 2-DGE | MALDI-TOF/TOF | Image Master 2D Platinum 6.0 software, MASCOT SwissProt, NCBInr and MSDB | 18 | Deeba et al. 2012 | |
| INDIA | Mangifera indica L. | 1-DGE, OFF-gel based fractionation, strong cation exchange chromatography | Q-TOF-MS | SEQUEST, NCBI, PepNovo algorithm | 1001 | Renuse et al. 2012 |
| Oryza sativa L. | 2-DGE | 2-DGE | PDQuest software version 8.0, MASCOT, NCBInr, SwissProt | 21 | Hakeem et al. 2012a | |
| Anabaena species | 2-DGE | MALDI-TOF/LC-MS | PDQuest version 7.1, MASCOT | 45 | Pandey et al. 2012 | |
| Arabidopsis thaliana | 2-DGE | MALDI-TOF | MASCOT & NCBI | 39 | Gill et al. 2012 | |
| Chickpea (Cicer arietinum L.) | 2-DGE | MALDI-TOF/TOF and LC-ESI-MS/MS | PDQuest version 7.2.0, MASCOT, MSDB, Ludwig NR | 91 | Jaiswal et al. 2012 | |
| Chickpea (Cicer arietinum L.) | 2-DGE | nESI-LC-MS/MS | PDQuest version 7.2.0, MASCOT, SignalP-NN &SignalP-HMM, iPSORT, Signal-3 L, SecretomeP 2.0 & Multi Experiment Viewer software | 81 | Bhushan et al. 2011 | |
| Mungbean (Vigna radiata L.) | 2-DGE | MALDI-TOF/TOF, PMF | Image Master 2D Platinum software, MASCOT, NCBI, Swissport & Viridiplantae (green plants) | 25 | Sengupta et al. 2011 | |
| Black gram (Vigna mungo L.) | 2-DGE | MALDI-TOF/TOF | PDQuest Advanced™ 2D analysis software version 8.0, MASCOT, NCBI & Swissport | 29 | Kundu et al. 2011 | |
| Mint (Mentha arvensis L.) | 2-DGE | MALDI TOF/TOF | PD Quest software version 8.0.1, MASCOT, KEGG (Kyoto Encyclopedia of Genes and Genomes) & EXPASy | 45 | Sinha and Chattopadhyay 2011 | |
| Grasspea (Lathyrus sativus L.) | 2-DGE | LC-MS/TOF | Analyst Software v.1.4.1, MASCOT v.2.1 & Viridiplantae (green plants) | 48 | Chattopadhyay et al. 2011 | |
| INDIA | Chlamydomonas species | 2-DGE | MALDI-TOF | PDQuest basic 8.0.1 & MASCOT | NA | Yadavalli et al. 2011 |
| Chickpea (Cicer arietinum L.) | 1-DGE, 2-DGE & HPAEC | MALDI-TOF, HPAEC-PAD | MASCOT & NCBI | NA | Agrawal et al. 2011b | |
| Garlic (Allium sativum L.) | Affinity chromatography, 1-DGE | PMF, MALDI-TOF/TOF | MASCOT, NCBI & Swissport | NA | Upadhyay et al. 2010 | |
| Potato (Solanum tuberosum L.) | 2-DGE | Not mentioned | PDQuest analysis | NA | Chakraborty et al. 2010 | |
| Hot pepper (Capsicum annuum L.) | SDS-PAGE | MALDI-TOF-MS | Not mentioned | NA | Mishra et al. 2010 | |
| Rice (Oryza sativa L.) | 2-DGE | LC-MS/TOF | PDQuest version 7.2.0, MASCOT & NCBI. | 94 | Pandey et al. 2010 | |
| Peanut (Arachis hypogaea L.) | Affinity chromatography, 2-DGE | MALDI-TOF & MALDI-MS/MS | MASCOT & NCBI. | NA | Agrawal et al. 2010 | |
| Wild rice (Porteresia coarctate L.) | 2-DGE | MALDI-TOF | MASCOT & NCBI. | NA | Ray et al. 2010 | |
| Dhaincha (Sesbania aculeata L.) | Sephadex G-50 affinity chromatography & 2D-HPAEC-PAD | MALDI-TOF | Not mentioned | NA | Biswas et al. 2009 | |
| Mustard (Brassica juncea L.) | 2-DGE | PMF or LC-MS/MS (MALDI-TOF) | AlphaImager EC, ImageMaster 2D Platinum software & MASCOT | 20 | Abat and Deswal 2009 | |
| Black gram (Vigna mungo L.) | RP-HPLC | MALDI TOF | Not mentioned | 16 | Mandal et al. 2009 | |
| INDIA | Rice (Oryza sativa L.) | 2-DGE | LC-MS/TOF | PDQuest version 7.2.0 & MASCOT. | 109 | Choudhary et al. 2009 |
| Wild rice (Porteresia coarctata L.) | 2-DGE | MALDI-TOF | PDQuest 8.0,MASCOT, MS-Fit from Protein Prospector & Aldente | 16 | Sengupta and Majumder 2009 | |
| Rue (Ruta graveolens L.) | RP-HPLC | ESI-MS & MALDI-TOF | Not mentioned | NA | Raghav et al. 2007 | |
| Chickpea (Cicer arietinum L.) | 2-DGE | LC-MS/TOF | PD Quest version 7.2.0, Analyst Software v.1.4.1, MASCOT v.2.1 & NCBI | 147 | Pandey et al. 2008 | |
| Potato (Solanum tuberosum L.) | 2-DGE | LC-TOF or TOF/TOF | PD Quest software version 7.2.0, MASCOT & Viridiplantae (green plants) | 97 | Agrawal and Rakwal 2008 | |
| Setaria italica L. cv. Prasad | 2-DGE | MALDI-TOF-MS | Vilber Lourmat Gel doc Photo Capt MW, MASCOT, NCBInr | 29 | Veeranagamallaiah et al. 2008 | |
| Chickpea (Cicer arietinum L.) | 2-DGE | LC-MS/TOF | PDQuest version 7.2.0, MASCOT & Viridiplantae (green plants) | 80 | Bhushan et al. 2007 | |
| Peanut (Arachis hypogaea L.) | 1-DGE | MALDI-TOF | Not mentioned | NA | Pathak et al. 2006 | |
| Chickpea (Cicer arietinum L.) | 2-DGE | nESI-LC-MS/MS | PD Quest version 7.2.0, MASCOT, Viridiplantae (green plants) & NCBI | 150 | Pandey et al. 2006 | |
| Chickpea (Cicer arietinum L.) | 2-DGE | MALDI-TOF & MS/MS. | PDQuest version 7.2.0, MASCOT, Viridiplantae (green plants) & Sequest | NA | Bhushan et al. 2006 | |
| Peanut (Arachis hypogaea L.) | 2-DGE | ESI-Q-TOF | PDQuest software, Mascot V NCBI. | NA | Jain et al. 2006 | |
| Rice (Oryza sativa L.) | 2-DGE | Edman degradation | 2 | Chourey et al. 2003 | ||
| INDIA & NEPAL | Wheat (Triticum aestivum L.) | 1-DGE & 2-DGE | Q-TOF-MS/MS &nESI-LC-MS/MS | Ludesi’s proprietary image analysis software, MASCOT & NCBI. | 20 | Sarkar et al. 2010 |
| Sugarcane (Saccharum officinarum L.) | 2-DGE | eLD-IT-TOF-MS/MS &nESI-LC-MS/MS | Image Master platinum 2D software version 6.0, MASCOT version 2.1, NCBI & Swiss-Prot | 36 | Amalraj et al. 2010 | |
| NEPAL | Zea mays, Glycine max, Arabidopsis species, Oryza sativa | 2-DGE | MALDI-TOF-TOF | – | – | Kim et al. 2013b |
| Oryza sativa and Magnaporthe Oryzae | 2-DGE and MudPIT | MALDI-TOF-MS and/or nESI-LC–MS/MS | ImageMaster 2D Platinum imaging software ver. 6.0, MASCOT & NCBI, TurboSequest, SignalP, SigPred, SigCleave, RPSP, PSortII, TargetP 1.1, SecretomeP 2.0 | 732 | Kim et al. 2013a | |
| Arabidopsis species | 1-DGE (SDS-PAGE) | GeLC-MS/MS, LC-MS/MS | BioWorks version 3.3.1 SP1 (SEQUEST) & Scaffold. | NA | Swatek et al. 2011 | |
| Rice (Oryza sativa L.) | 2-DGE | MALDI-TOF & μLC-ESI-MS/MS | ImageMaster 2D Platinum imaging software ver. 6.0, ProteinProspector & MASCOT. | 21 | Kim et al. 2009 | |
| Soybean (Glycine max) | 2-DGE and Sec-MudPIT | LC-MS/MS | NCBI, SEQUEST, BioWorks, TargetP, iPSORT, Predotar | 478 | Agrawal and Rakwal 2008 | |
| Rice (Oryza sativa L.) | 2-DGE | nESI-LC-MS/MS | EasyPro-2D software, Agilent Spectrum Mill & MASCOT. | 192 | Jung et al. 2008 | |
| Rice (Oryza sativa L.) | 2-DGE | LC-MS/MS | Ludesi’s proprietary image analysis software, Agilent Spectrum Mill & NCBI. | 21 | Cho et al. 2008 | |
| NEPAL | Rice (Oryza sativa L.) | 2-DGE | nLC-IT-TOF-MS/MS &nESI-LC-MS/MS | ImageMaster 2D Platinum software ver. 5.0, Agilent Spectrum Mill, NCBI & Swiss-Prot | 108 | Cho et al. 2007 |
| Bean (Phaseolus vulgaris L.) and Maize (Zea mays L.) | 1-DGE & 2-DGE | 10 (Phaseolus vulgaris) & 9 (Zea mays) | Torres et al. 2007 | |||
| Rice (Oryza sativa L.) | 1-DGE & 2-DGE | nESI-LC-MS/MS | ImageMaster 2D Platinum imaging software ver. 5.0, Agilent Spectrum Mill, NCBI & Swiss-Prot | 26 | Jung et al. 2006 | |
| Oilseed Rape (Brassica napus L.) | 2-DGE & MudPIT | nESI-LC-MS/MS | SEQUEST & NCBI. | NA | Katavic et al. 2006 | |
| Oilseed Rape (Brassica napus L.) | 2-DGE | LC-MS/MS | ImageMaster 2D Platinum software version 5, BioWorks 3.1SR1 (SEQUEST) & NCBI. | 70 | Agrawal and Thelen 2006 | |
| Rice (Oryza sativa L.) | 2-DGE | nESI-LC-MS/MS | ImageMaster 2D Platinum software version 5, Agilent Spectrum Mill, Swiss-Prot & EST databases from NCBI. | 25 | Kim et al. 2005 | |
| Rice (Oryza sativa L.) | 2-DGE | Edman degradation | NCBI | 56 | Agrawal et al. 2002 | |
| Rice (Oryza sativa L.) | 2-DGE | Edman degradation | MPsrch-pp protein-protein database | 33 | Hajduch et al. 2001 | |
| Rice (Oryza sativa L.) | 2-DGE | Edman degradation | Not mentioned | 12 | Rakwal et al. 1999 |
Fig. 1.
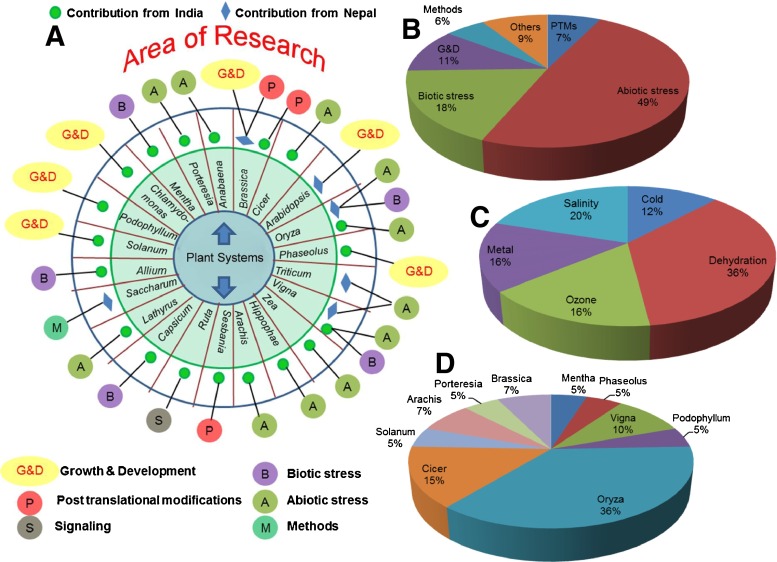
a Different laboratories dedicated to plant proteomics research in India and Nepal. b Total number of INPPO members in different states of India and Nepal at the time of review writing (as of April, 2013)
There has been a spurt of activities in plant proteomics research and publications over the past few years (Fig. 2). Major research areas of the plant proteomics scientific community in India and Nepal are biotic and abiotic stresses, plant-pathogen interactions, disease resistance, and growth and development (Fig. 3, Table 1). Proteome-based studies on Arabidopsis, as a model plant, have provided an impetus to work on other plant systems of economic importance including crop and medicinal plants. Rice, brassica, wheat, chickpea, sugarcane, etc. have been used largely to elucidate physiological processes at the molecular level (Fig. 3).
Fig. 2.
The number of publications in plant proteomics from India and Nepal in last 5 years. The number of publications for the year 2013 was calculated by tripling the 4 month value
Fig. 3.
a Overview of plant proteomics research areas and plant systems being used. b, c, d Pie charts showing (b) focus area of research, (c) different areas of abiotic stress research, (d) different plant systems being used
Summary of the research work carried out in India and Nepal
Abiotic stress
Dehydration stress
Dehydration is one of the most important abiotic stresses, which impairs almost all physiological processes and greatly influences the geographical distribution of many crop species. For analysis of dehydration stress, proteomes of the extracellular matrix (ECM) and nucleus have been analyzed in rice (Pandey et al. 2010) and chickpea (Bhushan et al. 2011).
For analyzing the effect of dehydration stress, a total of 8 varieties of the chickpea were compared for their dehydration stress tolerance. Analysis of relative water content, proline content, lipid peroxidation, relative ion leakage and photosynthetic pigments from 24–92 h of dehydration showed that the JG-62 variety is relatively more dehydration tolerant than others. ECM proteome analysis of JG-62 revealed 186 proteins, out of which 134 were identified. In case of rice, 2D gel profile of ECM proteins revealed 192 differentially modulated spots. Functional categorization of these proteins revealed their involvement in a variety of functions, including carbohydrate metabolism, cell defense and rescue, cell wall modification, cell signaling, and molecular chaperones. A comparison of the drought stress induced differentially modulated targets in rice, chickpea, and maize led to the identification of some of the common targets including chitinase, glyoxalase 1, superoxide dismutase (SOD), thioredoxin, and ascorbate peroxidase (APX) suggesting their pivotal roles in providing dehydration stress tolerance.
Analysis of nuclear proteome of rice and chickpea during dehydration stress showed differential expression of the proteins involved in transcriptional regulation and chromatin remodeling, signaling and gene regulation, cell defense and rescue, and protein degradation. Furthermore a comparison between the dehydration responsive nuclear proteome of rice and chickpea, showed some of the common proteins including receptor-like protein kinase, WRKY DNA binding domain-containing protein, and probable SOD (Cu-Zn) precursor. However, most of the dehydration responsive proteins were plant specific suggesting an evolutionary divergence in dehydration response in different plants.
In addition, effect of dehydration stress was also analyzed in the roots of Vigna radiata (Sengupta et al. 2011) and leaves of cotton (Deeba et al. 2012). Effect of dehydration stress on root proteome of V. radiata was analyzed after 3 and 6 days of dehydration stress as well as 6 days after re-watering. Estimation of the relative water content (RWC) showed a decline from 78 % in control to 66 and 56 % in 3 and 6 days after stress, respectively. In the recovery experiment, RWC increased to 70 % suggesting roots were able to recover after drought stress. Analysis of several other photosynthetic gas exchange parameters including photosynthetic rate, stomatal conductance, transpiration rate, and internal CO2 showed a gradual decrease from control to 3 and 6 days after stress and their recovery after re-watering. In addition, primary root growth inhibition and induction of lateral roots abscission was also observed during drought stress. More than 500 spots were reproducibly detected in the root proteome of V. radiata out of which 34 spots showing differential abundances were identified by MALDI-TOF-MS. Glycoproteins like lectins and oxidative stress-related proteins were upregulated while cytoskeletal related proteins showed downregulation after 3 days but their expression was regained after 6 days of stress. In cotton leaves, a similar decrease in gas-exchange parameters of net photosynthesis, stomatal conductance, effective transpiration, quantum yield of PSII, and electron transport rates was also observed. In addition, increased level of hydrogen peroxide (H2O2) and malondialdehyde (MDA) was also seen indicating higher oxidative damage during drought stress. Proteome analysis of control and stressed leaves showed 22 differentially modulated protein involved in photosynthesis, metabolism, lipid biosynthesis, and reactive oxygen species (ROS) metabolism.
Salinity stress
Effect of salt stress was investigated in Porteresia coarctata (Sengupta and Majumder 2009) and Setaria italica (Veeranagamallaiah et al. 2008). P. coarctata is a halophytic rice that is relatively more salt tolerant than domesticated rice (Oryza sativa). A comparison between P. coarctata and O. sativa showed lesser accumulation of sodium ion and conservation RCW in P. coarctata during salinity stress. Proteomic analysis of IR64 and Pokkali varieties of rice showed ~1,088 and ~1,076 spots, which decreased to ~482 and ~654 after 200 mM of salt (NaCl) treatment. In case of P. coarctata, ~700 spots were detected in the control, and the protein loss after salt stress was not as severe as observed in other rice varieties. At 200 mM salt treatment, 40 % of the proteins did not change, 39 % were downregulated, and 13 % showed increased abundance. Identification of these proteins showed their major involvement in photosynthesis.
In case of S. italic (foxtail millet), ~175 spots were reproducibly detected in the 2D gel of whole seedling proteome out of which 34 spots showed altered abundance after 100, 150 or 200 mM of salt stress. Identification of 29 differentially modulated proteins showed their major involvement in photosynthesis, signal transduction, cell wall biogenesis, stress-related, and metabolism.
Low temperature stress
For analyzing the effect of low temperature (LT) stress, the apoplast proteome/secretome of Hippophae rhamnoides, a cold hardy shrub, was analyzed. Extracellular proteins, isolated from cold acclimated and sub-zero acclimated seedlings, were resolved on 2-DGE. Approximately 255 spots were reproducible detected in the 2-D gels out of which 61 were differentially modulated in one or more LT treatments. Identification of 34 LT-induced spots showed their involvement in signaling, defense, and redox regulation. LT induced the activities of SOD and chitinase to 1.5 and 4 fold respectively, suggesting their significant roles in LT stress tolerance. In addition, increased abundances of calmodulin 1, calcium dependent protein kinase 23, and GTPase activating protein suggests activation of calcium and G-protein signalling during cold stress. Besides, accumulation of antifreeze proteins (AFPs) in the secretome during cold and sub-zero acclimation was also observed. AFPs are a class of polypeptides that bind to and inhibit the growth of ice crystals, thereby minimizing the freezing stress-induced damage. A polygalacturonase inhibitor protein showing antifreeze activity was purified by ice adsorption chromatography (Gupta and Deswal 2012). These AFPs are further being analyzed for their biotechnological applications.
Ozone stress
Effect of elevated ozone (O3) was studied on two high-yielding cultivars of tropical wheat (Sarkar et al. 2010). Exposure to elevated O3 resulted in foliar injury in both the cultivars (Sonalika and HUW 510). In addition, elevated O3 caused decreased photosynthetic rate, stomatal conductance, and chlorophyll fluorescence kinetics (Fv/Fm) in test cultivars. Biochemical characterization further showed decrease in photosynthetic pigments and induction in antioxidant system ROS detoxifying enzymes. Degradation of both the subunits of ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO), decreased abundance of energy metabolism-related proteins and induction of stress/defense-related proteins was observed by elevated O3.
Heavy metal stress
Effect of heavy metals, copper and arsenic, was analyzed in transgenic Arabidopsis overexpressing the SOD gene from Potentilla atrosanguinea (PaSOD) (Gill et al. 2012) and Anabaena sp. PCC7120 (Pandey et al. 2012), respectively. Copper stress resulted in altered abundances of 39 spots, identification of which showed their major involvement in oxidative stress, detoxification, germination, intermediary metabolism and regulation. In case of arsenic stress, a decrease in growth, carbon fixation, nitrogenase activity and chlorophyll content was observed in Anabaena after 1 day and recovery after 15 days of treatment. Proteome analysis of control and 1 and 15 days arsenic exposed Anabaena showed 45 differentially modulated proteins. Interestingly, enzymes related to glycolysis, pentose phosphate pathway, and Calvin cycle were downregulated under stress conditions, but recovered during recovery experiments. In addition, upregulation of ROS-detoxifying enzymes like catalase, peroxiredoxin, thioredoxin, and oxidoreducates was also observed during arsenic stress suggesting their pivotal roles in overcoming the heavy metal-induced stress.
Biotic stress
Proteomics based plant-pathogen interactions studies have provided insight into combating plant diseases by exploiting identified target proteins to engineer plants resistant to pathogens. One such study investigated the effect of Alternaria alternata infection on mint leaves (Sinha and Chattopadhyay 2011). Total proteins were isolated from control and infected leaves and resolved by 2-DGE. A total of 210 spots were detected in colloidal coomassie brilliant blue stained gels out of which 67 spots showed altered abundances. MS based identification of 45 differentially modulated spots showed their major association with energy and metabolism, stress and defense, cell structure, signal transduction, protein synthesis and turnover. A recently published study examined the effect of A. alternata on transgenic mint overexpressing the tomato γ-glutamyl-cysteine synthetase (Leγ-ECS) gene. γ-ECS gene encodes a rate limiting enzyme in glutathione biosynthesis that is involved in detoxification of ROS and reactive nitrogen species (Sinha et al. 2013). A comparison of the proteomes of wild-type and transgenic mint, infected with A. alternata and their respective controls showed a total of 250 and 500 spots, respectively. Interestingly, accumulation of PR-1 like protein and disease resistance protein was observed during infection with A. alternata, suggesting their roles in biotic stress tolerance.
The proteomics of salicylic acid (SA) induced resistance to Mungbean Yellow Mosaic India Virus (MYMIV) in Vigna mungo has also been the focus of study (Kundu et al. 2011). Plants treated with 100 μM SA showed no visible symptoms of infection while control plants showed characteristic yellow mosaic symptoms when infected with MYMIV, suggesting the SA treatment prior to viral inoculation may be involved in providing resistance to MYMIV. In addition, an increase in chlorophyll, total proteins, phenolics, and H2O2 was observed in the plants treated with SA. 2D gels of control and SA-treated leaves showed approximately 350 spots out of which 50 spots showed increased abundance after 72 h of SA treatment. Identification of 29 upregulated spots revealed their involvement in stress responses, metabolism, photosynthesis, transport, and signal transduction (Kundu et al. 2011).
Rice secretome was investigated to analyze the rice-Magnaporthe oryzae interaction (Kim et al. 2013a). Analysis of in vivo secreted proteins of rice leaves infected with incompatible (KJ401) and compatible (KJ301) races of M. oryzae led to the identification of 732 secretory proteins of both rice and M. oryzae. MudPIT coupled with MALDI-TOF-MS and/or nESI-LC-MS/MS identification of these secretory proteins showed that rice proteins were mainly involved in stress response, ROS, and energy metabolism, while M. oryzae proteins were associated with metabolism and cell wall hydrolyses.
Growth and development
Nutritive development of crops such as potato has been studied through proteome analysis. Proteome analysis of potato tuberization showed induction of ROS detoxifying enzymes such as SOD, APX, and catalase, suggesting their possible role in tuber formation (Agrawal et al. 2008b).
In addition to potato tuberization, changes in Podophyllum hexandrum cell proteome potentially related to podophyllotoxin (PTOX) accumulation in response to methyl jasmonate (MeJA) elicitation has been investigated (Bhattacharayya et al. 2012). PTOX is derived from the phenolpropanoid pathway and is the precursor for cancer therapeutics like etoposide, teniposide, and etophos. Elicitation of P. hexandrum cells with 100 μM MeJA resulted in 7–8 fold higher accumulation of PTOX. Proteome analysis of elicited and control cells showed modulation of enzymes involved in phenylpropanoid and monolignol pathway including chalcone synthase, polyphenol oxidase, caffeoyl CoA 3-O-methyltransferase, S-adenosyl-L-methionine-dependent methyltransferases, caffeic acid-O-methyl transferase, etc. Besides, the proteomes of P. hexandrum and Aconitum heterophyllum seeds were also analyzed to identify proteins involved in seed germination (Dogra et al. 2013; Rana and Sreenivasulu 2013). Comparative analysis of 2D gels of dormant and germinating Podophyllum seeds showed 113 differentially modulated proteins involved in metabolism, abscisic acid (ABA) and/or gibberellic acid (GA) signaling, and stress. Data from MS/MS and RT-PCR showed upregulation of β-1,3-glucanase and xyloglucan endotransglycosylase. Activities of these enzymes weaken the thick walled micropylar endosperm, which may cause radicle emergence from the seeds.
The sugarcane stalk proteome was a novel study that developed a protein extraction procedure from the stalk tissues from which proteins are difficult to isolate due to presence of high levels of oxidative enzymes, phenolic compounds, and carbohydrates (Amalraj et al. 2010). Out of five different methods tried, phenol extraction method followed by 2-D cleanup kit showed the best results. 2D gel of proteins extracted by phenol method and phenol method in combination with 2-D cleanup kit showed 390 and 445 spots, respectively. Identification of 36 non-redundant proteins showed that sugarcane stalk proteins are mainly involved in sugar metabolism (20 %), nucleic acid metabolism (13.33 %), protein metabolism, cell growth and development (10 %), structure and defense (6.67 %), and secondary metabolism (6.67 %), while about 16.67 % proteins were unknown or uncharacterized (Amalraj et al. 2010).
Methods were also optimized to remove the RuBisCO, major leaf protein, which interferes with the deep analysis of the leaf proteome, using a novel protamine sulfate (PS) precipitation (Kim et al. 2013b). Results from western blotting and 1-DGE showed that 0.1 % (w/v) PS was sufficient to precipitate both the subunits of RuBisCO. This method of RuBisCO depletion was simple, fast, and economical. In addition, this method is applicable to both dicot and monocot plants and is suitable for down-stream proteomics analysis.
Spectral counting based quantitative proteomic analysis of plastids from developing embryos and leaves of Brassica napus showed that plastids from leaves were rich in light reaction proteins while Calvin cycle enzymes were more prominent in plastids isolated from developing embryo (Demartini et al. 2011). Interestingly, enzymes related to de novo fatty acid and amino acid biosynthesis were detected in embryoplasts but not in chloroplasts.
Post-translational modifications
Analysis of S-nitrosylation (a NO based PTM) has been done in Brassica juncea (Abat and Deswal 2009) and Kalanchoe pinnata (Abat et al. 2008). In addition, the phosphoproteome of Brassica napus has also been analyzed during seed filling (Agarwal and Thelen 2006). S-nitrosylation analysis in Kalanchoe pinnata resulted in identification of some novel targets including kinesin-like protein, glycolate oxidase, putative UDP glucose 4-epimerase and putative DNA topoisomerase II. Interestingly, equivalent inhibition of RuBisCO was shown by S-nitrosoglutathione and cold stress which was reversed by DTT/GSH, suggesting RuBisCO inactivation by S-nitrosylation (Abat et al. 2008) in B. juncea. LT stress induced RuBisCO inhibition (~40 %) (Abat and Deswal 2009) might explain the LT induced productivity loss to some extent. Analysis of the phosphoproteome of developing rapeseed revealed proteins mainly associated with energy (25.7 %), metabolism (18.6 %), protein destination (12.9 %), signaling (11.4 %) and defense (8.6 %). To further validate phosphorylation of the identified proteins, phosphorylation sites of 16 non-redundant proteins including 14-3-3 and annexin were mapped by LC-MS/MS. This was the first quantitative large scale phosphoproteome analysis in plants (Agrawal and Thelen 2006).
Others
The Arachis hypogaea stem lectin (SL-I) isoform variants and its tryptic digested peptides have been analyzed by MALDI-TOF-MS. Results showed that the SL-I of peanut is an isoform of glucose/mannose binding lectin. Further characterization of SL-I using 2-DE-MS in combination with western blotting showed presence of its six isoforms in peanut (Agrawal et al. 2010).
Efforts have also been made to analyze the mango leaf proteome. For proteome analysis, both gel-based and gel-free approaches led to the identification of 1,001 peptides which correspond to 538 proteins. This study is the first high-throughput analysis of mango leaf proteome (Renuse et al. 2012).
The above mentioned research and the list of publications clearly emphasize that plant proteomics scientific community of the Indian sub-continent is growing at a fast pace and with a noticeable contribution at the global stage. In above reported publications, the gel-based proteomics approaches (1-DGE and/or 2-DGE) coupled with various MS instruments (i.e., MALDI-TOF, MALDI-TOF/TOF, and LC-MS/MS) have primarily been utilized to establish proteomes and address the biological questions. Shotgun proteomics approaches (i.e., MudPIT) and enrichment techniques are yet to be fully utilized for comprehensive proteome investigation.
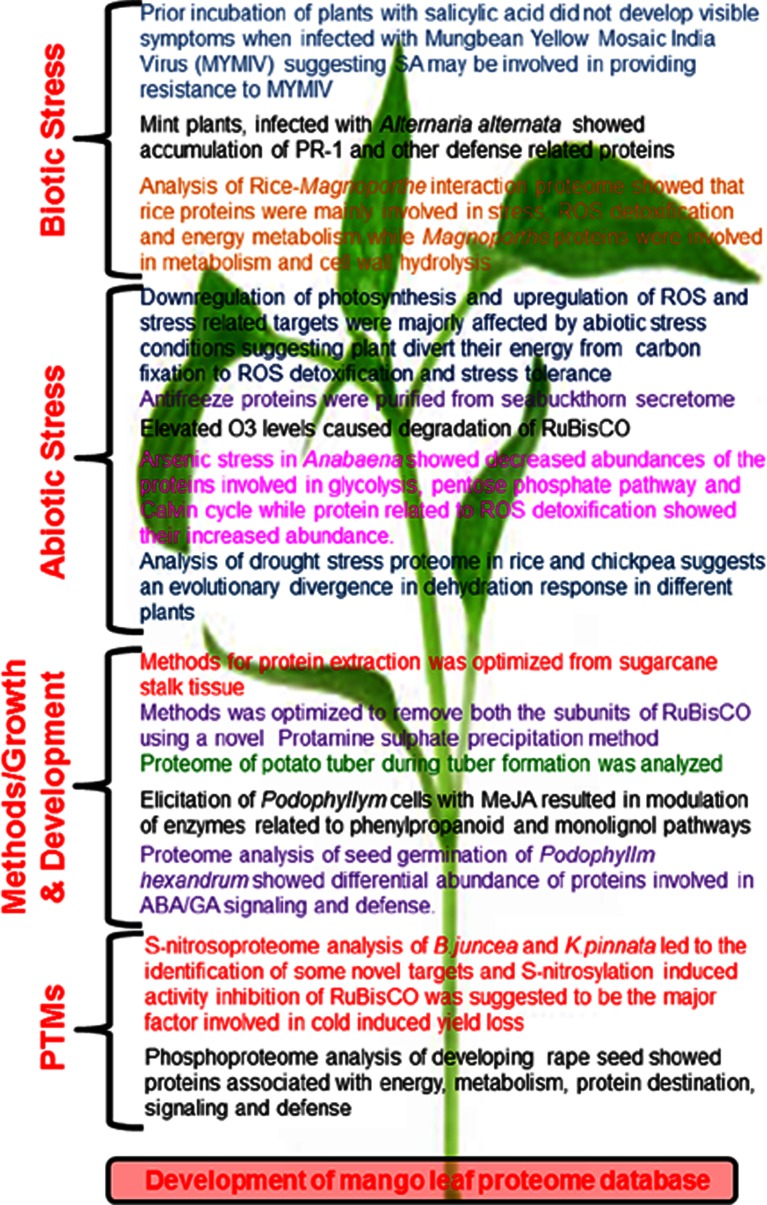
Proteomics research is majorly focused on biotic and abiotic stress, development of protein extraction procedures from the recalcitrant plants/tissues and analysis of post-translational modifications. Results from different studies showed that biotic and abiotic stress conditions results in decreased photosynthesis rate, stomatal conductance, carbon fixation and chlorophyll content, suggesting overall decrease in the photosynthesis. In addition, increased abundances of ROS-detoxifying enzymes and defense associated enzymes during stress conditions revealed their major involvement in stress tolerance. These results suggest that plants divert their energy from normal growth and photosynthesis to defense and redox regulation during stress conditions, leading to tolerance to the particular stress. In addition, protein extraction procedures from sugarcane stalk and seabuckthorn seedlings are also optimized to get good quality protein with negligible contamination. Development of proteome database of mango leaf is a noteworthy contribution (Fig. 4). Besides these research publications, recent advances on plant proteome research have been published as reviews to present the current scenario of this newly emerging area of plant science (Hakeem et al. 2012b; Agrawal et al. 2013; Sehrawat et al. 2013; Narula et al. 2013).
Fig. 4.
Figure highlighting some of the important contributions in plant protoemics from India and Nepal
Challenges ahead for plant proteomics research
Studying proteomes is not straightforward as one may imagine. This is due to some limitations associated with proteomics technology, dynamic proteome, and huge diversity in plant species. Besides, the plant science research community is not fully aware of the power and benefits of the proteomics technology and application, at least in the developing countries. On the other hand, how proteins perform their functions is a continuously and of late, a rapidly evolving research area. This limits the vision on innovations in proteomics that would allow a comprehensive listing of proteins used/needed by a plant or its parts, to survive under normal or aberrant environmental situations. This in turn limits a full-fledged understanding of the interaction networks that may allow addressing broader issues of crop productivity and yield gaps or barriers holistically. Indeed, another major limitation is to have easy access to proteomics facilities or to share the existing proteomics facilities under certain guidelines or understandings. Even under situations where raw data can be obtained through outsourced services; there is a critical dearth of trained personnel who can, not just interpret the data in terms of categories and bins, but also diligently make biological sense of it. Hence, there are a number of challenges ahead as discussed below.
The proteome coverage is one major limitation. It is still not feasible to study proteins on a scale equivalent to that of the nucleic acids. We take a look at the most common technical issues affecting plant proteomics research. For example, the analysis of low-abundance proteins (LAPs) remains a major challenge due to the unavailability of any technique equivalent to PCR for proteins. The abundant proteins usually mask the detection of LAPs of equal importance. Best examples of LAPs are, but not limited to, transcription factors, protein kinases, and other regulatory proteins. Pre-fractionation of a complex protein is one strategy to enrich LAPs. Another strategy is to use affinity approach to enrich rare or desired class of proteins. Recent development in this area is the use of combinatorial peptide ligand libraries (CPLL), which has been used to detect LAPs from a variety of biological samples (Righetti et al. 2006, 2011), including plants (Frohlich et al. 2012) .
Development of plant specific proteomics protocols is another major challenge. Proteins are dynamic molecules and no one method can be suitably utilized for extraction and analysis due to vast differences in their abundance. Since these proteins are functional molecules, the half-life varies significantly among the different proteins. Tissue-specific expression and differences in plants contributes to difficulty in applying and/or developing universal protocols for protein extraction. Unavailability of efficient protocols for protein extraction, like those available for animal tissues, has resulted in limited exploration of plant proteomes.
Although a number of successful strategies have been developed to overcome these issues, including MS-compatible extraction procedures designed for plant tissues, reproducibility of these methods in other plants is always questionable (Heazlewood 2011). The major challenge in plant proteomics is the inability to measure the entire proteome. It is often important to obtain a relatively pure (about 95–99 %) and saturated proteome to obtain meaningful biological results, to address specific questions, and to enhance dynamic resolution. In spite of the significant advances to the MS technologies, accomplishment of a saturated proteome and the number of proteins that can be reproducibly identified from a single sample is limited.
The ability to deliver large-scale protein quantification that permits comparative proteomics studies is a fundamental challenge for proteomics. Only a comprehensive proteome comparison would enable the examination of global protein changes during processes such as plant development or stress responses, and provide a robust biological application to the technology (Van Wijk 2001; Cánovas et al. 2004). Current proteomic technologies need genomic information for identification of proteins. Relatively large number of genes present in plant systems offers functional diversity, however, poses problems for correct identification of proteins.
In addition to these few technical challenges mentioned above, certain other bigger questions also need to be addressed by the plant research community. For example: (i) how plant proteomics research groups take the challenges in a united way to prioritize and address these; (ii) how to openly share the proteomics information or make it easily available; (iii) how to have an integrated plant proteomics database or develop one; and maybe most importantly (iv) how to bring awareness about proteomics and its applications.
One major step taken in addressing some of the issues outlined above is the establishment of the International Plant Proteomics Organization (INPPO). This organization initiated the process of a collective vision on how to overcome the technical and other stumbling blocks in the way of plant proteomics, especially in relation to facilitating cutting edge proteomics on the subcontinent (Agrawal et al. 2011a). Achievements of INPPO in a short period of its activities were recently highlighted (Agrawal et al. 2012b).
Establishment of INPPO-India-Nepal plant proteomics chapter and the work ahead
INPPO (www.inppo.com) was formally founded in 2011, with a key aim towards having a shared global platform for the plant proteomics community (for details, see INPPO history at http://www.inppo.com/inppohistory.jsp). A total of 10 initiatives of INPPO were outlined (see INPPO viewpoint paper, Agrawal et al. 2011a, 2012b). Besides the objectives detailed in Fig. 5 and on the INPPO website, one important goal is to develop interactions with other proteomic initiatives [like HUPO (the Human Proteome Organization) and MASCP (the Multinational Arabidopsis Steering Committee for Proteomics)], and help address plant biological questions that have societal implications such as food security. To keep the momentum and inform the INPPO members of on-going INPPO activities, the INPPO Public Relation Committee publishes the INPPO Express, News & Views (http://www.inppo.com/newsletter.jsp).
Fig. 5.
Objectives and future strategies of INPPO-India-Nepal Plant Proteomics Chapter (INPPC) to enhance collaboration and capacity building in plant proteomics
As expected, the advent of INPPO has stimulated interaction between researchers at both personal and professional levels, locally and globally. Moreover, one of the major objectives of INPPO is to highlight national plant proteomics issues via common regional platforms. In November 2011, one scientist (Associate Professor Renu Deswal, Department of Botany, University of Delhi) from India attended a proteomics conference in Tsukuba, Japan. As Dr. Deswal is an active member of the Executive Council of INPPO and the Chairperson of INPPO Development Committee, Dr. Rakwal as the Vice President of INPPO had an opportunity to meet her in person and discuss about INPPO development. One key outcome of discussions was to establish INPPO national chapters in developing countries. One outstanding reason was to take stock and address the lack of proteomics infrastructure and funding in this cutting edge field of biological research in developing countries. This meeting resulted in the establishment of an ‘INPPO-India-Nepal Plant Proteomics Chapter (INPPC; www.inppo.com/chapter) with inputs from many plant researchers to facilitate close interaction of researchers from the two countries. An important aspect of this was to potentiate Nepalese researchers with interesting plant/crop science questions and interesting materials, to collaborate with Indian scientists who have access to proteomics facilities.
The establishment of INPPC is a step towards the goal of networking plant proteomics researchers at the national and regional levels. It would lead the way for INPPO’s vision to bring together all plant proteomers and plant biologists, in general, to a common platform to discuss the urgent and expedient problems in refining the proteomics technologies to the betterment of plant and agricultural sciences thus positively impacting food and livelihood generation.
An important element of INPPC is to encourage and mentor the younger plant biologists especially through their direct involvement in running the affairs of INPPC, so that they are well networked for technical, scientific and career guidance, with senior members who could even provide research opportunities to deserving candidate from among the young pool of members. Equally, the senior members will be cognizant of their commitment to render support.
The proposed objectives (http://www.inppo.com/indonepalobjective.jsp) and future strategies (http://www.inppo.com/indonepalobjective.jsp) of INPPC are detailed in Fig. 5. INPPC envisaged national level/nation-wide coordinated projects funded by national and international funding agencies under INPPO umbrella. The creation of national plant proteomics hub in proper coordination with INPPO GATOR was envisioned. Chapter meetings and national-level conferences organized every 2 years by INPPC at different locations of the two countries hope to attract the interest and momentum of younger generation. INPPC also envisaged conducting national workshops, generating scholarships, and funding options for young researchers to attend these workshops. Last but not the least, promoting the utilization of plant proteomics techniques for finding sustainable solutions for nation-wide major thrust areas such as food security, biosafety and biodiversity, etc., will remain an overriding concern of INPPC as well as INPPO.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 36 kb)
(DOC 62 kb)
Acknowledgments
Authors would like to thank Mr. Raj Agrawal (Database & Webpage Administrator, INPPO) for constantly updating our members on the INPPC information through the INPPO website (www.inppo.com). We would also like to thank the team of INPPO supporting staff for their help and support during the development of INPPC. We would like to express our thanks to Dominique Job for presenting INPPO initiatives at the French-Indian proteomics workshop (2013) in Bangalore. RD thanks Department of Biotechnology and R & D grant from University of Delhi for partial financial support for the work mentioned in the review.
Contributor Information
Renu Deswal, Email: rdeswal@botany.du.ac.in.
Ganesh Kumar Agrawal, Email: gkagrawal123@gmail.com.
Randeep Rakwal, Email: plantproteomics@gmail.com.
References
- Abat JK, Deswal R. Differential modulation of S-nitrosoproteome of Brassica juncea by low temperature: Change in S-nitrosylation of Rubisco is responsible for the inactivation of its carboxylase activity. Proteomics. 2009;9:4368–4380. doi: 10.1002/pmic.200800985. [DOI] [PubMed] [Google Scholar]
- Abat JK, Deswal R. Nitric oxide modulates the expression of proteins and promotes epiphyllous bud differentiation in Kalanchoe pinnata. J Plant Growth Regul. 2012;32:92–101. doi: 10.1007/s00344-012-9279-3. [DOI] [Google Scholar]
- Abat JK, Mattoo AK, Deswal R. S-nitrosylated proteins of a medicinal CAM plant Kalanchoe pinnata- ribulose-1,5-bisphosphate carboxylase/oxygenase activity targeted for inhibition. FEBS J. 2008;275:2862–2872. doi: 10.1111/j.1742-4658.2008.06425.x. [DOI] [PubMed] [Google Scholar]
- Agrawal GK Rakwal R (2008) Plant proteomics: technologies, strategies, and applications. In: Agrawal GK, Rakwal R (eds.), John Wiley & Sons, Inc., Hoboken
- Agrawal GK, Thelen JJ. Large scale identification and quantitative profiling of phosphoproteins expressed during seed filling in oilseed rape. Mol Cell Proteomics. 2006;5:2044–2059. doi: 10.1074/mcp.M600084-MCP200. [DOI] [PubMed] [Google Scholar]
- Agrawal GK, Rakwal R, Yonekura M, Kubo A, Saji H. Proteome analysis of differentially displayed proteins as a tool for investigating ozone stress in rice (Oryza sativa L.) seedlings. Proteomics. 2002;2:947–959. doi: 10.1002/1615-9861(200208)2:8<947::AID-PROT947>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Agrawal GK, Hajduch M, Graham K, Thelen JJ. In-depth investigation of the soybean seed-filling proteome and comparison with a parallel study of rapeseed. Plant Physiol. 2008;148:504–518. doi: 10.1104/pp.108.119222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal L, Chakraborty S, Jaiswal DK, Gupta S, Datta A, Chakraborty N. Comparative proteomics of tuber induction, development and maturation reveal the complexity of tuberization process in potato (Solanum tuberosum L.) J Proteome Res. 2008;7:3803–3817. doi: 10.1021/pr8000755. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Kumar S, Das HR. Mass spectrometric characterization of isoform variants of peanut (Arachis hypogaea) stem lectin (SL-I) J Proteome Res. 2010;73:1573–1586. doi: 10.1016/j.jprot.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Agrawal GK, Job D, Zivy M, Agrawal VP, Bradshaw RA, Dunn MJ, Haynes PA, van Wijk KJ, Kikuchi S, Renaut J, Weckwerth W, Rakwal R. Time to articulate a vision for the future of plant proteomics - a global perspective: an initiative for establishing the International Plant Proteomics Organization (INPPO) Proteomics. 2011;11:1559–1568. doi: 10.1002/pmic.201000608. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Kumar S, Jaiswal YK, Das HR, Das RH. A Mesorhizobium lipopolysaccharide (LPS) specific lectin (CRL) from the roots of nodulating host plant, Cicer arietinum. Biochimie. 2011;93:440–449. doi: 10.1016/j.biochi.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Agrawal GK, Pedreschi R, Barkla BJ, Bindschedler LV, Cramer R, Sarkar A, Renaut J, Job D, Rakwal R. Translational plant proteomics: a perspective. J Proteomics. 2012;75:4588–4601. doi: 10.1016/j.jprot.2012.03.055. [DOI] [PubMed] [Google Scholar]
- Agrawal GK, Sarkar A, Agrawal R, Ndimba BK, Tanou G, Dunn MJ, Kieselbach T, Cramer R, Wienkoop S, Chen S, Rafudeen MS, Deswal R, Barkla BJ, Weckwerth W, Heazlewood JL, Renaut J, Job D, Chakraborty N, Rakwal R. Boosting the globalization of plant proteomics through INPPO: current developments and future prospects. Proteomics. 2012;12:359–368. doi: 10.1002/pmic.201290018. [DOI] [PubMed] [Google Scholar]
- Agrawal GK, Sarkar A, Righetti PG, Pedreschi R, Carpentier S, Wang T, Barkla BJ, Kohli A, Ndimba BK, Bykova NV, Rampitsch C, Zolla L, Rafudeen MS, Cramer R, Bindschedler LV, Tsakirpaloglou N, Ndimba RJ, Farrant JM, Renaut J, Job D, Kikuchi S, Rakwal R. A decade of plant proteomics and mass spectrometry: translation of technical advancements to food security and safety issues. Mass Spectrom Rev. 2013 doi: 10.1002/mas.21365. [DOI] [PubMed] [Google Scholar]
- Amalraj RS, Selvaraj N, Veluswamy GK, Ramanujan RP, Muthurajan R, Palaniyandi M, Agrawal GK, Rakwal R, Viswanathan R. Sugarcane proteomics: establishment of a protein extraction method for 2-DE in stalk tissues and initiation of sugarcane proteome reference map. Electrophoresis. 2010;31:1959–1974. doi: 10.1002/elps.200900779. [DOI] [PubMed] [Google Scholar]
- Bhattacharayya D, Sinha R, Ghanta S, Chakraborty A, Hazra S, Chattopadhyay S. Proteins differentially expressed in elicited cell suspension culture of Podophyllum hexandrum with enhanced podophyllotoxin content. Proteome Sci. 2012;10:34. doi: 10.1186/1477-5956-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan D, Pandey A, Chattopadhyay A, Choudhary MK, Chakraborty S, Datta A, Chakraborty N. Extracellular matrix proteome of chickpea (Cicer arietinum L.) illustrates pathway abundance, novel protein functions and evolutionary perspect. J Proteome Res. 2006;5:1711–1720. doi: 10.1021/pr060116f. [DOI] [PubMed] [Google Scholar]
- Bhushan D, Pandey A, Choudhary MK, Datta A, Chakraborty S, Chakraborty N. Comparative proteomics analysis of differentially expressed proteins in Chickpea extracellular matrix during dehydration stress. Mol Cell Proteomics. 2007;6:1868–1884. doi: 10.1074/mcp.M700015-MCP200. [DOI] [PubMed] [Google Scholar]
- Bhushan D, Jaiswal DK, Ray D, Basu D, Datta A, Chakraborty S, Chakraborty N. Dehydration-responsive reversible and irreversible changes in the extracellular matrix: comparative proteomics of chickpea genotypes with contrasting tolerance. J Proteome Res. 2011;10:2027–2046. doi: 10.1021/pr200010f. [DOI] [PubMed] [Google Scholar]
- Biswas S, Agrawal P, Saroha A, Das HR. Purification and mass spectrometric characterization of Sesbania aculeata (Dhaincha) stem lectin. Protein J. 2009;28:391–399. doi: 10.1007/s10930-009-9206-z. [DOI] [PubMed] [Google Scholar]
- Cánovas FM, Dumas-Gaudot E, Recorbet G, Jorrin J, Mock HP, Rossignol M. Plant proteome analysis. Proteomics. 2004;4:285–298. doi: 10.1002/pmic.200300602. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Chakraborty N, Agrawal L, Ghosh S, Narula K, Shekhar S, Naik PS, Pande PC, Chakrborti SK, Datta A. Next-generation protein-rich potato expressing the seed protein gene AmA1 is a result of proteome rebalancing in transgenic tuber. Proc Natl Acad Sci USA. 2010;107:17533–17538. doi: 10.1073/pnas.1006265107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A, Subba P, Pandey A, Bhushan D, Kumar R, Datta A, Chakraborty S, Chakraborty N. Analysis of the grasspea proteome and identification of stress-responsive proteins upon exposure to high salinity, low temperature, and abscisic acid treatment. Phytochemistry. 2011;72:1293–1307. doi: 10.1016/j.phytochem.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Cho K, Agrawal GK, Shibato J, Jung YH, Kim YK, Nahm BH, Jwa NS, Tamogami S, Han O, Kohda K, Iwahashi H, Rakwal R. Survey of differentially expressed proteins and genes in jasmonic acid treated rice seedling shoot and root at the proteomics and transcriptomics levels. J Proteome Res. 2007;6:3581–3603. doi: 10.1021/pr070358v. [DOI] [PubMed] [Google Scholar]
- Cho K, Shibato J, Agrawal GK, Jung YH, Kubo A, Jwa NS, Tamogami S, Satoh K, Kikuchi S, Higashi T, Kimura S, Saji H, Tanaka Y, Iwahashi H, Masuo Y, Rakwal R. Integrated transcriptomics, proteomics, and metabolomics analyzes to survey ozone responses in the leaves of rice seedling. J Proteome Res. 2008;7:2980–2998. doi: 10.1021/pr800128q. [DOI] [PubMed] [Google Scholar]
- Choudhary MK, Basu D, Datta A, Chakraborty N, Chakraborty S. Dehydration-responsive nuclear proteome of rice (Oryza sativa L.) illustrates protein network, novel regulators of cellular adaptation, and evolutionary perspective. Mol Cell Proteomics. 2009;8:1579–1598. doi: 10.1074/mcp.M800601-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourey K, Ramani S, Apte SK. Accumulation of LEA proteins in salt (NaCl) stressed young seedlings of rice (Oryza sativa L.) cultivar Bura Rata and their degradation during recovery from salinity stress. J Plant Physiol. 2003;160:1165–1174. doi: 10.1078/0176-1617-00909. [DOI] [PubMed] [Google Scholar]
- Deeba F, Pandey AK, Ranjan S, Mishra A, Singh R, Sharma YK, Shirke PA, Pandey V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol Biochem. 2012;53:6–18. doi: 10.1016/j.plaphy.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Demartini DR, Jain R, Agrawal G, Thelen JJ. Proteomic comparison of plastids from developing embryos and leaves of Brassica napus. J Proteome Res. 2011;10:2226–2237. doi: 10.1021/pr101047y. [DOI] [PubMed] [Google Scholar]
- Dogra V, Ahuja PS, Sreenivasulu Y. Change in protein content during seed germination of a high altitude plant Podophyllum hexandrum Royle. J Proteomics. 2013;78:26–38. doi: 10.1016/j.jprot.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Frommer WB. New technologies for 21st century plant science. Plant Cell. 2012;00:1–21. doi: 10.1105/tpc.111.093302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . How to feed the world in 2050, high-level expert forum. Rome: Food and Agriculture Organization of the United Nations; 2009. [Google Scholar]
- FAO (2010) The State of Food Insecurity in the World: addressing food insecurity in protracted crises. ISBN 978-92-5-106610-2, 2010, Food and Agriculture Organization of the United Nations, Rome
- Frohlich A, Gaupels F, Sarioglu H, Holzmeister C, Spannagl M, Durner J, Lindermayr C. Looking deep inside: detection of low-abundance proteins in leaf extracts of Arabidopsis and phloem exudates of pumpkin. Plant Physiol. 2012;159:902–914. doi: 10.1104/pp.112.198077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T, Dogra V, Kumar S, Ahuja PS, Sreenivasulu Y. Protein dynamics during seed germination under copper stress in Arabidopsis over-expressing Potentilla superoxide dismutase. J Plant Res. 2012;125:165–172. doi: 10.1007/s10265-011-0421-2. [DOI] [PubMed] [Google Scholar]
- Gupta R, Deswal R. Low temperature stress modulated secretome analysis and purification of antifreeze protein from Hippophae rhamnoides, a Himalayan wonder plant. J Proteome Res. 2012;11:2684–2696. doi: 10.1021/pr200944z. [DOI] [PubMed] [Google Scholar]
- Hajduch M, Rakwal R, Agrawal GK, Yonekura M, Pretova A. High-resolution two-dimensional electrophoresis separation of proteins from metal-stressed rice (Oryza sativa L.) leaves: drastic reductions/fragmentation of ribulose-1,5-bisphosphate carboxylase/oxygenase and induction of stress related proteins. Electrophoresis. 2001;22:2824–2831. doi: 10.1002/1522-2683(200108)22:13<2824::AID-ELPS2824>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hakeem KR, Chandna R, Ahmad A, Qureshi MI, Iqbal M. Proteomic analysis for low and high nitrogen-responsive proteins in the leaves of rice genotypes grown at three nitrogen levels. Appl Biochem Biotechnol. 2012;168:834–850. doi: 10.1007/s12010-012-9823-4. [DOI] [PubMed] [Google Scholar]
- Hakeem KR, Chandna R, Ahmad P, Iqbal M, Ozturk M. Relevance of proteomic investigations in plant abiotic stress physiology. OMICS. 2012;16:621–635. doi: 10.1089/omi.2012.0041. [DOI] [PubMed] [Google Scholar]
- Heazlewood JL. The green proteome: challenges in plant proteomics. Front Plant Sci. 2011;2:6. doi: 10.3389/fpls.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Srivastava S, Sarin NB, Kav NN. Proteomics reveals elevated levels of PR 10 proteins in saline-tolerant peanut (Arachis hypogaea) calli. Plant Physiol Biochem. 2006;44:253–259. doi: 10.1016/j.plaphy.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Jaiswal DK, Ray D, Subba P, Mishra P, Gayali S, Datta A, Chakraborty S, Chakraborty N. Proteomic analysis reveals the diversity and complexity of membrane proteins in chickpea (Cicer arietinum L.) Proteome Sci. 2012;10:59. doi: 10.1186/1477-5956-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YH, Rakwal R, Agrawal GK, Shibato J, Kim JA, Lee MO, Choi PK, Jung SH, Kim SH, Koh HJ, Yonekura M, Iwahashi H, Jwa NS. Differential expression of defense/stress-related marker proteins in leaves of a unique rice blast lesion mimic mutant (blm) J Proteome Res. 2006;5:2586–2598. doi: 10.1021/pr060092c. [DOI] [PubMed] [Google Scholar]
- Jung YH, Jeong SH, Kim SH, Singh R, Lee JE, Cho YS, Agrawal GK, Rakwal R, Jwa NS. Systematic secretome analyzes of rice leaf and seed callus suspension-cultured cells: workflow development and establishment of high-density two-dimensional gel reference maps. J Proteome Res. 2008;7:5187–5210. doi: 10.1021/pr8005149. [DOI] [PubMed] [Google Scholar]
- Katavic V, Agrawal GK, Hajduch M, Harris SL, Thelen JJ. Protein and lipid composition analysis of oil bodies from two Brassica napus cultivars. Proteomics. 2006;6:4586–4598. doi: 10.1002/pmic.200600020. [DOI] [PubMed] [Google Scholar]
- Kim DW, Rakwal R, Agrawal GK, Jung YH, Shibato J, Jwa NS, Iwahashi Y, Iwahashi H, Kim DH, Shim S, Usui K. A hydroponic rice seedling culture model system for investigating proteome of salt stress in rice leaf. Electrophoresis. 2005;26:4521–4539. doi: 10.1002/elps.200500334. [DOI] [PubMed] [Google Scholar]
- Kim ST, Kang YH, Wang Y, Wu J, Park ZY, Rakwal R, Agrawal GK, Lee SY, Kang KY. Secretome analysis of differentially induced proteins in rice suspension-cultured cells triggered by rice blast fungus and elicitor. Proteomics. 2009;9:1302–1313. doi: 10.1002/pmic.200800589. [DOI] [PubMed] [Google Scholar]
- Kim SG, Wang Y, Lee KH, Park ZY, Park J, Wu J, Kwon SJ, Lee YH, Agrawal GK, Rakwal R, Kim ST, Kang KY. In-depth insight into in vivo apoplastic secretome of rice-Magnaporthe oryzae interaction. J Proteomics. 2013;78:58–71. doi: 10.1016/j.jprot.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Lee HM, Wang Y, Wu J, Kim SG, Kang KY, Park KH, Kim YC, Choi IS, Agrawal GK, Rakwal R, Kim ST. Depletion of abundant plant RuBisCO protein using the protamine sulfate precipitation method. Proteomics. 2013 doi: 10.1002/pmic.201200555. [DOI] [PubMed] [Google Scholar]
- Kumar S, Verma AK, Sharma A, Kumar D, Tripathi A, Chaudhari BP, Das M, Jain SK, Dwivedi PD. Phytohemagglutinins augment red kidney bean (Phaseolus vulgaris L.) induced allergic manifestations. J Proteomics. 2013 doi: 10.1016/j.jprot.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Kundu S, Chakraborty D, Pal A. Proteomic analysis of salicylic acid induced resistance to Mungbean Yellow Mosaic India Virus in Vigna mungo. J Proteomics. 2011;74:337–349. doi: 10.1016/j.jprot.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Kundu S, Chakraborty D, Kundu A, Pal A. Proteomics approach combined with biochemical attributes to elucidate compatible and incompatible plant-virus interactions between Vigna mungo and Mungbean Yellow Mosaic India Virus. Proteome Sci. 2013;11:15. doi: 10.1186/1477-5956-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JP, Ethier M, Smith JC, Figeys D. Proteomics: from gel based to gel free. Anal Chem. 2005;77:3771–3788. doi: 10.1021/ac050586d. [DOI] [PubMed] [Google Scholar]
- Mandal SM, Mandal M, Pati BR, Das AK, Ghosh AK. Proteomics view of a Rhizobium isolate response to arsenite [As(III)] stress. Acta Microbiol Immunol Hung. 2009;56:157–167. doi: 10.1556/AMicr.56.2009.2.4. [DOI] [PubMed] [Google Scholar]
- McDonald H, Friedman D. Leverging technologies: DIGE and MudPIT. J Biomol Tech. 2010;21:S10. [Google Scholar]
- Mishra M, Tamhane VA, Khandelwal N, Kulkarni MJ, Gupta VS, Giri AP. Interaction of recombinant CanPIs with Helicoverpa armigera gut proteases reveals their processing patterns, stability and efficiency. Proteomics. 2010;10:2845–2857. doi: 10.1002/pmic.200900853. [DOI] [PubMed] [Google Scholar]
- Narula K, Datta A, Chakraborty N, Chakraborty S. Comparative analyses of nuclear proteome: extending its function. Frontiers Plant Sci. 2013 doi: 10.3389/fpls.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A, Choudhary MK, Bhushan D, Chattopadhyay A, Chakraborty S, Datta A, Chakraborty N. The nuclear proteome of Chickpea (Cicer arietinum L.) reveals predicted and unexpected proteins. J Proteome Res. 2006;5:3301–3311. doi: 10.1021/pr060147a. [DOI] [PubMed] [Google Scholar]
- Pandey A, Chakraborty S, Datta A, Chakraborty N. Proteomics approach to identify dehydration responsive nuclear proteins from Chickpea (Cicer arietinum L.) Mol Cell Proteomics. 2008;7:88–107. doi: 10.1074/mcp.M700314-MCP200. [DOI] [PubMed] [Google Scholar]
- Pandey A, Rajamani U, Verma J, Subba P, Chakraborty N, Datta A, Chakraborty S, Chakraborty N. Identification of extracellular matrix proteins of rice (Oryza sativa L.) involved in dehydration-responsive network: a proteomic approach. J Proteome Res. 2010;9:3443–3464. doi: 10.1021/pr901098p. [DOI] [PubMed] [Google Scholar]
- Pandey S, Rai R, Rai LC. Proteomics combines morphological, physiological and biochemical attributes to unravel the survival strategy of Anabaena sp. PCC7120 under arsenic stress. J Proteomics. 2012;75:921–937. doi: 10.1016/j.jprot.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Pathak M, Singh B, Sharma A, Agrawal P, Pasha SB, Das HR, Das RH. Molecular cloning, expression, and cytokinin (6-benzylaminopurine) antagonist activity of peanut (Arachis hypogaea) lectin SL-I. Plant Mol Biol. 2006;62:529–545. doi: 10.1007/s11103-006-9038-6. [DOI] [PubMed] [Google Scholar]
- Raghav SK, Gupta B, Shrivastava A, Das HR. Inhibition of lipopolysaccharide-inducible nitric oxide synthase and IL-1β through suppression of NF-κB activation by 3-(1′-1′-dimethyl-allyl)-6-hydroxy-7-methoxy-coumarin isolated from Ruta graveolens L. Eur J Pharmacol. 2007;560:69–80. doi: 10.1016/j.ejphar.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Rakwal R, Agrawal GK, Yonekura M. Separation of proteins from stressed rice (Oryzae sativa L.) leaf tissues by two-dimensional polyacrylamide gel electrophoresis: induction of pathogenesis-related and cellular protectant proteins by jasmonic acid, UV irradiation and copper chloride. Electrophoresis. 1999;20:3472–3478. doi: 10.1002/(SICI)1522-2683(19991101)20:17<3472::AID-ELPS3472>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rana B, Sreenivasulu Y. Protein changes during ethanol induced seed germination in Aconitum heterophyllum. Plant Sci. 2013;198:27–38. doi: 10.1016/j.plantsci.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Ray S, Patra B, Das-Chatterjee A, Ganguli A, Majumder AL. Identification and organization of chloroplastic and cytosolic L-myo-inositol 1-phosphate synthase coding gene(s) in Oryza sativa: comparison with the wild halophytic rice, Porteresia coarctata. Planta. 2010;231:1211–1227. doi: 10.1007/s00425-010-1127-8. [DOI] [PubMed] [Google Scholar]
- Renuse S, Harsha HC, Kumar P, Acharya PK, Sharma J, Goel R, Kumar GS, Raju R, Prasad TS, Slotta T, Pandey A. Proteomic analysis of an unsequenced plant–Mangifera indica. J Proteomics. 2012;75:5793–5796. doi: 10.1016/j.jprot.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Righetti PG, Boschetti E, Lomas L, Citterio A. Protein equalizer technology: the quest for a “democratic proteome”. Proteomics. 2006;6:3980–3992. doi: 10.1002/pmic.200500904. [DOI] [PubMed] [Google Scholar]
- Righetti PG, Boschetti E, Fasoli E. Capturing and amplifying impurities from recombinant therapeutic proteins via combinatorial peptide libraries: a proteomic approach. Curr Pharm Biotechnol. 2011;12:1537–1547. doi: 10.2174/138920111798357285. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Rakwal R, Agrawal SB, Shibato J, Ogawa Y, Yoshida Y, Agrawal GK, Agrawal M. Investigating the impact of elevated levels of ozone on tropical wheat using integrated phenotypical, physiological, biochemical, and proteomics approaches. J Proteome Res. 2010;9:4565–4584. doi: 10.1021/pr1002824. [DOI] [PubMed] [Google Scholar]
- Sehrawat A, Gupta R, Deswal R. Nitric oxide-cold stress signalling crosstalk-evolution of a novel regulatory mechanism. Proteomics. 2013 doi: 10.1002/pmic.201200445. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Majumder AL. Insight into the salt tolerance factors of a wild halophytic rice, Porteresia coarctata: a physiological and proteomic approach. Planta. 2009;229:911–929. doi: 10.1007/s00425-008-0878-y. [DOI] [PubMed] [Google Scholar]
- Sengupta D, Kannan M, Reddy AR. A root proteomics-based insight reveals dynamic regulation of root proteins under progressive drought stress and recovery in Vigna radiata (L.) Wilczek. Planta. 2011;233:1111–1127. doi: 10.1007/s00425-011-1365-4. [DOI] [PubMed] [Google Scholar]
- Sinha R, Chattopadhyay S. Changes in the leaf proteome profile of Mentha arvensis in response to Alternaria alternata infection. J Proteomics. 2011;74:327–336. doi: 10.1016/j.jprot.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Sinha R, Bhattacharyya D, Majumdar AB, Datta R, Hazra S, Chattopadhyay S. Leaf proteome profiling of transgenic mint infected with Alternaria alternata. J Proteomics. 2013 doi: 10.1016/j.jprot.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Swatek KN, Graham K, Agrawal GK, Thelen JJ. The 14-3-3 isoforms chi and epsilon differentially bind client proteins from developing Arabidopsis seed. J Proteome Res. 2011;10:4076–4087. doi: 10.1021/pr200263m. [DOI] [PubMed] [Google Scholar]
- Thelen JJ, Peck S. Quantitative proteomics in plants: choices in abundance. Plant Cell. 2007;19:3339–3346. doi: 10.1105/tpc.107.053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiellement H, Zivy M, Damerval C, Mechin V (2007) Plant proteomics: methods and protocols. Thiellement H (ed.), vol. 355, Humana Press.
- Torres NL, Cho K, Shibato J, Hirano M, Kubo A, Masuo Y, Iwahashi H, Jwa NS, Agrawal GK, Rakwal R. Gel-based proteomics reveals potential novel protein markers of ozone stress in leaves of cultivated bean and maize species of Panama. Electrophoresis. 2007;28:4369–4381. doi: 10.1002/elps.200700219. [DOI] [PubMed] [Google Scholar]
- Upadhyay SK, Mishra M, Singh H, Ranjan A, Chandrashekar K, Verma PC, Singh PK, Tuli R. Interaction of Allium sativum leaf agglutinin with midgut brush border membrane vesicles proteins and its stability in Helicoverpa armigera. Proteomics. 2010;10:4431–4440. doi: 10.1002/pmic.201000152. [DOI] [PubMed] [Google Scholar]
- Van Wijk KJ. Challenges and prospects of plant proteomics. Plant Physiol. 2001;126:501–508. doi: 10.1104/pp.126.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranagamallaiah G, Jyothsnakumari G, Thippeswamy M, Reddy PCO, Surabhi G-K, Sriranganayakulu G, Mahesh Y, Rajasekhar B, Madhurarekha C, Sudhakar C. Proteomic analysis of salt stress responses in foxtail millet (Setaria italica L. cv. Prasad) seedlings. Plant Sci. 2008;175:631–641. doi: 10.1016/j.plantsci.2008.06.017. [DOI] [Google Scholar]
- Washburn MP, Wolters D, Yates JR., III Large-scale analysis of the proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- Wilkins MR, Sanchez J-C, Gooley AA, Appel RD, Humphery-Smith I, Hochstrasser DF, Williams KL. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. 1995;13:19–50. doi: 10.1080/02648725.1996.10647923. [DOI] [PubMed] [Google Scholar]
- Yadavalli V, Nellaepalli S, Subramanyam R. Proteomic analysis of thylakoid membranes. Methods Mol Biol. 2011;684:159–170. doi: 10.1007/978-1-60761-925-3_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 36 kb)
(DOC 62 kb)