Abstract
In the past, we showed that exposure to abiotic and biotic stresses changes the homologous recombination frequency (HRF) in somatic tissue and in the progeny. In current work we planned to answer the following question: do stress intensity/duration and time during exposure influence changes in somatic HRF and transgenerational changes in HRF? Here, we tested the effects of exposure to UV-C, cold and heat on HRF at 7, 14, 21 and 28 days post germination (dpg). We found that exposure at 14 and 21 dpg resulted in a higher increase in HRF as compared to exposure at 7 dpg; longer exposure to UV-C resulted in a higher frequency of HR, whereas prolonged exposure to cold or heat, especially at later developmental stages, had almost no effect on somatic HRF. Exposure at 7 dpg had a positive effect on somatic growth of plants; plants exposed to stress at this age had larger leaves. The analysis of HRF in the progeny showed that the progeny of plants exposed to stress at 7 dpg had an increase in somatic HRF and showed larger sizes of recombination spots on leaves. The progeny of plants exposed to UV-C at 7 dpg and the progeny of plants exposed to cold or heat at 28 dpg had larger leaves as compared to control plants. To summarize, our experiments showed that changes in somatic and transgenerational HRF depend on the type of stress plants are exposed to, time of exposure during development and the duration of exposure.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-013-0197-z) contains supplementary material, which is available to authorized users.
Keywords: Abiotic stress, Arabidopsis thaliana, Development time, Homologous recombination frequency, Transgenerational response
Introduction
Plants cannot move away and thus are not able to avoid stress. As a result, plants developed a variety of mechanisms of acclimation and adaptation allowing them to cope with prolonged stress (Hauser et al. 2011; Kranner et al. 2010; Tuba and Lichtenthaler 2007). Abiotic and biotic stresses are known to destabilize the plant genome, although only a few of them, such as UV-C, ionizing radiation and certain chemicals, damage DNA directly (Boyko et al. 2010b; Kovalchuk et al. 2000). The great majority of stresses, such as water availability, salt, temperature fluctuations or infection with pathogens, are not known to damage DNA directly, but they may do so through free radicals, signaling, changes in DNA methylation and chromatin structure and the efficiency of DNA repair (Boyko et al. 2010a; Roldan-Arjona and Ariza 2009; Zhang et al. 2009).
One of the DNA repair mechanisms that can be substantially influenced by changes in chromatin structure is homologous recombination. HR repairs DNA single- and double-strand breaks, and the readily accessible regions of homology are required for its activity (Puchta 2005). Stresses that may change the chromatin structure either via changes in DNA methylation or histone modifications may also result in altered HR frequency (Bilichak et al. 2012; Endo et al. 2006; Gao et al. 2012; Kirik et al. 2006; Mirouze et al. 2012).
Many stresses were shown to alter the frequency of somatic homologous recombination (HRF), including gamma- and X-rays, exposures to UV-C and UV-B, treatments with chemicals causing oxidative stress, herbicides as well as changes in temperature and water regimes and infection with pathogens (Besplug et al. 2004; Boyko et al. 2005, 2010a, b; Filkowski et al. 2004; Kathiria et al. 2010; Kovalchuk et al. 2000; Lucht et al. 2002; Molinier et al. 2005; Pecinka et al. 2009; Ries et al. 2000; Yao and Kovalchuk 2011). The influence of stress on the frequency of homologous recombination cannot be underestimated since HR is able to rearrange the plant genome substantially, thus leading to loss of heterozygocity. HR is also a mechanism of crossing-over and therefore can make a significant contribution to plant evolution. Exposure to stress is also known to influence the frequency of meiotic recombination (Kovalchuk et al. 2003; Ries et al. 2000). Moreover, several experiments showed that the progeny of stressed plants exhibited higher levels of somatic HRF without being exposed to any stress (Boyko et al. 2007, 2010a; Kathiria et al. 2010; Molinier et al. 2006; Pecinka et al. 2009).
Previous experiments showed that the intensity or severity of the applied stress is not always directly proportional to an increase in HRF (Boyko et al. 2010a, b; Ries et al. 2000). We demonstrated that DNA damage and changes in HRF are not directly proportional to the received dose of ionizing radiation (Kovalchuk et al. 2000). In the past, we also showed that plants exposed to stress early during development exhibit the highest increase in HRF (Boyko et al. 2006b). Similarly to what was observed for changes in somatic HRF, increase in HRF in the progeny of stressed plants also depended on the severity of stress obtained in parental generation. The most pronounced changes in the progeny were observed when parents were exposed to 25 mM but not to 75 or 100 mM NaCl. In fact, the progeny of plants exposed to 100 mM NaCl did not show any increase in HRF. Exposure to 100 mM NaCl causes noticeable effect on plant physiology, whereas exposure to 25 and 75 mM NaCl does not. It is possible that transgenerational changes in HRF may only occur when parents are exposed to mild stress. Unfortunately, no other evidence exists to confirm this hypothesis.
Thus, in the current work, we attempted to answer the following questions: Do changes in HRF in the exposed somatic cells depend on the time of exposure and the duration/severity of stress exposure? Do transgenerational changes in HRF depend on the time of exposure and stress duration/severity in parental generation? To answer these questions, we exposed Arabidopsis thaliana plants to different levels of heat, cold and UV-C at 7, 14, 21, or 28 days post germination and analyzed HRF in somatic tissue and in the progeny. Changes in HRF in somatic cells were higher in plants exposed to higher levels of stress, although the most intense stress often resulted in lower changes in HRF or no changes at all. The progeny of plants exposed to heat exhibited the highest levels of HRF when parents were exposed early during development, whereas in the progeny of plants exposed to UV-C or cold, an increase in HRF was more pronounced when parental generation was exposed later during development.
Materials and methods
Plants used for experiments
In the experiments, we used Arabidopsis thaliana plants (line 15D8). This transgenic line is homozygous for two non-functional fragments of the luciferase transgene, one without a promoter and ATG, and another without stop codon and 3′ UTR (Figure S1). Strand breaks in one of the overlapping regions can be repaired via homologous recombination, thus restoring transgene structure. The luciferase activity is then visualized in vivo using a long exposure CCD camera (Ilnytskyy et al. 2004).
Growth condition
Seeds from Arabidopsis thaliana (line 15D8) were incubated in soil at 4 °C for 3 days. Plants were then transferred to a growth chamber for germination, and 3 days after germination, they were transplanted into the standard 5 × 5 cm plastic pots with compost soil. Plants were grown at 22 °C (16 h day)/18 °C (8 h night).
Experimental setup
For UV-C exposure, the Arabidopsis plants were irradiated with an intensity of 100 ergs/cm2/s of UV-C. The plants were exposed at 7, 14, 21, and 28 days post germination for 15 s, 30 s, 2 min and 4 min receiving 3,000 ergs (3.0 J/m2), 12,000 ergs (12 J/m2) and 24,000 ergs (24 J/m2), respectively.
For heat stress exposure, two groups of plants were exposed to high temperatures: the heat of different temperature (HDT) group and the heat of different duration (HDD) group. The HDT group was exposed to 28 °C or 37 °C for 2 h at 7, 14, 21 or 28 days post germination. The HDD group was exposed to 50 °C for 30 min, 2 h or 4 h at the same development times.
For cold exposure, plants were exposed to 4 °C for 3, 6, 12, 24, and 48 h at 7, 14, 21 or 28 dpg. The trays with plants exposed to heat or cold were covered with a plastic lid. Control plants were grown at 22 °C.
In all cases, we measured HRF (average number of recombination events per plant), leaf size and the size of recombination events 10 days after stress application: at 17 dpg in plants exposed to stress at 7 dpg, at 24 dpg in plants exposed to stress at 14 dpg, and at 31 dpg in plants exposed to stress at 21 dpg. All measurements were done using a CCD camera and SpotCounter software (https://sourceforge.net/projects/spotcounter/). The software was developed by us for the specific application of counting the number of recombination events (HRF), measuring the size of recombination events (arbitrary units, reflecting size in pixels) and the size of leaves (arbitrary units, reflecting size in pixels). We were not able to measure HRF in plants that were exposed to stress at 28 dpg because at 10 days after stress (38 dpg), plants were too large for measurements with a CCD camera, and we were afraid to damage plants that otherwise would be needed for collecting seeds. The seeds from all experimental groups (including the 28 dpg group) were harvested and stored at room temperature. The analysis of HRF, leaf size and the size of recombination events in the progeny was done at 20 dpg.
The analysis of the frequency of homologous recombination
HRF in the parental generation (F0) was analyzed 10 days after stress exposure and in the progeny of stressed plants (F1)—at the age of 20 days. Prior to the analysis, F1 plants were grown under normal conditions. For the analysis of HRF, the plants were sprayed with luciferin, and 1 h later, luminescent spots were analyzed using a long exposure CCD camera (Boyko et al. 2006a, b). Spots were counted using SpotCounter software (https://sourceforge.net/projects/spotcounter/). HRF was calculated as an average number of recombination events in a population of 16 plants per treatment group—from two independent experiments, each consisting of 4 technical repeats with 4 plants per repeat.
The statistical treatment of the data
In all cases, two independent experiments, each consisting of 4 technical repeats, were performed. Each technical repeat consisted of 4 plants. In each case, the data from an individual technical repeat were prorated to the data from the corresponding control group and expressed as a percentage of control (set as 100 %). The averages standard errors (SE) were calculated from 8 data points (4 technical duplicates from 2 independent experiments). The statistical significance was analyzed by performing Student’s t-test (two-tailed, paired or non-paired type 3) using MS Excel software. The asterisks in each graph indicate p < 0.05. The statistical significance of experiments was also confirmed using the Analysis of Variance (ANOVA).
Results
Stress exposure results in changes in HRF, the average size of recombination events, and the average plant size
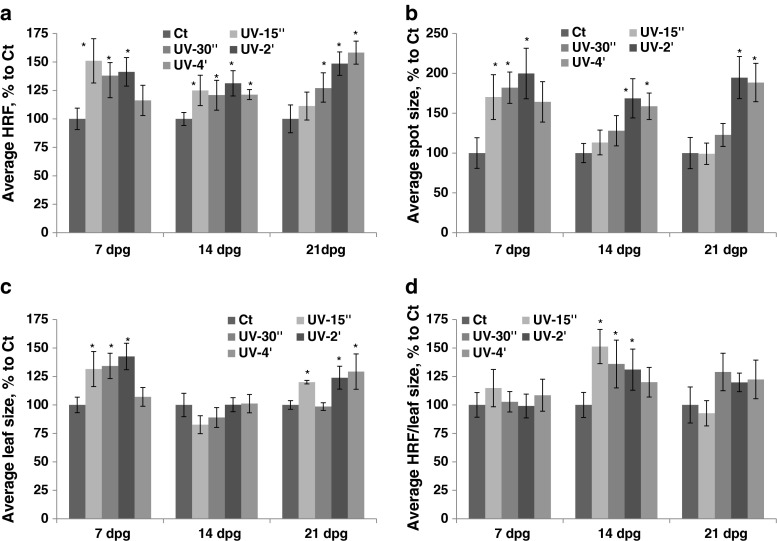
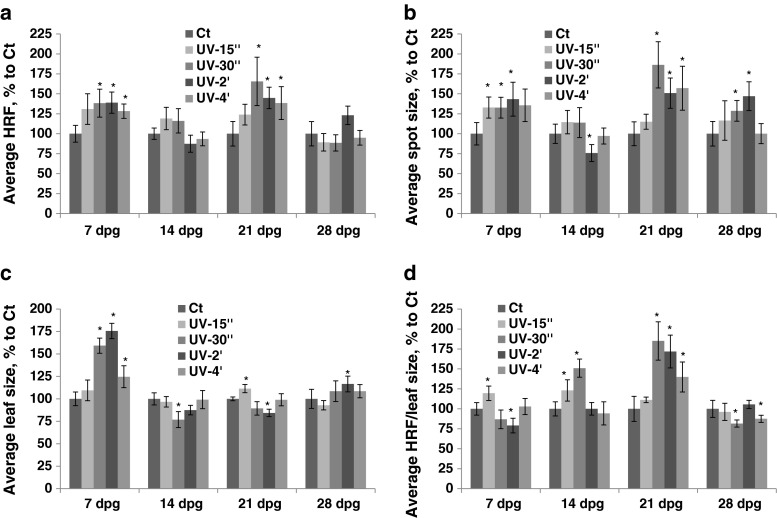
An increase in HRF was observed in plants exposed to UV-C; exposure at 7 dpg resulted in a lower increase in HRF after prolonged exposure, whereas exposure at 21 dpg led to an increase in HRF that was proportional to a UV-C dose applied (Fig. 1a, Table S1). The average spot size increased in parallel with an increase in duration of UV-C exposure at all developmental stages (7, 14 and 21 dpg) (Fig. 1b). The plants exposed to UV-C at 7 or 21 dpg increased the size of their leaves more or less proportionally to a UV-C dose received, except those that were exposed for 4 min at 7 dpg; leaf size did not change if plants were exposed at 14 dpg (Fig. 1c). If HRF was prorated according to leaf size, an increase was observed in plants exposed at 14 dpg (Fig. 1d).
Fig. 1.
The analysis of HRF, the size of recombination events, leaf size and HRF per leaf size in plants exposed to UV-C. The plants received different doses of UV-C at 7, 14, 21 and 28 dpg. All measurements were done in somatic tissues 10 days after stress application. The data are shown as a percentage relative to the control taken as 100 %. The error bars indicate a standard error calculated from 8 repeats (2 biological repeats, each with 4 technical repeats). The asterisks show a significant difference compared to the control (p < 0.05). a. Average HRF. b. Average spot size. c. Average leaf size. d. Average HRF related to leaf size
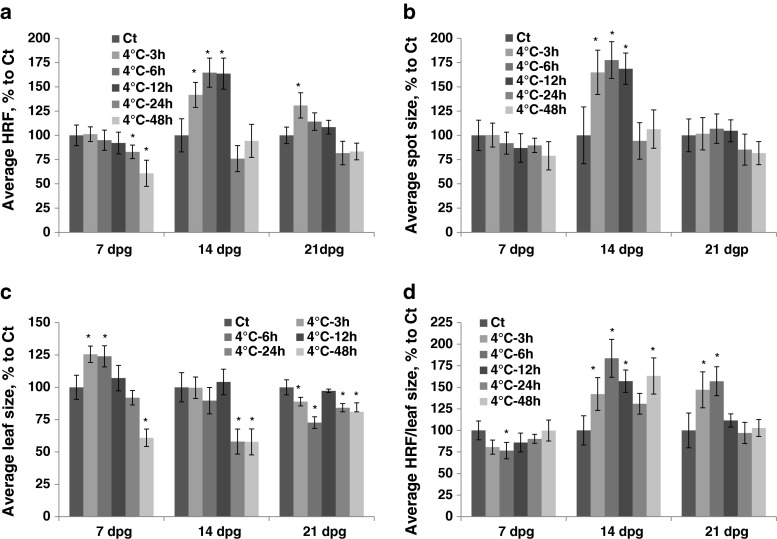
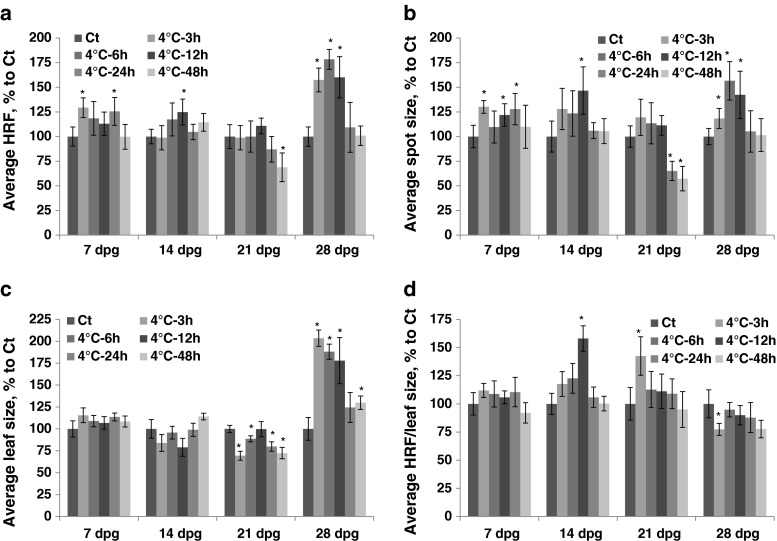
Exposure to cold at 7 dpg resulted in a decrease in HRF that was proportional to the length of exposure (Fig. 2a, Table S1). Exposure at 14 dpg caused a significant increase in HRF after short duration (3–12 h), but it did not change HRF after long exposure. (Fig. 2a). The size of recombination events increased only after 3–12 h of exposure to cold at 14 dpg (Fig. 2b). The average leaf size increased after short-term exposure (3–6 h) to cold at 7 dpg and decreased after prolonged exposure at all three developmental stages (Fig. 2c). There was an increase in HRF prorated according to leaf size in plants exposed to cold at 14 and 21 dpg (Fig. 2d).
Fig. 2.
The analysis of HRF, the size of recombination events, leaf size and HRF per leaf size in plants exposed to cold. The plants were exposed to cold at 7, 14, 21 and 28 dpg. All measurements were done in somatic tissues 10 days after stress application. The data are shown as a percentage relative to the control taken as 100 %. The error bars indicate a standard error calculated from 8 repeats (2 biological repeats, each with 4 technical repeats). The asterisks show a significant difference compared to the control (p < 0.05). a. Average HRF. b. Average spot size. c. Average leaf size. d. Average HRF related to leaf size
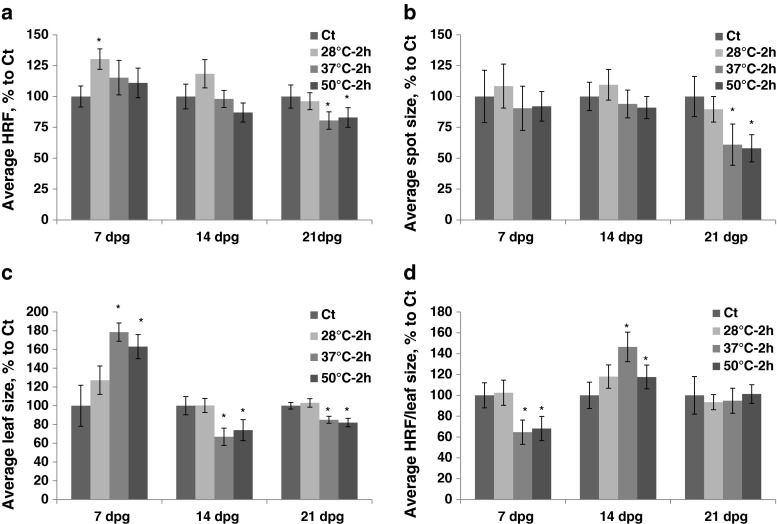
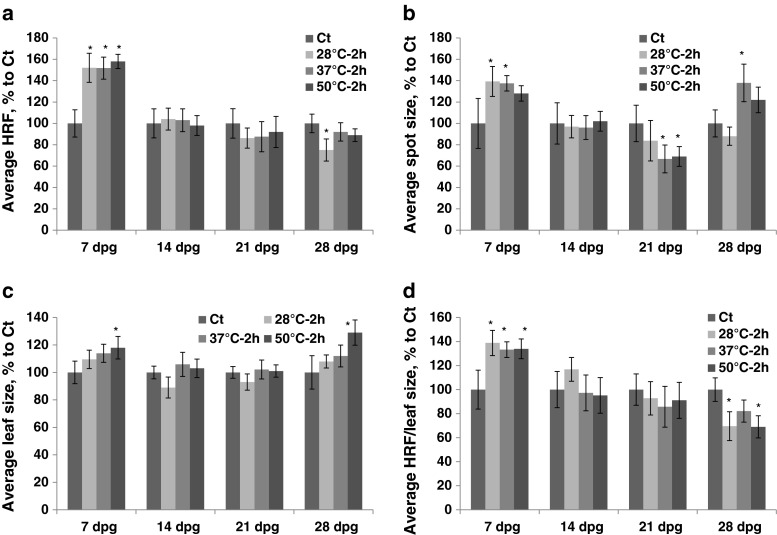
Exposure to heat caused an increase in HRF (the HDT group) if plants were exposed at 7 dpg. Exposure to 37 °C and 50 °C at 21 dpg led to a decrease in HRF (Fig. 3a, Table S1). The size of recombination events decreased after exposure of 21 dpg plants to 37 °C and 50 °C (Fig. 3b). Exposure to 37 °C and 50 °C increased leaf size if plants were exposed at 7 dpg and decreased it if plants were exposed at 14 or 21 dpg (Fig. 3c).
Fig. 3.
The analysis of HRF, the size of recombination events, leaf size and HRF per leaf size in plants exposed to different heat temperatures. The plants were exposed for 2 h to 28 °C, 37 °C and 50 °C at 7, 14, 21 and 28 dpg. All measurements were done in somatic tissues 10 days after stress application. The data are shown as a percentage relative to the control taken as 100 %. The error bars indicate a standard error calculated from 8 repeats (2 biological repeats, each with 4 technical repeats). The asterisks show a significant difference compared to the control (p < 0.05). a. Average HRF. b. Average spot size. c. Average leaf size. d. Average HRF related to leaf size
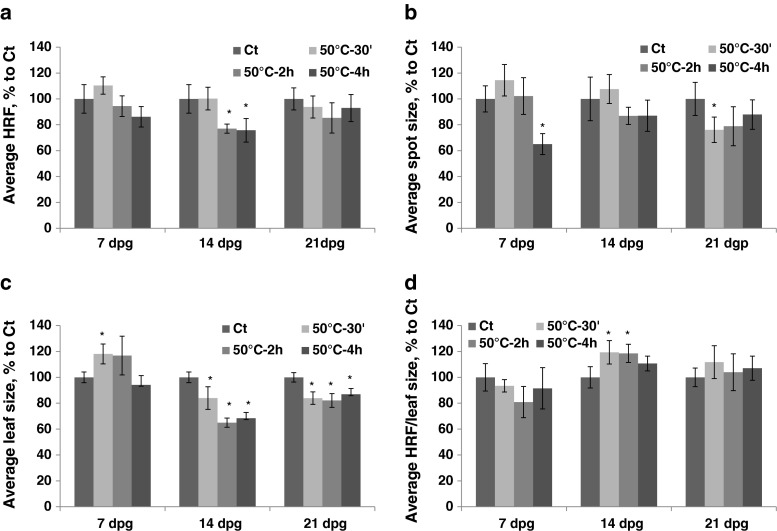
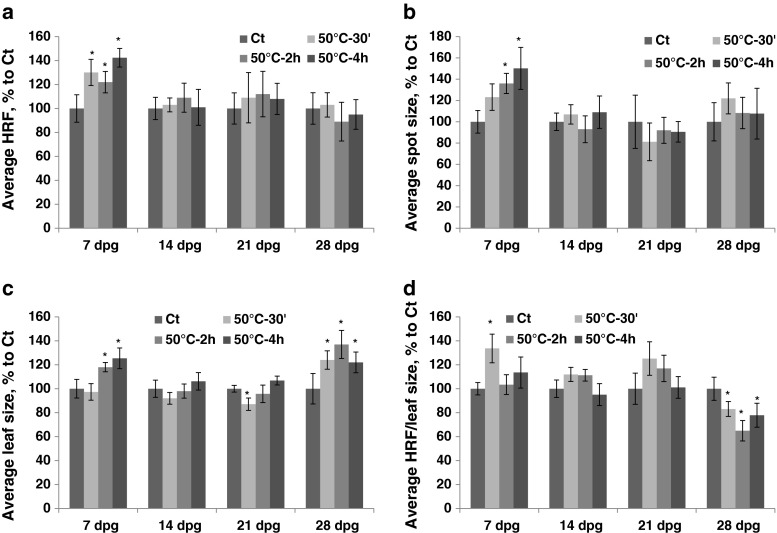
Exposure to 50 °C for different durations of time (the HDD group) resulted in a decrease in HRF upon prolonged exposure (2–4 h) (Fig. 4a, Table S1). The size of recombination events also decreased after prolonged exposure to 50 °C (Fig. 4b). Leaf size increased after 30 min–2 h of exposure at 7 dpg and decreased after exposure at 14 and 21 dpg (Fig. 4c). There was an increase in HRF prorated according to leaf size after 30 min–2 h of exposure at 14 dpg (Fig. 4d).
Fig. 4.
The analysis of HRF, the size of recombination events, leaf size and HRF per leaf size in plants exposed to 50 °C for different duration of time. The plants were exposed to 50 °C for 30 min, 2 h and 4 h at 7, 14, 21 and 28 dpg. All measurements were done in somatic tissues 10 days after stress application. The data are shown as a percentage relative to the control taken as 100 %. The error bars indicate a standard error calculated from 8 repeats (2 biological repeats, each with 4 technical repeats). The asterisks show a significant difference compared to the control (p < 0.05). a. Average HRF. b. Average spot size. c. Average leaf size. d. Average HRF related to leaf size
Exposure to stress alters HRF, plant size and the size of recombination events in the progeny
In the progeny of plants exposed to UVC, spontaneous HRF was found to be increased in the 7 and 21 dpg groups, but not in 14 or 28 dpg groups (Fig. 5a, Table S2). The average size of recombination events also increased in the progeny of plants exposed to UV-C at 7, 21 and 28 dpg (Fig. 5b). The progeny of plants exposed to UV-C at 7 dpg had larger leaves (Fig. 5c). If HRF was prorated according to leaf size, an increase was observed in the 14 and 21 dpg groups, and a decrease in the 28 dpg group (Fig. 5d).
Fig. 5.
The analysis of HRF, the size of recombination events, leaf size and HRF per leaf size in the progeny of plants exposed to UV-C. The parental generation received different doses of UV-C at 7, 14, 21 and 28 dpg. All measurements in the progeny were done in somatic tissues at 20 dpg. The data are shown as a percentage relative to the control taken as 100 %. The error bars indicate a standard error calculated from 8 repeats (2 biological repeats, each with 4 technical repeats). The asterisks show a significant difference compared to the control (p < 0.05). a. Average HRF. b. Average spot size. c. Average leaf size. d. Average HRF related to leaf size
In the progeny of plants exposed to cold, HRF was higher in the 7 and 28 dpg groups (Fig. 6a, Table S2). The progeny of plants exposed to cold for a long period of time (48 h) had HRF that was either similar or lower than that in the control group, regardless of exposure time. The progeny of plants exposed to cold at 7, 14 and 28 dpg had larger recombination events than control plants (Fig. 6b). The average leaf size increased in the progeny of plants exposed to cold at 28 dpg and decreased in the 21 dpg group (Fig. 6c).
Fig. 6.
The analysis of HRF, the size of recombination events, leaf size and HRF per leaf size in the progeny of plants exposed to cold. The parental generation was exposed to cold at 7, 14, 21 and 28 dpg. All measurements in the progeny were done in somatic tissues at 20 dpg. The data are shown as a percentage relative to the control taken as 100 %. The error bars indicate a standard error calculated from 8 repeats (2 biological repeats, each with 4 technical repeats). The asterisks show a significant difference compared to the control (p < 0.05). a. Average HRF. b. Average spot size. c. Average leaf size. d. Average HRF related to leaf size
There was a significant increase in HRF in the progeny of plants exposed to heat (the HDT group) at 7 dpg (Fig. 7a, Table S2). The size of recombination events was also larger in the progeny of plants exposed to heat at 7 dpg (Fig. 7b). The progeny of plants exposed to heat at 7 or 28 dpg had larger leaves (Fig. 7c). The progeny of plants exposed to heat at 7 dpg had also higher HRF prorated according to leaf size, whereas those exposed at 28 dpg had lower (Fig. 7d).
Fig. 7.
The analysis of HRF, the size of recombination events, leaf size and HRF per leaf size in the progeny of plants exposed to different heat temperatures. The parental generation was exposed for 2 h to 28 °C, 37 °C and 50 °C at 7, 14, 21 and 28 dpg. All measurements in the progeny were done in somatic tissues at 20 dpg. The data are shown as a percentage relative to the control taken as 100 %. The error bars indicate a standard error calculated from 8 repeats (2 biological repeats, each with 4 technical repeats). The asterisks show a significant difference compared to the control (p < 0.05). a. Average HRF. b. Average spot size. c. Average leaf size. d. Average HRF related to leaf size
Finally, in the HDD group, exposure to 50 °C for 30 min–4 h resulted in an increase in HRF in the progeny of plants exposed at 7 dpg but not at later developmental stages (Fig. 8a, Table S2). These plants also had similar changes in the size of recombination events—it increased only in the 7 dpg group (Fig. 8b). Leaf size increased in the progeny of plants exposed to 50 °C at 7 and 28 dpg (Fig. 8c). If HRF was prorated according to leaf size, an increase in HRF was observed only in the progeny of the 7 dpg group upon 30 min exposure (Fig. 8d).
Fig. 8.
The analysis of HRF, the size of recombination events, leaf size and HRF per leaf size in the progeny of plants exposed to 50 °C for different durations of time. The parental generation was exposed to 50 °C for 30 min, 2 h and 4 h at 7, 14, 21 and 28 dpg. All measurements in the progeny were done in somatic tissues at 20 dpg. The data are shown as a percentage relative to the control taken as 100 %. The error bars indicate a standard error calculated from 8 repeats (2 biological repeats, each with 4 technical repeats). The asterisks show a significant difference compared to the control (p < 0.05). a. Average HRF. b. Average spot size. c. Average leaf size. d. Average HRF related to leaf size
Discussion
The experiments reported here showed that plants exposed to different levels of UV-C, heat or cold exhibited changes in HRF, in the size of recombination events and leaf size in plants exposed to the aforementioned stresses and their progeny. The changes observed depended on the age of plants, the duration and type of stress applied.
Changes in somatic tissues
In somatic tissues, changes in HRF varied substantially depending on the type of stress. The most prominent increase in HRF was observed in response to UV-C; in general, there was no dependence on the age of exposed plants or the dose received, although older plants had a tendency towards a more prominent increase in HRF when exposed to higher doses. In contrast, the size of recombination events clearly depended on the dose received—the largest spots were observed in plants that received a higher dose. It seems that higher doses do not necessarily cause more recombination events but rather trigger their earlier appearance after exposure—possibly immediately after exposure. Therefore, in cells where such events occurred, there is more time for cell division, which leads to the identification of larger spots (Boyko et al. 2006a). Since smaller spots appear later after exposure and plants that are exposed to lower doses of UV-C have more of them (they have fewer large spots), it is possible that lower doses trigger the delayed effects that are possibly caused by indirect DNA damage and changes in chromatin structure. This phenomenon is called a non-linear response to radiation exposure and is partially related to the bystander effect (Filkowski et al. 2004; Li et al. 2010; Oudalova et al. 2002).
A dose-dependent increase in HRF upon exposure to UV-C and ionizing radiation was reported before (Kovalchuk et al. 1998; Ries et al. 2000). Although these studies clearly demonstrated higher HRF in plants that received higher doses of UV-C and ionizing radiation, the effect was not linear—a more drastic effect was observed at lower doses. Increasing the radiation dose resulted in HRF reaching a plateau, and a further increase caused a negative effect on an increase in HRF. These reports, however, did not analyze changes in HRF as a function of plant age.
One study reported the effect of age on changes in HRF. Boyko et al. (2006b) analyzed changes in HRF in Arabidopsis thaliana and Nicotiana tabacum plants exposed to ionizing radiation (IR) and UV-B. They found that older plants indeed had much smaller changes in HRF, whereas younger plants responded more dramatically to IR and UV-B. They also showed that an increase in HRF is positively correlated with the severity of stress. Our current study showed that higher doses of UV-C resulted in larger spots, whereas the number of spots was not dose-dependent.
Finally, we found that there was often a substantial difference between HRF and HRF prorated according to leaf size. This is an interesting finding since none of the previous studies took into consideration plant size when measuring HRF. However, it should be noted that in the past, we prorated HRF according to the total number of genomes in the plant under analysis, thus calculating recombination rate (RR). We indeed found that in many cases, changes in HRF and RR in stressed plants were different (Boyko et al. 2006b). Larger leaves often mean more cells, but this is not always the case. Therefore, it is important that future studies analyze either plant size or DNA content (the number of genomes).
Changes in the progeny
The progeny of plants exposed to all types of stresses exhibited an increase in HRF at least in one experimental group. Curiously, the size of recombination events also increased in the progeny of stressed plants. All experimental groups that exhibited an increase in the number of recombination events also showed an increase in the size of these events. This suggests that in the progeny of stressed plants, recombination events occur earlier during development even without any stress application.
Various stresses were shown to alter HRF in the progeny, including exposures to UV-C, temperature changes, salt and paraquat as well as infection with pathogens (Boyko et al. 2010a; Kathiria et al. 2010; Molinier et al. 2006; Pecinka et al. 2009). The majority of these stresses were applied to plants at a young age (7 dpg), although several of them were applied at 12 dpg or later (Pecinka et al. 2009). In the latter case, changes in HRF in the progeny were very inconsistent, and many of them, including exposure to UV-B, UV-C and temperature shifts, did not result in transgenerational changes in HRF. Our current study actually confirms this trend and shows that if plants are exposed at 7 dpg, they have changes in HRF in the progeny, but if they are exposed at 14 dpg, no changes are observed.
In some of the groups, average leaf size also changed. In our recent work (unpublished), we found that stress has effects on leaf shape, namely, leaf length and width increased, and this trait was passed on to the progeny. The study by Bos et al. (2000) demonstrated that the temperatures at which maize was grown had a substantial effect on the speed of leaf elongation and the final leaf size/weight; higher temperatures accelerated both leaf elongation and changes in leaf width, although the effects of temperatures higher than 28 °C were not tested. As shown by our work, it is possible that this trait may be inherited by the progeny.
If HRF was prorated according to leaf size, trends of increasing HRF disappeared due to a positive effect of stress on leaf size in the progeny, and it became apparent that the progeny of plants exposed to more intensive stresses at 28 dpg had lower HRF prorated according to leaf size. Changes in plant phenotype, including changes to plant biomass in the progeny of stressed plants, have been reported before. The progeny of plants exposed to various heavy metal salts had longer roots if grown under non-induced conditions or exposed to heavy metal salts over again (Rahavi et al. 2011). The progeny of tobacco plants infected with oilseed rape mosaic virus was larger in size and tolerated stress better than the progeny of non-stressed plants (Kathiria, et al. 2010). The progeny of infected tobacco plants also appeared to accumulate more metabolites, especially sugars and amino acids, as compared to the progeny of control plants (Mandal et al. 2012).
Transgenerational changes in HRF, the size of recombination events and leaf size observed in our work are indications of the existence of transgenerational memory. An increase in the HRF in the progeny is unlikely due to the higher number of spontaneous strand breaks. Although we have not analyzed the DNA strand break levels in this work, we previously showed that the progeny of stressed plants do not have higher level of breaks even so they have higher HRF (Boyko et al. 2010a, b). Higher HRF could be due to a number of factors, including more frequent use of HR repair machinery rather than non-homologous end joining (NHEJ) for repair of endogenous strand breaks, higher activity of HR enzymes, as well as changes in the chromatin structure of the transgene locus, due to changes in DNA methylation or histone modifications.
Transgenerational stress memory may allow plants to prepare their immediate progeny for being able to withstand stress better. In several recent publications, it has been demonstrated that the progeny of stressed plants can better cope with similar and different stresses (Boyko et al. 2010a, b; Kathiria et al. 2010; Luna et al. 2012; Rasmann et al. 2012; Slaughter et al. 2012). Transgenerational memory has an epigenetic component, and it likely depends on the activity of small interfering RNAs, differential patterns of DNA methylation and histone modification in gametes and embryos (Boyko et al. 2007; Boyko and Kovalchuk 2011; Ito et al. 2011; Mirouze and Paszkowski 2011). This indicates that changes in gametes and progeny are likely to escape reprogramming providing the progeny with better opportunities to withstand stress exposures. Although epigenetic components have not been a major focus of our study, several reports suggest that such regulation may take place (Bilichak et al. 2012; Boyko et al. 2010a; Ito et al. 2011). However, the importance of our current work is that we have been able to demonstrate that the age of plants exposed to stress plays a crucial role in passing this memory on to the progeny. Since exposure to stress at 7 dpg seems to cause the best and uniform transgenerational response, it can be hypothesized that at this developmental stage, plants contain meristematic cells that are more flexible in their programming and are able to accommodate changes caused by stress in their epigenomes. Future research will show the sustainability of this hypothesis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PPTX 60 kb)
(DOCX 15 kb)
(DOCX 15 kb)
Acknowledgments
We would like to acknowledge Alberta Innovates Biosolutions and National Science and Engineering Research Council of Canada for funding this research. We declare no conflict of interest. We also thank Valentina Titova for proofreading the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- CCD
Charge-coupled device
- DPG
Days post germination
- HDT
Heat of different temperature
- HDD
Heat of different duration
- HR
Homologous recombination
- HRF
Homologous recombination frequency
- SE
Standard error
References
- Besplug J, Filkowski J, Burke P, Kovalchuk I, Kovalchuk O. Atrazine induces homologous recombination but not point mutation in the transgenic plant-based biomonitoring assay. Arch Environ Contam Toxicol. 2004;46:296–300. doi: 10.1007/s00244-003-3075-9. [DOI] [PubMed] [Google Scholar]
- Bilichak A, Ilnystkyy Y, Hollunder J, Kovalchuk I. The progeny of Arabidopsis thaliana plants exposed to salt exhibit changes in DNA methylation, histone modifications and gene expression. PLoS One. 2012;7:e30515. doi: 10.1371/journal.pone.0030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos HJ, Tijani-Eniola T, Struik PC. Morphological analysis of leaf growth of maize: responses to temperature and light intensity. Neth J Agric Sci. 2000;48:181–198. [Google Scholar]
- Boyko A, Kovalchuk I. Genome instability and epigenetic modification–heritable responses to environmental stress? Curr Opin Plant Biol. 2011;14:260–266. doi: 10.1016/j.pbi.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Boyko A, Filkowski J, Kovalchuk I. Homologous recombination in plants is temperature and day-length dependent. Mutat Res. 2005;572:73–83. doi: 10.1016/j.mrfmmm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Boyko A, Hudson D, Bhomkar P, Kathiria P, Kovalchuk I. Increase of homologous recombination frequency in vascular tissue of Arabidopsis plants exposed to salt stress. Plant Cell Physiol. 2006;47:736–742. doi: 10.1093/pcp/pcj045. [DOI] [PubMed] [Google Scholar]
- Boyko A, Zemp F, Filkowski J, Kovalchuk I. Double-strand break repair in plants is developmentally regulated. Plant Physiol. 2006;141:488–497. doi: 10.1104/pp.105.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A, Kathiria P, Zemp FJ, Yao Y, Pogribny I, Kovalchuk I. Transgenerational changes in the genome stability and methylation in pathogen-infected plants. Nucleic Acids Res. 2007;35:1714–1725. doi: 10.1093/nar/gkm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A, Blevins T, Yao Y, Golubov A, Bilichak A, Ilnytskyy Y, et al. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS One. 2010;5:e9514. doi: 10.1371/journal.pone.0009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A, Golubov A, Bilichak A, Kovalchuk I. Chlorine ions but not sodium ions alter genome stability of Arabidopsis thaliana. Plant Cell Physiol. 2010;51:1066–1078. doi: 10.1093/pcp/pcq048. [DOI] [PubMed] [Google Scholar]
- Endo M, Ishikawa Y, Osakabe K, Nakayama S, Kaya H, Araki T, et al. Increased frequency of homologous recombination and T-DNA integration in Arabidopsis CAF-1 mutants. EMBO J. 2006;25:5579–5590. doi: 10.1038/sj.emboj.7601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkowski J, Yeoman A, Kovalchuk O, Kovalchuk I. Systemic plant signal triggers genome instability. Plant J. 2004;38:1–11. doi: 10.1111/j.1365-313X.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- Gao J, Zhu Y, Zhou W, Molinier J, Dong A, Shen WH. NAP1 family histone chaperones are required for somatic homologous recombination in arabidopsis. Plant Cell. 2012;24:1437–1447. doi: 10.1105/tpc.112.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MT, Aufsatz W, Jonak C, Luschnig C. Transgenerational epigenetic inheritance in plants. Biochim Biophys Acta. 2011;1809:459–468. doi: 10.1016/j.bbagrm.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilnytskyy Y, Boyko A, Kovalchuk I. Luciferase-based transgenic recombination assay is more sensitive than beta-glucoronidase-based. Mutat Res. 2004;559:189–197. doi: 10.1016/j.mrgentox.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Ito H, Gaubert H, Bucher E, Mirouze M, Vaillant I, Paszkowski J. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature. 2011;472:115–119. doi: 10.1038/nature09861. [DOI] [PubMed] [Google Scholar]
- Kathiria P, Sidler C, Golubov A, Kalischuk M, Kawchuk LM, Kovalchuk I. Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial, and fungal pathogens in the progeny of infected tobacco plants. Plant Physiol. 2010;153:1859–1870. doi: 10.1104/pp.110.157263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik A, Pecinka A, Wendeler E, Reiss B. The chromatin assembly factor subunit FASCIATA1 is involved in homologous recombination in plants. Plant Cell. 2006;18:2431–2442. doi: 10.1105/tpc.106.045088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk I, Kovalchuk O, Arkhipov A, Hohn B (1998) Transgenic plants are sensitive bioindicators of nuclear pollution caused by the Chernobyl accident. Nat Biotechnol 1054–9 [DOI] [PubMed]
- Kovalchuk O, Arkhipov A, Barylyak I, Karachov I, Titov V, Hohn B, et al. Plants experiencing chronic internal exposure to ionizing radiation exhibit higher frequency of homologous recombination than acutely irradiated plants. Mutat Res. 2000;449:47–56. doi: 10.1016/S0027-5107(00)00029-4. [DOI] [PubMed] [Google Scholar]
- Kovalchuk I, Kovalchuk O, Kalck V, Boyko V, Filkowski J, Heinlein M, et al. Pathogen-induced systemic plant signal triggers DNA rearrangements. Nature. 2003;423:760–762. doi: 10.1038/nature01683. [DOI] [PubMed] [Google Scholar]
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010;188:655–673. doi: 10.1111/j.1469-8137.2010.03461.x. [DOI] [PubMed] [Google Scholar]
- Li F, Liu P, Wang T, Bian P, Wu Y, Wu L, et al. The induction of bystander mutagenic effects in vivo by alpha-particle irradiation in whole Arabidopsis thaliana plants. Radiat Res. 2010;174:228–237. doi: 10.1667/RR2052.1. [DOI] [PubMed] [Google Scholar]
- Lucht JM, Mauch-Mani B, Steiner H-Y, Metraux J-P, Ryals J, Hohn B. Pathogen stress increases somatic recombination frequency in Arabidopsis. Nat Genet. 2002;30:311–314. doi: 10.1038/ng846. [DOI] [PubMed] [Google Scholar]
- Luna E, Bruce TJ, Roberts MR, Flors V, Ton J. Next-generation systemic acquired resistance. Plant Physiol. 2012;158:844–853. doi: 10.1104/pp.111.187468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal R, Kathiria P, Psychogios N, Bouatra S, Krishnamurthy R, Wishart D, Kovalchuk I. Progeny of tobacco mosaic virus-infected Nictoiana tabacum plants exhibit trans-generational changes in metabolic profiles. Biocat Agric Biotechnol. 2012;1(2):115–123. [Google Scholar]
- Mirouze M, Paszkowski J. Epigenetic contribution to stress adaptation in plants. Curr Opin Plant Biol. 2011;14:267–274. doi: 10.1016/j.pbi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Mirouze M, Lieberman-Lazarovich M, Aversano R, Bucher E, Nicolet J, Reinders J, et al. Loss of DNA methylation affects the recombination landscape in Arabidopsis. Proc Natl Acad Sci U S A. 2012;109:5880–5885. doi: 10.1073/pnas.1120841109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinier J, Oakeley EJ, Niederhauser O, Kovalchuk I, Hohn B. Dynamic response of plant genome to ultraviolet radiation and other genotoxic stresses. Mutat Res Fundam Mol Mech Mutagen. 2005;571:235–247. doi: 10.1016/j.mrfmmm.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Molinier J, Ries G, Zipfel C, Hohn B. Transgeneration memory of stress in plants. Nature. 2006;442:1046–1049. doi: 10.1038/nature05022. [DOI] [PubMed] [Google Scholar]
- Oudalova AA, Geras’kin SA, Dikarev VG, Nesterov Y, Dikareva NS. Induction of chromosome aberrations is non-linear within the low dose region and depends on dose rate. Radiat Prot Dosim. 2002;99:245–248. doi: 10.1093/oxfordjournals.rpd.a006774. [DOI] [PubMed] [Google Scholar]
- Pecinka A, Rosa M, Schikora A, Berlinger M, Hirt H, Luschnig C, et al. Transgenerational stress memory is not a general response in Arabidopsis. PLoS One. 2009;4:e5202. doi: 10.1371/journal.pone.0005202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta H. The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J Exp Bot. 2005;56:1–14. doi: 10.1093/jxb/eri123. [DOI] [PubMed] [Google Scholar]
- Rahavi MR, Migicovsky Z, Titov V, Kovalchuk I. Transgenerational adaptation to heavy metal salts in Arabidopsis. Front Plant Sci. 2011;2:91. doi: 10.3389/fpls.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmann S, De Vos M, Casteel CL, Tian D, Halitschke R, Sun JY, et al. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012;158:854–863. doi: 10.1104/pp.111.187831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries G, Heller W, Puchta H, Sandermann H, Seidlitz HK, Hohn B. Elevated UV-B radiation reduces genome stability in plants. Nature. 2000;406:98–101. doi: 10.1038/35017595. [DOI] [PubMed] [Google Scholar]
- Roldan-Arjona T, Ariza RR. Repair and tolerance of oxidative DNA damage in plants. Mutat Res. 2009;681:169–179. doi: 10.1016/j.mrrev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 2012;158:835–843. doi: 10.1104/pp.111.191593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuba Z, Lichtenthaler HK. Long-term acclimation of plants to elevated CO2 and its interaction with stresses. Ann N Y Acad Sci. 2007;1113:135–146. doi: 10.1196/annals.1391.021. [DOI] [PubMed] [Google Scholar]
- Yao Y, Kovalchuk I. Abiotic stress leads to somatic and heritable changes in homologous recombination frequency, point mutation frequency and microsatellite stability in Arabidopsis plants. Mutat Res. 2011;707:61–66. doi: 10.1016/j.mrfmmm.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Y, Xing D, Gao C. Characterization of mitochondrial dynamics and subcellular localization of ROS reveal that HsfA2 alleviates oxidative damage caused by heat stress in Arabidopsis. J Exp Bot. 2009;60:2073–2091. doi: 10.1093/jxb/erp078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX 60 kb)
(DOCX 15 kb)
(DOCX 15 kb)