Abstract
Triterpenoid saponins are the class of secondary metabolites, synthesized via isoprenoid pathway. Oxidosqualene cyclases (OSCs) catalyzes the cyclization of 2, 3-oxidosqualene to various triterpene skeletons, the first committed step in triterpenoid biosynthesis. A full-length oxidosqualene cyclase cDNA from Bacopa monniera (BmOSC) was isolated and characterized. The open reading frame (ORF) of BmOSC consists of 2,292 bp, encoding 764 amino acid residues with an apparent molecular mass of 87.62 kDa and theoretical pI 6.21. It contained four QxxxxxW motifs, one Asp-Cys-Thr-Ala-Glu (DCTAE) motif which is highly conserved among the triterpene synthases and another MWCYCR motif involved in the formation of triterpenoid skeletons. The deduced amino acid sequence of BmOSC shares 80.5 % & 71.8 % identity and 89.7 % & 83.5 % similarity with Olea europaea mixed amyrin synthase and Panax notoginseng dammarenediol synthase respectively. Phylogenetic analysis revealed that BmOSC is closely related with other plant OSCs. Quantitative real-time PCR (qRT-PCR) data showed that BmOSC is expressed in all tissues examined with higher expression in stem and leaves as compared to roots and floral parts.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-013-0195-1) contains supplementary material, which is available to authorized users.
Keywords: Bacopa monniera, Differential expression, Isoprenoid pathway, Oxidosqualene cyclases, Triterpenoid saponin
Introduction
Various plant species have been used traditionally as a brain tonic in Indian ayurvedic medicine. Bacopa monniera, also known as Bacopa monnieri is one of the most popular small medicinal herb growing in marshy areas throughout the Indian subcontinent (Tiwari et al. 2006). It contains alkaloids (brahmine and herpestin), glycoside (asiaticoside), flavonoids (apigenin and luteolin) and saponins such as bacosides, considered to be the major active constituent of the plant (Mathew et al. 2010). Bacopa extract is mainly used for the treatment of anxiety and improvement of intelligence and memory (Singh and Dhawan 1997). In addition, it also possesses anti-inflammatory, antipyretic, analgesic, sedative, free radical scavenging and anti-lipid peroxidative activities (Anbarasi et al. 2005; Kishore and Singh 2005). The main active chemical constituent of this plant are triterpenoid saponins (Garai et al. 1996) and the pharmacological properties are mainly attributed to the triterpenoid saponin compounds present in the plant extract (Sivaramakrishna et al. 2005).
Different type of triterpenes and plant sterols are synthesized via isoprenoid pathway by oxidosqualene cyclases (Abe et al. 1993). Till date, OSCs cDNA involved in sterol and triterpenes biosynthesis have been cloned and characterized from various organisms including fungi, yeasts, rat, human and plants. For example, β-amyrin synthase and cycloartenol synthase from P. ginseng (Kushiro et al. 1998), five OSCs from Kalanchoe daigremontiana (Wang et al. 2010), dammaranediol synthase from Centella asiatica (Kim et al. 2009), β-amyrin synthase from Aster sedifolius and Arabidopsis thaliana (Shibuya et al. 2009) and OSC from Aster tataricus and tomato (Wang et al. 2011; Sawai et al. 2011), have been cloned and functionally characterized in mutant yeast strain GIL77, having lanosterol synthase deficiency. The catalytic functions of several plants OSCs have been studied using the S. cerevisiae yeast mutants and Pichia pastoris strains (Kajikawa et al. 2005; Ito et al. 2011; Xue et al. 2012; Sun et al. 2013). Confalonieri et al. (2009) over-expressed the Aster sodifolius β-amyrin synthase (AsOXA1) in Medicago truncatula and observed significantly higher amount of some triterpenes accumulation in leaf and roots. Transgenic lines of soybean with RNAi construct of β-amyrin synthase cDNA fragment with seed specific promoter, exhibited stable reduction in seed saponin content, correlating with β-amyrin synthase mRNA reduction (Takagi et al. 2011). This suggests that OSCs may play an important role in enhancement of triterpenes and saponin production.
Triterpenoid saponins from B. monniera (bacosides) have immense medicinal importance but yield of such compounds from the plant is very low. Therefore, development of transgenic lines with improved bacosides content by alteration of biosynthetic pathway may offer the solution. This is the first report on cloning and characterization of an oxidosqualene cyclase gene from B. monniera, the first committed step for biosynthesis of triterpenod saponins (Wang et al. 2010).
Materials and methods
Plant material, RNA isolation and cDNA synthesis
B. monniera (L.), maintained in a green house at National Chemical Laboratory (NCL) premises under standard conditions were used for RNA isolation. Total RNA was isolated from B. monniera leaves by Trizol method (Sigma, USA) as per manual instruction and 1.0 μg of total RNA was reverse-transcribed by AMV-RT (Promega, USA) with oligo (dT)15 primer. The reaction was carried out at 42 °C for 90 min and final denaturation step at 70 °C for 10 min.
Oxidosqualene cyclase gene isolation
Sequences for OSC available at NCBI GenBank database were aligned with Clustal W program. Primers were designed from conserved regions (Supplementary Fig. S1) and PCR was done using cDNA as template for partial BmOSC gene amplification. The PCR was carried out in a thermal cycler (BIO-RAD, USA) under the following conditions: 1 cycle of 94 °C (5 min), 35 cycles of 94 °C (30 s), 55 °C (30 s) and 68 °C (1 min 45 s), and final extension at 68 °C (7 min). The PCR product amplified with primers OSC F3 5′-TTCCTATGCACCCAGC(T/A)AAAATGTGG-3′ and OSC R4 5′-ATCACCATCTTCCATCTGAGA(G/A)TTGAT-3′ was analyzed on 1 % agarose gel and cloned in T/A cloning vector pGEM-T easy (Promega, USA). The ligated product was transformed into E. coli (XL10 Gold, Stratagene, USA) competent cells and plated on LB agar plates containing ampicillin (100 μg/mL). Plasmids were isolated from selected clones and subjected to sequencing with T7 and SP6 promoter primers from both the ends of the insert.
The RACE PCR protocol was implemented to isolate full-length gene. Two forward and two reverse gene specific primers were designed from the partial BmOSC sequence are as follows: RaceOSC F 5′-CACTCAAAATGAGGAAGGTGGATGG-3′, RaceOSC Nested F 5′-ATC GCACCAATCTTGTGCAGACTG-3′ for 3′ RACE and RaceOSC R 5′-AGGCTTGAC ATAAATTTCTTGCCTGA-3′, RaceOSC Nested R 5′- GGTCCATGGTATCTCTTTCCA TAGA-3′ for 5′ RACE. GeneRacer Kit (Invitrogen, USA) was used for RACE PCR and 3′ RACE and 5′RACE ready cDNA was prepared from total RNA as per manual instructions. Primary PCR for 3′RACE and 5′RACE was done using primers RaceOSC F/GeneRacer 3′ primer (provided with the kit) and RaceOSC R/GeneRacer 5′ primer (provided with the kit) respectively. The 3′ and 5′ RACE nested PCR products were cloned in pGEM-T easy vector and sequenced. Based on 3′ and 5′ RACE sequence data, the primers from start and stop codon, OSC full F (5′-ATGTGGAGGCTAAAGATTGC-3′) and OSC full R (5′- CTATTTCATCAATTTGTTTACGAGTATC-3′) were designed to amplify open reading frame (ORF) of the gene as a single PCR product under the conditions: 94 °C (5 min), 35 cycles of 94 °C (30 s), 55 °C (30 s), 68 °C (2 min 30 s) and final extension of 68 °C for 10 min. A single PCR product (2,295 bp) was cloned in T/A cloning vector pGEM-T easy and sequenced.
Bioinformatics and phylogenetic tree analysis
The partial and full-length BmOSC sequences were analyzed using online bioinformatics tools (http://www.ncbi.nlm.nih.gov). The deduction of the amino acid sequences, calculation of theoretical molecular mass and pI, was performed with ExPASy Proteomic tools provided at http://www.expasy.ch/tools/. Conserved domains in BmOSC were detected using Conserved Domain Database search tool (CDD) on NCBI server (http://www.ncbi.nlm.nih.gov/structure/cdd/wrpsb.cgi). Multiple alignments of the amino acid sequences were carried out with the Clustal W1.8 program (http://www.ebi.ac.uk/clustalw/). Global alignment of two amino acid sequences and percentages of identity was calculated using EMBOSS-Needle Pairwise Sequence Alignment (http://www.ebi.ac.uk/Tools/psa/). Phylogenetic tree was obtained using MEGA 4.0.2 program by Neighbor-Joining method (Tamura et al. 2007) and reliability of nodes has been tested with 500 bootstrap replicates.
Gene expression analysis (Quantitative Real-time PCR)
Semi-quantitative and quantitative real-time PCR (qRT-PCR) were used to assess the distribution and expression of BmOSC transcripts in different plant parts, including stem, leaf, root, sepal, petal and pedicel. Total RNA was isolated from different tissue samples and 1.0 μg of total RNA was used for first strand cDNA synthesis using cDNA synthesis kit (Promega). qRT-PCR reaction was performed using diluted synthesized cDNAs and gene specific primers qOSC F (5′- GCATGTGGAATGCACTGCTTCTGT-3′) and qOSC R (5′- TGCCTTCGCCACGGAGATTTCTAT-3′). All the reactions were normalized using 18S rRNA gene amplification as an internal control (18S F 5′- GCACGCGCGCTACACCGAAG -3′; 18S R 5′- GTCTGTACAAAGGGCAGGGACG -3′) (Vishwakarma et al. 2012). MxP3000 instrument (Stratagene, USA) and Brilliant SYBR Green QPCR master mix (Stratagene) were used for detection, and the PCR condition was as follows: 94 °C (10 min), 40 cycles of 94 °C (30 s), 55 °C (30 s), and 72 °C (30 s). All reactions were run in triplicate and repeated twice. The relative expression of gene was analyzed using comparative Ct method (2-ΔΔCt).
Results and discussion
Cloning and characterization of oxidosqualene cyclase gene of B. monniera
A 1,407 bp long partial fragment was obtained by PCR with primers OSC F3/R4 from B. monniera leaves. The partial sequence BLAST query at NCBI showed 73 %–77 % identity with reported plant OSCs. Gene specific primers were designed from this fragment and 3′ and 5′ RACE PCRs were performed to obtain full-length BmOSC cDNA (Supplementary Fig. S2). The 3′RACE nested PCR product of approximately 500 bp with RaceOSC Nested F/GeneRacer 3′nested primer and approximately 1 kb 5′ RACE nested PCR product with RaceOSC Nested R/ 5′ GeneRacer nested primer, were cloned and sequenced. BLAST analysis of 3′ RACE (491 bp) and 5′ RACE (1,084 bp) sequences showed the identity with reported oxidosqualene cyclases. After confirmation as oxidosqualene cyclase by BLAST analysis, the full-length cDNA sequence (2,765 bp) contained ORF (2,292 bp), 5′ UTR (260 bp) and 3′ UTR (188 bp), was designated as BmOSC and submitted to NCBI GenBank database (Acc. No. HM769762). The BmOSC ORF encodes polypeptide of 764 amino acid residues with apparent molecular mass and pI of 87.61 kDa and 6.21 respectively. EMBOSS-Needle Pairwise Sequence Alignment indicated that BmOSC is sharing 80.5 % & 71.8 % identity and 89.7 % & 83.5 % similarity with O. europaea and P. notoginseng respectively. CDD search revealed that BmOSC belongs to ISOPREN_C2_like super family and showed specific hits with squalene cyclase domain subgroup 1 (SQCY_1). Different active site cavities and highly conserved aspartic acid (Asp) residue, function as a catalytic acid (Wendt et al. 1997) were observed, correspond to Asp481 in case of BmOSC sequence (Fig. 1a and b). The secondary structure prediction of BmOSC by SOPMA (Geourjon and Deleage 1995) revealed that BmOSC was predominantly consisted of α-helices (43.32 %) and random coils (39.92 %), with very few sheets (10.60 %) and β-turn (6.15 %).
Fig. 1.
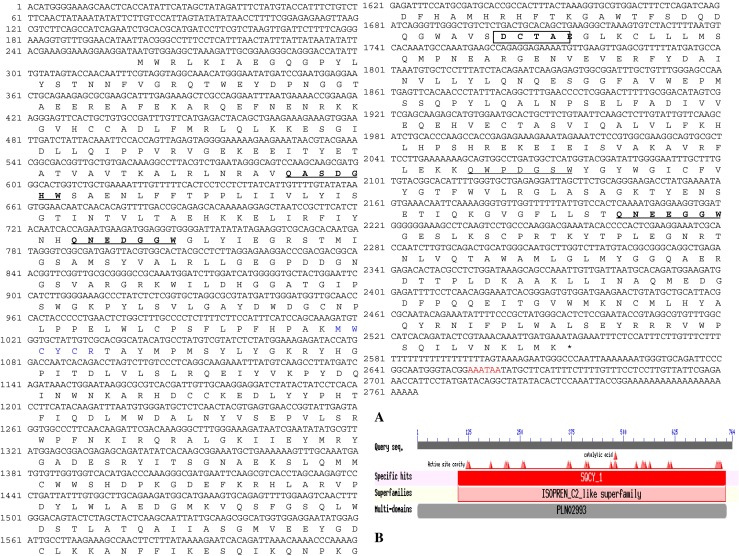
a Nucleotide and predicted deduced amino acid sequence of BmOSC gene from B. monniera. The deduced amino acid showed below the corresponding nucleotide and the remaining are 5′ UTR (260 bp) and 3′ UTR (188 bp) followed by poly (A) tail. Four QxxxxxW motifs are underlined and DCTAE motif is shown inside rectangle. Conserved sequence “MYCYCR” is shown in blue colour. The putative polyadenylation site is shown in red colour. b Image of Conserved Domain Database (CDD) search for BmOSC on NCBI server
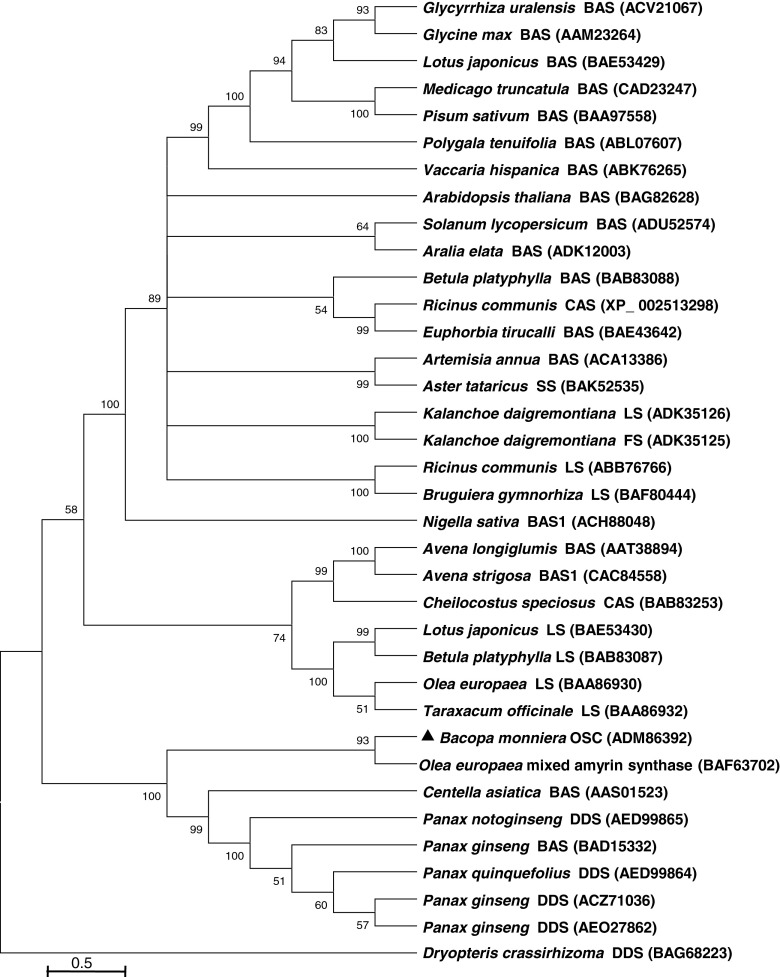
In addition, the multiple sequence alignment of Bacopa BmOSC with other reported OSC sequences showed consensus sequences including four QxxxxxW motifs, stabilizing the OSCs structure. The highly conserved DCTAE motif (481DCTAE485 in BmOSC) plays a key role in substrate binding and protonation (Wang et al.2011). Recently, mutation study in the DCTAE motif of Euphorbia tirucalli OSC revealed, DCTAE as a putative initiation site for the polycyclization reaction (Ito et al. 2013). Kushiro et al. (2000) demonstrated that the tryptophan residue in the MWCYCR motif of β-amyrin synthase from pea plays an important role in synthesis of β-amyrin. The BmOSC amino acid sequence also contains the 253MWCYCR258 motif (Fig. 2), suggesting that the enzyme may be involved in formation of cyclic backbone leads to formation of bacosides in B. monniera. Phylogenetic tree analysis showed that BmOSC is closely related to a number of other dicot OSCs and grouped in the cluster of O. europaea, P. notoginseng and Centella asiatica (Fig. 3).
Fig. 2.

Multiple-Alignment of deduced amino acid sequences of B. monniera BmOSC (ADM86392) with four other plants OSC amino acid sequences; Panax quinquefolius (AED99864), Panax ginseng (ACZ71036), Centella asiatica (AAS01523) and Olea europaea (BAF63702). QxxxxxW motifs are shown in red colour conserved DCTAE motif is in green colour. MWCYCR motif is shown in blue colour, tryptophan (W) of this motif play an important role in triterpene backbone synthesis
Fig. 3.
Un-rooted phylogenetic tree containing the deduced amino acid sequence of BmOSC (ADM86392) with 35 other plants OSC amino acid sequences. The tree was generated with MEGA4.0.2 using the neighbor-joining method and ClustalX2 from a consensus of 500 bootstrap replicates. Accession numbers are shown in brackets. BAS (β-amyrin synthase), LS (Lupeol synthase), DDS (Dammarenediol synthase), FDS (Friedelin synthase), CAS (Cycloartenol synthase), SS (Shionone synthase)
Expression analysis of BmOSC transcripts in different tissue of B. monniera (qRT-PCR)
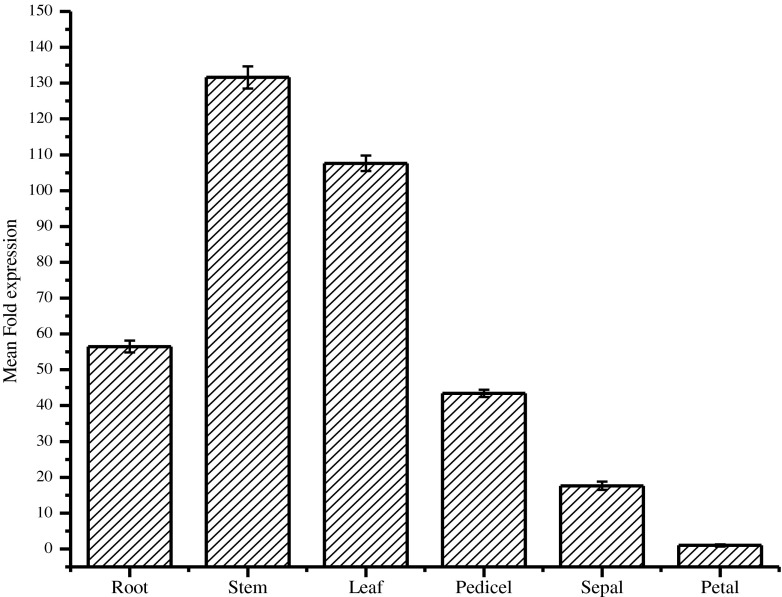
qRT-PCR results showed that BmOSC was expressed highly in stems and leaves (130 and 105 fold respectively higher than petals) than roots and floral parts (Fig. 4). These data were also supported by semi-quantitative PCR (Supplementary Fig. S3). BmOSC expression pattern in Bacopa is similar to the earlier reports from M. truncatula and Pisum sativum, where OSC expression was higher in stem (Iturbe-Ormaetxe et al. 2003). In Catharanthus roseus, qRT-PCR results revealed that two OSCs were expressed in all the aerial tissues with the highest expression levels in the leaves, but not in the roots and the gene expression data were consistent with the triterpenoid accumulation pattern (Huang et al. 2012). Recently, it has been reported that bacoside A in B. monneira accumulates mostly in vegetative parts as compared to the floral parts (Naik et al. 2012), and in our study, BmOSC mRNA expression is consistent with bacoside A content accumulation. Thus, we can say BmOSC may be associated with triterpenoid saponin bacosides biosynthesis in Bacopa. In contrast, the expression level was highest in flower, and roots and hypocotyls in case of Nigella sativa (Scholz et al. 2009) and Glycyrrhiza glabra (Hayashi et al. 2001) respectively. In other reports, the accumulation of transcripts was higher in leaves of Gentiana straminea (Liu et al. 2009) and C. asiatica (Kim et al. 2005). The accumulation of asiaiticosides in Centella was higher in leaves and agreement with OSC transcript accumulation.
Fig. 4.
Relative expression analysis of BmOSC transcript in different tissues by qRT-PCR (The data are presented as mean±SE from three experimental analyses)
In conclusion, the present study of cloning and characterization of oxidosqualene cyclase cDNA will provide a potential avenue for developing transgenic Bacopa plants which over express this gene for enhanced production of important triterpene saponins.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 71 kb)
(DOC 1047 kb)
(DOC 77 kb)
Acknowledgements
Authors are thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, India for funding the project under grant NWP0009.
Abbreviations
- AMV-RT
Avian myeloblastosis virus-reverse transcriptase
- OSC
Oxidosqualene cyclase
- PCR
Polymerase chain reaction
- RACE
Rapid amplification of cDNA ends
References
- Abe I, Rohmer M, Prestwich GD. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem Rev. 1993;93:2189–2206. doi: 10.1021/cr00022a009. [DOI] [Google Scholar]
- Anbarasi K, Vani G, Balakrishna K, Devi CSS. Creatine kinase isoenzyme patterns upon chronic exposure to cigarette smoke: protective effect of Bacoside A. Vasc Pharmacol. 2005;42:57–61. doi: 10.1016/j.vph.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Confalonieri M, Cammareri M, Biazzi E, Pecchia P, Fevereiro MP, Balestrazzi A, Tava A, Conicella C. Enhanced triterpene saponin biosynthesis and root nodulation in transgenic barrel medic (Medicago truncatula Gaertn.) expressing a novel beta-amyrin synthase (AsOXA1) gene. Plant Biotechnol J. 2009;7:172–182. doi: 10.1111/j.1467-7652.2008.00385.x. [DOI] [PubMed] [Google Scholar]
- Garai S, Mahato SB, Ohtani K, Yamasaki K. Dammarane-type triterpenoid saponins from Bacopa monniera. Phytochemistry. 1996;42:815–820. doi: 10.1016/0031-9422(95)00936-1. [DOI] [PubMed] [Google Scholar]
- Geourjon C, Deleage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Huang P, Kirakosyan A, Inoue K, Hiraoka N, Ikeshiro Y, Kushiro T, Shibuya M, Ebizuka Y. Cloning and characterization of a cDNA encoding beta-amyrin synthase involved in glycyrrhizin and soyasaponin biosyntheses in licorice. Biol Pharm Bull. 2001;24:912–916. doi: 10.1248/bpb.24.912. [DOI] [PubMed] [Google Scholar]
- Huang L, Li J, Ye H, Li C, Wang H, Liu B, Zhang Y. Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus. Planta. 2012;236(5):1571–1581. doi: 10.1007/s00425-012-1712-0. [DOI] [PubMed] [Google Scholar]
- Ito R, Mori K, Hashimoto I, Nakano C, Sato T, Hoshino T. Triterpene cyclases from Oryza sativa L.: cycloartenol, parkeol and achilleol B synthases. Organic Lett. 2011;13:2678–2681. doi: 10.1021/ol200777d. [DOI] [PubMed] [Google Scholar]
- Ito R, Masukawa Y, Hoshino T. Purification, kinetics, inhibitors and CD for recombinant β-amyrin synthase from Euphorbia tirucalli L and functional analysis of the DCTA motif, which is highly conserved among oxidosqualene cyclases. FEBS J. 2013;280(5):1267–1280. doi: 10.1111/febs.12119. [DOI] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Haralampidis K, Papadopoulou K, Osbourn AE. Molecular cloning and characterization of triterpene synthases from Medicago truncatula and Lotus japonicus. Plant Mol Biol. 2003;51:731–743. doi: 10.1023/A:1022519709298. [DOI] [PubMed] [Google Scholar]
- Kajikawa M, Yamato KT, Fukuzawa H, Sakai Y, Uchida H, Ohyama K. Cloning and characterization of a cDNA encoding β-amyrin synthase from petroleum plant Euphorbia tirucalli L. Phytochemistry. 2005;66:1759–1766. doi: 10.1016/j.phytochem.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Kim OT, Kim MY, Huh SM, Bai DG, Ahn JC, Hwang B. Cloning of a cDNA probably encoding oxidosqualene cyclase associated with asiaticoside biosynthesis from Centella asiatica (L.) Urban. Plant Cell Rep. 2005;24:304–311. doi: 10.1007/s00299-005-0927-y. [DOI] [PubMed] [Google Scholar]
- Kim OT, Lee JW, Bang KH, Kim YC, Hyun DY, Cha SW, Choi YE, Jin ML, Hwang B. Characterization of a dammarenediol synthase in Centella asiatica (L.) Urban. Plant Physiol Biochem. 2009;47:998–1002. doi: 10.1016/j.plaphy.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Kishore K, Singh M. Effect of bacosides, alcoholic extract of Bacopa monniera Linn. (brahmi), on experimental amnesia in mice. Indian J Exp Biol. 2005;43:640–645. [PubMed] [Google Scholar]
- Kushiro T, Shibuya M, Ebizuka Y. Beta-amyrin synthase-cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur J Biochem. 1998;256(1):238–244. doi: 10.1046/j.1432-1327.1998.2560238.x. [DOI] [PubMed] [Google Scholar]
- Kushiro T, Shibuya M, Masuda K, Ebizuka Y. Mutational studies on triterpene synthases: engineering lupeol synthase into β-amyrin synthase. J Am Chem Soc. 2000;122:6816–6824. doi: 10.1021/ja0010709. [DOI] [Google Scholar]
- Liu Y, Cai Y, Zhao Z, Wang J, Li J, Xin W, Xia G, Xiang F. Cloning and functional analysis of a beta-amyrin synthase gene associated with oleanolic acid biosynthesis in Gentiana straminea MAXIM. Biol Pharm Bull. 2009;32(5):818–824. doi: 10.1248/bpb.32.818. [DOI] [PubMed] [Google Scholar]
- Mathew J, Paul J, Nandhu MS, Paulose CS. Bacopa monniera and Bacoside-A for ameliorating epilepsy associated behavioral deficits. Fitoterapia. 2010;81:315–322. doi: 10.1016/j.fitote.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Naik PM, Manohar SH, Praveen N, Upadhya V, Murthy HN. Evaluation of bacoside A content in different accessions and various organs of Bacopa monnieri (L.) Wettst. J Herbs Spices Med Plants. 2012;18(4):387–395. doi: 10.1080/10496475.2012.725456. [DOI] [Google Scholar]
- Sawai S, Uchiyama H, Mizuno S, Aoki T, Akashi T, Ayabe S, Takahashi T. Molecular characterization of an oxidosqualene cyclase that yields shionone, a unique tetracyclic triterpene ketone of Aster tataricus. FEBS Lett. 2011;585:1031–1036. doi: 10.1016/j.febslet.2011.02.037. [DOI] [PubMed] [Google Scholar]
- Scholz M, Lipinski M, Leupold M, Luftmann H, Harig L, Ofir R, Fischer R, Prufer D, Muller KJ. Methyl jasmonate induced accumulation of kalopanaxsaponin I in Nigella sativa. Phytochemistry. 2009;70:517–522. doi: 10.1016/j.phytochem.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Katsube Y, Otsuka M, Zhang H, Tansakul P, Xiang T, Ebizuka Y. Identification of a product specific beta-amyrin synthase from Arabidopsis thaliana. Plant Physiol Biochem. 2009;47:26–30. doi: 10.1016/j.plaphy.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Singh HK, Dhawan BN. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi) Indian J Pharmacol. 1997;29:359–365. [Google Scholar]
- Sivaramakrishna C, Rao CV, Trimurtulu G, Vanisree M, Subbaraju GV. Triterpenoid glycosides from Bacopa monniera. Phytochemistry. 2005;66:2719–2728. doi: 10.1016/j.phytochem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Sun J, Xu X, Xue Z, Snyder JH, Qi X. Functional analysis of a rice oxidosqualene cyclase through total gene synthesis. Mol Plant. 2013 doi: 10.1093/mp/sst038. [DOI] [PubMed] [Google Scholar]
- Takagi K, Nishizawa K, Hirose A, Kita A, Ishimoto M. Manipulation of saponin biosynthesis by RNA interference-mediated silencing of beta-amyrin synthase gene expression in soybean. Plant Cell Rep. 2011;30(10):1835–1846. doi: 10.1007/s00299-011-1091-1. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Tiwari K, Deo Singh B. Shoot bud regeneration from different explants of Bacopa monniera (L.) Wettst. by trimethoprim and bavistin. Plant Cell Rep. 2006;25:629–635. doi: 10.1007/s00299-006-0126-5. [DOI] [PubMed] [Google Scholar]
- Vishwakarma RK, Ruby, Singh S, Sonawane PD, Srivastava S, Kumari U, Santosh Kumar RJ, Khan BM. Molecular cloning, biochemical characterization, and differential expression of an acetyl-CoA C-Acetyltransferase gene (AACT) of Brahmi (Bacopa monniera) Plant Mol Biol Rep. 2012 [Google Scholar]
- Wang Z, Yeats T, Han H, Jetter R. Cloning and characterization of oxidosqualene cyclases from Kalanchoe daigremontiana: enzymes catalyzing up to 10 rearrangement steps yielding friedelin and other triterpenoids. J Biol Chem. 2010;285:29703–29712. doi: 10.1074/jbc.M109.098871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Guhling O, Yao R, Li F, Yeats TH, Rose JK, Jetter R. Two oxidosqualene cyclases responsible for biosynthesis of tomato fruit cuticular triterpenoids. Plant Physiol. 2011;155:540–552. doi: 10.1104/pp.110.162883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt KU, Poralla K, Schulz GE. Structure and function of a squalene cyclase. Science. 1997;277:1811–1815. doi: 10.1126/science.277.5333.1811. [DOI] [PubMed] [Google Scholar]
- Xue Z, Duan L, Liu D, Guo J, Ge S, Dicks J, ÓMáille P, Osbourn A, Qi X. Divergent evolution of oxidosqualene cyclases in plants. New Phytol. 2012;193:1022–1038. doi: 10.1111/j.1469-8137.2011.03997.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 71 kb)
(DOC 1047 kb)
(DOC 77 kb)