Abstract
Chronic pain is thought to be partly caused by a loss of GABAergic inhibition and resultant neuronal hyperactivation in the central pain-modulating system, but the underlying mechanisms for pain-modulating neurons in the brain are unclear. In this study, we investigated the cellular mechanisms for activation of brainstem descending pain facilitation in rats under persistent pain conditions. In the nucleus raphe magnus (NRM), a critical relay in the brain’s descending pain-modulating system, persistent inflammatory pain induced by complete Freund’s adjuvant decreased the protein level of K+–Cl− cotransporter (KCC2) in both total and synaptosomal preparations. Persistent pain also shifted the equilibrium potential of GABAergic inhibitory postsynaptic current (EIPSC) to a more positive level and increased the firing of evoked action potentials selectively in μ-opioid receptor (MOR)–expressing NRM neurons, but not in MOR-lacking NRM neurons. Microinjection of brain-derived neurotrophic factor (BDNF) into the NRM inhibited the KCC2 protein level in the NRM, and both BDNF administration and KCC2 inhibition by furosemide mimicked the pain-induced effects on EIPSC and excitability in MOR-expressing neurons. Furthermore, inhibiting BDNF signaling by NRM infusion of tyrosine receptor kinase B–IgG or blocking KCC2 with furosemide prevented these pain effects in MOR-expressing neurons. These findings demonstrate a cellular mechanism by which the hyperactivity of NRM MOR-expressing neurons, presumably responsible for descending pain facilitation, contributes to pain sensitization through the signaling cascade of BDNF-KCC2-GABA impairment in the development of chronic pain.
Introduction
The brainstem nucleus raphe magnus (NRM) is a crucial supraspinal site for pain modulation and opioid analgesia (Fields, 2004). It also plays an important role in maintaining a behavioral state of sensitized pain in chronic pain conditions (Porreca et al., 2002). As a pivotal relay in the brainstem descending pain-modulating system, the NRM functions by receiving integrated inputs from higher brain sites through connections with the periaqueductal gray (PAG) and by projecting directly to pain-transmitting neurons in the spinal cord dorsal horn (Basbaum et al., 2009).
Several different types of NRM neurons have been characterized in vivo and in vitro, and as shown in previous studies, each type of NRM neuron can exhibit a distinct inhibitory or facilitatory effect on spinal pain transmission (Fields et al., 1983; Pan et al., 1990; Fields, 2004). One type of NRM neurons, defined by its inhibitory response to opioid analgesics as agonists of the μ-opioid receptor (MOR) and by its membrane expression of functional MOR, is thought to facilitate spinal pain transmission (Pan et al., 1997; Fields, 2004). Our recent study shows that this neuronal type is selectively activated in persistent inflammatory and neuropathic pain states (Zhang et al., 2011). Thus, it appears that hyperactivity of the MOR-expressing NRM neurons is important in mediating and maintaining pain sensitization in persistent and chronic pain states. However, the cellular mechanisms underlying pain-induced maladaptive activation of these NRM neurons are unknown.
Loss of GABAergic inhibition in pain-signaling pathways has been proposed as a primary mechanism for pain-induced maladaptive responses termed central sensitization, a key process in the development of acute pain to chronic pain (Moore et al., 2002; Knabl et al., 2008; Costigan et al., 2009; Munro et al., 2009). Coull et al. (2003) demonstrated a novel mechanism for this loss of GABA inhibition in spinal dorsal horn neurons by showing that nerve injury downregulates the K+–Cl– cotransporter (KCC2), which controls the intracellular Cl− gradient in neurons, causing a depolarizing shift in the equilibrium potential of Cl− (ECl) currents such that inhibitory GABAA currents become depolarizing and excitatory. This reduced KCC2 function in maintenance of neuronal Cl− homeostasis has been reported later by other studies on spinal cord neurons and dorsal root ganglion cells after peripheral inflammation and nerve injury (Price et al., 2005). A later study by Coull et al. (2005) showed that brain-derived neurotrophic factor (BDNF) from microglia also caused impairment of GABA inhibition in spinal neurons under neuropathic pain conditions. Recently, the same group reported that a similar mechanism involving a KCC2-induced shift in neuronal Cl− homeostasis is also responsible for repeated morphine-induced hyperalgesia (pain sensitization) (Ferrini et al., 2013).
All the previous studies were of peripheral or spinal cord neurons, so the mechanisms underlying potential loss of GABA inhibition in brain neurons during the pain development have not been characterized. Because the NRM has an important role in descending pain modulation under sensitized pain conditions, we investigated the function of KCC2 in equilibrium potential of GABA synaptic currents and its adaptive changes in NRM neurons from a rat model of persistent inflammatory pain. We also identified BDNF as an upstream protein that regulated the KCC2 function in the pain model.
Materials and Methods
Animals and the Pain Model.
Male Wistar rats, 9 to 14 days old, and adult rats weighing 200–300 g were used (Charles River, Wilmington, MA). A rat model of persistent inflammatory pain was induced by a single injection of complete Freund’s adjuvant (CFA; 40 μl) into a hind paw. All procedures involving the use of animals conformed to the guidelines of the University of Texas MD Anderson Cancer Center Animal Care and Use Committee.
Brian Slice Preparations and Whole-Cell Recording.
We performed visualized whole-cell voltage-clamp recordings of NRM neurons in slice preparations, with general methods described previously elsewhere (Zhang and Pan, 2010, 2012; Zhang et al., 2011). Neonatal rats were used in visualized whole-cell recording experiments due to the limited visibility and quality of cells in NRM slices from older rats. Both similarities and differences between neonatal and adult NRM neurons in their responses to pain have been described previously elsewhere (Zhang and Hammond, 2010), and those differential responses could potentially confound the data interpretation. Despite these recognized limitations, we found the findings of cellular recording from neonatal rats to be a useful and efficient model to help understand the cellular mechanisms of pain behavior in adult rats (Pan et al., 1997; Bie et al., 2005; Ma et al., 2006; Zhang and Pan, 2010; Zhang et al., 2011).
NRM slices (200 μm thick) were cut, and a single slice was perfused in a recording chamber with preheated (35°C) physiologic saline containing the following (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 11 glucose, and 25 NaHCO3, saturated with 95% O2 and 5% CO2, at pH 7.2–7.4. Recordings were obtained from visually identified NRM neurons with a glass pipette (resistance 3–5 MΩ) filled with a solution containing the following (in mM): 126 K-gluconate, 10 NaCl, 1 MgCl2, 11 EGTA, 10 HEPES, 2 ATP, and 0.25 GTP, at pH adjusted to 7.3 with KOH and osmolarity 280–290 mOsm/l. The ECl estimated from the Nernst equation under these conditions was −65 mV.
For perforated patch recordings, pipette tips were filled with internal solution containing 150 mM K-gluconate and 10 mM HEPES, and then were backed with internal solution containing gramicidin D (50 μg/ml; Sigma-Aldrich, St. Louis, MO). Gramicidin-perforated patches in the whole-cell recording configuration allow recording of membrane currents by gramicidin-generated membrane pores exclusively permeable to monovalent cations without anion permeability or consequent change in intracellular Cl− concentration (Kyrozis and Reichling, 1995).
Electrical stimulus from constant current (0.25 milliseconds, 0.2–0.4 mA) was used to evoke GABA-mediated inhibitory postsynaptic currents (IPSCs) with a bipolar stimulating electrode placed within the nucleus. With a holding potential of −60 mV, GABA IPSCs were recorded in the presence of glutamate receptor antagonists D-2-amino-5-phosphonopentanoic acid (50 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM), and were completely blocked by the GABAA receptor antagonist bicuculline (10 μM). Perforated patch recording was used on some cells, with the results presented in Fig. 1. We did not observe a difference in basic cell properties between the experiments performed with perforated patch and whole-cell recordings; therefore, the results of perforated patch and regular whole-cell recordings were pooled in Fig. 1. The reversal potential of IPSCs was determined by measuring the IPSC amplitudes at different holding potentials from −80 to −30 mV in 5–10 mV increments. The current-voltage relationship was calculated by linear regression. Furosemide (100 μM) was applied to slices for at least 10 minutes before data collection.
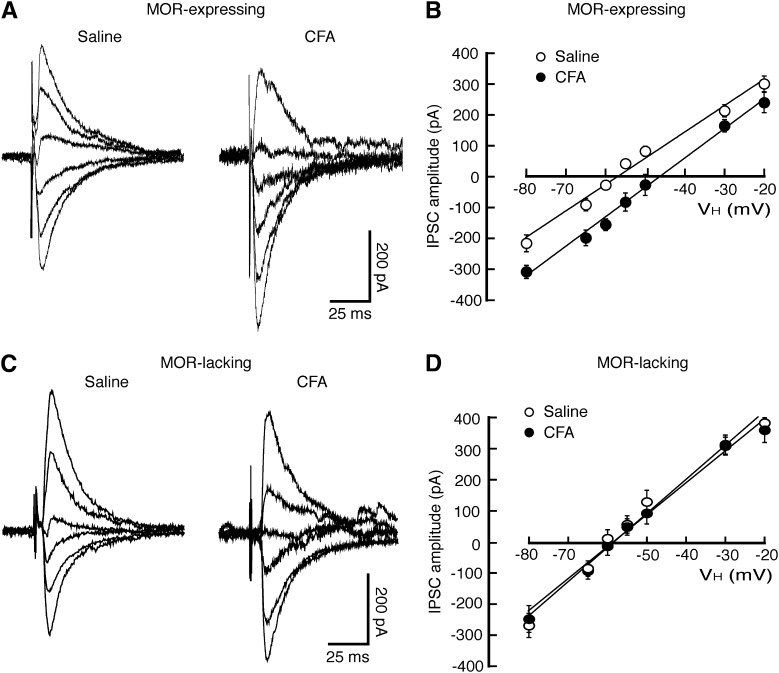
Fig. 1.
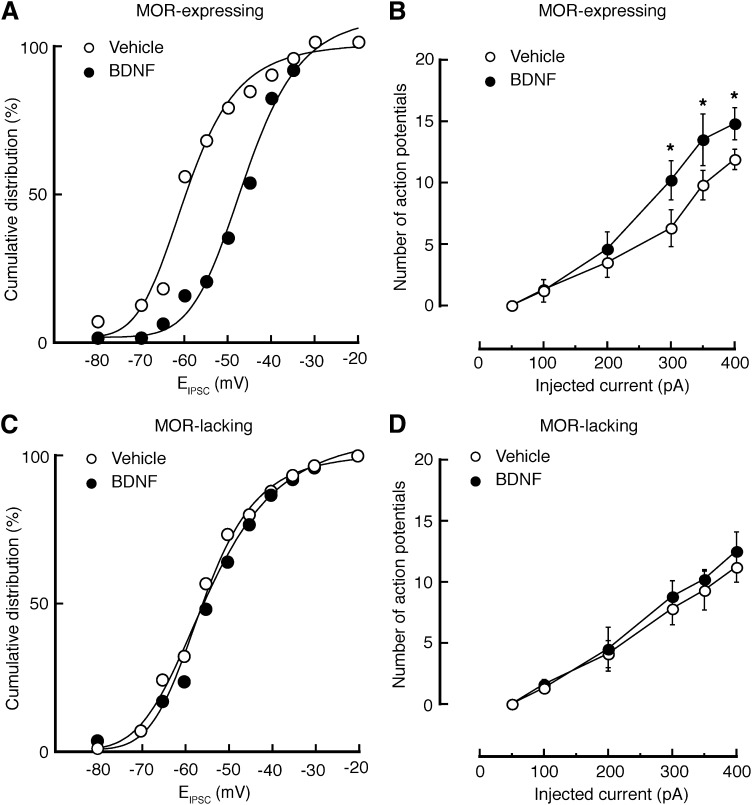
Persistent inflammatory pain induces a depolarizing shift in the reversal potential of GABAergic inhibitory postsynaptic current (EIPSC) selectively in MOR-expressing neurons from NRM. (A and B) Representative traces (A) and plots of amplitudes (B) of GABAergic IPSCs evoked at various holding potentials in MOR-expressing neurons from saline-injected control rats or CFA-injected rats. (C and D) Similar data of IPSC traces (C) and amplitude plots (D) in MOR-lacking neurons from the two groups of rats. n = 17–30 cells in each group. VH, holding potential.
Synaptosome Preparations and Western Blot Analysis.
The synaptosome preparations and Western blot analysis were described in our previous reports (Bie et al., 2012). For synaptosome preparations, NRM tissues from saline- or CFA-injected rats were gently homogenized in ice-cold 0.32 M sucrose buffer at pH 7.4 and then were centrifuged for 10 minutes at 1000g (4°C). The supernatant was collected and centrifuged for 20 minutes at 10,000g (4°C), then the synaptosomal pellet was resuspended in the lysis buffer (0.1% Triton X-100, 150 mM NaCl, 25 mM KCl, 10 mM Tris-HCl, pH 7.4, with protease inhibitors) at 4°C for 10 minutes. The protein concentrations were determined using the Bio-Rad (Hercules, CA) protein assay kit.
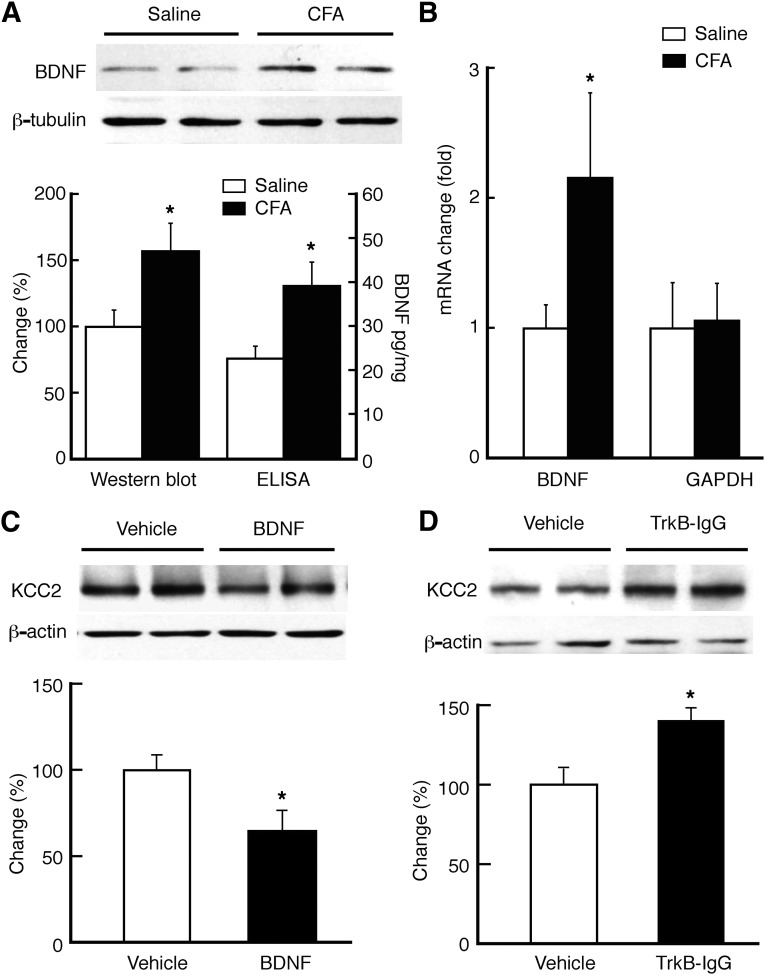
For Western blotting, total proteins were prepared after tissue lysis and centrifugation for SDS-polyacrylamide gel electrophoresis. The protein was mixed with SDS sample buffer, heated to 95°C for 10 minutes, separated under reducing conditions on a 12 or 5% SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane. The membrane was incubated with a polyclonal rabbit antibody for KCC2 (1:2000; Millipore, Billerica, MA), BDNF (1:250; Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin (1:1000; Santa Cruz Biotechnology) with agitation overnight at 4°C, and then with horseradish peroxidase-linked secondary antibody (Santa Cruz Biotechnology) for 1 hour at room temperature. The bands were detected using enhanced chemiluminescence (GE Healthcare Biosciences, Piscataway, NJ). BDNF protein assays were performed with the ChemiKine BDNF sandwich enzyme-linked immunosorbent assay kit (Millipore).
Real-Time Polymerase Chain Reaction.
RNA was extracted from rat NRM with the RNAqueous-4 polymerase chain reaction (PCR) kit (Applied Biosystems, Foster City, CA). Reverse transcription was performed with the RETROscript kit (Applied Biosystems). cDNA was quantified by real-time PCR as we described previously elsewhere (Ma et al., 2006). The following primers (Invitrogen, Carlsbad, CA) were used to amplify specific cDNA regions of the transcripts: BDNF, 5′-CCATAAGGACGCGGACTTGTAC-3′ and 5′-AGACATGTTTGCGGCATCCAGG-3′; GAPDH, 5′-AACGACCCCTTCATTGAC-3′ and 5′-TCCACGACATACTCAGCAC-3′. We used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) quantification as an internal control for normalization. Fold differences of mRNA levels over vehicle control were calculated by ΔCt. Each PCR reaction was repeated at least twice independently.
Microinjection and Behavioral Experiments.
Adult rats were used for NRM microinjection and behavioral tests, as described in our previous reports (Zhang and Pan, 2010, 2012; Zhang et al., 2011). A rat was implanted with a 26-gauge double guide cannula (Plastics One, Roanoke, VA) aimed at the NRM (anteroposterior, −10.0 mm from the Bregma; lateral, 0; dorsoventral, 10.5 mm from the dura). Drugs were microinjected into the NRM in a total volume of 1 μl through a 33-gauge double injector with an infusion pump at a rate of 0.2 μl/min. All NRM microinjection sites were histologically verified after the experiment by injecting 0.5 μl of a blue dye, and controls of off-site injections were performed as described elsewhere (Bie et al., 2005). The confined effect of an injected drug within the NRM with this microinjection method has been demonstrated in our previous studies (Zhang and Pan, 2010, 2012; Zhang et al., 2011).
The behavioral effect of microinjected furosemide or [(dihydroindenyl)oxy]alkanoic acid (DIOA) was measured 20–30 minutes after administration. The pain threshold was measured every 5 minutes by the paw-withdrawal test on a freely moving rat by use of a Hargreaves analgesia instrument (Stoelting, Wood Dale, IL). The heat intensity was set to elicit stable baseline latencies with a cutoff time of 12 seconds.
Statistical Analyses and Drugs.
Numerical data of protein and mRNA assays from control and treatment groups were statistically analyzed and compared with Student’s t test (paired or unpaired, two-tailed). Behavioral and electrophysiologic results with multiple comparisons were statistically analyzed by analysis of variance (ANOVA) for repeated measures and the Tukey-Kramer test of post-hoc analysis. Data are presented as mean ± S.E.M. P < 0.05 was considered statistically significant. All drugs were purchased from Sigma-Aldrich or Tocris Bioscience (Ellisville, MO).
Results
Persistent Pain Shifts Reversal Potential of GABA Synaptic Currents.
Under whole-cell voltage-clamp recordings with a holding potential of −60 mV, NRM neurons were identified as two types: MOR-expressing cells hyperpolarized by the MOR agonist [d-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO; 1 μM), and MOR-lacking cells with no response to DAMGO, as described previously elsewhere (Pan et al., 1990). To identify the effect of CFA-induced persistent pain sensitization on the properties of GABA synaptic transmission, we compared the reversal potential of GABAergic IPSCs (EIPSC) in the two NRM cell types from saline-injected control rats and from CFA-injected rats 3 days after injection. In MOR-expressing cells, we found that the CFA-induced pain condition induced a statistically significant depolarizing shift in EIPSC by an average of ∼11 mV (control, −58.8 ± 3.1 mV; CFA, −47.9 ± 2.6 mV; P < 0.05) (Fig. 1, A and B); in contrast, it did not statistically significantly alter the EIPSC in the MOR-lacking cells (control, −61.1 ± 2.1 mV; CFA, −59.3 ± 2.2 mV; P > 0.05) (Fig. 1, C and D). This result indicates that the inhibitory function of GABA neurotransmission is impaired by the pain condition selectively in MOR-expressing cells, consistent with our previous report that the persistent pain-induced increase in neuronal excitability mainly occurs in this neuron type in the NRM (Zhang et al., 2011).
Pain Reduction of KCC2 Impairs Inhibitory Function of GABA Synapses.
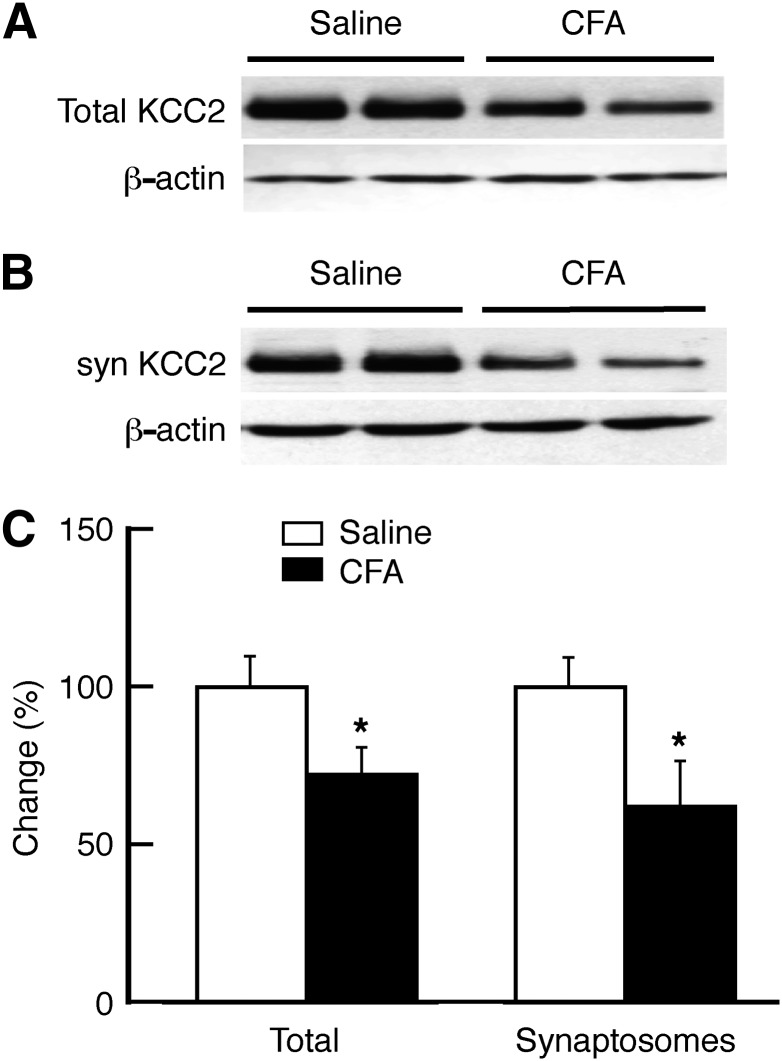
Given the critical role of KCC2 in maintaining the anion equilibrium potential of GABA synapses (Coull et al., 2003), we determined the changes in KCC2 protein level in the NRM under the pain condition. Three days after the CFA injection, the total KCC2 protein was reduced to about 72% of control in the NRM (Fig. 2). To identify KCC2 changes specifically in the synaptic structures, we conducted similar experiments with NRM preparations of synaptosomes, which contain mostly proteins and structures of synaptic membrane with nearly no cell body contents and greatly reduced intraterminal contents (Dunkley et al., 2008). As shown in Fig. 2, the amount of synaptosomal KCC2 protein was also significantly decreased to 60% of control in the NRM from CFA-injected rats when compared with that from saline-injected control rats. These results suggest that KCC2 downregulation may contribute to the pain-induced depolarizing shift of EIPSC in the GABA synapses of the NRM neurons. To test that hypothesis, we examined whether pharmacologic inhibition of KCC2 would produce a similar EIPSC shift.
Fig. 2.
Persistent pain downregulates the K+–Cl− cotransporter KCC2 in the NRM. (A and B) Representative Western blot lanes of total (A) and synaptosomal (syn; B) KCC2 and β-actin proteins in NRM tissues harvested from a saline-injected rat and a CFA-injected rat 3 days after injection. (C) Summarized Western blots data of total and synaptosomal KCC2, normalized to β-actin, in NRM tissues from saline-injected rats (n = 5) and CFA-injected rats (n = 6). *P < 0.05.
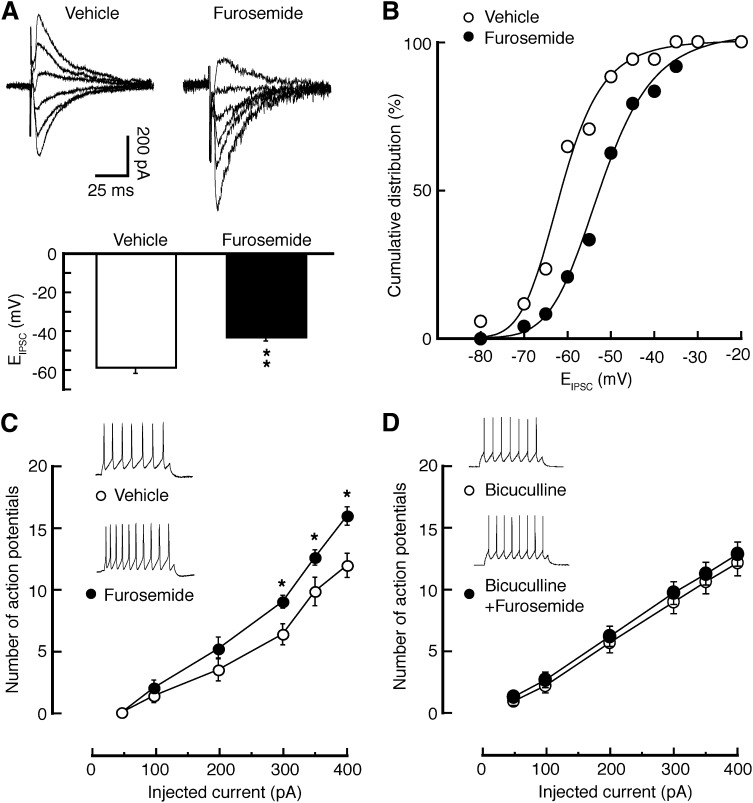
In MOR-expressing neurons from naïve rats, application of the KCC2 inhibitor furosemide (100 μM) caused a marked shift of about 15 mV in EIPSC toward more positive levels when compared with vehicle-treated controls (Fig. 3, A and B). The KCC2 inhibition also statistically significantly increased the firing of evoked action potentials in MOR-expressing neurons (Fig. 3C). To determine whether the excitatory effect of furosemide involved tonic, GABAA receptor-mediated inhibition in these neurons, we examined the furosemide effect after blockade of GABAA receptors with bicuculline (10 μM). As shown in Fig. 3D, under such conditions, furosemide was no longer effective on the excitability of these neurons, indicating that the excitatory effect of furosemide is at least partially mediated by reducing tonic GABAA inhibition in these neurons. However, such effects of KCC2 inhibition by furosemide were not observed either on EIPSC or on evoked firing in MOR-lacking neurons from naïve rats (Fig. 4, A–C).
Fig. 3.
Inhibition of KCC2 induces a depolarizing shift in EIPSC and an increase in excitability selectively in MOR-expressing neurons. (A) Traces of IPSCs evoked at various holding potentials (top) and averaged EIPSC (bottom) in MOR-expressing neurons in NRM slices from naïve rats and treated with vehicle or furosemide (100 μM). (B) Cumulative distribution of EIPSC for the data shown in A. (C and D) Number of action potentials evoked by depolarizing current pulses of various amplitudes in MOR-expressing neurons in vehicle- or furosemide-treated NRM slices (C) and in slices treated with bicuculline or bicuculline + furosemide (D). Insets are traces of action potentials from the indicated slice groups. n = 15–24 cells each group. *P < 0.05; **P < 0.01.
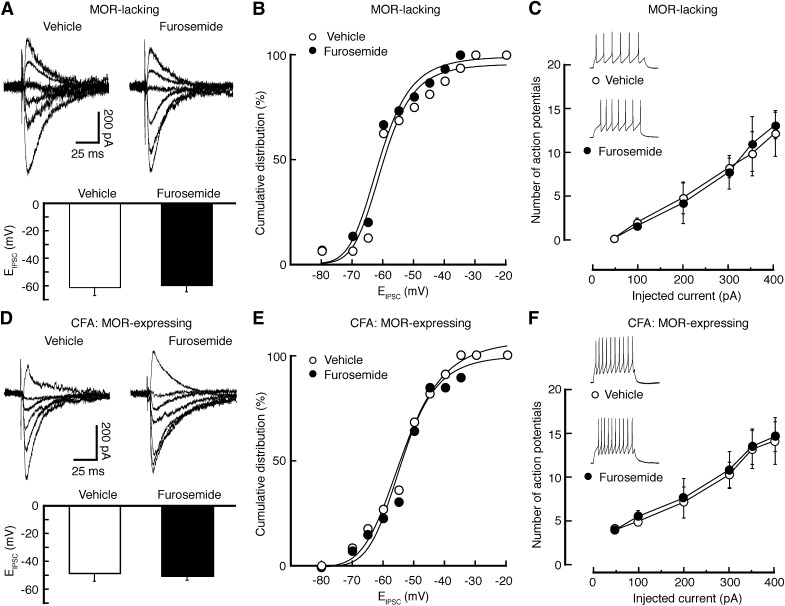
Fig. 4.
Pain occludes the effect of furosemide through KCC2 inhibition in MOR-expressing neurons. (A–C) Effects of furosemide on EIPSC (A and B) and on evoked firing activity (C) in MOR-lacking neurons. (D–F) Similar data of furosemide effects on EIPSC (D and E) and on firing (F) in MOR-expressing neurons from CFA-injected rats. n = 10–24 cells in each group.
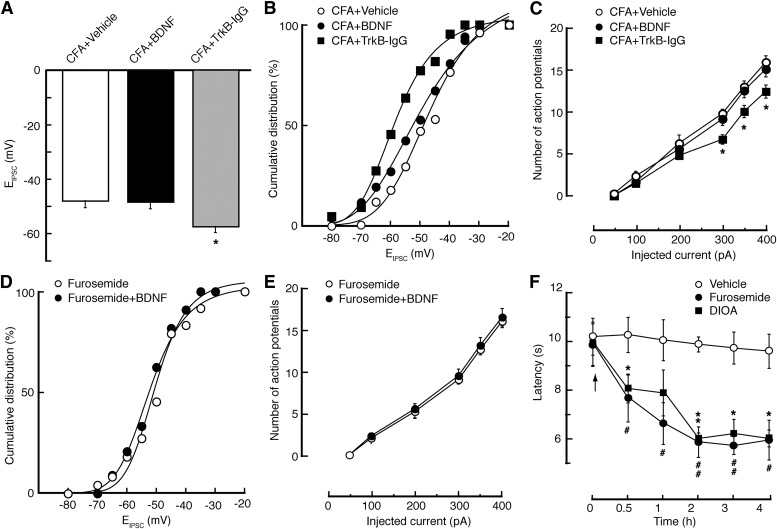
In MOR-expressing neurons from CFA-injected rats, we found that similar KCC2 inhibition by furosemide was no longer able to induce a statistically significant shift in EIPSC (Fig. 4, D and E); it also failed to change the firing rate of evoked action potentials (Fig. 4F), suggesting that pain-induced KCC2 downregulation might have occluded the effects of KCC2 inhibition by furosemide. Thus, it is likely that persistent pain downregulates KCC2, which shifts the EIPSC to more positive potentials, reducing the inhibitory function of GABA synapses selectively in MOR-expressing neurons and consequently increasing the excitability of these neurons in the NRM.
BDNF Contributes to KCC2 Downregulation in Pain Sensitization.
We then investigated what was responsible for the KCC2 downregulation induced by the pain condition. A strong candidate is BDNF, as it has been shown to downregulate KCC2 in hippocampal cells (Rivera et al., 2002; Wardle and Poo, 2003). Our results of both Western blotting and enzyme-linked immunosorbent assay showed a statistically significant increase in the level of BDNF protein in the NRM at 3 days after CFA injection when compared with that in saline-injected control rats (Fig. 5A); a similar increase was observed in the BDNF mRNA level (Fig. 5B). Microinjection of BDNF (1 μg in 1 μl) into the NRM of naïve rats statistically significantly reduced the KCC2 protein level at 6 hours after BDNF microinjection when compared with the vehicle group (Fig. 5C). In addition, in CFA-injected rats, inhibition of BDNF signaling by NRM microinjection of tyrosine receptor kinase B (TrkB)–IgG (1 μg in 1 μl), an infusion protein of the BDNF receptor TrkB-IgG that sequesters endogenously released BDNF, increased KCC2 protein level in NRM (Fig. 5D). These findings indicate that persistent pain downregulates KCC2 by increasing BDNF level and signaling in NRM.
Fig. 5.
Pain upregulates BDNF and BDNF downregulates KCC2 in the NRM. (A) Western blots (top) and normalized data of Western blots and enzyme-linked immunosorbent assay (bottom) of BDNF protein in NRM tissues from saline- (n = 5) and CFA-injected rats (n = 6). (B) Pain-induced change in BDNF mRNA level (n = 6 rats). (C) Western blots (top) and summarized data (bottom) of KCC2 protein in NRM tissues from naïve rats after NRM microinjection of vehicle (n = 4 rats) or BDNF (1 μg in 1 μl, n = 6 rats). (D) Western blots (top) and summarized data (bottom) of KCC2 protein in NRM tissues from CFA-injected rats after NRM microinjection of vehicle (n = 4 rats) or TrkB-IgG (1 μg in 1 μl, n = 6 rats). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05.
To further confirm this BDNF role, we determined the effect of BDNF signaling on EIPSC and cell firing in the NRM. After treatment of NRM slices from naïve rats with BDNF (100 ng/ml) for at least 1 hour, MOR-expressing cells displayed a depolarizing shift of about 13 mV in EIPSC when compared with those cells in the vehicle-treated control slices (vehicle, −58.8 ± 7.1 mV; BDNF, −46.2 ± 2.0 mV; P < 0.01) (Fig. 6A). The BDNF treatment also caused a statistically significant increase in the firing of evoked action potentials in these cells (Fig. 6B). However, these BDNF effects were not observed in MOR-lacking cells, either on EIPSC (vehicle, −61.1 ± 2.1 mV; BDNF, −57.4 ± 3.1 mV; P > 0.05) or on cell firing (Fig. 6, C and D).
Fig. 6.
BDNF induces a depolarizing shift in EIPSC and an increase in excitability selectively in MOR-expressing neurons. (A and B) Cumulative distribution of EIPSC (A) and number of firing action potentials (B) in MOR-expressing neurons from NRM slices treated with vehicle or BDNF (50 ng/ml) (n = 17–27 cells per group). (C and D) Similar data of EIPSC (C) and firing (D) in MOR-lacking neurons (n = 12–17 per group). *P < 0.05.
Next, we further examined the functional relationship between BDNF and KCC2 in the regulation of EIPSC and neuronal excitability under the pain condition. In MOR-expressing cells from CFA-injected rats, we found that, similar to the effect of KCC2 inhibition, the BDNF treatment could no longer alter the EIPSC or the firing rate (Fig. 7, A–C); conversely, inhibiting BDNF signaling by NRM microinjection of TrkB-IgG reversed the pain effects on EIPSC, shifting it to more negative levels by about 10 mV (Fig. 7, A and B), and on the cell firing, decreasing their excitability in these cells (Fig. 7C). Moreover, after KCC2 inhibition by furosemide in MOR-expressing cells in naïve slices, BDNF induced no further change in either EIPSC (furosemide, −43.1 ± 2.1 mV; furosemide + BDNF, −44.2 ± 2.4 mV; P > 0.05) (Fig. 7D) or cell firing (Fig. 7E). These data further support the notion that activation of BDNF/TrkB signaling downregulates KCC2 and thereby causes a depolarizing shift in EIPSC and hyperexcitability of MOR-expressing neurons.
Fig. 7.
Downregulation of KCC2 by activation of BDNF signaling induces pain sensitization. (A–C) Averaged EIPSC (A), cumulative distribution of EIPSC (B), and neuronal firing (C) in MOR-expressing neurons of NRM slices from CFA-injected rats and treated with vehicle, BDNF (100 ng/ml), or TrkB-IgG (500 ng/ml). n = 22–27 cells per group. (D and E) Cumulative distribution of EIPSC (D) and neuronal firing (E) in MOR-expressing neurons of NRM slices from naïve rats and treated with furosemide or furosemide plus BDNF (n = 16–22 cells per group). (F) Time course of changes in thermal pain threshold in rats (n = 5 rats for each group) in vivo after NRM microinjection (at arrow) of vehicle, furosemide (0.3 μg), or DIOA (0.2 μg). *,#P < 0.05; **,##P < 0.01.
Finally, we conducted behavioral experiments in vivo to determine a causal role of KCC2 downregulation in pain sensitization. In naïve rats, we found that microinjection of the KCC2 inhibitor furosemide (0.3 μg) or DIOA (2 μg), but not vehicle as control, into the NRM induced a statistically significant and long-lasting decrease in the thermal pain threshold (Fig. 7F), suggesting that inhibiting KCC2 in NRM is sufficient to cause pain sensitization.
Discussion
In this study, we have shown that persistent inflammatory pain upregulates BDNF, which downregulates KCC2 and decreases KCC2 function of maintaining Cl− gradient for inhibitory GABA synapses, resulting in impaired GABA inhibition and hyperexcitability selectively in presumably pain-facilitating, MOR-expressing NRM neurons, and contributing to pain sensitization. It suggests a signaling cascade of BDNF–KCC2–GABA synapses in MOR-expression NRM neurons as a cellular mechanism for a behavioral state of pain sensitization.
Pain Downregulation of KCC2.
It has been shown that both neuropathic and inflammatory pain involves the mechanism of impaired GABA synaptic inhibition in the spinal dorsal horn where a pain signal is transmitted to the brain for pain perception (Knabl et al., 2008; Costigan et al., 2009). A well-characterized mechanism for GABA functional impairment is the disruption of trans-synaptic anion gradient in GABA synapses of spinal cord neurons (Coull et al., 2003). KCC2 maintains the Cl− gradient by extruding Cl− from a cell, keeping a low intracellular Cl− concentration and thus an inhibitory Cl− current flowing through GABAA receptors. A number of studies have reported a significant downregulation of KCC2 and disruption of neuronal Cl− homeostasis in inflammatory and neuropathic pain models (Coull et al., 2003; Price et al., 2005; Miletic and Miletic, 2008; Zhang et al., 2008; Boulenguez et al., 2010; Hasbargen et al., 2010). All these findings of KCC2 downregulation were made in spinal dorsal horn neurons, so it is unclear whether a KCC2-mediated mechanism is involved in brain neurons and particularly in neurons in the brainstem descending pain-modulating system that maintains the pain hypersensitivity under chronic pain conditions (Porreca et al., 2002). Our study provides direct evidence for pain-induced KCC2 downregulation in brainstem neurons involved in descending pain modulation. There is evidence showing highly colocalized KCC2 and GABAA receptors in central neurons (Williams et al., 1999). Our finding of KCC2 downregulation in NRM preparations of synaptosomes suggests that pain downregulates KCC2 in NRM synapses, a cellular location consistent with the KCC2 role in regulation of GABA synaptic function.
Our results further demonstrate a causal link between KCC2 downregulation and a depolarizing shift in ECl for GABA IPSCs under the persistent pain condition. That pharmacologic inhibition of NRM KCC2 caused a depolarizing shift in EIPSC, increased the excitability of neurons with GABA synaptic inputs, and enhanced the pain response in naïve animals suggests that the KCC2 action is physiologically tonic, actively maintaining proper GABA inhibitory function and its impact on spinal pain transmission. Our finding on the absence of these cellular and behavioral effects of KCC2 inhibition in animals with persistent pain may indicate that pain-induced effects on KCC2 expression and GABA neurotransmission occlude the effects of KCC2 antagonists, thereby pharmacologic inhibition of KCC2 has no further effect on already pain-shifted EIPSC and sensitized pain response in CFA-injected rats. Thus, KCC2 inhibition in the NRM is sufficient to cause impairment of GABA inhibition and pain sensitization.
A unique feature of central GABA synapses in development is their functional maturation with the associated ECl shifting from less negative potentials (excitatory) to more negative potentials (inhibitory) during the first postnatal weeks (Ben-Ari et al., 2012). As our recordings were made in NRM neurons from animals of the second postnatal week, it is important to consider possible effects of this GABA synaptic maturation on our results of pain-induced ECl shift. We believe that this effect, if any, would not be significant due to the following reasons: 1) the animals in control and treatment groups were age-matched and recordings were obtained from randomly selected animals within the same range of postnatal days for both groups after vehicle or drug treatment; 2) As shown in the cumulative distribution plots (Figs. 3, 4, and 6), data points of EIPSC for both groups were in the same range of distribution probability; 3) no change in EIPSC was observed in MOR-lacking neurons from animals of the same postnatal days selected also randomly; and 4) other nonrecording data from adult animals without the maturation issue were consistent with recording data from neonatal animals.
As we did not observe an effect of KCC2 inhibitors on EIPSC in MOR-lacking neurons, KCC2 roles in this type of NRM neurons remain to be investigated. Our current data cannot rule out KCC2 regulation of EIPSC or ECl in these cells and there are several possibilities for the differential KCC2 roles in the two types of NRM neurons, including 1) differential density of KCC2 expression; 2) different subtype of Cl– transporters that regulates ECl in MOR-lacking cells; and 3) differential responses to pain stimuli (CFA) with distinct adaptive changes in KCC2 expression and in GABAergic synaptic inputs.
BDNF Downregulation of KCC2.
BDNF has diverse trophic effects on structural modifications and functional plasticity of central synapses in the adult brain (Poo, 2001; Chao and Bothwell, 2002). BDNF was originally found to downregulate KCC2 through activation of its TrkB receptor and downstream signaling in hippocampal culture (Rivera et al., 2002). Both BDNF and inhibition of KCC2 produce similar effects in inverting inhibitory GABA synaptic currents in spinal dorsal horn neurons, representing the cellular mechanisms for impaired GABA inhibitory function and consequent pain sensitization (Coull et al., 2003, 2005). Later studies on spinal neurons provide more direct supporting evidence for BDNF downregulation of KCC2 as the signaling mechanism for loss of GABA inhibition under sensitized pain conditions (Miletic and Miletic, 2008; Zhang et al., 2008; Ferrini et al., 2013). However, this role of BDNF, an important neurotrophin in the brain, in the brain’s pain-modulating circuitry has not been described. The current study shows a marked increase in NRM BDNF protein by the pain condition and reversal of the pain effects by TrkB inhibition, suggesting the involvement of endogenous BDNF in KCC2-mediated GABA impairment and neuronal hyperexcitability in these neurons for the brainstem mechanisms of pain sensitization.
BDNF has a well-documented pronociceptive role at both spinal and supraspinal levels in inflammatory and neuropathic pain responses (Pezet and McMahon, 2006; Merighi et al., 2008). In the spinal cord, BDNF released from microglia functions as a signaling link between microglia and dorsal horn neurons for neuronal hyperexcitability under neuropathic pain conditions (Biggs et al., 2010; Ferrini et al., 2013). The role of brain BDNF in pain modulation has been much less studied. In the brainstem descending pain-modulating pathway, a study by Guo et al. (2006) shows that BDNF in the rostral ventromedial medulla (RVM) that contains the NRM is likely originated from BDNF-containing neurons in PAG, and that BDNF activation of TrkB signaling in RVM induces descending pain facilitation. However, how BDNF affects the activity of these brainstem neurons has been unknown. Our study illustrates a cellular mechanism for selective BDNF activation of a specific NRM cell type that is responsible for descending pain facilitation.
Descending Pain Facilitation.
Although peripheral nociceptors and neurons in dorsal root ganglion and spinal cord dorsal horn play important roles in pain transmission and in initiation and maintenance of sensitized pain responses in the development of chronic pain, the brainstem descending system of PAG-RVM-spinal dorsal horn is also important to sustain such sensitized pain responses in the pain states (Porreca et al., 2002; Fields, 2004). By direct projections to dorsal horn neurons, neurons in RVM including the NRM can either inhibit or facilitate spinal pain transmission under different behavioral states. Several lines of evidence in vivo and in vitro have demonstrated that NRM neurons expressing MOR and inhibited by analgesic opioids produce descending pain facilitation, increasing pain sensitivity under normal and pathologic conditions (Pan et al., 1997; Porreca et al., 2001; Fields, 2004). Our recent studies have shown an increased excitability of these MOR-expressing NRM neurons in persistent pain conditions (Zhang and Pan, 2010; Zhang et al., 2011). Whereas several neurotransmitters have been proposed for the activation of this descending pain facilitation (Porreca et al., 2002; Pan, 2004), the underlying cellular mechanism that links NRM neurons to the behavioral pain sensitization has been unexplored. Our current results reveal a BDNF and KCC2-mediated impairment of GABA synaptic inhibition for the activation of MOR-expressing and pain-facilitating NRM neurons, and consequently for a sensitized pain state. The source of endogenous BDNF in this process remains unknown in our study, and it is possible that BDNF is released from PAG inputs, as previously suggested elsewhere (Guo et al., 2006), or from local sources such as microglia in the NRM.
In summary, we have demonstrated that persistent pain activates the descending pain-facilitating pathway from the NRM by BDNF-mediated KCC2 downregulation, resulting in decreased GABA synaptic inhibition and disinhibition of a selected group of NRM neurons responsible for descending pain facilitation. This mechanism may play an important role in the process of central sensitization during the development of chronic pain.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CFA
complete Freund’s adjuvant
- DIOA
[(dihydroindenyl)oxy]alkanoic acid
- DAMGO
d-Ala2, N-MePhe4, Gly-ol
- EIPSC
reversal potential of IPSCs
- IPSCs
inhibitory postsynaptic currents
- KCC2
K+–Cl– cotransporter
- MOR
μ-opioid receptor
- NRM
nucleus raphe magnus
- PAG
periaqueductal grey
- PCR
polymerase chain reaction
- RVM
rostral ventromedial medulla
- TrkB
tyrosine receptor kinase B
Authorship Contributions
Participated in research design: Zhang, Pan.
Conducted experiments: Zhang, X. Wang, W. Wang, Lu.
Performed data analysis: Zhang, X. Wang, W. Wang, Lu, Pan.
Wrote or contributed to the writing of the manuscript: Zhang, Pan.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA023069 and DA027541] (to Z.Z.P.); and the National Natural Science Foundation of China [Grant 31100802] (to Z.Z.).
References
- Basbaum AI, Bautista DM, Scherrer G, Julius D. (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. (2012) The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 18:467–486 [DOI] [PubMed] [Google Scholar]
- Bie B, Peng Y, Zhang Y, Pan ZZ. (2005) cAMP-mediated mechanisms for pain sensitization during opioid withdrawal. J Neurosci 25:3824–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B, Wang Y, Cai YQ, Zhang Z, Hou YY, Pan ZZ. (2012) Upregulation of nerve growth factor in central amygdala increases sensitivity to opioid reward. Neuropsychopharmacology 37:2780–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs JE, Lu VB, Stebbing MJ, Balasubramanyan S, Smith PA. (2010) Is BDNF sufficient for information transfer between microglia and dorsal horn neurons during the onset of central sensitization? Mol Pain 6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, et al. (2010) Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 16:302–307 [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell M. (2002) Neurotrophins: to cleave or not to cleave. Neuron 33:9–12 [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438:1017–1021 [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424:938–942 [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Robinson PJ. (2008) A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc 3:1718–1728 [DOI] [PubMed] [Google Scholar]
- Ferrini F, Trang T, Mattioli TA, Laffray S, Del’Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, et al. (2013) Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nat Neurosci 16:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. (2004) State-dependent opioid control of pain. Nat Rev Neurosci 5:565–575 [DOI] [PubMed] [Google Scholar]
- Fields HL, Vanegas H, Hentall ID, Zorman G. (1983) Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature 306:684–686 [DOI] [PubMed] [Google Scholar]
- Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. (2006) Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci 26:126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbargen T, Ahmed MM, Miranpuri G, Li L, Kahle KT, Resnick D, Sun D. (2010) Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Ann N Y Acad Sci 1198:168–172 [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, et al. (2008) Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451:330–334 [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. (1995) Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods 57:27–35 [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. (2006) Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol Pharmacol 69:1137–1145 [DOI] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. (2008) BDNF as a pain modulator. Prog Neurobiol 85:297–317 [DOI] [PubMed] [Google Scholar]
- Miletic G, Miletic V. (2008) Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain 137:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. (2002) Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 22:6724–6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G, Ahring PK, Mirza NR. (2009) Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends Pharmacol Sci 30:453–459 [DOI] [PubMed] [Google Scholar]
- Pan ZZ. (2004) An intensified descending pain-facilitating pathway. Drug Discov Today Dis Models 1:121–125 [Google Scholar]
- Pan ZZ, Tershner SA, Fields HL. (1997) Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature 389:382–385 [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, Osborne PB. (1990) Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J Physiol 427:519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. (2006) Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29:507–538 [DOI] [PubMed] [Google Scholar]
- Poo MM. (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2:24–32 [DOI] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. (2001) Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci 21:5281–5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. (2002) Chronic pain and medullary descending facilitation. Trends Neurosci 25:319–325 [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y. (2005) Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem 5:547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, et al. (2002) BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol 159:747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle RA, Poo MM. (2003) Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci 23:8722–8732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Sharp JW, Kumari VG, Wilson M, Payne JA. (1999) The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J Biol Chem 274:12656–12664 [DOI] [PubMed] [Google Scholar]
- Zhang L, Hammond DL. (2010) Cellular basis for opioid potentiation in the rostral ventromedial medulla of rats with persistent inflammatory nociception. Pain 149:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu LY, Xu TL. (2008) Reduced potassium-chloride co-transporter expression in spinal cord dorsal horn neurons contributes to inflammatory pain hypersensitivity in rats. Neuroscience 152:502–510 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. (2011) Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 17:1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Pan ZZ. (2010) Synaptic mechanism for functional synergism between delta- and mu-opioid receptors. J Neurosci 30:4735–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Pan ZZ. (2012) Signaling cascades for δ-opioid receptor-mediated inhibition of GABA synaptic transmission and behavioral antinociception. Mol Pharmacol 81:375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]