Abstract
Kalirin-7 (Kal7) is a Rho-guanine nucleotide exchange factor that is localized in neuronal postsynaptic densities. Kal7 interacts with the NR2B subunit of the NMDA receptor and regulates aspects of dendritic spine dynamics both in vitro and in vivo. Chronic treatment with cocaine increases dendritic spine density in the nucleus accumbens (NAc) of rodents and primates. Kal7 mRNA and protein are upregulated in the NAc following cocaine treatment, and the presence of Kal7 is necessary for the normal proliferation of dendritic spines following cocaine use. Mice that constitutively lack Kal7 [Kalirin-7 knockout mice (Kal7KO)] demonstrate increased locomotor sensitization to cocaine and a decreased place preference for cocaine. Here, using an intravenous cocaine self-administration paradigm, Kal7KO mice exhibit increased administration of cocaine at lower doses as compared with wild-type (Wt) mice. Analyses of mRNA transcript levels from the NAc of mice that self-administered saline or cocaine reveal that larger splice variants of the Kalrn gene are increased by cocaine more dramatically in Kal7KO mice than in Wt mice. Additionally, transcripts encoding the NR2B subunit of the NMDA receptor increased in Wt mice that self-administered cocaine but were unchanged in similarly experienced Kal7KO mice. These findings suggest that Kal7 participates in the reinforcing effects of cocaine, and that Kal7 and cocaine interact to alter the expression of genes related to critical glutamatergic signaling pathways in the NAc.
Introduction
Addiction is a disorder marked by persistent compulsive behaviors despite prolonged periods of abstinence and appropriate treatment (Hyman et al., 2006). While a great deal of work has been performed to elucidate the underlying pathophysiology of this disorder, the molecular mechanisms and neural circuits that result in such long-standing behavioral changes have yet to be fully clarified (Nestler, 2005; Kauer and Malenka, 2007; Kalivas, 2009). One potential mechanism underlying the chronic nature of addiction is morphologic changes in regions of the brain that are important for reward-related learning, such as the nucleus accumbens (NAc) and prefrontal cortex (Koob and Volkow, 2010). A multitude of animal studies have demonstrated that prolonged treatment with psychostimulants (e.g., cocaine or amphetamine) or opiates (e.g., morphine) cause long-lasting changes in dendritic spine density and morphology in these regions (Robinson and Kolb, 1997; Robinson et al., 2001; Lee et al., 2006; Russo et al., 2010). Changes in spine density and morphology have also been implicated in other chronic psychiatric/neurologic conditions, such as schizophrenia, autism, and mental retardation (Hutsler and Zhang, 2010; Lin and Koleske, 2010; van Spronsen and Hoogenraad, 2010).
Kalirin-7 (Kal7) is a Rho-guanine nucleotide exchange factor that is localized to the postsynaptic density of excitatory synapses and plays an important role in the normal formation and maintenance of dendritic spines both in vitro (Ma et al., 2003, 2008a) and in vivo (Ma et al., 2008b). At baseline, NAc spine density is normal in mice that constitutively lack Kal7 [Kalirin-7 knockout mice (Kal7KO)], but spine length and spine area are reduced. In wild-type (Wt) mice, levels of Kal7 protein and mRNA increase in the NAc following chronic administration of cocaine, and both spine density and spine area increase (Kiraly et al., 2010b; Mains et al., 2011). In Kal7KO mice, chronic cocaine administration fails to increase NAc spine density and paradoxically decreases spine area (Kiraly et al., 2010b; Mains et al., 2011). Behavioral experiments demonstrate that Kal7KO mice exhibit increased locomotor sensitization to cocaine but decreased cocaine place preference (Kiraly et al., 2010b).
The glutamate hypothesis of cocaine action posits that many of the short- and long-term behavioral, morphologic, and biochemical adaptations to cocaine exposure are mediated, in major part, through alterations in glutamatergic neurotransmission (Knackstedt and Kalivas, 2009; Eipper-Mains et al., 2013). Biochemical analyses of Kal7KO mice reveal a decrease in the expression, surface localization, and function of the NR2B subunit of the NMDA receptor, which interacts directly with Kal7 (Ma et al., 2008b; Kiraly et al., 2011). The literature suggests that NR2B function is important for the strengthening and stabilization of new synapses (Lee et al., 2010; Lee and Dong, 2011). An increase in silent synapses expressing NR2B in the NAc has been noted following cocaine treatment (Huang et al., 2009), and NR2B function is thought to be critical for the development of drug-induced locomotor sensitization and conditioned place preference (Brown et al., 2011; Ma et al., 2011; Pascoli et al., 2011). Similarly, the decrease in NR2B function observed in Kal7KO mice is thought to contribute to their decreased conditioned place preference for cocaine. The role of decreased NR2B function in the increased locomotor sensitization response observed in Kal7KO mice has not yet been studied (Kiraly et al., 2011).
While these previous studies have provided important information about the role of Kal7 in cocaine-related morphologic and behavioral changes, the most directly translational model of addiction is that of drug self-administration (Thomsen and Caine, 2005). Here we demonstrate that Kal7KO mice exhibit increased self-administration of cocaine at lower doses while exhibiting normal responses for a higher dose of cocaine and for food reinforcement. Since Kal7 has been shown to play a role in signaling pathways triggered by receptor tyrosine kinases, G protein–coupled receptors, ligand-gated ion channels, and homophilic adhesion molecules, we used quantitative polymerase chain reaction (qPCR) assays to search for alterations in the ability of Kal7KO mice to regulate the transcriptional response to self-administered cocaine. While a heightened transcriptional response was observed for the Kalrn gene, the ability of self-administered cocaine to increase expression of transcripts encoding NR2B was eliminated in Kal7KO mice. Differences in baseline expression of transcripts encoding vesicular glutamate transporter 1 (Vglut1) and Gria1 in the NAc of Wt and Kal7KO mice may contribute to their different responses to self-administered cocaine.
Materials and Methods
Animals
Kal7KO mice were generated as described previously (Ma et al., 2008b) and back-crossed to a C57BL/6 background for ≥15 generations. All experiments were performed using only male littermate controls from 2 to 5 months of age (25–35 g). Animals were shipped from the University of Connecticut Health Center to Arizona State University under climate-controlled conditions with abundant food and water available. Upon arrival, animals were quarantined for 6 to 8 weeks and then transferred to a temperature- and humidity-controlled colony room on a reversed 12-hour light/dark cycle (lights off at 7:00 AM). Animals were given ad libitum access to food and water except where specified. All experimental procedures were conducted with the approval of the University of Connecticut Health Center and Arizona State University Institutional Animal Care and Use Committees and in accordance with National Institutes of Health procedures for animal care.
Surgical Procedures
Mice were anesthetized with isoflurane (2% v/v) vaporized in medical-grade oxygen at a flow rate of 2 l/min. The skin overlying the dorsum between the scapulae as well as that overlying the jugular vein was shaved and scrubbed with iodine. A 1-cm incision was made to expose the jugular vein, and a sterile silicone catheter (0.67 mm and 1.0 mm internal diameters at venous and distal ends, respectively; Access Technologies, Skokie, IL) was implanted into the right jugular vein. The catheter was inserted 11 mm into the vein and was sutured in place and to the surrounding tissue. The distal end of the catheter was tunneled subcutaneously to exit the skin between the scapulae via a 1-cm incision. The catheter was attached to a threaded vascular access port (Plastics One, Roanoke, VA), which was then sutured to the surrounding tissue. Both wounds were sutured closed, and mice were treated daily with 10 U/ml heparin (0.05 ml volume) to maintain catheter patency and 6.67 mg/ml ticarcillin–clavulanate (0.05 ml volume) to protect against infection. The surgery site was treated with topical lidocaine and triple antibiotic ointment to facilitate healing of the wound, and mice were allowed 3 to 5 days to recover prior to initiation of drug self-administration procedures. All veterinary supplies and pharmaceuticals were obtained from Butler Schein Animal Health (Dublin, OH).
Behavioral Experiments
Testing Chambers.
Drug and food self-administration procedures were conducted in operant self-administration chambers (ENV-307; Med Associates, St. Albans, VT). Each self-administration chamber was located inside a sound-attenuating cubicle equipped with a house light and exhaust fan designed to mask external noise and odors, and the chamber was interfaced with a computer. Each chamber was also equipped with two infrared nosepoke apertures located approximately 2 cm above the chamber floor. Syringe pumps were located outside of the self-administration chambers, and both were interfaced with a computer. Drug solutions were delivered via the syringe pump through a single-channel liquid swivel mounted atop the chamber, which was connected to the vascular access port.
Cocaine Self-Administration.
Cocaine hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO) and was dissolved in sterile saline for intravenous self-administration. All self-administration procedures were conducted during the dark phase of the light/dark cycle. Mice were allowed to spontaneously acquire self-administration of cocaine (0.5 mg/kg/infusion) or saline in 2-hour daily sessions. Cocaine or saline solution (infusion volume 0.02 ml over a 1-second period) was delivered to the animal following an active aperture response on a fixed ratio 1 (FR1) schedule of reinforcement. Each infusion was followed by a time-out period of 20 seconds during which additional responses were recorded but did not result in activation of the syringe pump. Self-administration sessions were conducted on consecutive days. Body weights were measured every 5 to 7 days to assess weight loss as a result of drug self-administration and to adjust the concentration of cocaine in the infusion syringe as needed. Responses on the designated inactive aperture were recorded but produced no programmed consequences.
All cocaine self-administration experiments began with 10 daily FR1 sessions at a dose of 0.5 mg/kg/infusion. No shaping or prior operant training was performed to encourage increased responding. Following this initial phase, animals then went through another 10 days of FR1 sessions at either 1.0 or 0.25 mg/kg/infusion. Following the 20 days of FR1 training, animals were either given two days of abstinence and then a progressive ratio (PR) session or were sacrificed. For PR sessions, a single cocaine infusion was earned with an escalating number of correct responses (e.g., 1, 2, 4, 8, 16, 32); the session was terminated when an animal went 60 minutes without enough responses to initiate an additional infusion.
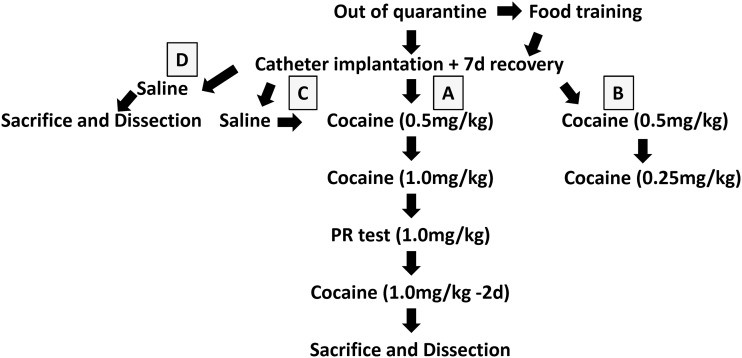
Due to the vagaries of animal breeding to produce male littermates, and the time- and effort-intensive nature of these experiments, it was necessary to use some animals in more than one behavioral assay. Figure 1 outlines the different paradigms that were used. With the exception of the PR test, all behavioral procedures were 10 days in length. After quarantine, some mice first experienced operant food self-administration. All animals then underwent catheter implantation surgery and 7 days of recovery. The majority of animals were then used in Path A (indicated by the light gray box in Fig. 1), wherein they moved from the 0.5 → 1.0-mg/kg/infusion training before having a PR test and sacrifice. The other group of mice, Path B, moved to a lower dose (0.25 mg/kg/infusion) after the first 10 days. Animals that started with saline infusions were either used in Path C, where they then went to cocaine self-administration, or in Path D, where they were sacrificed without receiving cocaine to serve as controls for qPCR. For animals with prior operant experience, the active aperture was switched prior to transitioning to cocaine. Additionally, we performed an analysis comparing animals that had received prior treatment with those who started with cocaine training. Repeated measures analysis of variance (ANOVA) of the first 10 days of cocaine training reveals no difference between the different pathways (P ≥ 0.44; Supplemental Fig. 1) so all groups were included in the same analysis. To ensure that results were not biased by animals who never administered enough drug to form an operant association (i.e., low responders) animals that received <2 infusions/day at the 0.5-mg/kg or 1.0-mg/kg dose or <1 infusion/day at the 0.25-mg/kg dose were not included in longitudinal behavioral analyses. Similar numbers of Wt and Kal7KO animals were excluded using these criteria.
Fig. 1.
Paradigm for behavioral testing. Due to breeding difficulties and lengths of experiments, some mice were used for multiple tests. Before surgery, some mice received operant conditioning for food reward. After catheter implantation, mice were used in one of four pathways. Animals assigned to Path A administered 0.5-mg/kg infusions for 10 days followed by 1.0-mg/kg infusions for 10 days, then underwent a progressive ratio test and were sacrificed for dissection. Those in Path B received the lower dose (0.25 mg/kg/infusion) after the first 10 days. Path C animals had 10 days of saline self-administration followed by cocaine. Animals in Path D had 10 days of saline self-administration and were then sacrificed and dissected to serve as molecular biology controls for the mice from Path A.
Food Self-Administration Experiments.
To test for motivation to self-administer a nondrug reinforcer (food), mice were food-restricted for 5 days prior to experimentation as described previously (Kiraly et al., 2010b). Daily weights were monitored to ensure the animals did not lose >15–20% of their total body weight. For these experiments, animals were placed in the operant conditioning chambers for 30 minutes each day. The active nosepoke hole was programmed to deliver a 20-mg grain pellet (Bio-Serv) on a FR1 schedule with a 20-second timeout. The inactive aperture had no programmed consequences. Animals were given free access to food for 90 minutes/day 1–6 hours after the completion of the session. As with cocaine self-administration, very low responders (average of <1 reward/session) were excluded from longitudinal behavioral analyses. This resulted in considerably more Wt mice being excluded (10 Wt and 3 Kal7KO); this issue is addressed in the Results and Discussion sections.
Molecular Biology
Following cocaine self-administration training and progressive ratio tasks, animals were given two additional 2-hour sessions with access to 0.5-mg/kg/infusion cocaine and were sacrificed 24 hours after the final exposure. Brains were dissected on ice, and a mouse brain block was used to obtain the 2-mm slice containing the NAc. Using the anterior commissure as a guide, a tissue punch (d = 1.5 mm) was used to remove the NAc. Samples were homogenized in ice-cold Trizol (Invitrogen, Carlsbad, CA) using a Polytron homogenizer (Kinematica, Luzerne, Switzerland). RNA was prepared as described (Mains et al., 2011), and cDNA was synthesized using iScript (Bio-Rad, Hercules, CA) with random primers; the 5-minute 65°C step before chilling and adding the enzyme gives much more reproducible qPCR data for very long transcripts. Real-time qPCR was performed as described (Mains et al., 2011). Data were calculated with respect to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for each sample within an assay; these values with respect to GAPDH for each transcript were consistent across assays and sets of mice over a 3-year period. The primer sets all yielded products that were ∼120 nt long, with melt temperatures of 61 ± 1°C (Mains et al., 2011; Eipper-Mains et al., 2013).
Statistical Analyses
Statistical analysis was performed using GraphPad Prism software (GraphPad, La Jolla, CA). For longitudinal behavioral experiments, repeated-measures two-way ANOVA analyses were used. Cumulative distributions were compared using a Kolmogorov-Smirnov test, and progressive ratio data were compared using a two-tailed t test. The qPCR data were compared using two-way ANOVA analyses for main effects with a Holm-Sidak post-hoc test, corrected for multiple comparisons.
Results
Cocaine Self-Administration.
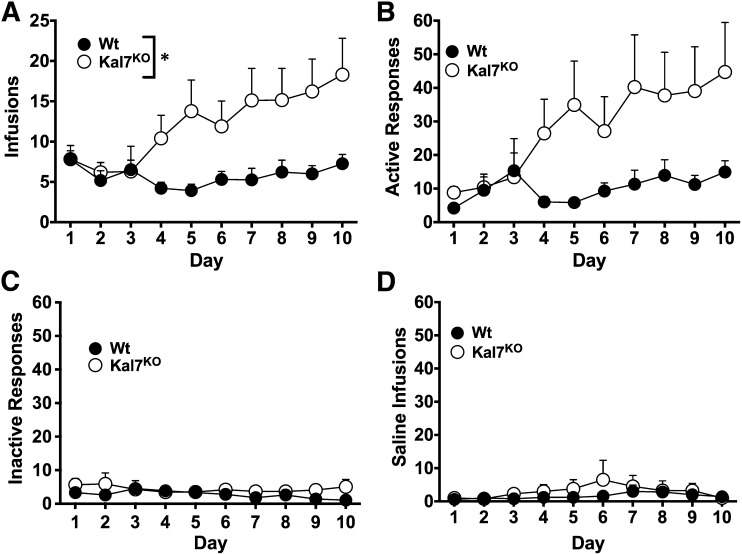
All cocaine self-administration experiments were performed with littermate male mice and began with with 10 daily sessions of 0.5-mg/kg/infusion cocaine reinforced on an FR1 schedule (Fig. 1). Wt and Kal7KO mice exhibited similar rates of administration over the first 3 days of conditioning, but while Wt mice remained relatively stable in their rate of administration, the Kal7KO mice increased their number of infusions over time (Fig. 2A). A two-way repeated-measures ANOVA examining the number of infusions revealed main effects of genotype (F(1,48) = 5.34, P = 0.025) and day (F(9,432) = 2.7, P = 0.005), as well as a genotype x day interaction (F(9,432) = 2.69, P = 0.005). Examination of the number of active responses made by each genotype demonstrated the same pattern as the number of infusions, with a strong effect of treatment day (F(9,342) = 3.65, P < 0.001), but there was no significant effect of genotype due to increases in variability (F(1,38) = 2.96, P = 0.09) (Fig. 2B). Analysis of responding on the inactive aperture (Fig. 2C) and of responding for saline infusions (Fig. 2D) demonstrated low levels of responding for both, with no main effects of genotype, day, or any interactions by repeated measures ANOVA (P ≥ 0.19 for all analyzed).
Fig. 2.
Cocaine self-administration at 0.5 mg/kg/infusion and saline. (A) When given daily training on an FR1 schedule for cocaine infusions, male Kal7KO mice earned a significantly higher level of infusions than their Wt littermates (repeated-measures two-way ANOVA: genotype F(1,48) = 5.34, *P = 0.025) with additional main effects of day (F(9,432) = 2.7, P = 0.005) and a significant genotype x time interaction (F(9,432) = 2.69, P = 0.005). (B) The pattern of responding on the active aperture (active responses) was similar to that seen with infusions, with a main effect of day (F(9,342) = 3.65, P < 0.001), but no significant effect of genotype was observed due to increased variability (F(1,38)=2.96, P = 0.09). Both Wt and Kal7KO mice exhibited low levels of responding for both the inactive aperture (C) and for infusions of saline (D), with no statistically significant differences by repeated-measures two-way ANOVA on either measure (P ≥ 0.19 for all). A total of 50 animals received cocaine (Wt, N = 23; Kal7KO, N = 27) and 10 received saline (Wt, N = 5; Kal7KO, N = 5).
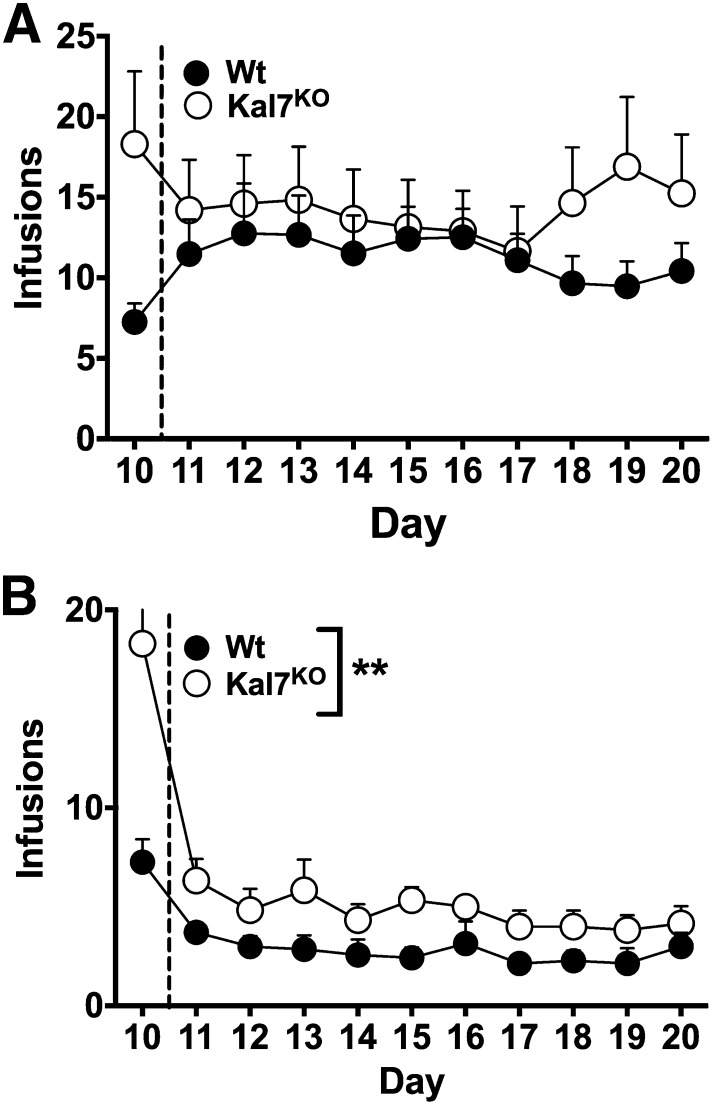
After completion of 10 days of training at a dose of 0.5 mg/kg, animals were transferred to a new dose of cocaine for the next 10 days. The first group had their dose increased to 1.0 mg/kg (shown in Path A in Fig. 1). In these animals, there was no significant difference between genotype in the number of infusions earned (Fig. 3A, repeated measures two-way ANOVA effect of genotype: F(1,39)=0.76, P = 0.39). Likewise, there was no main effect of day for this cohort (F(9,351)=0.38, P = 0.95) or a genotype x day interaction (F(9,351) = 1.02, P = 0.42). The animals that were trained at the 1.0-mg/kg dose then went on to a 1-day progressive ratio task, which also demonstrated no genotypic difference in the number of infusions earned (graph not shown: Wt 6.92 ± 1.4; Kal7KO 5.49 ± 1.3; results of a two-tailed t test showed t = 0.78, df = 51, P = 0.44). Higher doses of cocaine per infusion were not tested, as previous studies have shown 1.0 mg/kg to be the dose at which C57/bl6 mice typically plateau (Thomsen and Caine, 2007).
Fig. 3.
Cocaine self-administration at other doses. After completing the initial 10-day acquisition period at 0.5 mg/kg/infusion, Wt and Kal7KO mice were tested with different doses of cocaine. (A) At a dose of 1.0 mg/kg (Wt, N = 21; Kal7KO, N = 20), the Kal7KO mice maintained a trend toward increased infusions, but there were no statistically significant results (repeated-measures two-way ANOVA: genotype F(1,39) = 0.76, P = 0.39; Day F(9,351) = 0.38, P = 0.95; interaction F(9,351) = 1.02, P = 0.42). (B) At the 0.25-mg/kg dose (Wt, N = 7; Kal7KO, N = 6), Kal7KO mice retained a significantly higher number of infusions (repeated-measures two-way ANOVA effect of genotype F(1,11) = 14.16, **P = 0.003). There was no main effect of day (F(9,99) = 1.46, P = 0.17) or any genotype x day interaction (F(9,99) = 0.31, P = 0.97).
Given that the Kal7KO mice administered more cocaine at 0.5 mg/kg/infusion but no statistically significant genotypic difference was observed at 1.0 mg/kg/infusion, the next cohort of mice was moved to a lower dose (0.25 mg/kg/infusion) following the initial 10 days of training (shown in Path B of Fig. 1). While both Wt and Kal7KO mice exhibited a decrease in the number of infusions earned at this dose, the Kal7KO mice administered at a significantly higher level than did their Wt counterparts (Fig. 3B; repeated-measure two-way ANOVA effect of genotype: F(1,11) = 14.16, P = 0.003). As with the 1.0-mg/kg dose, there was no main effect of day (F(9,99) = 1.46, P = 0.17) or genotype x day interaction (F(9,99) = 0.31, P = 0.97).
Population of High-Responding Mice Among Kal7KO.
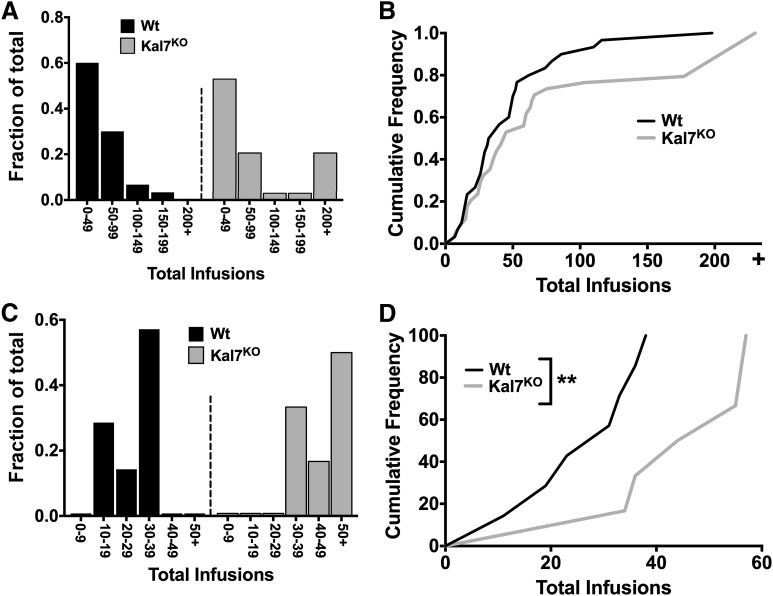
Following analysis of the longitudinal group data for cocaine self-administration (Figs. 2 and 3), we examined the data to determine how the behavior of individual animals contributed to the overall genotype effect at 0.25- and 0.5-mg/kg infusions. Figure 4A presents a histogram of the total number of infusions earned by individual mice in the 0.5-mg/kg/infusion experiment (all mice, including low responders, are included in Fig. 4). As can be seen, the Wt animals included a large population responding in the lower range, with a monotonic decline in the percentage of animals earning higher numbers of infusions. However, when the corresponding results were plotted for the Kal7KO mice, a secondary peak representing a population of very high responders was observed. The same data plotted as a cumulative frequency plot demonstrated a rightward shift in the curve (Fig. 4B); Kolmogorov-Smirnov analysis did not demonstrate statistical significance to this shift (P = 0.33).
Fig. 4.
Analysis of high-responder populations in Kal7KO mice. The distribution of total levels of responding for cocaine among mice was analyzed. (A) Histogram showing the fraction of each genotype earning a set number of infusions. A group of very high–level responders is observed for the Kal7KO mice but not for the Wt mice. (B) Cumulative frequency plot of the data from A demonstrates the expected right-shift in the Kal7KO curve. Kolmogorov-Smirnov analysis indicates that the shift does not reach statistical significance (P = 0.33). Histogram (C) and Kolmogorov-Smirnov (D) plots for 0.25-mg/kg/infusion data. A right-shift (higher responding) for the Kal7KO mice is apparent on both the histogram and cumulative frequency plots. At this dose, Kolmogorov-Smirnov analysis shows a statistically significant difference between genotypes (**P = 0.039).
Figure 4C presents a histogram of the total number of infusions earned by individual mice receiving 0.25 mg/kg/infusion. Although the shapes of the Wt and Kal7KO distributions were nearly identical, the Kal7KO distribution was shifted to the right; 4% of the Wt mice earned 10 to 29 infusions; none of the Kal7KO mice earned fewer than 30 infusions. Fully 67% of the Kal7KO mice earned more total infusions than the highest responding Wt mouse. Cumulative distribution plot and Kolmogorov-Smirnov analysis revealed this to be a statistically significant shift in the curve between genotypes (P = 0.039), with more of the Kal7KO mice receiving more total infusions (Fig. 4D).
Food Self-Administration.
Previous studies have shown that Kal7KO mice exhibited normal radial arm maze learning for a food reward (Ma et al., 2008b; Kiraly et al., 2010b), as well as other food-motivated behaviors (Ma et al., 2008b; Kiraly et al., 2010b). When tested for operant food self-administration, a two-way repeated measures ANOVA revealed that there was an overall increase in responding across days (Fig. 5A; F(9,135) = 3.51; P < 0.001) but no significant difference between genotypes (F(1,15) = 0.02; P = 0.90) or a genotype x day interaction (F(9,135) = 0.99; P = 0.45).
Fig. 5.
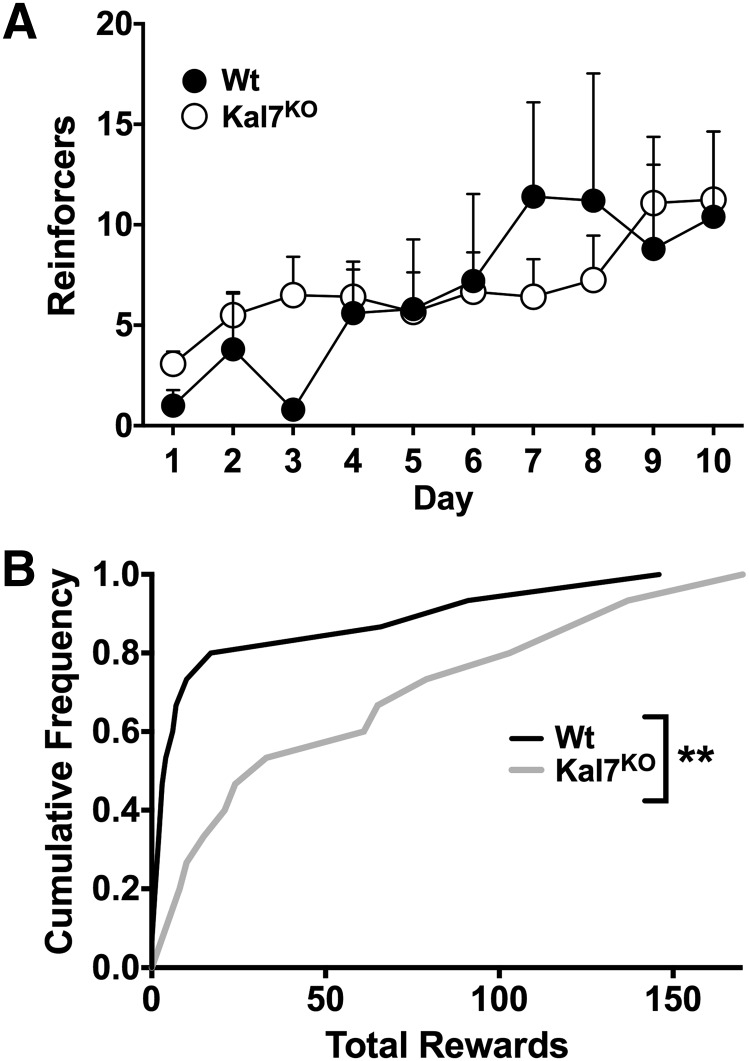
Instrumental responding for food. Cocaine-naïve animals that were food restricted for 5 days (Wt, N = 5; Kal7KO, N = 12) were then tested for operant responding for a grain pellet reward on an FR1 schedule for 10 days. When the number of reinforcers earned is examined by day (A), there are no differences in responding by genotype (repeated-measures two-way ANOVA: F(1,15) = 0.02; P = 0.90). There is a significant increase in responding across days (F(9,135) = 3.51; P < 0.001) but no genotype by day interaction (F(9,135) = 0.99; P = 0.45). (B) Plot of the total number of reinforcers earned by each animal as a cumulative distribution plot shows a strong right shift of the Kal7KO curve, indicating that a larger segment of the Kal7KO population garners a greater number of food rewards over time. Additionally, the large population of low responders (Wt, N = 15; Kal7KO, N = 15) excluded from the analysis in A is evident from the course of the Wt line. These results are significant based on Kolmogorov-Smirnov analysis (**P = 0.002).
As for cocaine self-administration, we examined the individual data for total levels of food responding. Interestingly, far more Wt animals fell into the low-responder category (<1 response/session: 10/15 in the Wt group, 3/15 in the Kal7KO group) and were excluded from the longitudinal analysis in Fig. 5A. This difference was quantified using a cumulative frequency curve of the total number of food rewards earned for all animals followed by Kolmogorov-Smirnov analysis. Figure 5B shows a distinct rightward shift in the Kal7KO curve, which was driven by both a population of low-responding Wt mice and a population of high-responding Kal7KO mice; 27% of the Kal7KO mice earned >100 total rewards, whereas only 7% of the Wt mice reached this level. Kolmogorov-Smirnov analysis of these data revealed a strongly significant difference between genotypes (P = 0.003). While there was no genotypic difference among the cohort of mice that responded to the task, the Kal7KO mice had a much greater propensity to show adequate levels of responding for the food reward.
qPCR Analysis of NAc Transcripts.
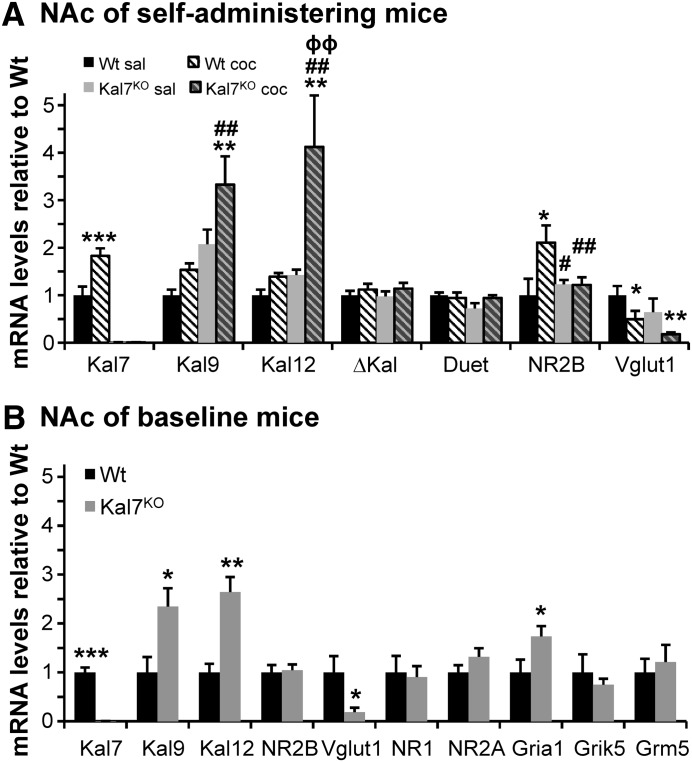
Chronic cocaine exposure produces a wide variety of changes in gene expression, and Kalrn has been identified as a cocaine-responsive gene (Kiraly et al., 2010b; Mains et al., 2011; Ma et al., 2012). Kalrn is a large, alternatively spliced gene that yields multiple protein products (Kiraly et al., 2010a); Kal7 and ΔKal7 are absent in Kal7KO mice, whereas expression of the larger isoforms, Kal9 and Kal12, is increased when the single exon unique to Kal7 and ΔKal7 is deleted (Ma et al., 2008b). To begin to identify the molecular changes underlying the behavioral alterations noted in Kal7KO mice, we performed qPCR analysis of transcripts from the NAc of Wt and Kal7KO mice that had self-administered either cocaine or saline (Fig. 1, Path A versus Path D). For these experiments, transcript levels were normalized to GAPDH within each group, and levels were consistent among multiple groups across experiments over a 3-year period. For ease of viewing, these data are presented here as fold change from Wt Saline levels (Fig. 6A); statistics and actual levels of expression are provided in Table 1. Similar to prior analyses of Kal7 RNA and protein expression in rodents receiving experimenter administered cocaine (Kiraly et al., 2010b; Mains et al., 2011; Ma et al., 2012), analysis of Kal7 transcripts in Wt mice revealed an increase after cocaine self-administration, with no signal in Kal7KO mice (Fig. 6A; Table 1). Examination of Kal9 transcript levels revealed a strong effect of genotype, with Kal7KO mice expressing higher levels of Kal9 transcript and a main effect of cocaine treatment. Similarly, Kal12 transcript levels showed a strong effect of genotype and cocaine, as well as a significant genotype x treatment interaction (Fig. 6A; Table 1). Transcripts encoding Kal9 and Kal12 did not increase significantly in Wt animals self-administering cocaine. Despite starting at a higher level in Kal7KO mice, levels of Kal12 transcript increased following cocaine self-administration in Kal7KO mice. Cocaine self-administration was without effect on expression of transcripts initiated at the ΔKal promoter or at the rarely used Duet promoter.
Fig. 6.
Results of qPCR. (A) RNA prepared from the NAc of cocaine and saline self-administering Wt and Kal7KO mice was subjected to qPCR analyses with data averaged across all sets of experiments and expressed with respect to internal GAPDH values. For ease of viewing, all transcripts are graphed as fold-change from Wt saline; statistical analyses and expression levels are provided in Table 1. Kal7, Kal9, and Kal12 reflect the use of different 3′-untranslated regions; ΔKal and Duet measure transcripts derived from alternate promoters. Cocaine self-administration results in an increase in NAc levels of NR2B transcript in Wt mice, but not in Kal7KO animals; Vglut transcript levels decline in response to self-administered cocaine in both genotypes. No significant changes with genotype or drug treatment are seen for a number of other transcripts known to be regulated by experimenter-administered cocaine (Eipper-Mains et al., 2013): NR1, NR2A, Gria1, Grik5, Grm5, Drd1a, Drd2, Mef2A, Arhgap32, Ppp1r1b (not shown). Symbols represent Holm-Sidak post-hoc results following two-way ANOVAs tabulated in Table 1. *Significant compared with Wt-Sal; #Significant compared with Wt-Coc; ϕSignificant compared with KO-Sal. (B) Comparison of NAc gene expression in naïve male Wt and Kal7KO mice; data are plotted as fold-change from Wt. *Significant compared with naïve Wt NAc. For both A and B: one symbol *,#P < 0.05; two symbols **,##,ϕϕP < 0.01; three symbols ***P < 0.001.
TABLE 1.
Two-way ANOVAs for qPCR analysis of transcript levels in self-administering mice
The full two-way ANOVA datasets for the mRNA analyses from the NAc of cocaine and saline self-administering Wt and Kal7KO mice graphed in Fig. 6 are presented. Transcript levels normalized to GAPDH are presented for saline-administering Wt and Kal7KO mice.
| Transcript | Test | F(DFn,DFd) | P Value | Wt Sal/GAPDH | Kal7KO Sal/GAPDH |
|---|---|---|---|---|---|
| Kal7 | Genotype | (1,39) = 107.7 | <0.0001*** | 0.0311 ± 0.0057 | 0.00026 ± 0.00007 |
| Treatment | (1,39) = 9.37 | 0.004** | |||
| Interaction | (1,39) = 9.30 | 0.004** | |||
| Kal9 | Genotype | (1,54) = 12.00 | 0.001** | 0.0134 ± 0.0016 | 0.0277 ± 0.0041 |
| Treatment | (1,54) = 4.67 | 0.04* | |||
| Interaction | (1,54) = 0.76 | 0.38 | |||
| Kal12 | Genotype | (1,41) = 46.10 | <0.001*** | 0.0052 ± 0.0006 | 0.0074 ± 0.0006 |
| Treatment | (1,41) = 43.03 | <0.001*** | |||
| Interaction | (1,41) = 16.81 | <0.001*** | |||
| NR2B | Genotype | (1,52) = 1.12 | 0.29 | 0.0179 ± 0.0062 | 0.0220 ± 0.0016 |
| Treatment | (1,52) = 3.06 | 0.09 | |||
| Interaction | (1,52) = 4.42 | <0.04* | |||
| Vglut1 | Genotype | (1,46) = 2.93 | 0.09 | 0.0183 ± 0.0035 | 0.0118 ± 0.0052 |
| Treatment | (1,46) = 12.74 | <0.001*** | |||
| Interaction | (1,46) = 0.32 | 0.57 |
P < 0.05; **P < 0.01; ***P < 0.001.
The heightened effect of cocaine self-administration on Kalrn expression in Kal7KO mice led us to examine several other transcripts known to increase following experimenter-administered cocaine (Eipper-Mains et al., 2013) (Fig. 6A; Table 1). Since Kal7 interacts directly with the NR2B subunit of the NMDA receptor, we examined this transcript. Levels of NR2B mRNA increased in Wt mice self-administering cocaine, but did not increase in cocaine self-administering Kal7KO mice; levels of NR2B transcript were indistinguishable in Wt and Kal7KO mice self-administering saline. Levels of the mRNA encoding Vglut1 decreased in both Wt and Kal7KO mice self-administering cocaine. These changes in NR2B and Vglut1 expression are similar to the changes seen in Wt mice receiving experimenter-administered cocaine (Eipper-Mains et al., 2013).
The fact that self-administration of cocaine affects NR2B transcript levels in the NAc differently in Wt and Kal7KO mice (Fig. 6A) led us to examine gene expression in naïve male Wt and Kal7KO mice (Fig. 6B). As expected, Kal7 transcripts were not detectable in the NAc of Kal7KO mice, and levels of Kal9 and Kal12 transcripts were significantly increased over Wt levels. NR2B transcript levels in Wt and Kal7KO NAc were indistinguishable (Fig. 6B). We next compared levels of several transcripts previously shown to respond to experimenter-administered cocaine (Eipper-Mains et al., 2013) in the NAc of naive Wt and Kal7KO mice (Fig. 6B). Levels of Vglut1 are diminished more than 5-fold in Kal7KO NAc. As for NR2B mRNA, the levels of transcripts encoding NMDA receptor subunits NR1 and NR2A did not differ in Wt and Kal7KO NAc. Levels of AMPA receptor subunit AMPA1 (Gria1) transcript were doubled in the NAc of Kal7KO mice, whereas levels of kainate receptor subunit kainate 5 (Grik5) and metabotropic glutamate receptor Grm5 transcript are unaltered (Fig. 6B). Neither Vglut1 nor AMPA1 transcript levels differ in RNA prepared from the motor cortex of the same Wt and Kal7KO mice (not shown).
Discussion
Behavioral Results.
Previous studies of Kal7KO mice have demonstrated an altered behavioral response to experimenter-administered cocaine. When given a sensitizing dose of cocaine, male Kal7KO mice demonstrate a higher degree of locomotor sensitization than Wt mice (Kiraly et al., 2010b). Additionally, Kal7KO mice exhibit decreased conditioned place preference for cocaine but normal place preference for food (Kiraly et al., 2010b). The present study represents the first examination of cocaine self-administration in Kal7KO mice. While direct comparisons between these three models are difficult, some conclusions can be drawn. Kal7KO mice self-administered low doses of cocaine at higher rates than Wt mice; this difference was not observed with higher doses of cocaine (Figs. 2 and 3; Supplemental Fig. 2). Previous studies have demonstrated a plateau in responses by C57BL/6 mice at 1.0 mg/kg/infusion (Thomsen and Caine, 2007), and our data concur (Supplemental Fig. 2). In our animals, we saw differences at lower doses that were not present at the 1.0-mg/kg dose and therefore chose not to escalate dosing beyond this level. It is noteworthy that Kal7KO mice maintained a higher level of response at the 0.25-mg/kg dose that elicited little response from Wt mice (Figs. 3 and 4). This suggests that Kal7KO mice have increased sensitivity to the rewarding properties of cocaine, in line with the increased locomotor sensitization response noted previously (Kiraly et al., 2010b). A connection between failure to upregulate NAc spine density after cocaine and increased locomotor sensitization and food-seeking was noted in Kal7KO mice and in mice lacking Cdk5, SynCAM1, Mef2, or Dnmt3a (Norrholm et al., 2003; Benavides et al., 2007; Meyer et al., 2008; Pulipparacharuvil et al., 2008; LaPlant et al., 2010; Giza et al., 2013; Rothenfluh and Cowan, 2013). As other models are examined, it will be interesting to see the possible connection between blunted spine plasticity, heightened locomotor sensitization, and increased self-administration.
The fact that Kal7KO mice only self-administered at higher rates for lower doses of cocaine gives perspective to the decrease in conditioned place preference (Kiraly et al., 2010b). The conditioned place preference task is performed with a single large bolus of cocaine, similar to the total amount administered over 2 hours in the 1.0-mg/kg/infusion experiments (Fig. 3A). If Kal7KO mice are more sensitive to the behavioral effects of cocaine, the large bolus dosing for the conditioned place preference test may be less reinforcing than for Wt mice, whereas response-contingent infusions at low doses may be more motivating for Kal7KO mice than for their Wt counterparts.
Examination of data from individual mice revealed that increased self-administration in Kal7KO mice was partly driven by a few high responders (Fig. 4). This is particularly interesting, as addiction is a disorder in which only a subset of individuals who use a drug escalate to the point of dependency and loss of control (SAMSHA, 2012). Studies in self-administering rats mimicking addiction conditions (e.g., cocaine administration paired with negative consequence, such as a foot shock) demonstrated that all rats self-administer cocaine but only ∼20% of them meet these “addiction” criteria (Deroche-Gamonet et al., 2004). Interestingly, approximately the same percentage of very high responders was observed in Kal7KO mice at the 0.5-mg/kg dose (Fig. 4A). Like many psychiatric disorders, addiction is believed to be a gene x environment interaction in which environmental influences and genetic risks become important contributing factors (Ducci and Goldman, 2012; Guo et al., 2012). On the basis of our results, one would predict that decreased Kal7 expression or function would contribute to increased vulnerability to cocaine addiction. Interestingly, a genome-wide linkage scan (Yang et al., 2011) found the region of chromosome 3 containing KALRN to have a very high LOD (logarithm of the odds) score for African-American families with cocaine dependence distinct from major depressive disorder.
Additional experiments are needed to elucidate the role of Kal7 in cocaine self-administration. The extinction-reinstatement model, which best recapitulates the abstinence-relapse paradigm seen in human addicts (Schmidt et al., 2005; Epstein et al., 2006; Knackstedt and Kalivas, 2009), could determine if lack of Kal7 affects sensitivity to stress, drug, or context-induced reinstatement. Additionally, studies examining NR2B inhibition in cocaine self-administration in Wt and Kal7KO animals will be important to understanding the significance of the direct interaction of Kal7 and NR2B (Kiraly et al., 2011), the failure of self-administered cocaine to increase NAc NR2B expression in Kal7KO mice, and the critical role of NR2B in the behavioral response to cocaine (Ma et al., 2006; Huang et al., 2009; Mao et al., 2009; Brown et al., 2011; Kiraly et al., 2011; Pascoli et al., 2011).
In addition to cocaine self-administration, we also characterized operant self-administration for food. Previous experiments showed normal radial arm maze learning (Ma et al., 2008b) and conditioned place preference for food (Kiraly et al., 2010b) in Kal7KO mice. Food-restricted Kal7KO mice had similar levels of overall responding for food reward pellets, without the population of low responders seen in Wt mice (Fig. 5). The previously studied behaviors are much more dependent on the pairing of an overall context with food rather than the pairing of an operant response (e.g., nose poke) with food reinforcement. Instrumental responding for reinforcers is largely dependent on the striatum, whereas context pairing is more dependent on the hippocampus (Kelley and Berridge, 2002; Hyman et al., 2006; Shiflett and Balleine, 2011). The effects of eliminating Kal7 expression on spine morphology are distinctly different in hippocampal CA1 pyramidal neurons and NAc medium spiny neurons (Ma et al., 2008b; Kiraly et al., 2010b). A systematic characterization of Kal7-mediated changes in dendritic spine morphology and gene expression in specific cell types is needed to determine mechanisms underlying genotypic differences in food reward and reinforcement place conditioning and operant self-administration paradigms.
Molecular Biology.
In Wt mice, Kal7 protein and transcript increase following experimenter-administered cocaine (Kiraly et al., 2010b; Mains et al., 2011). After prolonged cocaine self-administration, there was an increase in Kal7 mRNA levels in Wt mice similar in magnitude to that produced by experimenter-administered cocaine (Fig. 6A). Although the Kal7KO mice do not express Kal7 and ΔKal7, the larger isoforms of Kalirin (e.g., Kal9 and Kal12) are present in higher amounts at baseline (Ma et al., 2008b) (Fig. 6B). Less is understood about the functions of these larger isoforms, but they are known to promote process outgrowth by sympathetic neurons (May et al., 2002) and are expressed in adult forebrain neurons (Ma et al., 2001; Mains et al., 2011). Interestingly, there was a dramatic increase in Kal9 and Kal12 transcript levels in Kal7KO mice (Fig. 6A), likely corresponding to the elevated levels in naive animals (Fig. 6B).
On the basis of analyses of its role in specific signaling pathways and its extensive phosphorylation, Kal7 is a participant in multiple signaling pathways. Prior research has shown the cortex of Kal7KO mice has decreased levels of NR2B protein at the postsynaptic density and decreased surface expression of this receptor (Ma et al., 2008b; Kiraly et al., 2011; Lemtiri-Chlieh et al., 2011), but this is the first examination of NR2B expression in the NAc of Kal7KO mice; NR2B transcript levels did not differ in the NAc of naïve Wt and Kal7KO mice. An increase in NAc levels of NR2B mRNA, seen following experimenter-administered cocaine (Eipper-Mains et al., 2013), was also observed in Wt mice self-administering cocaine; strikingly, no increase in NAc NR2B expression was observed in Kal7KO mice self-administering cocaine (Fig. 6A). Although the molecular mechanisms underlying the lack of NR2B regulation in Kal7KO mice are unknown, NR2B expression is clearly regulated by the REST transcription factor (Rodenas-Ruano et al., 2012), histone acetylation (Myers et al., 1999; Qiang et al., 2011; Fujita et al., 2012), the CREB protein (Myers et al., 1999; Rani et al., 2005; Brown et al., 2011), the neuron-restrictive silencer factor NRSF (Qiang et al., 2005), and Sp1 (Myers et al., 1999), a transcription factor thought to bind to the Kalrn promoter (Miyatake et al., 2002; Mains et al., 2011).
A subset of GABAergic neurons in the NAc express both D1 and D2 dopamine receptors, plus Vglut1 (Gangarossa et al., 2013). Expression of Vglut1, although markedly reduced in the NAc of Kal7KO mice, is still diminished in response to self-administered cocaine, as in Wt mice. Vglut1 transcript levels are much higher in motor cortex than in NAc and are independent of genotype (not shown). The doubling of AMPA1 subunit mRNA observed in the NAc of Kal7KO versus Wt mice is another indication that glutamatergic transmission is altered in the NAc of naïve Kal7KO mice. Identification of the NAc neurons in which Vglut1 and AMPA1 expression is altered in Kal7KO mice will be an essential part of elucidating the pathways leading to enhanced cocaine self-administration in these mice.
Conclusions.
These studies represent the first behavioral and biochemical examination of Kal7KO mice using a cocaine self-administration paradigm. They make it clear that the presence of Kal7 at the synapse plays a critical role in normal self-administration behavior. Given that self-administration of low doses of cocaine is increased substantially in Kal7KO mice, our data suggest that augmenting Kal7 function might serve to diminish this response. Additionally, our data reveal a role for Kal7 in baseline glutamatergic signaling in the NAc and in the transcriptional response to cocaine self-administration.
Supplementary Material
Acknowledgments
The authors thank Darlene D’Amato for untiring laboratory assistance.
Abbreviations
- ANOVA
analysis of variance
- FR1
fixed ratio 1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- Kal7
Kalirin-7
- Kal7KO
Kalirin-7 knockout mouse
- NAc
nucleus accumbens
- PR
progressive ratio
- qPCR
quantitative polymerase chain reaction
- Vglut1
vesicular glutamate transporter 1
- Wt
wild-type
Authorship Contributions
Participated in research design: Kiraly, Olive, Eipper, Mains.
Conducted experiments: Nemirovsky, LaRese, Tomek, Yahn, Eipper, Mains.
Performed data analysis: Kiraly, LaRese, Olive, Eipper, Mains.
Wrote or contributed to the writing of the manuscript: Kiraly, LaRese, Olive, Eipper, Mains.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA-15464, DA-23082, DA-26706, and DA-24355]; and the Daniel Schwartzberg Charitable Fund.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, Olausson P, Yan Z, Taylor JR, Bibb JA. (2007) Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci 27:12967–12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, et al. (2011) A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci 31:8163–8174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. (2004) Evidence for addiction-like behavior in the rat. Science 305:1014–1017 [DOI] [PubMed] [Google Scholar]
- Ducci F, Goldman D. (2012) The genetic basis of addictive disorders. Psychiatr Clin North Am 35:495–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper-Mains JE, Kiraly DD, Duff MO, Horowitz MJ, McManus CJ, Eipper BA, Graveley BR, Mains RE. (2013) Effects of cocaine and withdrawal on the mouse nucleus accumbens transcriptome. Genes Brain Behav 12:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Morinobu S, Takei S, Fuchikami M, Matsumoto T, Yamamoto S, Yamawaki S. (2012) Vorinostat, a histone deacetylase inhibitor, facilitates fear extinction and enhances expression of the hippocampal NR2B-containing NMDA receptor gene. J Psychiatr Res 46:635–643 [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, de Kerchove d’Exaerde A, El Mestikawy S, Gerfen CR, Hervé D, Girault JA, Valjent E. (2013) Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits 7:22 DOI: 10.3389/fncir.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza JI, Jung Y, Jeffrey RA, Neugebauer NM, Picciotto MR, Biederer T. (2013) The synaptic adhesion molecule SynCAM 1 contributes to cocaine effects on synapse structure and psychostimulant behavior. Neuropsychopharmacology 38:628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Liu Z, Wang X, Zhang H. (2012) Large scale association analysis for drug addiction: results from SNP to gene. ScientificWorldJournal 2012:939584 DOI: 10.1100/2012/939584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, et al. (2009) In vivo cocaine experience generates silent synapses. Neuron 63:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H. (2010) Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res 1309:83–94 [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598 [DOI] [PubMed] [Google Scholar]
- Kalivas PW. (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572 [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8:844–858 [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Eipper-Mains JE, Mains RE, Eipper BA. (2010a) Synaptic plasticity, a symphony in GEF. ACS Chem Neurosci 1:348–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Lemtiri-Chlieh F, Levine ES, Mains RE, Eipper BA. (2011) Kalirin binds the NR2B subunit of the NMDA receptor, altering its synaptic localization and function. J Neurosci 31:12554–12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Ma XM, Mazzone CM, Xin X, Mains RE, Eipper BA. (2010b) Behavioral and morphological responses to cocaine require kalirin7. Biol Psychiatry 68:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. (2009) Glutamate and reinstatement. Curr Opin Pharmacol 9:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez SD, et al. (2010) Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci 13:1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Dong Y. (2011) Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology 61:1060–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. (2006) Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA 103:3399–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Yasuda R, Ehlers MD. (2010) Metaplasticity at single glutamatergic synapses. Neuron 66:859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Zhao L, Kiraly DD, Eipper BA, Mains RE, Levine ES. (2011) Kalirin-7 is necessary for normal NMDA receptor-dependent synaptic plasticity. BMC Neurosci 12:126 DOI: 10.1186/1471-2202-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Koleske AJ. (2010) Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci 33:349–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. (2008a) Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J Neurosci 28:711–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Huang JP, Wang Y, Eipper BA, Mains RE. (2003) Kalirin, a multifunctional Rho GEF, is necessary for maintenance of hippocampal pyramidal neuron dendrites and dendritic spines. J Neurosci 23:10593–10603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Huang JP, Xin X, Yan Y, Mains RE, Eipper BA. (2012) A role for kalirin in the response of rat medium spiny neurons to cocaine. Mol Pharmacol 82:738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Johnson RC, Mains RE, Eipper BA. (2001) Expression of kalirin, a neuronal GDP/GTP exchange factor of the trio family, in the central nervous system of the adult rat. J Comp Neurol 429:388–402 [DOI] [PubMed] [Google Scholar]
- Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim E-J, Levine ES, Eipper BA, Mains RE. (2008b) Kalirin-7 is required for synaptic structure and function. J Neurosci 28:12368–12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Guo CY, Yu P, Lee DY, Han JS, Cui CL. (2006) The role of NR2B containing NMDA receptor in place preference conditioned with morphine and natural reinforcers in rats. Exp Neurol 200:343–355 [DOI] [PubMed] [Google Scholar]
- Ma YY, Yu P, Guo CY, Cui CL. (2011) Effects of ifenprodil on morphine-induced conditioned place preference and spatial learning and memory in rats. Neurochem Res 36:383–391 [DOI] [PubMed] [Google Scholar]
- Mains RE, Kiraly DD, Eipper-Mains JE, Ma XM, Eipper BA. (2011) Kalrn promoter usage and isoform expression respond to chronic cocaine exposure. BMC Neurosci 12:20 DOI: 10.1186/1471-2202-12-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, et al. (2009) Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci 12:602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Schiller MR, Eipper BA, Mains RE. (2002) Kalirin Dbl-homology guanine nucleotide exchange factor 1 domain initiates new axon outgrowths via RhoG-mediated mechanisms. J Neurosci 22:6980–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DA, Richer E, Benkovic SA, Hayashi K, Kansy JW, Hale CF, Moy LY, Kim Y, O’Callaghan JP, Tsai LH, et al. (2008) Striatal dysregulation of Cdk5 alters locomotor responses to cocaine, motor learning, and dendritic morphology. Proc Natl Acad Sci USA 105:18561–18566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake R, Furukawa A, Suwaki H. (2002) Identification of a novel variant of the human NR2B gene promoter region and its possible association with schizophrenia. Mol Psychiatry 7:1101–1106 [DOI] [PubMed] [Google Scholar]
- Myers SJ, Dingledine R, Borges K. (1999) Genetic regulation of glutamate receptor ion channels. Annu Rev Pharmacol Toxicol 39:221–241 [DOI] [PubMed] [Google Scholar]
- Nestler EJ. (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8:1445–1449 [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. (2003) Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience 116:19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Besnard A, Hervé D, Pagès C, Heck N, Girault JA, Caboche J, Vanhoutte P. (2011) Cyclic adenosine monophosphate-independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellular signal-regulated kinase activation. Biol Psychiatry 69:218–227 [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al. (2008) Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 59:621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Denny A, Lieu M, Carreon S, Li J. (2011) Histone H3K9 modifications are a local chromatin event involved in ethanol-induced neuroadaptation of the NR2B gene. Epigenetics 6:1095–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Rani CS, Ticku MK. (2005) Neuron-restrictive silencer factor regulates the N-methyl-D-aspartate receptor 2B subunit gene in basal and ethanol-induced gene expression in fetal cortical neurons. Mol Pharmacol 67:2115–2125 [DOI] [PubMed] [Google Scholar]
- Rani CS, Qiang M, Ticku MK. (2005) Potential role of cAMP response element-binding protein in ethanol-induced N-methyl-D-aspartate receptor 2B subunit gene transcription in fetal mouse cortical cells. Mol Pharmacol 67:2126–2136 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. (2001) Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 39:257–266 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. (1997) Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 17:8491–8497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas-Ruano A, Chávez AE, Cossio MJ, Castillo PE, Zukin RS. (2012) REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nat Neurosci 15:1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfluh A, Cowan CW. (2013) Emerging roles of actin cytoskeleton regulating enzymes in drug addiction: actin or reactin’? Curr Opin Neurobiol 23:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. (2010) The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 33:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSHA (2012) Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-44.
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. (2005) Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol 526:65–76 [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW. (2011) Molecular substrates of action control in cortico-striatal circuits. Prog Neurobiol 95:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. (2005) Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci 32:9.20.1–9.20.40 DOI: 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. (2007) Intravenous drug self-administration in mice: practical considerations. Behav Genet 37:101–118 [DOI] [PubMed] [Google Scholar]
- van Spronsen M, Hoogenraad CC. (2010) Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep 10:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BZ, Han S, Kranzler HR, Farrer LA, Gelernter J. (2011) A genomewide linkage scan of cocaine dependence and major depressive episode in two populations. Neuropsychopharmacology 36:2422–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.