Abstract
Therapeutic and toxic response to low-dose methotrexate (MTX) in the treatment of autoimmune disease continues to be highly variable, resulting in a critical need to identify predictive biomarkers of response. Biomarker development has been hampered by an incomplete understanding of the molecular pharmacology of low-dose MTX. To address this issue, accumulation of the substrates for aminoimidazole carboxamide ribonucleotide transformylase (AICART) and thymidylate synthase (TS) was measured as markers of pharmacological activity of MTX in an erythroblastoid cell line. A 115-fold increase in the AICART substrate and anti-inflammatory mediator, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate (ZMP), was observed following exposure to 10 nM MTX but subsequently decreased with increasing MTX concentrations, declining to baseline levels with 1000 nM MTX. In contrast, the TS substrate, 2′-deoxyuridine 5′-monophosphate disodium salt (dUMP), displayed concentration-dependent accumulation, increasing 29-, 342-, and 471-fold over baseline with 10, 100, and 1000 nM MTX, respectively. Cellular levels of dUMP correlated with levels of the parent drug (MTX-PG1; r = 0.66, P < 0.001) and its polyglutamates (MTX-PG2–6) (r = 0.81, P < 0.001), whereas cellular levels of ZMP were only moderately correlated with MTX-PG1 (r = 0.34, P < 0.01). In contrast, accumulation of ZMP at 10 nM MTX was associated with a 2.9-fold increase in the AICART inhibitor dihydrofolate (DHF), represented primarily by long-chain DHF polyglutamates. Selectivity, defined as the ratio of ZMP to dUMP, was maximal following exposure to 6 nM MTX. Characterizing the range of MTX concentrations that selectively promote ZMP accumulation while preserving pyrimidine biosynthesis may lead to optimization of low-dose MTX therapy.
Introduction
Low-dose methotrexate (MTX) has become a commonly used therapeutic option as an anti-inflammatory in the treatment of various autoimmune diseases, including rheumatoid arthritis, lupus, psoriasis, and juvenile idiopathic arthritis (Weinblatt et al., 1985, 1994; Giannini et al., 1992; Fortin et al., 2008; Kalb et al., 2009). Despite the widespread use of MTX, response to low-dose MTX therapy is highly variable and currently unpredictable, with failure to achieve disease remission occurring in 40% or more of adult and pediatric arthritis patients (Lambert et al., 2004; Ruperto et al., 2004). Hepatic, gastrointestinal, and hematologic toxicities are commonly reported and often therapy limiting (Weinblatt, 1985; Becker et al., 2010). In addition, several months of therapy are often required before response can be accurately judged. For patients who fail to respond to MTX therapy, this delay in disease control results in potential disease progression and irreversible joint damage. Thus, early identification of clinical biomarkers that predict optimal response or risk of toxicity will have important clinical implications.
Recent investigations have included genetic, pharmacokinetic, and pharmacodynamic markers of response (Halilova et al., 2012). It is noteworthy that the use of erythrocyte measurements of MTX and its polyglutamates has been proposed to reflect both a pharmacokinetic and a pharmacodynamic metric for clinical use (Dervieux et al., 2004, 2005). The importance of the erythrocyte as a pharmacokinetic component of this measurement is reflected in the short plasma half-life of MTX that makes plasma measurements impractical (Kremer et al., 1986). The pharmacodynamic hypothesis is based on the notion that the polyglutamates of MTX are the biologically active form of the drug and, therefore, may be predictive of pharmacological activity. Metabolism of MTX to its polyglutamated forms has been found to result in increasingly potent inhibition of enzymes of nucleotide biosynthesis, including thymidylate synthase (TS), phosphoribosylglycinamide formyltransferase (GART), aminoimidazole carboxamide ribonucleotide transformylase (AICART), and amido phosphoribosyltransferase (ATase) (Allegra et al., 1985a,b; Baram et al., 1988; Sant et al., 1992). However, such a correlation between MTX polyglutamation and inhibition of these enzymes in a cellular system has not been experimentally evaluated.
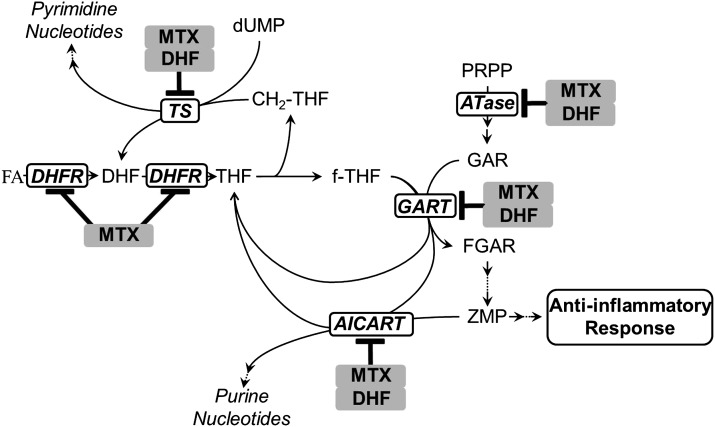
To date, biomarker development has been hampered by an incomplete understanding of the mechanism of action of low-dose MTX (Chan and Cronstein, 2010). The antiproliferative and anti-inflammatory effects of MTX are believed to be distinct, occurring through the inhibition of different biochemical pathways. Specifically, the antiproliferative activity of MTX is primarily attributed to inhibition of purine and pyrimidine nucleotide biosynthesis through depletion of intracellular reduced-folate cofactors, via inhibition of dihydrofolate reductase (DHFR), and direct inhibition of TS and enzymes of de novo purine biosynthesis, including ATase, GART, and AICART (Borsa and Whitmore, 1969b; Pinedo et al., 1976). In addition, inhibition of DHFR by MTX results in the cellular accumulation of dihydrofolate (DHF), which also has varying potency in inhibition of these enzymes (Allegra et al., 1985b, 1987; Baram et al., 1988; Chu et al., 1990; Sant et al., 1992). The anti-inflammatory mechanism of MTX activity remains controversial, but current evidence supports the inhibition of the purine synthesis pathway through direct inhibition of AICART (Fig. 1) (Baggott et al., 1998, 1999; Morgan et al., 2004; Baggott and Morgan, 2007). Inhibition of AICART has been found to result in the accumulation of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate (ZMP) and its metabolites, which inhibit adenosine deaminase and AMP deaminase, resulting in an increase in adenosine and adenine nucleotide levels (Cronstein et al., 1991; Baggott et al., 1993; Morabito et al., 1998). Elevated levels of extracellular adenosine, through adenosine receptor activation, result in a reduction in inflammation and may depend on MTX polyglutamate formation (Baggott et al., 1986; Cronstein et al., 1993; Urakawa et al., 2000; Dolezalova et al., 2005; You et al., 2013). Selective toxicity toward T cells and activation of AMP-activated protein kinase by ZMP has also been proposed as mechanisms of activity of MTX in autoimmune disorders (Taisun and Baggott, 1994; Genestier et al., 1998; Fairbanks et al., 1999; Johnston et al., 2005; Beckers et al., 2006; Katerelos et al., 2010).
Fig. 1.
A diagram of the hypothesized biochemical basis for the antiproliferative and anti-inflammatory activities of MTX. Inside the cell, MTX and its polyglutamate metabolites bind with high affinity to DHFR, inhibiting the reduction of folic acid (FA) and DHF to tetrahydrofolate (THF), resulting in depletion of the reduced folate pool, including THF, methylene-tetrahydrofolate (CH2-THF), and formyl-tetrahydrofolate (f-THF). Inhibition of enzymes of nucleotide biosynthesis, including ATase, GART, AICART, and TS, is inhibited through the following: 1) depletion of their respective reduced folate cofactors, 2) direct inhibition by MTX and its polyglutamate metabolites, and 3) direct inhibition by DHF. The antiproliferative activity of MTX is attributed to inhibition of purine and pyrimidine biosynthesis, whereas the anti-inflammatory activity is hypothesized to occur through inhibition of AICART, resulting in the accumulation of its substrate and anti-inflammatory mediator, ZMP. FGAR, formyl-glycinamide ribonucleotide; GAR, glycinamide ribonucleotide; PRPP, phosphoribosyl pyrophosphate.
Despite experimental evidence that MTX is a potent inhibitor of purified AICART, no study has thoroughly evaluated the exposure-dependent accumulation of ZMP in a cellular system following exposure to MTX. Therefore, a major focus of this study was to understand the relationship between low-dose MTX and the accumulation of this potentially important biochemical in mammalian cells.
Materials and Methods
Cell Culture.
K562 cells (catalog no. GM05372; erythroblastoid, human) were obtained from Coriell Cell Repositories (Camden, NJ) and grown in RPMI 1640 medium (61870-127; Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (S11150; Atlanta Biologicals, Lawrenceville, GA). Unless otherwise stated, cells were maintained under normal growth conditions in a 37°C and 5% CO2 controlled incubator at a density between 2 × 105 and 1 × 106 cells/ml to maintain cells in a logarithmic growth phase. All experiments were conducted within six passages following removal from cryopreservation.
Cell Growth/Cytotoxicity Studies.
K562 cells under normal growth conditions were exposed to 1000 nM MTX and monitored over a 96-hour period for changes in cell growth and viability as determined by the trypan blue exclusion assay and routine cell counting on a hemocytometer. In addition, cell growth and cytotoxicity were measured using reagents provided in the Live/Dead Viability/Cytotoxicity kit (L-3324; Invitrogen, Carlsbad, CA). In brief, K562 cells seeded into a 96-well plate were treated with MTX concentrations between 1 nM and 30 mM in triplicate for 24 hours under normal growth conditions. Live cell controls consisted of untreated cells, and dead cell controls consisted of cells pretreated with 70% ethanol. After MTX treatment, calcein-AM and ethidium homodimer-1 were added to each well at a final concentration of 4 μM and incubated at room temperature for 45 minutes. Fluorescence signal was determined for each sample using a BioTek Synergy HT fluorescence plate reader (BioTek, Winooski, VT) equipped with excitation/emission filters set at 485/528 nm and 530/645 nm. Percentage viability and cytotoxicity were determined as the fluorescence signal relative to the untreated control.
MTX Treatment.
K562 cells under normal culture conditions were counted using a hemocytometer, and for each experiment, a sample of approximately 2.5 × 106 cells was obtained for baseline measurements and denoted the zero hour sample. After resuspension at a density of 2.5 × 105 cells/ml in fresh growth media, a volume of 5 ml/well was added to 6-well cell culture grade polystyrene plates. MTX (16.411; Schircks Laboratories, Jona, Switzerland) in Dulbecco’s phosphate-buffered saline (D-PBS) or D-PBS alone (untreated control) was added to each well from sterile-filtered 100× stock solutions. Each sample was mixed gently and maintained under normal growth conditions for up to 72 hours. Cell samples containing approximately 1 × 105 to 5 × 106 cells were obtained through routine sampling at specified time points and washed in duplicate with 1 ml of 4°C D-PBS. A 25-μl aliquot was removed preceding the second wash step and retained for analysis of cellular protein content using the micro bicinchoninic acid method (23235; Thermo Scientific, Waltham, MA). The remaining cellular pellet was stored at −80°C prior to analysis.
Sample Preparation.
Cell samples were removed from −80°C storage and maintained on ice during processing. Frozen cell pellets were resuspended in 50 μl of extraction buffer consisting of 40% acetonitrile, 40% methanol, and 20% 0.1 M phosphate-buffered ultra pure water at pH 7.4 containing 0.1% 2-mercaptoethanol and 1% sodium ascorbate. Samples were vortexed for 30 seconds and centrifuged at 16,100g for 3 minutes to remove proteins. The resulting supernatant was subsequently analyzed by ultra-performance liquid chromatography tandem mass spectrometry (UPLC/MS/MS).
Analytical Methodology.
MTX and its polyglutamated metabolites were measured according to an earlier published procedure involving ion-pair UPLC/MS/MS (van Haandel et al., 2009, 2011). DHF polyglutamates were measured by UPLC/MS/MS using an appended version of a previously described method (van Haandel et al., 2012). ZMP (A611705; Toronto Research Chemicals, Toronto, ON, Canada) and 2′-deoxyuridine 5′-monophosphate disodium salt (dUMP, D3876; Sigma,St. Louis, MO) were measured with an additional UPLC/MS/MS assay. Separation was conducted on a Waters BEH HILIC chromatography column (100 × 2.1 mm packed with 1.7-μM particles; Waters, Milford, MA). A linear gradient with an isocratic hold for 0.2 minutes at 2.5% mobile phase A, 70% A at 2.2 minutes, and 2.5% A at 2.3 minutes with a re-equilibration time of 2 minutes was used to elute the analytes. Mobile phase A consisted of 20 mM ammonium acetate (pH 10.0), and mobile phase B consisted of acetonitrile with a flow rate of 0.3 ml/min. The mass spectrometer was equipped with an electrospray ionization source operating in negative ion mode. Selected reaction monitoring was used to detect ZMP (337 → 79) and dUMP (307 → 195), and collision energies were 15 and 20 V, respectively. Quantitation occurred by interpolation of the sample signal on a 5-point calibration curve ranging from 0.1 to 100 μM constructed for each analyte. Measured ZMP and dUMP concentrations were normalized for protein content and expressed in peak area (PA) or femtomoles per microgram of cellular protein.
Results
Cellular Uptake of MTX and Its Polyglutamate Metabolites.
The choice of the K562 cell line in these studies was based on the following two criteria: 1) the erythroblastoid properties of the cells (Andersson et al., 1979) with respect to the current use of erythrocyte MTX content as a biomarker of MTX toxicity and response, and 2) the documented capacity of K562 cells to actively transport (Matherly et al., 1991) and polyglutamate (Koizumi, 1988) MTX. Preliminary studies with qualitative analysis were conducted to determine the optimum range of MTX concentrations and exposure times for subsequent quantitative analysis.
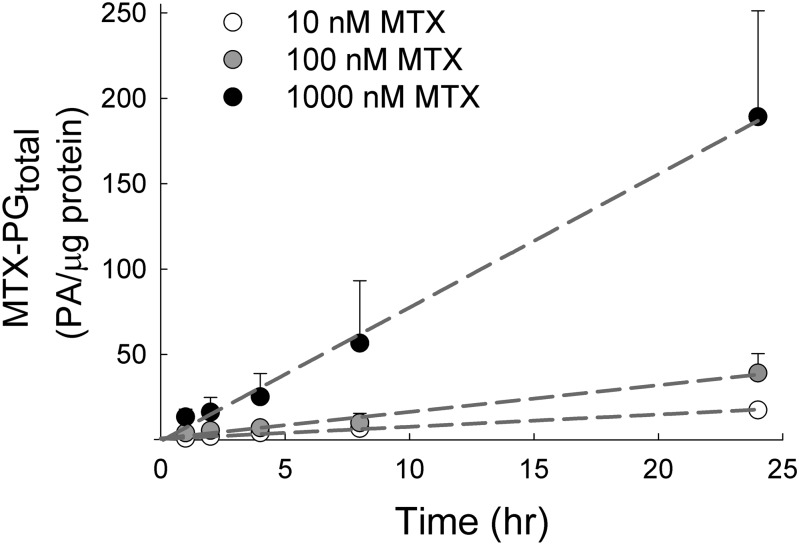
Uptake of MTX in the K562 cell line has been reported to be primarily mediated through the reduced folate carrier with a half-maximal saturation constant of 4.64 μM (Matherly et al., 1991), which is well above the maximum concentration of 1000 nM used in this study. Under these conditions, cellular uptake of MTX was linear and concentration-dependent over the 24-hour period, with mean (± S.D.) uptake velocities of 0.76 (± 0.13), 1.62 (± 0.49), and 7.78 (± 2.75) PA/μg protein per hour following exposure to 10, 100, and 1000 nM MTX, respectively (Fig. 2). Total MTX uptake at the end of the 24-hour exposure was found to be 18 (± 3), 39 (± 11), and 189 (± 62) PA/μg protein for the 10, 100, and 1000 nM MTX-treated cells, respectively. Significant cellular toxicity was not observed with MTX concentrations of up to 1000 nM for 24 hours, as determined by trypan blue exclusion and analysis with the fluorescent probes calcein-AM and ethidium homodimer-1 fluorescence (Supplemental Fig. 1).
Fig. 2.
Kinetic analysis of MTX accumulation in an erythroblastoid cell line. K562 cells maintained under normal culture conditions were treated with MTX at extracellular concentrations of 10, 100, and 1000 nM for 0, 1, 2, 4, 8, and 24 hours. Cellular levels of MTX and its polyglutamate metabolites (MTX-PGtotal) were determined by liquid chromatography–tandem mass spectrometry and normalized to cellular protein content. MTX-PGtotal, expressed in PA per microgram of protein, is presented as the mean ± S.D. from three independent experimental evaluations. Data were fit by linear regression, and the resulting slopes were expressed as the calculated rate of MTX-PGtotal accumulation under each treatment condition.
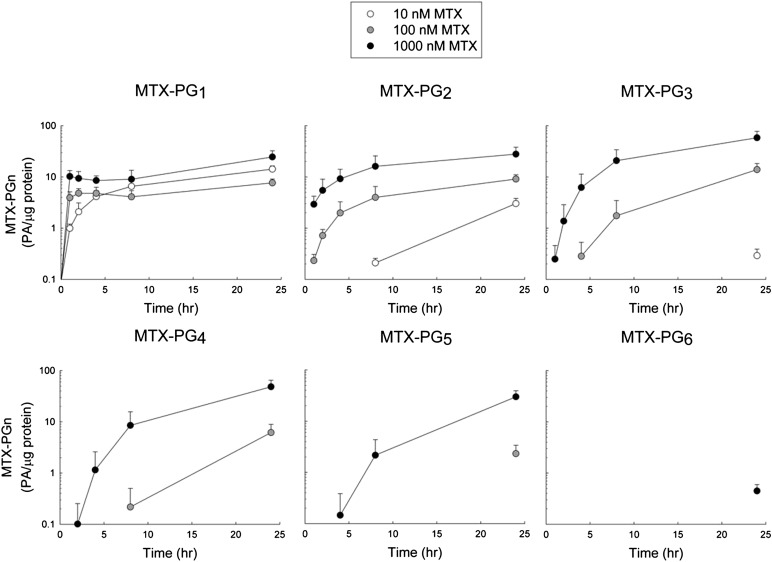
As the pharmacological activity of MTX is also dependent on the formation of polyglutamate metabolites because of their increased cellular retention and inhibitory activity against several target enzymes (Jolivet et al., 1982), the cellular accumulation of the parent compound, MTX (i.e., MTX-PG1), and its polyglutamated metabolites, containing between two (i.e., MTX-PG2) and six (i.e., MTX-PG6) glutamate residues, was also determined over the 24-hour drug exposure (Fig. 3). The initial rate of MTX-PG1 uptake was rapid with higher extracellular MTX concentrations (100 and 1000 nM), with near steady-state levels achieved within 1 hour. In contrast, when exposed to an extracellular concentration of 10 nM MTX, uptake of MTX-PG1 continued to increase throughout the treatment period. At the end of the treatment period, MTX-PG1 levels varied by 3-fold at most and were found to be 14.2 (± 2.2), 7.7 (± 1.4), and 24.5 (± 7.9) PA/μg protein for the cells treated with 10, 100, and 1000 nM MTX, respectively.
Fig. 3.
Kinetic analysis of cellular accumulation of MTX and its polyglutamate metabolites. K562 cells were exposed to MTX at extracellular concentrations of 10, 100, or 1000 nM for 0, 1, 2, 4, 8, and 24 hours under normal culture conditions. Cellular levels of the parent drug (i.e., MTX-PG1) and each polyglutamate metabolite (i.e., MTX-PG2 through MTX-PG6) were determined by liquid chromatography–tandem mass spectrometry and expressed in PA per microgram of protein. Each sample is presented as the mean ± S.D. from three independent experimental evaluations.
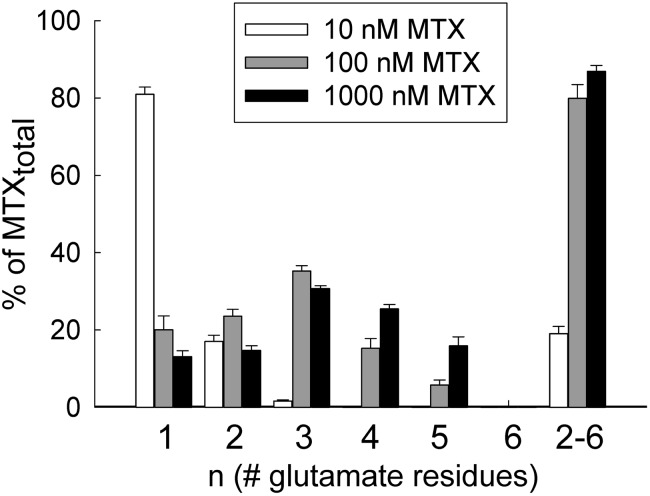
In contrast to the parent compound, the accumulation of MTX polyglutamates was found to display a higher degree of dependence on MTX concentration and exposure time. MTX-PG2 and MTX-PG3 were detected with all three MTX treatment concentrations and varied by as much as 9- and 200-fold, respectively. MTX-PG4 and MTX-PG5 were only detected at the two higher concentrations of MTX, with an 8-fold difference observed between the two concentrations for both species. MTX-PG6 was only detected following the 24-hour exposure to the highest concentration of MTX (Fig. 3). At the end of the treatment period, mean (± S.D.) concentrations of the polyglutamated species (i.e., MTX-PG2–6) were found to be 3.4 (± 0.9), 31.6 (± 10.1), and 164.7 (± 54.6) PA/μg protein for the cells treated with 10, 100, and 1000 nM MTX, respectively. Further analysis of the MTX polyglutamate distribution was conducted by determining the percentage of total MTX that was accounted for by each polyglutamate species (Fig. 4). Of note, MTX-PG1 accounted for 81.0% (± 1.9%), 20.1% (± 3.5%), and 13.1% (±1.5%) of total cellular MTX in the cells treated with 10, 100, and 1000 nM MTX, respectively. In contrast, higher-order MTX polyglutamates (MTX-PG2–6) accounted for 19.0% (± 1.9%), 79.9% (± 3.6%), and 86.9% (± 1.5%) of total cellular MTX in the cells treated with 10, 100, and 1000 nM MTX, respectively.
Fig. 4.
Effect of MTX concentration on cellular MTX polyglutamate distribution. Following a 24-hour exposure to MTX at concentrations of 10, 100, and 1000 nM, the percentage of total cellular MTX represented by the parent drug (i.e., MTX-PG1), each of its polyglutamate metabolites (i.e., MTX-PG2 through MTX-PG6), and the sum total of the polyglutamate metabolites (MTX-PG2-6) was determined. Each sample is presented as the mean ± S.D. from three independent experimental evaluations.
Accumulation of Substrates for TS and AICART.
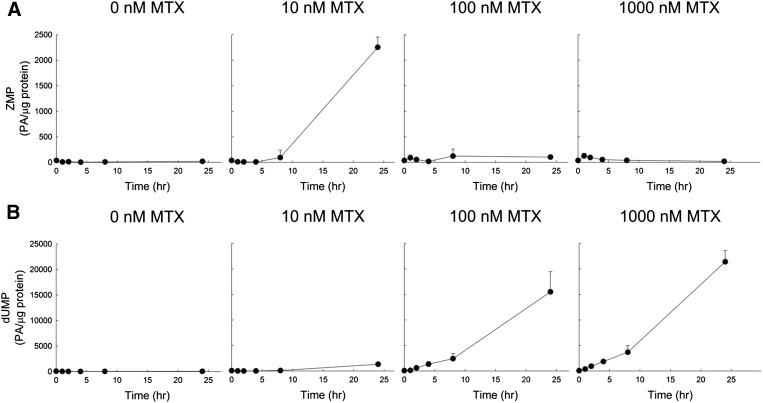
The concentration- and time-dependent inhibition of TS and AICART was monitored by measuring intracellular levels of their respective substrates, ZMP and dUMP (Fig. 5). Levels of dUMP increased with increasing concentrations of MTX in a time-dependent manner. Following a 24-hour exposure to 10, 100, and 1000 nM MTX, dUMP reached levels of 1327 (± 83), 15,511 (± 3952), and 21,404 (± 2213) PA/μg protein, respectively, representing a 29-, 342-, and 471-fold increase over untreated control levels of 45 (± 9) PA/μg protein, reflecting concentration-dependent inhibition of TS. In contrast, intracellular ZMP levels did not display the same exposure-dependent response. In the initial 8 hours of drug exposure, ZMP levels appeared mostly unchanged under all treatment conditions. However, after 24 hours of exposure, ZMP levels in the 10, 100, and 1000 nM MTX samples measured 2253 (± 199), 103 (± 34), and 19 (± 8) PA/μg protein, respectively. Compared with untreated control ZMP measurements of 19 (± 11) PA/μg protein, these measurements represent a 115- and 5-fold increase in ZMP levels following the 10 and 100 nM MTX treatments, respectively, and no change in levels following the 1000 nM MTX treatment.
Fig. 5.
Kinetic analysis of accumulation of substrates for AICART and TS following exposure to MTX. K562 cells were exposed to MTX at extracellular concentrations of 0, 10, 100, or 1000 nM for 0, 1, 2, 4, 8, and 24 hours under normal culture conditions. Cellular levels of the AICART substrate (ZMP) (A) and the TS substrate (dUMP) (B) were determined by liquid chromatography–tandem mass spectrometry. Analyte levels, expressed in PA per microgram of protein, are presented as the mean ± S.D. from three independent experimental evaluations.
Pearson’s pairwise comparisons between the cellular accumulation of dUMP or ZMP and the accumulation of MTX and its polyglutamates were conducted (Table 1). Intracellular levels of dUMP significantly correlated with total uptake of MTX (MTX-PGtotal); however, this was not observed for ZMP. In addition, further analysis showed that intracellular levels of dUMP significantly correlated with each of the measured polyglutamate species, and most strongly observed with MTX-PG3 (r = 0.81, P < 0.001). In contrast, ZMP levels were only moderately correlated with levels of the parent drug (MTX-PG1; r = 0.34, P < 0.01) and not with any of the measured polyglutamate species.
TABLE 1.
Pearson’s pairwise correlations (r values) for the relationship between intracellular MTX polyglutamates and levels of ZMP and dUMP in MTX-treated K562 cells
| MTX-PGtotal | MTX-PG1 | MTX-PG2 | MTX-PG3 | MTX-PG4 | MTX-PG5 | MTX-PG6 | MTX-PG2–6 | |
|---|---|---|---|---|---|---|---|---|
| ZMP | −0.01 | 0.34** | −0.02 | −0.08 | −0.07 | −0.07 | −0.14 | −0.07 |
| dUMP | 0.81*** | 0.66*** | 0.75*** | 0.81*** | 0.79*** | 0.78*** | 0.69*** | 0.81*** |
P < 0.01; ***P < 0.001.
Time- and Concentration-Dependent Accumulation of ZMP and dUMP.
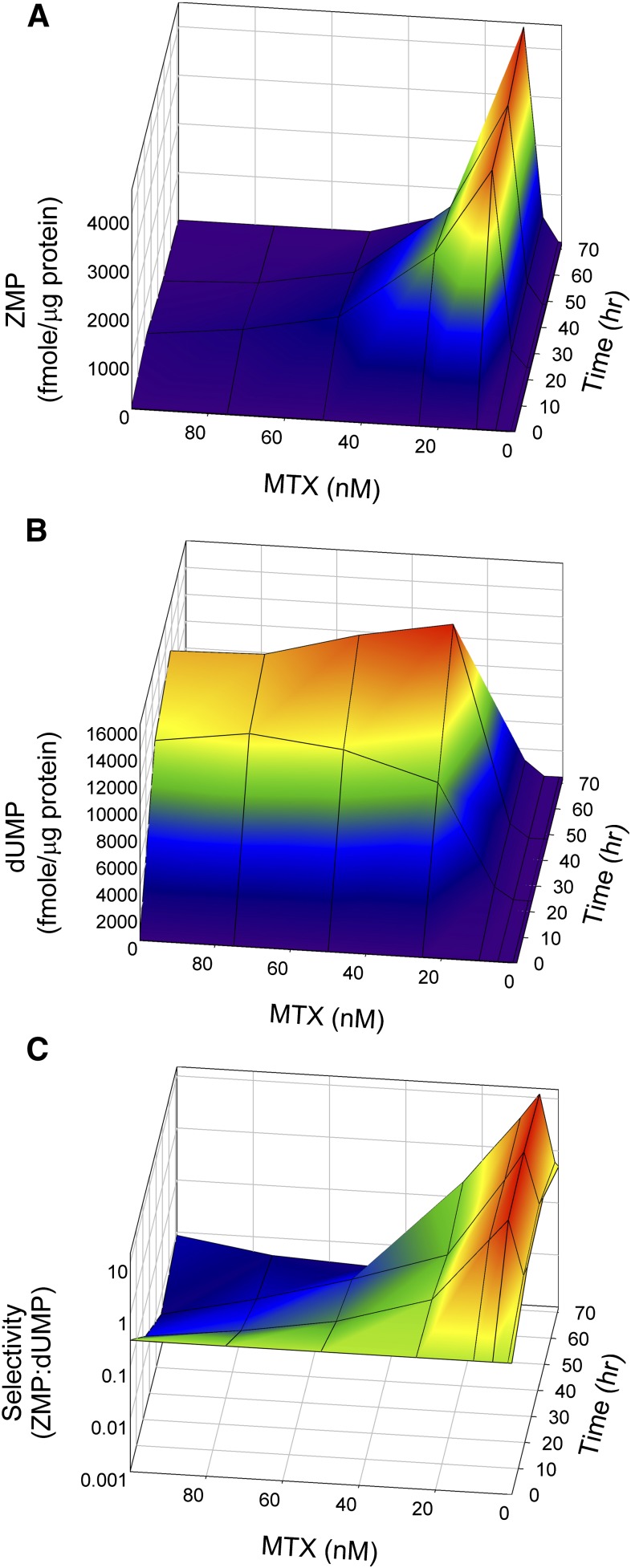
Given the discordance between MTX effects on ZMP and dUMP accumulation, subsequent studies were designed to elucidate the response over a narrower concentration range with longer exposure times. K562 cells were exposed to MTX concentrations ranging from 1 to 100 nM and untreated control (0 nM MTX) for up to 72 hours. Intracellular levels of ZMP, dUMP, and the ratio of the analytes were determined as a function of both concentration and time (Fig. 6). Analysis was conducted on a calibrated analytical system allowing for absolute quantification of ZMP and dUMP.
Fig. 6.
Surface plots of ZMP and dUMP accumulation following exposure to MTX. K562 cells under normal culture conditions were exposed to extracellular MTX concentrations of 0, 10, 25, 50, 75, and 100 nM for 0, 24, 48, and 72 hours. Cellular levels of ZMP (A) and dUMP (B) were determined by liquid chromatography–tandem mass spectrometry and expressed in femtomoles per microgram of protein. (C) The ratio of cellular ZMP to dUMP, termed selectivity, was determined for each sample.
In agreement with the previous set of experiments, ZMP levels were maximal following exposure to 10 nM MTX and were sustained throughout the 72-hour exposure, reaching levels 170-fold greater than the untreated control (4421 vs. 26 fmol/μg protein). At 5 nM MTX, ZMP levels progressively increased with time, reaching a level of 523 fmol/μg protein that was 20-fold greater than control values. In contrast, MTX concentrations greater than 10 nM resulted in an initial increase in ZMP concentrations that subsequently returned to control levels over the 72-hour period of exposure.
Intracellular dUMP levels were also highly responsive to MTX concentration and exposure time. A slight increase in dUMP was observed at the 10 nM MTX concentration, reaching levels up to 40-fold greater than the untreated control (1128 vs. 52 fmol/μg protein). The maximum response was observed at 25 nM MTX (15,633 fmol/μg protein) and represented a 620-fold increase over control. Interestingly, at concentrations greater than 10 nM MTX, dUMP concentrations continued to increase, reaching a maximum at 48 hours and declining thereafter.
To assess the relative effect of low concentrations of MTX on ZMP and dUMP accumulation, the ratio of intracellular ZMP to dUMP was also determined. Although the maximum ZMP accumulation was observed at the 10 nM MTX concentration, maximum selectivity for inhibition of AICART relative to TS occurred at 5 nM MTX with a ZMP:dUMP ratio of 14.3, representing a 48-fold increase in selectivity over the untreated baseline value of 0.3. At 10 nM MTX, a favorable ratio of up to 4.4 was observed, representing a 15-fold increase in selectivity. However, selectivity continued to decrease at higher MTX concentrations due to both a decrease in ZMP accumulation and a pronounced increase in dUMP levels with selectivity ratios as low as 0.004, 75-fold lower than the untreated control and 3575-fold lower than the maximum observed selectivity at 5 nM MTX.
Fine-Scale Optimization of ZMP Accumulation.
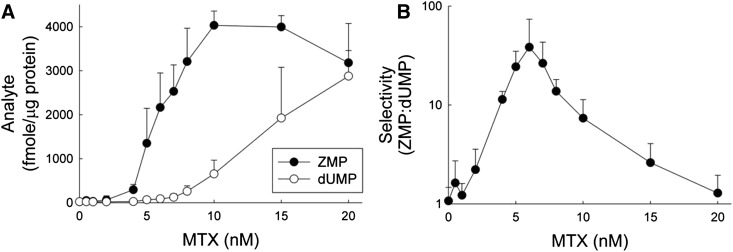
To determine the concentration of MTX that provides the maximum selectivity for accumulation of ZMP without significant inhibition of TS in K562 cells, cells were exposed to 12 different MTX concentrations between 0.5 and 20 nM and untreated control (0 nM) for 24 hours. Replicate experiments were conducted on three separate days (Fig. 7).
Fig. 7.
Concentration-dependent selectivity for ZMP and dUMP accumulation with low concentrations of MTX. K562 cells under normal culture conditions were exposed to extracellular MTX concentrations of 0, 0.5, 1, 2, 4, 5, 6, 7, 8, 10, 15, and 20 nM for 24 hours, and (A) cellular levels of ZMP and dUMP were determined by liquid chromatography–tandem mass spectrometry and expressed in femtomoles per microgram of protein. (B) Selectivity, defined as the ratio of cellular ZMP to dUMP, was determined for each sample. Data are presented as the mean ± S.D. from three independent experimental evaluations.
In support of the previous findings, ZMP levels increased in a concentration-dependent fashion, reaching levels of 4032 (± 326) fmol/μg protein in the cells treated with 10 nM MTX, representing a 194-fold increase over the untreated control levels of 21 (± 6) fmol/μg protein. ZMP levels remained significantly elevated after exposure to the 15 and 20 nM MTX concentrations; however, a trend toward declining ZMP levels was apparent at these higher MTX concentrations. The data were fit to a 3-parameter Hill equation, and the effective concentration resulting in 50% of the maximal response (EC50) was determined to be 6.1 (± 1.2) nM. Maximal increase in dUMP was observed with the highest tested concentration (i.e., 20 nM MTX) reaching 2878 (± 1196) fmol/μg protein, which represents a 129-fold increase over control levels of 22 (± 14) fmol/μg protein. Selectivity, expressed as the ratio of ZMP to dUMP concentrations, was maximal following exposure to 6 nM MTX, with a 36-fold increase in the ZMP-to-dUMP ratio.
DHF Polyglutamate Accumulation at Low MTX Concentrations.
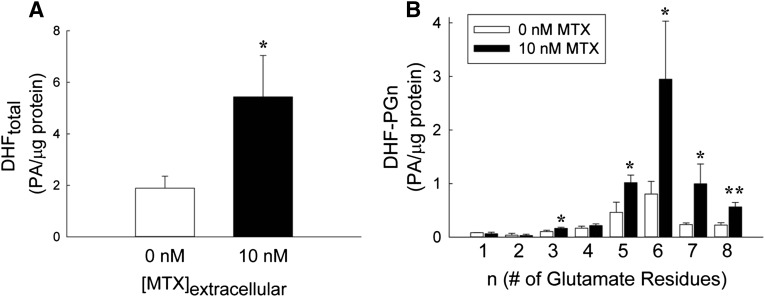
To determine the effect of low concentrations of MTX on cellular levels of DHF, K562 cells were exposed to 10 nM MTX for 24 hours, and the changes in cellular DHF were determined. MTX caused a significant increase in DHF to levels 2.9-fold greater than the untreated controls (Fig. 8A). Furthermore, MTX exposure resulted in a shift in DHF polyglutamation to long-chain polyglutamates, with the weighted average glutamate distribution shifting from a mean (± S.D.) of 5.52 (± 0.05) to 5.95 (± 0.08) glutamate residues per molecule of DHF (P < 0.01) (Fig. 8B). This effect was most pronounced for DHF-PG6, which was increased 4.3-fold over control.
Fig. 8.
DHF accumulation and polyglutamate distribution with low concentrations of MTX. K562 cells maintained under normal culture conditions were treated with or without 10 nM MTX for 24 hours. Cellular levels of DHF (DHFtotal) (A) and each of its polyglutamated forms (DHF-PGn) (B) were determined by liquid chromatography–tandem mass spectrometry, normalized to cellular protein content, and expressed in PA per microgram of protein. Data are presented as the mean ± S.D. from three independent experimental evaluations (*P < 0.05; **P < 0.01 by Student’s t test).
Discussion
In this study, exposure of erythroblastoid cells to low concentrations of MTX resulted in the accumulation of the AICART substrate and anti-inflammatory mediator ZMP. Accumulation of ZMP did not correlate with the formation of MTX polyglutamates and was observed at concentrations below those necessary for inhibition of TS. Together, these findings substantiate the selective accumulation of ZMP with low-dose MTX and do not support a relationship between levels of MTX polyglutamates and ZMP. However, the finding that DHF accumulates at low MTX concentrations may be indicative of its role in the selective accumulation of ZMP. These results will be discussed in regard to the biochemical pharmacology of MTX and pharmacodynamic markers of MTX activity, with therapeutic implications in the context of the hypothesized role of ZMP as the mediator of the anti-inflammatory effects of low-dose MTX therapy.
Intact cellular transport and metabolism of MTX is evidenced by the kinetics of MTX accumulation (Fig. 2) and polyglutamte formation (Fig. 3). MTX accumulation was time- and concentration-dependent with no evidence of saturation. The initial accumulation of MTX-PG1 appears to reach a rapid equilibrium consistent with high-affinity binding of MTX-PG1 (Goldman, 1974), with subsequent accumulation occurring primarily through the formation of higher-order glutamates. This is perhaps best illustrated by the formation of MTX-PG2 in the cells treated with 10 nM MTX only when MTX-PG1 approached saturating levels after 8 hours. Further formation of the higher-order polyglutamates (MTX-PG3–6) did not appear to display the same saturation-limited formation, but were highly dependent on MTX exposure. The MTX polyglutamate distribution at the end of the 24-hour treatment differed with MTX concentration, MTX-PG1 being the predominant species following the 10 nM MTX treatment, compared with the predominantly polyglutamated species (MTX-PG2–6) following the 100 and 1000 nM MTX treatments (Fig. 4).
Drug activity was measured through accumulation of substrates for the MTX-targeted enzymes AICART and TS. Despite the ability of MTX to inhibit various enzymes, we focused on AICART and TS to explore the balance of the hypothesized anti-inflammatory and antiproliferative effects of MTX. ZMP is the proposed mediator of the anti-inflammatory effects of MTX, whereas the TS substrate, dUMP, is a byproduct of inhibition of pyrimidine synthesis, which is important for the antiproliferative activity of MTX (Borsa and Whitmore, 1969a). The elevated ZMP concentrations observed only after exposure to low concentrations of MTX (Figs. 5–7) may be explained by a selective inhibition of AICART relative to other upstream enzymes in the purine biosynthesis pathway (Fig. 1), including ATase and GART (Lyons and Christopherson, 1991). These findings suggest that ZMP measurements may not provide a useful marker for inhibition of purine biosynthesis, but may provide an important biochemical marker for the anti-inflammatory effects of MTX. In vivo, levels of ZMP and its metabolites have been observed to increase intracellularly in splenocytes of MTX-treated mice, as well as in animal and human biologic fluids with MTX therapy, but a relationship with therapeutic response has not been established (Cronstein et al., 1993; Baggott et al., 1999; Smolenska et al., 1999; Baggott and Morgan, 2007). Further in vivo clinical studies are needed to define the relationship between ZMP and the anti-inflammatory activity of MTX before its value as a biomarker can be determined.
Although MTX polyglutamates are potent inhibitors of AICART in purified enzyme preparations (Allegra et al., 1985b), cellular levels of MTX polyglutamates were not significantly associated with ZMP levels in the cellular system used in the current study (Table 1). In an intact cellular system, MTX and its polyglutamates as well as endogenous folate inhibitors, such as DHF, would be expected to inhibit not only AICART, but also upstream enzymes in the purine biosynthesis pathway, as illustrated in Fig. 1, providing an explanation for the poor correlation between cellular MTX polyglutamate and ZMP concentrations.
Although modest, the correlation between the accumulation of MTX-PG1 and ZMP suggests that the parent compound may be active in the inhibition of AICART (Table 1). However, it can be seen that low levels of MTX-PG2, MTX-PG3, and saturating levels of MTX-PG1 (Fig. 3) correspond to increased levels of ZMP at the 10 nM MTX concentration (Fig. 5A). This observation suggests that short-chain polyglutamates or saturation of high-affinity binding sites (i.e., DHFR) by MTX-PG1 contributes to the accumulation of ZMP. The former would be in accordance with recent findings that clinical response in rheumatoid arthritis correlates with erythrocyte levels of MTX-PG2 (Hobl et al., 2012). Although the relationship between ZMP accumulation and therapeutic response remains to be established, the finding that ZMP accumulates without significant MTX polyglutamate formation suggests that efforts to measure cellular MTX polyglutamates as a predictor of therapeutic response may not be predictive of ZMP accumulation.
Selective inhibition of AICART by MTX or its short-chain polyglutamates is not supported by the reported inhibition constants (Ki) for these compounds (Table 2). Specifically, MTX-PG1, MTX-PG2, and MTX-PG3 are 11-, 32-, and 4-fold more potent inhibitors of TS than AICART, and therefore, low concentrations of MTX would be expected to have an observable effect on dUMP accumulation prior to any detectable effect on ZMP. In contrast, DHF-PG5 is reported to be 12-fold more potent as an inhibitor of AICART compared with TS, supporting DHF as being responsible for the observed concentration-dependent selectivity of ZMP accumulation at low MTX concentrations (Table 3). The observed accumulation of DHF, predominantly in the form of DHF-PG6, following exposure to low MTX concentrations (Fig. 8) would support the hypothesized role of DHF polyglutamates in the selective inhibition of AICART (Fig. 7).
TABLE 2.
Inhibition constants (Ki) of enzymes involved in nucleotide biosynthesis for MTX and its polyglutamates (Allegra et al., 1985a,b; Drake et al., 1987; Baram et al., 1988; Sant et al., 1992)
| Ki | MTX-PG1 | MTX-PG2 | MTX-PG3 | MTX-PG4 | MTX-PG5 |
|---|---|---|---|---|---|
| µM | |||||
| DHFR | 11.0 × 10−5 | 17.0 × 10−5 | 8.0 × 10−5 | 8.0 × 10−5 | |
| TS | 13.0 | 0.17 | 0.14 | 0.13 | 0.047 |
| AICART | 143.9 | 5.47 | 0.56 | 0.056 | 0.057 |
| GART | 80 | 57 | 7.1 | 5.1 | 2.5 |
| ATase | ND | 550 | |||
ND, no inhibition was detected.
TABLE 3.
Inhibition constants (Ki) of enzymes involved in nucleotide biosynthesis for DHF and DHF-PG5 (Allegra et al., 1985b; Baram et al., 1988; Sant et al., 1992)
| Ki | DHF-PG1 | DHF-PG5 |
|---|---|---|
| μM | ||
| TS | 77.7 | 0.5 |
| AICART | 63.3 | 0.043 |
| GART | 25.3 | 21.9 |
| ATase | 312 | 3.41 |
In contrast to ZMP, the cellular accumulation of dUMP correlates with the cellular accumulation of MTX and its polyglutamated metabolites (Table 1). In agreement with the reported Ki values (Table 2), dUMP accumulation correlates more strongly with MTX polyglutamates than with cellular levels of the parent drug. Given the complexity of a cellular system, it may be possible that TS is directly or indirectly inhibited by MTX and its polyglutamates. However, the strong relationship between polyglutamate formation and dUMP accumulation does provide evidence to support the role of MTX polyglutamates in the direct inhibition of TS. Therefore, in contrast to AICART, clinical measurements of intracellular MTX polyglutamates would be expected to correspond with inhibition of TS and may represent a potential biomarker for inhibition of the pyrimidine synthesis pathway and its downstream effects.
The pronounced accumulation of ZMP despite a minimal increase in dUMP after prolonged exposure to low concentrations of MTX suggests that low drug doses can be used to selectively target the accumulation of the anti-inflammatory mediator without significantly inhibiting TS activity (Fig. 7). Hence, sustained exposure to low levels of MTX may result in improved anti-inflammatory activity without the effects associated with inhibition of pyrimidine biosynthesis. Clinically, these findings would suggest that therapeutic response may also be paradoxically related to dose, with prolonged exposure to lower doses resulting in more favorable response due to the selective accumulation of ZMP in conditions in which this result is sought, such as inflammatory arthritis. Supportive of this concept, stepwise increases in MTX dose in patients with juvenile idiopathic arthritis who were nonresponders to standard low-dose MTX did not result in improved clinical outcomes (Ruperto et al., 2004).
In conclusion, the data presented in this study indicate that increases in external MTX concentration are associated with cellular accumulation of MTX over time, and are accompanied by a shift toward higher-order MTX polyglutamation. As the external MTX concentration increases to approximately 10 nM, ZMP accumulates due to selective inhibition of AICART. As the external MTX concentration increases beyond 20 nM, ZMP accumulation declines, consistent with inhibitory effects of MTX on other cellular targets, including enzymes upstream of AICART in the purine biosynthesis pathway that would restrict ZMP formation as well as inhibition of TS characterized by increased accumulation of its substrate, dUMP, and decreased production of the product of the methylation reaction, DHF. In K562 cells, selective formation of ZMP was optimal at 6 nM, reflecting preferential inhibition of AICART at lower MTX concentrations while preserving TS activity. Increasing MTX beyond this point results in decreased formation of anti-inflammatory species and increased risk of toxicity because of inhibition of other cellular folate-dependent pathways. These data have clinical implications for the use of low-dose MTX as an anti-inflammatory agent. First, the data imply that the relative abundance of ZMP and dUMP may support their use as biomarkers for characterizing the potential for efficacy and toxicity of low-dose MTX. Second, the dose of MTX required to optimize the ZMP/dUMP ratio in individual patients may vary according to their unique genetic constitution and interactions with environmental factors, such as dietary folate and changing biologic demands for folate. Additional work is required to replicate our findings in additional cell lines, and to determine the functional consequence of allelic variation in key folate pathway genes to provide insight into the role of genetic variation in optimization of MTX treatment in patients with inflammatory conditions. An improved understanding of the complex biochemical pharmacology of MTX will prove paramount in the identification and evaluation of therapeutic biomarkers in the future, and may pave the way for individualization of therapy.
Supplementary Material
Abbreviations
- AICART
aminoimidazole carboxamide ribonucleotide transformylase
- ATase
amido phosphoribosyltransferase
- D-PBS
Dulbecco’s phosphate-buffered saline
- DHF
dihydrofolate
- DHFR
dihydrofolate reductase
- dUMP
2′-deoxyuridine 5′-monophosphate disodium salt
- GART
phosphoribosylglycinamide formyltransferase
- Ki
enzyme inhibition constant
- MTX
methotrexate
- MTX-PGn
methotrexate polyglutamate where n represents the number of attached glutamate residues
- MTX-PGtotal
sum total of methotrexate and its polyglutamates
- PA
peak area
- TS
thymidylate synthase
- UPLC/MS/MS
ultra-performance liquid chromatography tandem mass spectrometry
- ZMP
5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate
Authorship Contributions
Participated in research design: Funk, van Haandel, Becker, Leeder.
Conducted experiments: Funk, van Haandel.
Performed data analysis: Funk, van Haandel.
Wrote or contributed to the writing of the manuscript: Funk, van Haandel, Becker, Leeder.
Footnotes
This work was supported in part by the Children’s Mercy Hospital and Clinics; the American College of Rheumatology, Research and Education Foundation Rheumatology Investigator Award; and the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant T32-HD069038].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Allegra CJ, Chabner BA, Drake JC, Lutz R, Rodbard D, Jolivet J. (1985a) Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates. J Biol Chem 260:9720–9726 [PubMed] [Google Scholar]
- Allegra CJ, Drake JC, Jolivet J, Chabner BA. (1985b) Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc Natl Acad Sci USA 82:4881–4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra CJ, Hoang K, Yeh GC, Drake JC, Baram J. (1987) Evidence for direct inhibition of de novo purine synthesis in human MCF-7 breast cells as a principal mode of metabolic inhibition by methotrexate. J Biol Chem 262:13520–13526 [PubMed] [Google Scholar]
- Andersson LC, Nilsson K, Gahmberg CG. (1979) K562–a human erythroleukemic cell line. Int J Cancer 23:143–147 [DOI] [PubMed] [Google Scholar]
- Baggott JE, Morgan SL. (2007) Methotrexate and erythro-9-(2-hydroxynon-3-yl) adenine therapy for rat adjuvant arthritis and the effect of methotrexate on in vivo purine metabolism. Eur J Pharm Sci 31:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott JE, Morgan SL, Ha TS, Alarcón GS, Koopman WJ, Krumdieck CL. (1993) Antifolates in rheumatoid arthritis: a hypothetical mechanism of action. Clin Exp Rheumatol 11 (Suppl 8):S101–S105 [PubMed] [Google Scholar]
- Baggott JE, Morgan SL, Koopman WJ. (1998) The effect of methotrexate and 7-hydroxymethotrexate on rat adjuvant arthritis and on urinary aminoimidazole carboxamide excretion. Arthritis Rheum 41:1407–1410 [DOI] [PubMed] [Google Scholar]
- Baggott JE, Morgan SL, Sams WM, Linden J. (1999) Urinary adenosine and aminoimidazolecarboxamide excretion in methotrexate-treated patients with psoriasis. Arch Dermatol 135:813–817 [DOI] [PubMed] [Google Scholar]
- Baggott JE, Vaughn WH, Hudson BB. (1986) Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5′-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem J 236:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram J, Chabner BA, Drake JC, Fitzhugh AL, Sholar PW, Allegra CJ. (1988) Identification and biochemical properties of 10-formyldihydrofolate, a novel folate found in methotrexate-treated cells. J Biol Chem 263:7105–7111 [PubMed] [Google Scholar]
- Becker ML, Rosé CD, Cron RQ, Sherry DD, Bilker WB, Lautenbach E. (2010) Effectiveness and toxicity of methotrexate in juvenile idiopathic arthritis: comparison of 2 initial dosing regimens. J Rheumatol 37:870–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers A, Organe S, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, Waelkens E, Brusselmans K, Verhoeven G, Swinnen JV. (2006) Methotrexate enhances the antianabolic and antiproliferative effects of 5-aminoimidazole-4-carboxamide riboside. Mol Cancer Ther 5:2211–2217 [DOI] [PubMed] [Google Scholar]
- Borsa J, Whitmore GF. (1969a) Studies relating to the mode of action of methotrexate. 3. Inhibition of thymidylate synthetase in tissue culture cells and in cell-free systems. Mol Pharmacol 5:318–332 [PubMed] [Google Scholar]
- Borsa J, Whitmore GF. (1969b) Studies relating to the mode of action of methotrexate. II. Studies on sites of action in L-cells in vitro. Mol Pharmacol 5:303–317 [PubMed] [Google Scholar]
- Chan ES, Cronstein BN. (2010) Methotrexate—how does it really work? Nat Rev Rheumatol 6:175–178 [DOI] [PubMed] [Google Scholar]
- Chu E, Drake JC, Boarman D, Baram J, Allegra CJ. (1990) Mechanism of thymidylate synthase inhibition by methotrexate in human neoplastic cell lines and normal human myeloid progenitor cells. J Biol Chem 265:8470–8478 [PubMed] [Google Scholar]
- Cronstein BN, Eberle MA, Gruber HE, Levin RI. (1991) Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc Natl Acad Sci USA 88:2441–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Naime D, Ostad E. (1993) The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest 92:2675–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervieux T, Furst D, Lein DO, Capps R, Smith K, Caldwell J, Kremer J. (2005) Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann Rheum Dis 64:1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervieux T, Furst D, Lein DO, Capps R, Smith K, Walsh M, Kremer J. (2004) Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum 50:2766–2774 [DOI] [PubMed] [Google Scholar]
- Dolezalová P, Krijt J, Chládek J, Nemcová D, Hoza J. (2005) Adenosine and methotrexate polyglutamate concentrations in patients with juvenile arthritis. Rheumatology (Oxford) 44:74–79 [DOI] [PubMed] [Google Scholar]
- Drake JC, Allegra CJ, Baram J, Kaufman BT, Chabner BA. (1987) Effects on dihydrofolate reductase of methotrexate metabolites and intracellular folates formed following methotrexate exposure of human breast cancer cells. Biochem Pharmacol 36:2416–2418 [DOI] [PubMed] [Google Scholar]
- Fairbanks LD, Rückemann K, Qiu Y, Hawrylowicz CM, Richards DF, Swaminathan R, Kirschbaum B, Simmonds HA. (1999) Methotrexate inhibits the first committed step of purine biosynthesis in mitogen-stimulated human T-lymphocytes: a metabolic basis for efficacy in rheumatoid arthritis? Biochem J 342:143–152 [PMC free article] [PubMed] [Google Scholar]
- Fortin PR, Abrahamowicz M, Ferland D, Lacaille D, Smith CD, Zummer M, Canadian Network For Improved Outcomes in Systemic Lupus (2008) Steroid-sparing effects of methotrexate in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 59:1796–1804 [DOI] [PubMed] [Google Scholar]
- Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. (1998) Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest 102:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, Fink CW, Newman AJ, Cassidy JT, Zemel LS, The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children’s Study Group (1992) Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. N Engl J Med 326:1043–1049 [DOI] [PubMed] [Google Scholar]
- Goldman ID. (1974) The mechanism of action of methotrexate. I. Interaction with a low-affinity intracellular site required for maximum inhibition of deoxyribonucleic acid synthesis in L-cell mouse fibroblasts. Mol Pharmacol 10:257–274 [PubMed] [Google Scholar]
- Halilova KI, Brown EE, Morgan SL, Bridges SL, Jr, Hwang MH, Arnett DK, Danila MI. (2012) Markers of treatment response to methotrexate in rheumatoid arthritis: where do we stand? Int J Rheumatol 2012:978396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobl EL, Jilma B, Erlacher L, Duhm B, Mustak M, Bröll H, Högger P, Rizovski B, Mader RM. (2012) A short-chain methotrexate polyglutamate as outcome parameter in rheumatoid arthritis patients receiving methotrexate. Clin Exp Rheumatol 30:156–163 [PubMed] [Google Scholar]
- Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H. (2005) The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol 114:154–163 [DOI] [PubMed] [Google Scholar]
- Jolivet J, Schilsky RL, Bailey BD, Drake JC, Chabner BA. (1982) Synthesis, retention, and biological activity of methotrexate polyglutamates in cultured human breast cancer cells. J Clin Invest 70:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb RE, Strober B, Weinstein G, Lebwohl M. (2009) Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol 60:824–837 [DOI] [PubMed] [Google Scholar]
- Katerelos M, Mudge SJ, Stapleton D, Auwardt RB, Fraser SA, Chen CG, Kemp BE, Power DA. (2010) 5-aminoimidazole-4-carboxamide ribonucleoside and AMP-activated protein kinase inhibit signalling through NF-κB. Immunol Cell Biol 88:754–760 [DOI] [PubMed] [Google Scholar]
- Koizumi S. (1988) Impairment of methotrexate (MTX)-polyglutamate formation of MTX-resistant K562 cell lines. Jpn J Cancer Res 79:1230–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JM, Galivan J, Streckfuss A, Kamen B. (1986) Methotrexate metabolism analysis in blood and liver of rheumatoid arthritis patients. Association with hepatic folate deficiency and formation of polyglutamates. Arthritis Rheum 29:832–835 [DOI] [PubMed] [Google Scholar]
- Lambert CM, Sandhu S, Lochhead A, Hurst NP, McRorie E, Dhillon V. (2004) Dose escalation of parenteral methotrexate in active rheumatoid arthritis that has been unresponsive to conventional doses of methotrexate: a randomized, controlled trial. Arthritis Rheum 50:364–371 [DOI] [PubMed] [Google Scholar]
- Lyons SD, Christopherson RI. (1991) Antifolates induce primary inhibition of the de novo purine pathway prior to 5-aminoimidazole-4-carboxamide ribotide transformylase in leukemia cells. Biochem Int 24:187–197 [PubMed] [Google Scholar]
- Matherly LH, Czajkowski CA, Angeles SM. (1991) Identification of a highly glycosylated methotrexate membrane carrier in K562 human erythroleukemia cells up-regulated for tetrahydrofolate cofactor and methotrexate transport. Cancer Res 51:3420–3426 [PubMed] [Google Scholar]
- Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, Huie MA, Cronstein BN. (1998) Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest 101:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SL, Oster RA, Lee JY, Alarcón GS, Baggott JE. (2004) The effect of folic acid and folinic acid supplements on purine metabolism in methotrexate-treated rheumatoid arthritis. Arthritis Rheum 50:3104–3111 [DOI] [PubMed] [Google Scholar]
- Pinedo HM, Zaharko DS, Bull JM, Chabner BA. (1976) The reversal of methotrexate cytotoxicity to mouse bone marrow cells by leucovorin and nucleosides. Cancer Res 36:4418–4424 [PubMed] [Google Scholar]
- Ruperto N, Murray KJ, Gerloni V, Wulffraat N, de Oliveira SK, Falcini F, Dolezalova P, Alessio M, Burgos-Vargas R, Corona F, et al. Pediatric Rheumatology International Trials Organization (2004) A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum 50:2191–2201 [DOI] [PubMed] [Google Scholar]
- Sant ME, Lyons SD, Phillips L, Christopherson RI. (1992) Antifolates induce inhibition of amido phosphoribosyltransferase in leukemia cells. J Biol Chem 267:11038–11045 [PubMed] [Google Scholar]
- Smoleńska Z, Kaznowska Z, Zarówny D, Simmonds HA, Smoleński RT. (1999) Effect of methotrexate on blood purine and pyrimidine levels in patients with rheumatoid arthritis. Rheumatology (Oxford) 38:997–1002 [DOI] [PubMed] [Google Scholar]
- Taisun H, Baggott JE. (1994) 5-aminoimidazole-4-carboxamide ribotide (AICAR) and its metabolites: metabolic and cytotoxic effects and accumulation during methotrexate treatment. J Nutr Biochem 5:522–528 [Google Scholar]
- Urakawa K, Mihara M, Suzuki T, Kawamura A, Akamatsu K, Takeda Y, Kamatani N. (2000) Polyglutamation of antifolates is not required for induction of extracellular release of adenosine or expression of their anti-inflammatory effects. Immunopharmacology 48:137–144 [DOI] [PubMed] [Google Scholar]
- van Haandel L, Becker ML, Leeder JS, Williams TD, Stobaugh JF. (2009) A novel high-performance liquid chromatography/mass spectrometry method for improved selective and sensitive measurement of methotrexate polyglutamation status in human red blood cells. Rapid Commun Mass Spectrom 23:3693–3702 [DOI] [PubMed] [Google Scholar]
- van Haandel L, Becker ML, Williams TD, Leeder JS, Stobaugh JF. (2011) Measurement of methotrexate polyglutamates in human erythrocytes by ion-pair UPLC-MS/MS. Bioanalysis 3:2783–2796 [DOI] [PubMed] [Google Scholar]
- van Haandel L, Becker ML, Williams TD, Stobaugh JF, Leeder JS. (2012) Comprehensive quantitative measurement of folate polyglutamates in human erythrocytes by ion pairing ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 26:1617–1630 [DOI] [PubMed] [Google Scholar]
- Weinblatt ME. (1985) Toxicity of low dose methotrexate in rheumatoid arthritis. J Rheumatol Suppl 12 (Suppl 12):35–39 [PubMed] [Google Scholar]
- Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, Trentham DE. (1985) Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 312:818–822 [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Kaplan H, Germain BF, Block S, Solomon SD, Merriman RC, Wolfe F, Wall B, Anderson L, Gall E, et al. (1994) Methotrexate in rheumatoid arthritis. A five-year prospective multicenter study. Arthritis Rheum 37:1492–1498 [DOI] [PubMed] [Google Scholar]
- You X, Williams A, Dervieux T, He W, Cronstein BN. (2013) Fibroblasts from methotrexate-sensitive mice accumulate methotrexate polyglutamates but those from methotrexate-resistant mice do not. Clin Exp Rheumatol 31:433–435 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.