Abstract
A previous study showed that cocaine self-administration induced dopamine-independent reinforcing effects of σ agonists mediated by their selective actions at σ1 receptors (σ1Rs), which are intracellularly mobile chaperone proteins implicated in abuse-related effects of stimulants. The present study assessed whether the induction was specific to self-administration of cocaine. Rats were trained to self-administer the dopamine releaser, d-methamphetamine (0.01–0.32 mg/kg per injection), the μ-opioid receptor agonist, heroin (0.001–0.032 mg/kg per injection), and the noncompetitive N-methyl-d-aspartate receptor/channel antagonist ketamine (0.032–1.0 mg/kg per injection). As with cocaine, self-administration of d-methamphetamine induced reinforcing effects of the selective σ1R agonists PRE-084 [2-(4-morpholinethyl)1-phenylcyclohexanecarboxylate hydrochloride] and (+)-pentazocine (0.032–1.0 mg/kg per injection, each). In contrast, neither self-administration of heroin nor ketamine induced PRE-084 or (+)-pentazocine (0.032–10 mg/kg per injection, each) self-administration. Although the σ1R agonists did not maintain responding in subjects with histories of heroin or ketamine self-administration, substitution for those drugs was obtained with appropriate agonists (e.g., remifentanil, 0.1–3.2 µg/kg per injection, for heroin and (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine ((+)-MK 801; dizocilpine), 0.32–10.0 µg/kg per injection, for ketamine). The σR antagonist N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide (BD 1008; 1.0–10 mg/kg) dose-dependently blocked PRE-084 self-administration but was inactive against d-methamphetamine, heroin, and ketamine. In contrast, PRE-084 self-administration was affected neither by the dopamine receptor antagonist (+)-butaclamol (10–100 μg/kg) nor by the opioid antagonist (−)-naltrexone (1.0–10 mg/kg), whereas these antagonists were active against d-methamphetamine and heroin self-administration, respectively. The results indicate that experience specifically with indirect-acting dopamine agonists induces reinforcing effects of previously inactive σ1R agonists. It is further suggested that induced σ1R reinforcing mechanisms may play an essential role in treatment-resistant stimulant abuse, suggesting new approaches for the development of effective medications for its treatment.
Introduction
σ1 receptors (σ1Rs) are intracellular chaperone proteins that are expressed widely in the central and peripheral nervous systems (Hayashi and Su, 2007; Maurice and Su, 2009). With agonist actions or cellular stress, σ1Rs translocate from their primary endoplasmic reticulum localization to different subcellular compartments and can regulate ion channels and G-protein-coupled–receptor signaling (Aydar et al., 2002; Cormaci et al., 2007; Hayashi and Su, 2007). The widespread distribution of σ1Rs and their capacity to influence intracellular signaling suggest a substantial role in various physiologic functions. Drugs acting at these receptors have been studied for therapeutic effects in cancer, HIV infection, psychiatric disorders, and substance abuse (Maurice and Su, 2009; Katz et al., 2011).
σ1Rs also are expressed widely in dopaminergic brain regions (Hayashi et al., 2010), and drugs acting at these receptors have been shown to regulate dopaminergic function (e.g., Nuwayhid and Werling, 2003). Furthermore, σ1R antagonists have been shown to block several in vivo effects that are related to abuse and excessive intake of cocaine (McCracken et al., 1999; Romieu et al., 2000, 2002; Matsumoto et al., 2001; Matsumoto, 2009; Katz et al., 2011). However, several studies have demonstrated a lack of effects of a wide range of the selective σ1R antagonists on cocaine self-administration (Martin-Fardon et al., 2007; Hiranita et al., 2010, 2011a, 2013), although selective σ1R antagonists did block cocaine self-administration when combined with standard dopamine uptake inhibitors (Hiranita et al., 2011a). These results suggest a complex involvement of σ1Rs in cocaine self-administration.
Consistent with that potential complex involvement, a recent study demonstrated that experience with cocaine self-administration induced reinforcing effects of σ1R agonists that were absent in drug-naïve subjects or subjects with a history of food reinforcement (Hiranita et al., 2010, 2013). Furthermore, the induced reinforcing effects of the selective σ1R agonist PRE-084 [2-(4-morpholinethyl)1-phenylcyclohexanecarboxylate hydrochloride] were insensitive to pretreatments with dopamine receptor antagonists (Hiranita et al., 2013). In addition, another study found little, if any, cocaine-like discriminative-stimulus effects of PRE-084 in rats (Hiranita et al., 2011b). Although PRE-084 administration did dose-dependently stimulate dopamine levels in the nucleus accumbens shell of rats (Garcés-Ramírez et al., 2011), a brain region involved in the reinforcing effects of cocaine (Pontieri et al., 1995, 1996; Tanda et al., 1997), its potency was roughly 30-fold lower than that of cocaine and stimulation of dopamine was absent at self-administered doses (Garcés-Ramírez et al., 2011).
The present study was designed to address the pharmacological specificity of the experience with cocaine self-administration as an inducer of the reinforcing effects of σ1R agonists. The study by Hiranita et al. (2013) determined that experience with food reinforcement was ineffective as an inducer of σ1R agonist self-administration, despite the experimental conditions of the exposure being similar to those for cocaine. The present study assessed whether the induction of reinforcing effects of selective σ1R agonists was obtained after self-administration of the indirect dopamine agonist d-methamphetamine, the μ-opioid receptor agonist heroin, and the noncompetitive N-methyl-d-aspartate receptor/channel antagonist ketamine in rats, each representing different classes of abused drugs.
Materials and Methods
Drugs.
The following drugs were used in this study: d-methamphetamine hydrochloride (Sigma-Aldrich, St. Louis, MO); (−)-heroin hydrochloride (RTI International, Research Triangle Park, NC); (±)-ketamine hydrochloride (Fort Dodge Animal Health, Fort Dodge, Iowa/Bioniche Pharma, Lake Forest, IL); PRE-084 (Tocris Bioscience, Ballwin, MO); (+)-pentazocine succinate (National Institute on Drug Abuse); d-amphetamine sulfate (Sigma-Aldrich); remifentanil hydrochloride (Ultiva; Hospira, Inc., Lake Forest, IL); (+)-MK 801 hydrogen maleate [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine)] (Sigma-Aldrich); BD 1008 [N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide] (Tocris Bioscience); (+)-butaclamol hydrochloride (Sigma-Aldrich); (−)-naltrexone hydrochloride (Sigma-Aldrich); and haloperidol (used only for the σ2R binding assay; Sigma-Aldrich). Saline (0.9% sodium chloride, USP; Hospira, Inc. was used as vehicle for all compounds. Drugs used were administered intravenously [d-methamphetamine, (−)-heroin, (±)-ketamine, PRE-084, and (+)-pentazocine, remifentanil, (+)-MK 801, BD 1008, (+)-butaclamol, and (−)-naltrexone)] or intraperitoneally [BD 1008, (+)-butaclamol, and (−)-naltrexone]. BD 1008 and (−)-naltrexone were administered 5 minutes before sessions. (+)-Butaclamol was administered 30 minutes before sessions.
σR Binding.
Frozen whole-guinea pig brains (minus cerebellum) were thawed on ice, weighed, and homogenized (with a glass and Teflon homogenizer) in 10 mM Tris-HCl with 0.32 M sucrose, pH 7.4 (10 ml/g tissue). Guinea pig brain was used because of the relatively higher density of those receptors in that tissue compared with rat (Tam, 1983; Nguyen et al., 1996). The homogenate was centrifuged at 1000g for 10 minutes at 4°C. The supernatant was collected into a clean centrifuge tube, and the remaining pellet was resuspended by vortex in 10-ml buffer (tissue) and centrifuged again at 50,000g for 15 minutes at 4°C. The resulting pellet was resuspended in experimental buffer to 80 mg/ml original wet weight (OWW).
Ligand binding experiments were conducted in polypropylene assay tubes containing 0.5 ml of 50 mM Tris-HCl buffer, pH 8.0. For σ1R binding, each tube contained 3 nM [3H](+)-pentazocine (PerkinElmer Life and Analytical Sciences, Waltham, MA) and 8.0-mg tissue OWW. Nonspecific binding was determined using 10 μM haloperidol. For σ2R binding, each tube contained 3 nM [3H]1,3-di-o-tolylguanidine (DTG; PerkinElmer Life and Analytical Sciences), 200 nM (+)-pentazocine, and 8.0-mg tissue OWW. Nonspecific binding was determined using 100 μM haloperidol. The reaction was started with the addition of tissue and the tubes were incubated for 120 minutes at room temperature.
Incubations for all binding assays were terminated by rapid filtration through Whatman GF/B filters, presoaked in polyethylenimine, using a Brandel R48 filtering manifold (Brandel Instruments, Gaithersburg, MD). The filters were washed twice with 5 ml of ice-cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0 ml) was added, and the vials were counted the next day using a Beckman 6000 liquid scintillation counter (Beckman Coulter Instruments, Fullerton, CA) at 50% efficiency. Assays were typically conducted in at least three independent experiments, each performed in triplicate.
For the displacement of radioligand binding, IC50 values were computed using a nonlinear, least-squares regression analysis (Prism; GraphPad Software Inc., San Diego, CA). Affinities (Ki values) were calculated using the concentration of radioligand used in the assay.
Self-Administration Procedures.
Twenty-four male Sprague-Dawley rats (Taconic Farms, Germantown, NY), weighing approximately 300 g at the start of the study, served as subjects. Subjects were acclimated to a temperature- and humidity-controlled vivarium for at least 1 week with food (Scored Bacon Lover Treats; BIOSERV, Frenchtown, NJ) and tap water unrestrictedly available in their home cages under a 12:12-h light/dark cycle with lights on at 7:00 AM. After acclimation, rats were assigned to treatment conditions in a nonsystematic manner and thereafter maintained at approximately 320 g by adjusting their daily food ration. Subjects were surgically implanted under anesthesia (60.0 mg/kg i.p. ketamine and 12.0 mg/kg i.p. xylazine) in the right external jugular vein with a chronic indwelling catheter that exited at the midscapular region of the animal’s back. Catheters were infused daily with 0.1 ml of a sterile saline solution containing heparin (30.0 IU/ml) and penicillin G potassium (250,000 IU/ml) to minimize the likelihood of infection and the formation of clots or fibroids. All animals were allowed to recover from surgery for approximately 7 days before drug self-administration studies were initiated. Care of the subjects was in accordance with the guidelines of the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program Animal Care and Use Program, which is fully accredited by AAALAC International.
Experimental sessions were conducted daily with animals placed in operant-conditioning chambers (modified ENV-203; Med Associates, St. Albans, VT) that measured 25.5 × 32.1 × 25.0 cm and were enclosed within sound-attenuating cubicles equipped with a fan for ventilation and white noise to mask extraneous sounds. On the front wall of each chamber were two response levers, 5.0 cm from the midline and 4.0 cm above the grid floor. A downward displacement of a lever with a force approximating 20g defined a response and always activated a relay mounted behind the front wall of the chamber producing an audible “feedback” click. Three light-emitting diodes (LEDs) were located in a row above each lever. A receptacle for the delivery of 45-mg food pellets via a pellet dispenser (model ENV-203-20; Med Associates) was mounted on the midline of the front wall between the two levers and 2.0 cm above the floor but was unused in this study. A syringe infusion pump (model 22; Harvard Apparatus, Holliston, MA) placed above each chamber delivered injections of specified volumes from a 10-ml syringe. The syringe was connected by Tygon tubing to a single-channel fluid swivel (375 series single-channel swivels; Tygon, Plymouth Meeting, PA) that was mounted on a balance arm above the chamber. Tygon tubing from the swivel to the subject’s catheter was protected by a surrounding metal spring and completed the connection to the subject.
Experimental sessions started with the illumination of the LEDs above each lever and initially lasted for 120 minutes, during which saline (n = 6), d-methamphetamine (0.1 mg/kg per injection, n = 6), (−)-heroin (0.01 mg/kg per injection, n = 6), or (±)-ketamine (0.32 mg/kg per injection, n = 6) was delivered after responses. Each response on the right lever turned off the LEDs, produced an audible click, and activated the infusion pump for 10 seconds [fixed ratio (FR) or FR1 schedule] followed by a 20-second time-out (TO) period, during which LEDs were off and responding had no scheduled consequences. After the TO, the LEDs were illuminated and responding again had the scheduled consequences. Responses on the left lever were recorded but had no scheduled consequences. This condition remained in effect over all of the sessions. One group was studied with self-administration of saline injections only, for 14 sessions. For the d-methamphetamine, (−)-heroin, or (±)-ketamine self-administration groups, subjects were given an opportunity to respond for injections of the respective drugs for 14 sessions to assess reinforcing effects. On the 15th session, PRE-084 (0.32 mg/kg per injection) was substituted for the initially self-administered drug for 10 sessions. Subsequently, saline was substituted for PRE-084 for seven sessions. Subjects were returned to their home cages in the vivarium after each session.
The self-administration procedure was then modified for assessments of a range of self-administered doses to ensure that the outcomes of the single-dose assessments were generalizable over a range of doses, and to more fully characterize the pharmacological mechanisms of the substitution with PRE-084. Subjects were trained to self-administer d-methamphetamine (0.1 mg/kg per injection), (−)-heroin (0.01 mg/kg per injection), or (±)-ketamine (0.32 mg/kg per injection) under an FR 5 schedule of reinforcement until self-administration was consistent from one session to the next. The session was then divided into five 20-minute components, each preceded by a 2-minute TO period. This arrangement allowed the assessment of a range of self-administered doses in a single session (Hiranita et al., 2009). By adjusting infusion volumes and durations, the drug dose per injection was incremented in the five sequential components in an ascending dose order as follows: no injection (also referred to as extinction because responses had no scheduled consequences), 0.01, 0.03, 0.10, and 0.32 mg/kg per injection for d-methamphetamine; no injection, 0.001, 0.003, 0.01, and 0.032 mg/kg per injection for (−)-heroin; and no injection, 0.03, 0.10, 0.32, and 1.0 mg/kg per injection for (±)-ketamine. Infusion volumes (and durations) were respectively 0 μl (0 seconds), 5.6 μl (0.32 seconds), 18.0 μl (1.0 seconds), 56.0 μl (3.2 seconds), and 180 μl (10.0 seconds) based on a body weight of 0.32 kg. A sample injection of the drug at the corresponding dose occurred independently of responding at the end of the TO period that preceded each component except the first.
Training continued until response rates across three consecutive sessions differed by less than 20%. With these stable performances various compounds were substituted for each of the drugs to assess their self-administration. The compounds substituted for each of the self-administered drugs were as follows: PRE-084 and (+)-pentazocine, each across a low (0.032, 0.1, 0.32, and 1.0 mg/kg per injection) and high (0.32, 1.0, 3.2, and 10 mg/kg per injection) range of doses; and the antagonists, BD 1008 (0.03, 0.10, 0.32, and 1.0 mg/kg per injection), (+)-butaclamol (0.0001, 0.00032, 0.001, and 0.0032 mg/kg per injection), (−)-naltrexone (0.03, 0.10, 0.32, and 1.0 mg/kg per injection), and saline. In addition, the indirect-acting dopamine agonist d-amphetamine (0.01, 0.03, 0.1, and 0.32 mg/kg per injection) was substituted for d-methamphetamine, the μ-opioid receptor agonist remifentanil (0.0001, 0.00032, 0.001, and 0.0032 mg/kg per injection) was substituted for (−)-heroin, and the noncompetitive N-methyl-d-aspartate receptor/channel antagonist (+)-MK 801 (0.00032, 0.001, 0.0032, and 0.01 mg/kg per injection) was substituted for (±)-ketamine. Due to high rates of remifentanil self-administration (Panlilio and Schindler, 2000; Hutchinson et al., 2012), infusion durations of remifentanil were reduced to 0, 0.24, 0.75, 2.4, and 7.5 seconds to avoid excessive fluid intake and emptying of the syringe.
Presession injections (intraperitoneal) of the nonselective σR antagonist BD 1008, the nonselective dopamine receptor antagonist (+)-butaclamol, or the nonselective opioid receptor antagonist (−)-naltrexone, were assessed. These antagonists also were examined in the rats trained with d-methamphetamine when PRE-084 (0.03, 0.10, 0.32, and 1.0 mg/kg per injection) was substituted under otherwise identical conditions. The effects of presession treatments on respective drug self-administration were separated by a minimum of 72 hours. The antagonists were studied with a mixed order of drugs and doses.
Response rates were determined by dividing responses by elapsed time, excluding all TO periods. The significance of effects on response rates was assessed by analysis of variance (ANOVA), with post hoc Bonferroni t tests. A two-way repeated-measures ANOVA was used to assess the effects of successive response rates during drug self-administration (factors were session number and lever: right or left). A one-way repeated-measures ANOVA was used to assess the effects of drug substitution on successive response rates during drug self-administration (for data shown in Fig. 2). A two-way repeated-measures ANOVA was also used to assess effects of presession treatment with antagonists on self-administration with drug dose and component, no injection or drug pretreatment dose as factors (for data shown in Figs. 3 and 4). Presentation of the entire outcomes of the statistical analyses of results shown in Figs. 2–4 and their corresponding post hoc tests was judged to be cumbrous and impractical. Therefore, the present text only indicates for those experiments, which outcomes were significant at P < 0.05. Complete results of statistical analyses are available in Supplemental Tables 1 and 2.
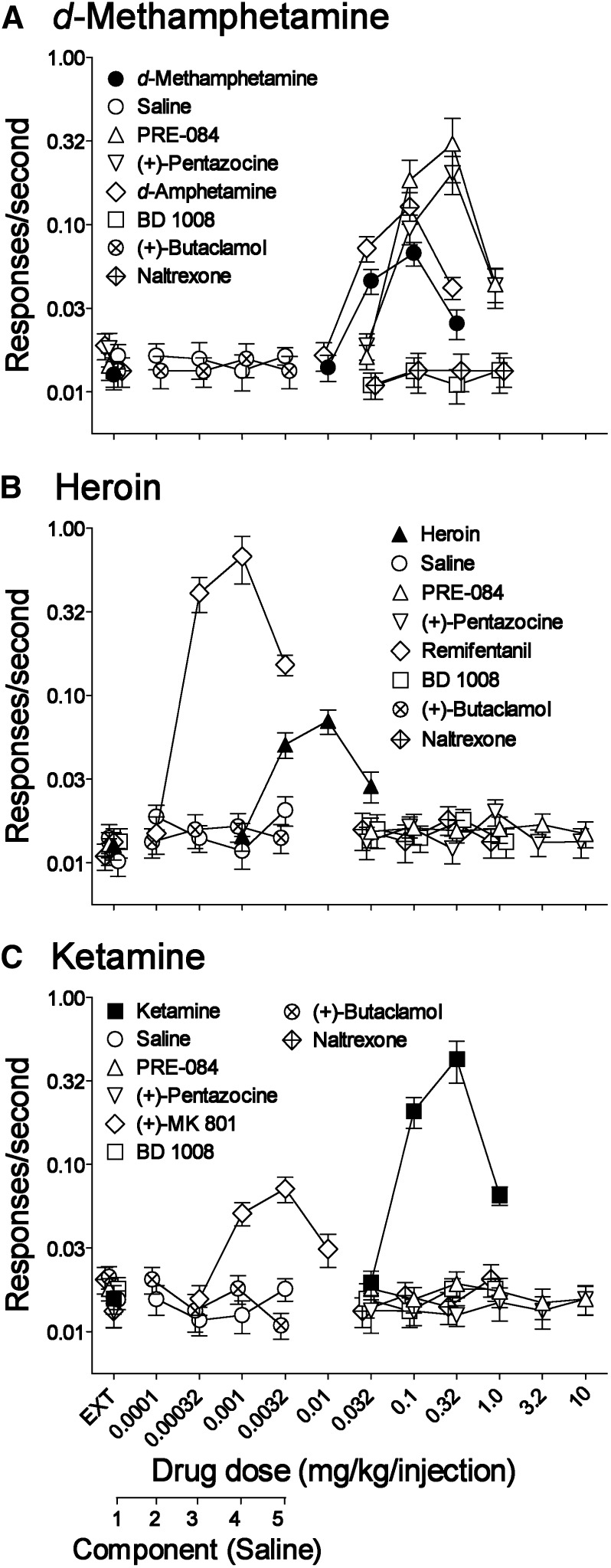
Fig. 2.
Substitution of various compounds in rats trained to self-administer either d-methamphetamine, heroin, or ketamine. Ordinates: responses per second, log scale; abscissae: drug dose (mg/kg per injection), log scale, or sequential component of the session (for saline). Each point represents the mean ± S.E.M. of six subjects. (A) Dose effects of various drugs in subjects trained to self-administer d-methamphetamine (●). Each dose-effect curve was determined only once except d-methamphetamine (shown as an average of 28 assessments) and PRE-084 (shown as an average of three assessments). Consecutive components with saline injections (○) did not maintain rates of responding greater than those obtained in the first component when response did not produce injections (EXT). (B) Dose effects of various drugs in subjects trained to self-administer heroin (▴). Each dose-effect curve was determined only once except heroin (an average of 22 assessments). Consecutive components with saline injections (○) did not maintain rates of responding greater than those obtained in the first component when response did not produce injections (EXT). For PRE-084 and (+)-pentazocine, their dose effects were assessed across two overlapping ranges of doses (0.032–1.0 and 0.32–10 mg/kg per injection) and their dose-effect curves are shown as a combination of the two determinations with an average of rates of responding maintained by no injections (EXT), or 0.32 or 1.0 mg/kg per injection of each drug. (C) Dose effects of various drugs in subjects trained to self-administer ketamine (▪). Consecutive components with saline injections (○) did not maintain rates of responding greater than those obtained in the first component when response did not produce injections (EXT). Each dose-effect curve was determined only once except ketamine (shown as an average of 27 assessments). For PRE-084 and (+)-pentazocine, dose effects were assessed with two overlapping but different ranges of doses (0.032–1.0 and 0.32–10 mg/kg per injection) and the dose-effect curves for these drugs are shown as described above. Complete results of statistical analyses are provided in Supplemental Tables 1 and 2. EXT, extinction.
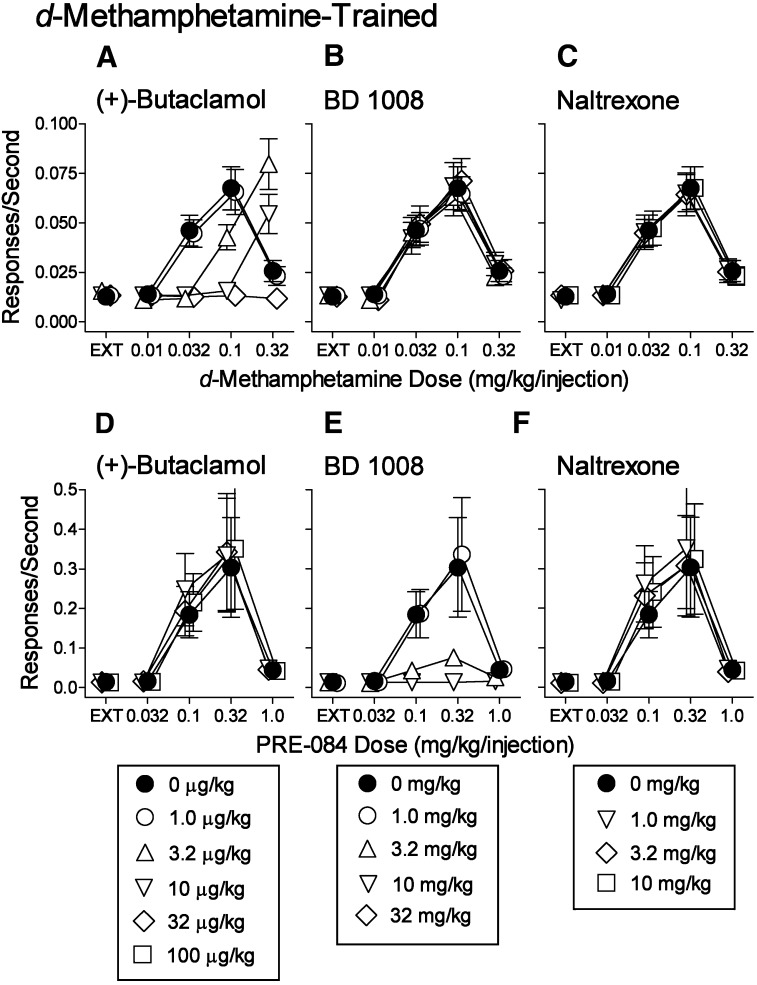
Fig. 3.
Effects of presession treatments with selective antagonists on self-administration of d-methamphetamine or PRE-084. Ordinates: responses per second; abscissae: drug dose (mg/kg per injection), log scale. Each point represents the mean ± S.E.M. of response rates on the active lever in six subjects. Injections of (+)-butaclamol were administered intraperitoneally 30 minutes before sessions. BD 1008 and naltrexone were administered intraperitoneally 5 minutes before sessions. Points labeled 0 mg/kg of the antagonist indicate vehicle pretreatments. (A–C) Effects of the antagonists on d-methamphetamine self-administration. (D–F) Effects of the antagonists on PRE-084 self-administration. Complete results of statistical analyses are provided in Supplemental Tables 1 and 2. EXT, extinction.
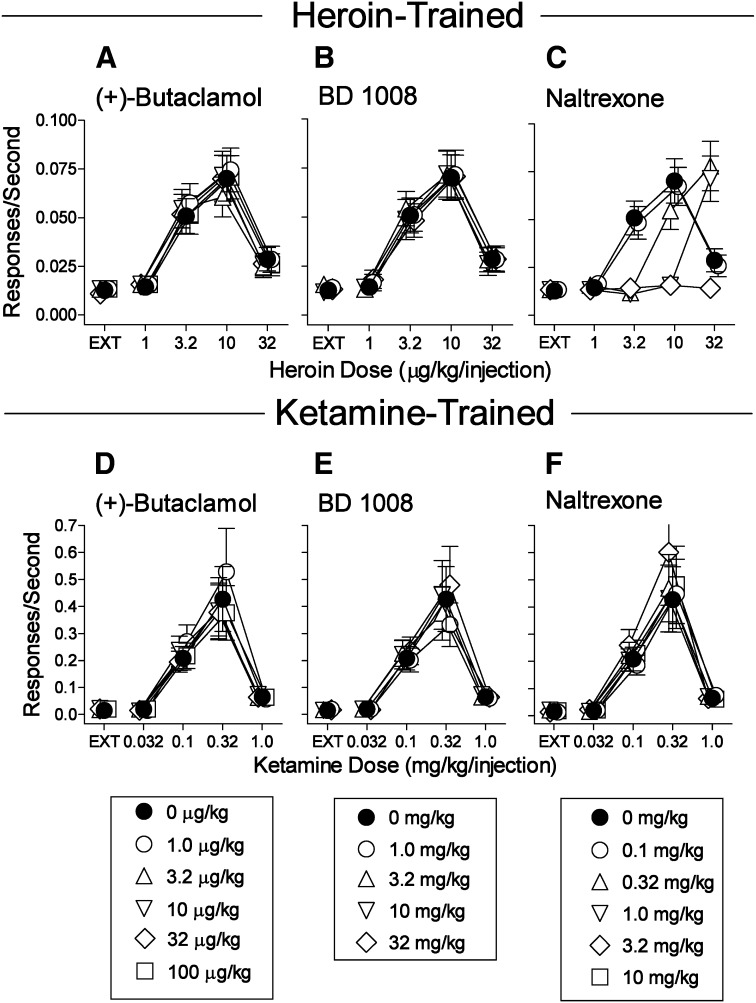
Fig. 4.
Effects of presession treatments with selective antagonists on self-administration of heroin or ketamine. Ordinates: responses per second; abscissae: drug dose (μg/kg per injection or mg/kg per injection, respectively), log scale. Each point represents the mean ± S.E.M. of response rates on the active lever in six subjects. Injections of (+)-butaclamol were administered intraperitoneally 30 minutes before sessions. BD 1008 and naltrexone were administered intraperitoneally 5 minutes before sessions. Points labeled 0 mg/kg of the antagonist indicate vehicle pretreatments. (A–C) Effects of the antagonists on heroin self-administration. (D–F) Effects of the antagonists on ketamine self-administration. Complete results of statistical analyses are provided in Supplemental Tables 1 and 2. EXT, extinction.
The ED50 values for the effects of antagonist pretreatments were calculated by linear regression (Snedecor and Cochran, 1967) of the response rates on dose of the antagonist, using rates from the dose of the self-administered drug that maintained maximal response rates after a saline pretreatment. From this analysis, ED50 values and their 95% confidence limits (95% Cls) were derived.
Results
Response rates on the active (right) lever remained infrequent over a series of 14 daily 2-hour sessions in drug-naïve rats (Fig. 1A) when each response on the active lever produced an injection of saline (an FR1 response schedule of reinforcement). In addition, responses on the alternate (left) lever that had no scheduled consequences remained infrequent. A two-way repeated-measures ANOVA (lever × sessions) indicated a nonsignificant effect of session number (F13,65 = 1.38; P = 0.193), lever position (F1,65 = 0.112; P = 0.752), and their interaction (F13,65 = 0.746; P = 0.711).
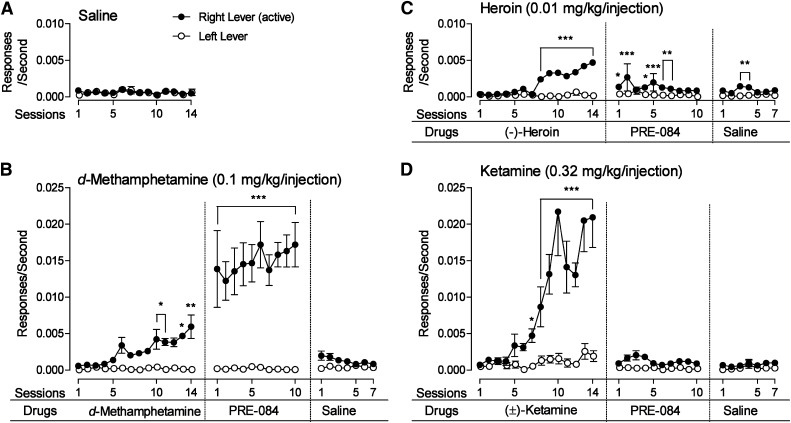
Fig. 1.
A specific induction of the reinforcing effects of the selective σ1R agonist PRE-084 in rats with a history of d-methamphetamine self-administration. Ordinates: responses per second; abscissae: sessions. Each point represents the mean ± S.E.M. of six subjects. Significant differences between response rates on the active versus inactive lever are shown as follows: *P < 0.05; **P < 0.01; ***P < 0.001, compared with responding on the inactive lever. (A) Lack of self-administration with saline injections. (B) Substitution of PRE-084 (0.32 mg/kg per injection) after self-administration of d-methamphetamine (0.1 mg/kg per injection) and absence of self-administration of saline injections. (C) Lack of substitution of PRE-084 (0.32 mg/kg per injection) after self-administration of heroin (0.01 mg/kg per injection) and absence of self-administration of saline injections. (D) Lack of substitution of PRE-084 (0.32 mg/kg per injection) after self-administration of ketamine (0.32 mg/kg per injection) and absence of self-administration of saline injections.
Responses on the active lever that produced d-methamphetamine injections (0.1 mg/kg per injection) increased in frequency (Fig. 1B) over the 14 sessions. In contrast, responses on the alternate (left) lever remained infrequent. Starting on session 15, when PRE-084 (0.32 mg/kg per injection) was substituted for d-methamphetamine, response rates immediately increased above those maintained by d-methamphetamine and further increased in frequency over 10 daily sessions (Fig. 1B). When saline was substituted for PRE-084 (for the next seven sessions), response rates decreased to low levels (Fig. 1B). A two-way repeated-measures ANOVA (lever × sessions) indicated a significant effect of session number (F30,150 = 14.3; P < 0.001), lever (F1,150 = 50.5; P < 0.001), and their interaction (F30,150 = 15.1; P < 0.001).
As with d-methamphetamine, responses on the active lever, which produced heroin injections (0.01 mg/kg per injection) increased in frequency over the first 14 sessions and those on the alternate (left) lever that had no scheduled consequences remained infrequent (Fig. 1C). In contrast to results with d-methamphetamine, response rates decreased to low levels when PRE-084 was substituted for heroin (Fig. 1C). Those levels were similar to levels obtained when saline was subsequently substituted for PRE-084 (Fig. 1C). A two-way repeated-measures ANOVA (lever × sessions) indicated a significant effect of session number (F30,150 = 6.84; P < 0.001), lever (F1,150 = 72.5; P < 0.001), and their interaction (F30,150 = 7.47; P < 0.001).
Responses on the active lever that produced ketamine injections (0.32 mg/kg per injection) dramatically increased in frequency over the 14 sessions of its initial availability (Fig. 1D). In contrast, responses on the alternate (left) lever that had no scheduled consequences remained infrequent. As with heroin, response rates decreased to low levels when PRE-084 was substituted for ketamine (Fig. 1D), and remained infrequent when saline was substituted for PRE-084 (Fig. 1D). A two-way repeated-measures ANOVA (lever × sessions) indicated a significant effect of session number (F30,150 = 17.2; P < 0.001), lever (F1,150 = 22.8; P = 0.005), and their interaction (F30,150 = 15.2; P < 0.001).
The dose-related effects of the compounds, substitutions of other compounds, and antagonist pretreatments were subsequently assessed after the schedule was modified to the FR5 schedule with separate components for evaluating a range of doses of the self-administered drugs. As expected, low to intermediate doses of d-methamphetamine (0.01–0.1 mg/kg per injection), heroin (0.001–0.01 mg/kg per injection), and ketamine (0.032–0.32 mg/kg per injection) produced dose-related increases in rates of responding (Fig. 2, A–C, filled symbols). Maximal self-administration of d-methamphetamine, heroin, and ketamine was obtained at doses of 0.1, 0.01, and 0.32 mg/kg per injection, respectively, with responding at those doses significantly greater than that obtained with saline injections.
For the subjects trained with d-methamphetamine, PRE-084 maintained self-administration across a range of doses, with maximal rates at the 0.32 mg/kg per injection dose greater than those maintained by saline and d-methamphetamine (Fig. 2A, upward triangles). In addition, the selective σ1R agonist, (+)-pentazocine, maintained response rates statistically greater than those with saline and greater than those maintained by d-methamphetamine across a similar range of doses (Fig. 2A, downward triangles). The indirect-acting dopamine agonist d-amphetamine substituted at rates greater than saline and with a potency similar to that of d-methamphetamine but with greater maximal effects (Fig. 2A, open diamonds). In contrast to the other compounds, responding occurred infrequently when saline was available for injection and rates of responding with the antagonists, BD 1008, (+)-butaclamol, or naltrexone, were typically not different from those with saline (Fig. 2A). The exception was 0.32 mg/kg per injection of BD 1008, at which response rates were slightly, but significantly, lower than those maintained by saline.
For the subjects trained with heroin, neither PRE-084 nor (+)-pentazocine maintained self-administration at levels appreciably greater than those obtained with saline (Fig. 2B), although a small (0.005 response/s) statistical increase above saline response rates was obtained at 3.2 mg/kg per injection of PRE-084 that was well below rates of responding maintained by remifentanil or heroin (Fig. 2B). With (+)-pentazocine small (≤0.009 responses/s) statistical increases above saline response rates were obtained at 0.1 and 1.0 mg/kg per injection that were also substantially less than those obtained with remifentanil and heroin. As expected, remifentanil was approximately 10-fold more potent than heroin and maintained the highest rates of responding among all of the compounds tested (Fig. 2B, compare filled symbols with open diamonds). As with subjects trained with d-methamphetamine, occasional small statistical increases (≤0.006 responses/s) in responding were obtained with a few doses of the antagonists, but these drugs did not maintain rates of responding comparable with those maintained by heroin or remifentanil (Fig. 2B).
Neither PRE-084 nor (+)-pentazocine maintained self-administration at levels comparable with those maintained with ketamine (Fig. 2C), although small statistical increases were obtained with 0.32 mg/kg per injection and 1.0 mg/kg per injection. In contrast, a biphasic dose-related and robust substitution for ketamine was obtained with (+)-MK 801 that was statistically greater than that obtained with saline (Fig. 2C). (+)-MK 801 was approximately 100-fold more potent than ketamine although the maximal rates of responding maintained by (+)-MK 801 were substantially lower than those maintained by ketamine (Fig. 2C). As with the other training drugs, the specific antagonists, BD 1008, (+)-butaclamol, and naltrexone, produced occasional small statistical increases (≤0.006 responses/s) in responding compared with those with saline, but response rates were uniformly low and approximated those obtained with saline (Fig. 2C).
It is currently unclear how much experience (in cumulative intake or time) is necessary or sufficient for the induction of reinforcing effects of selective σ1R agonists; consequently, the above-described negative outcomes might be altered by extended exposure to the primary self-administered drug. Therefore, the effects of PRE-084 substitutions were assessed again after the experiments shown in Fig. 3. For the subjects with a d-methamphetamine history, the dose-effect curve for the initially effective PRE-084 was shifted upward on its second assessment (after a total intake of about 165 mg/kg of d-methamphetamine) with maximal rates of responding maintained by PRE-084 increasing by a factor of 2.10 (Table 1). During the same period of time, there was no appreciable change in the maximal self-administration of d-methamphetamine (Table 1). Further exposure to d-methamphetamine, however, did not produce further change in the self-administration of PRE-084 (Table 1). In contrast, a greater than 6-fold increase in heroin or approximate 4.5-fold increase in ketamine intake did not induce self-administration of PRE-084 (Table 1).
TABLE 1.
Effects of extended history of drug self-administration on the induction of reinforcing effects of the σ1R agonist, PRE-084
| Training Drug | Cumulative Intake of the Training Drug | Maximal Response Rate Maintained by PRE-084 | Maximal Response Rate Maintained by the Training Drug |
|---|---|---|---|
| mg/kg | responses/sec at mg/kg per injection | ||
| d-Methamphetamine | 64.7 ± 6.75 | 0.184 ± 0.056 at 0.32 | 0.086 ± 0.015 at 0.10 |
| 163 ± 21.3 | 0.388 ± 0.177 at 0.32 | 0.068 ± 0.011 at 0.10 | |
| 243 ± 34.0 | 0.339 ± 0.145 at 0.32 | 0.068 ± 0.011 at 0.10 | |
| Heroin | 3.73 ± 0.292 | 0.021 ± 0.004 at 1.0 | 0.064 ± 0.012 at 0.01 |
| 23.7 ± 3.45 | 0.018 ± 0.004 at 3.2 | 0.070 ± 0.012 at 0.01 | |
| Ketamine | 367 ± 30.7 | 0.028 ± 0.007 at 0.32 | 0.376 ± 0.100 at 0.32 |
| 1630 ± 141 | 0.023 ± 0.004 at 0.32 | 0.427 ± 0.120 at 0.32 | |
A previous study indicated that the self-administration of PRE-084 was blocked by σR antagonists, but insensitive to dopamine receptor antagonists (Hiranita et al., 2013). Thus, the performances maintained by the various drugs were further compared with regard to their sensitivity to pharmacological antagonism. As expected, d-methamphetamine self-administration was dose-dependently blocked by the dopamine antagonist, (+)-butaclamol (Fig. 3A), with an ED50 value of 4.38 µg/kg (95% Cl 3.98–5.17). (+)-Butaclamol was used because it has low affinity for σRs (Table 2) compared with literature values of other DA antagonists (Andersen, 1988; Bristow et al., 1998). In contrast, neither the σR antagonist, BD 1008, nor the opioid antagonist, naltrexone, altered the self-administration of d-methamphetamine (Fig. 3, B and C).
TABLE 2.
Inhibition of the binding of radioligands labeling σ1 and σ2 receptors
Values in parentheses are 95% confidence limits.
| Compound | σ1R Ki Value Determined with [3H]Pentazocine | σ2R Ki Value Determined with [3H]DTG |
|---|---|---|
| nM | ||
| PRE-084 | 48.5 (39.8–59.2) | 12,700 (9290–17,300) |
| (+)-Pentazocine | 4.59 (4.26–4.97) | 224 (195–257) |
| d-Methamphetamine | 4390 (3740–5160) | 15,900 (11,700–21,500) |
| Heroin | N.D. | 68,600 (27,100–173,000) |
| Ketamine | 227,000 (53,400–963,000) | 36,900 (20,100–67,900) |
| (+)-Butaclamol | 4700 (4270–5170) | 2100 (1770–2498) |
| Naltrexone | N.D. | <50% displacement at 10 mM |
N.D., no displacement at concentrations up to 10 mM.
Compared with d-methamphetamine, the self-administration of PRE-084 was not appreciably altered (Fig. 3D) at doses of (+)-butaclamol that antagonized d-methamphetamine self-administration. Small statistical changes in rates of responding maintained by PRE-084 were obtained at a few doses of (+)-butaclamol that decreased d-methamphetamine self-administration. In contrast, PRE-084 self-administration was dose dependently blocked by BD 1008 (Fig. 3E) with an ED50 value of 3.45 mg/kg (95% Cl 2.68–4.52). BD 1008 was used because it is a σR antagonist with comparable affinity for both subtypes of σRs (Table 2). Neither (+)-butaclamol nor BD 1008 produced any appreciable shifts in the self-administration dose-effect curves for heroin or ketamine (Fig. 4, A–E), although small statistical decreases in rates compared with vehicle treatment were produced by 3.2 µg/kg (+)-butaclamol against 10.0 µg/kg per injection of heroin and 32.0 and 100.0 µg/kg (+)-butaclamol against 0.32 mg/kg per injection of ketamine compared with vehicle treatment. As expected, self-administration of heroin, but none of the other self-administered drugs, was blocked by naltrexone pretreatment (Figs. 3, C and F, and 4, C and F) with an ED50 value of 0.514 mg/kg (95% Cl 0.416–0.669).
Discussion
The results of the present study indicate that experience with d-methamphetamine self-administration induces σ1R-mediated reinforcing effects. Furthermore, comparable histories of either heroin or ketamine self-administration were insufficient as inducers of σ1R-mediated reinforcing effects. A previous study found a similar effect with cocaine self-administration (Hiranita et al., 2010, 2013). Together these results suggest that the induction of reinforcing effects of σ1R agonists is due to actions mediated by dopaminergic mechanisms and that this finding is consistent with previous studies indicating that qualitative and profound changes in the behavioral effects of drugs can be obtained due to experiential factors (e.g., Collins and Woods, 2007, 2009; Barrett, 2013).
Several previous findings may be related to the present induction of reinforcing effects of σ1R agonists. For example, passive exposure to cocaine increases levels of σ1R mRNA and protein in brain regions implicated in drug reinforcement, an effect sensitive to the preferential σ1R antagonist BD 1063 [1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrochloride] (Liu et al., 2005; Liu and Matsumoto, 2008). Moreover, the upregulation of σ1Rs by cocaine administration in vivo does not occur in mice with a genetic deletion of dopamine D1 receptors (Zhang et al., 2005). In rats actively self-administering d-methamphetamine, increases in midbrain levels of σ1R mRNA and protein have been found in the hippocampus and olfactory bulb, and were greater than those obtained in rats passively receiving the drug at the same doses and frequencies (i.e., “yoked” controls) (Stefanski et al., 2004; Hayashi et al., 2010). Further, the σ1Rs in the olfactory bulb were found to be colocalized with dopamine D1 receptors (Hayashi et al., 2010), and a linkage between D1 and σ1 receptors is further supported by studies suggesting that these proteins can form heterodimers (Navarro et al., 2010).
Dopamine D2 receptors may also be involved in the actions of σ1Rs. Stefanski et al. (1999) have shown that rats with d-methamphetamine self-administration histories showed a decrease in levels of dopamine D2 autoreceptors in the ventral tegmental area and the substantia nigra zona compacta as well as a downregulation of dopamine D1 receptors in the shell of the nucleus accumbens—effects not obtained in rats passively receiving d-methamphetamine or saline. More recently, Navarro et al. (2013) provided evidence for heteromers of σ1 and D2, but not D3 or D4, receptors in mouse striatum and that cocaine can inhibit signaling that is not obtained in tissue from mutant mice with the deletion of σ1Rs. Thus, current evidence points to an activation of dopaminergic effects, involving D1 and D2 dopamine receptors, as critical for triggering an upregulation of σ1Rs, which in turn may be involved in the induction of σ1R-agonist reinforcing effects. Further studies assessing the induction of these reinforcing effects with particular molecular targets and anatomic loci will further elucidate these potential pathways.
It remains possible that exposure to heroin or ketamine under other conditions (for example, longer exposures or greater cumulative intake) might more effectively induce σ1R agonist self-administration. However, after the initial assessment, further increases in the intake of heroin that were greater than 6-fold or ketamine that were approximately 4.5-fold did not induce self-administration of PRE-084. It appears that experiences with the dopamine indirect agonists d-methamphetamine (present study) or cocaine (Hiranita et al., 2010, 2013) are sufficient, whereas comparable experiences with other abused drugs are insufficient, to induce σ1R agonist self-administration. Both d-methamphetamine and cocaine exert their dopaminergic effects through actions at the dopamine transporter. At present, it appears that an indirect action mediated by the dopamine transporter is critical in the induction of reinforcing effects of σ1R agonists; however, the potential of direct-acting dopamine receptor agonists to induce σ1R agonist self-administration has not yet been assessed.
Once induced, the reinforcing effects of the selective σ1R agonists were independent of dopaminergic mechanisms in this study as well as a previous study (Hiranita et al., 2013). The lack of substantive dopaminergic mediation of the effects of σR agonists is not surprising in light of behavioral studies showing a failure of the σR agonists, PRE-084 or DTG, to substitute for cocaine in rats trained to discriminate cocaine from saline injections (Hiranita et al., 2011b), a procedure in which a number of indirect dopaminergic agonists fully substitute for cocaine (Witkin et al., 1991; Li et al., 2006). Furthermore, administration of intravenous doses of PRE-084 that maintained self-administration behavior did not significantly stimulate dopamine levels in the nucleus accumbens shell in rats that had self-administered cocaine and PRE-084, or in rats that were not previously exposed to cocaine or PRE-084 (Hiranita et al., 2010, 2013; Garcés-Ramírez et al., 2011). Doses of PRE-084 that were 18–30 times greater than the self-administered doses did increase nucleus accumbens dopamine, but the effect was less than that produced by cocaine and was not antagonized by the σR antagonist, BD 1063 (Garcés-Ramírez et al., 2011).
The present and similar past in vivo results are consistent with an independence from dopaminergic systems once σ1R-mediated reinforcing mechanisms are induced, and as such they present a picture different from that painted by the findings of Navarro et al. (2013) of cocaine inducing effects mediated through σ1-D2 heteromers. In contrast to the present findings, no particular behavioral history was necessary for the interactions with either D2 or D1 dopamine receptors obtained by Navarro et al. (2010, 2013). Thus, it is currently unclear whether or how those in vitro outcomes are related to the present in vivo findings. In addition, a previous study reported comparable stimulation of locomotor activity by methamphetamine in σ1R knockout mice and their wild-type controls (Fontanilla et al., 2009), suggesting that at least the acute stimulation produced by methamphetamine does not involve dopamine and σ1 receptor heteromeric complexes. The present results together with these published findings suggest initially distinct in vivo pharmacologically mechanisms of stimulant drugs and σ1R agonists that interact during experience with stimulant self-administration, followed by minimal if any involvement of dopamine systems after the reinforcing effects of the σ1R agonists are triggered.
There was a difference between the antagonism of σR agonist self-administration and that of heroin or methamphetamine in the present study. The antagonism of d-methamphetamine self-administration by (+)-butaclamol, as well as that of heroin by naltrexone, was mainly surmountable, with decreases in maximal effects only at the highest antagonist doses (see also Harrigan and Downs, 1978; Bertalmio and Woods, 1989; Bergman et al., 1990; Winger et al., 1992; Hiranita et al., 2013). In contrast, the antagonism of PRE-084 self-administration by BD 1008 appeared insurmountable, with prominent dose-related decreases in maximal effect (see also Winger et al., 1992; Hiranita et al., 2010, 2011a, 2013). The bitonic function for self-administration may reflect two different dose-related effects that are differentially sensitive to antagonism. For example, doses on the ascending limb of the dose-effect curve may be competitively antagonized by the σR antagonist with doses on the descending limb insensitive to antagonism, resulting in what appears to be a decrease in maximal effect. Differential mechanisms for ascending and descending limbs of bitonic dose-effect curves have been described in other systems (Collins et al., 2005). In addition, uniform direct effects on response rates by the antagonist may limit the degree to which it shifts the descending limb of the self-administration dose-effect curve. In that regard, it is interesting to note that the antagonism of cocaine and PRE-084 self-administration by haloperidol, which has well-known dopamine-antagonist effects but also is an antagonist at σRs (Tam, 1983; Iwamoto, 1989), shows surmountable or insurmountable antagonism, respectively. This finding suggests that at least for haloperidol, the insurmountable antagonism is not due to its own direct effects, which would be expected to decrease responding maintained by either cocaine or PRE-084. As such, studies of the nature of the antagonism of σR agonist self-administration might most profitably focus on the sensitivity of doses on the descending limb to antagonism rather than features of the antagonists.
It was recently reported that inhibition of the dopamine transporter coupled with antagonism of σRs selectively decreases cocaine self-administration (Hiranita et al., 2011a), a preclinical indication of potential efficacy as a cocaine-abuse treatment (Mello and Negus, 1996). Those effects appear to involve mechanisms similar to those responsible for the present findings. The present results may shed light on the etiology of that efficacy. If cocaine self-administration experience recruits σRs into reinforcement mechanisms such dual targeting may be a preferred approach to the discovery of effective stimulant-abuse treatments and may at least partly explain the intractability of stimulant abuse to various attempts at treatment (Gorelick et al., 2004; Vocci and Elkashef, 2005; Vocci et al., 2005), particularly medical treatments singly targeting dopamine systems.
Supplementary Material
Acknowledgments
The authors thank Maryann Carrigan for administrative assistance.
Abbreviations
- σR
σ-receptor
- 95% Cl
95% confidence limit
- ANOVA
analysis of variance
- BD 1008
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide
- BD 1063
1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrochloride
- DTG
1,3-di-o-tolylguanidine
- FR
fixed ratio
- LED
light-emitting diode
- (+)-MK 801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine)
- OWW
original wet weight
- PRE-084
2-(4-morpholinethyl)1-phenylcyclohexanecarboxylate hydrochloride
- TO
time-out
Authorship Contributions
Participated in research design: Hiranita, Kopajtic, Katz.
Conducted experiments: Hiranita, Kopajtic.
Performed data analysis: Hiranita, Kopajtic, Katz.
Wrote or contributed to the writing of the manuscript: Hiranita, Soto, Tanda, Kopajtic, Katz.
Footnotes
The research was supported by the Intramural Research Program of the National Institutes of Health [National Institute on Drug Abuse].
Portions of this work were previously presented at the following meeting: Hiranita T, Soto PL, Tanda G, and Katz JL (2013) Specificity of cocaine-induced dopamine-independent sigma agonist self-administration. Experimental Biology 2013; 2013 Apr 20–24; Boston, MA.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Andersen PH. (1988) Comparison of the pharmacological characteristics of [3H]raclopride and [3H]SCH 23390 binding to dopamine receptors in vivo in mouse brain. Eur J Pharmacol 146:113–120 [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. (2002) The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34:399–410 [DOI] [PubMed] [Google Scholar]
- Barrett JE. (2013) Behavioral and pharmacological determinants of pharmacological plasticity. Pharmacologist 55:35–44 [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. (1990) Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol 1:355–363 [DOI] [PubMed] [Google Scholar]
- Bertalmio AJ, Woods JH. (1989) Reinforcing effect of alfentanil is mediated by mu opioid receptors: apparent pA2 analysis. J Pharmacol Exp Ther 251:455–460 [PubMed] [Google Scholar]
- Bristow LJ, Cook GP, Patel S, Curtis N, Mawer I, Kulagowski JJ. (1998) Discriminative stimulus properties of the putative dopamine D3 receptor agonist, (+)-PD 128907: role of presynaptic dopamine D2 autoreceptors. Neuropharmacology 37:793–802 [DOI] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. (2005) Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther 314:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. (2007) Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther 323:599–605 [DOI] [PubMed] [Google Scholar]
- Collins GT, Woods JH. (2009) Influence of conditioned reinforcement on the response-maintaining effects of quinpirole in rats. Behav Pharmacol 20:492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormaci G, Mori T, Hayashi T, Su TP. (2007) Protein kinase A activation down-regulates, whereas extracellular signal-regulated kinase activation up-regulates sigma-1 receptors in B-104 cells: Implication for neuroplasticity. J Pharmacol Exp Ther 320:202–210 [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. (2009) The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 323:934–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcés-Ramírez L, Green JL, Hiranita T, Kopajtic TA, Mereu M, Thomas AM, Mesangeau C, Narayanan S, McCurdy CR, Katz JL, et al. (2011) Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biol Psychiatry 69:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. (2004) Agents in development for the management of cocaine abuse. Drugs 64:1547–1573 [DOI] [PubMed] [Google Scholar]
- Harrigan SE, Downs DA. (1978) Continuous intravenous naltrexone effects on morphine self-administration in rhesus monkeys. J Pharmacol Exp Ther 204:481–486 [PubMed] [Google Scholar]
- Hayashi T, Justinova Z, Hayashi E, Cormaci G, Mori T, Tsai SY, Barnes C, Goldberg SR, Su TP. (2010) Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharmacol Exp Ther 332:1054–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610 [DOI] [PubMed] [Google Scholar]
- Hiranita T, Mereu M, Soto PL, Tanda G, Katz JL. (2013) Self-administration of cocaine induces dopamine-independent self-administration of sigma agonists. Neuropsychopharmacology 38:605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Kohut SJ, Kopajtic T, Cao J, Newman AH, Tanda G, Katz JL. (2011a) Decreases in cocaine self-administration with dual inhibition of the dopamine transporter and σ receptors. J Pharmacol Exp Ther 339:662–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. (2009) Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. (2010) Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther 332:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. (2011b) Lack of cocaine-like discriminative-stimulus effects of σ-receptor agonists in rats. Behav Pharmacol 22:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, et al. (2012) Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32:11187–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto ET. (1989) Evidence for a model of activation of central sigma systems. Life Sci 44:1547–1554 [DOI] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, Kopajtic T, Tsai SY. (2011) A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals (Basel) 4:880–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Campbell BL, Katz JL. (2006) Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther 317:1088–1096 [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. (2005) Cocaine up-regulates Fra-2 and sigma-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther 314:770–779 [DOI] [PubMed] [Google Scholar]
- Liu Y, Matsumoto RR. (2008) Alterations in fos-related antigen 2 and sigma1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther 327:187–195 [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. (2007) Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology 32:1967–1973 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. (2009) Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert Rev Clin Pharmacol 2:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. (2001) Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting sigma1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol 419:163–174 [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. (2009) The pharmacology of sigma-1 receptors. Pharmacol Ther 124:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. (1999) Two novel sigma receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur J Pharmacol 370:225–232 [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424 [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J, Cortés A, Casadó V, Canela EI, Ortiz J, et al. (2010) Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci USA 107:18676–18681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Bonaventura J, Brugarolas M, Farré D, Aguinaga D, Mallol J, Cortés A, Casadó V, Lluís C, et al. (2013) Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS ONE 8:e61245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Kassiou M, Johnston GA, Christie MJ. (1996) Comparison of binding parameters of sigma 1 and sigma 2 binding sites in rat and guinea pig brain membranes: novel subtype-selective trishomocubanes. Eur J Pharmacol 311:233–240 [DOI] [PubMed] [Google Scholar]
- Nuwayhid SJ, Werling LL. (2003) Sigma1 receptor agonist-mediated regulation of N-methyl-D-aspartate-stimulated [3H]dopamine release is dependent upon protein kinase C. J Pharmacol Exp Ther 304:364–369 [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW. (2000) Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology (Berl) 150:61–66 [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA 92:12304–12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. (1996) Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382:255–257 [DOI] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. (2000) Involvement of the sigma1 receptor in the cocaine-induced conditioned place preference. Neuroreport 11:2885–2888 [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. (2002) Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology 26:444–455 [DOI] [PubMed] [Google Scholar]
- Snedecor G and Cochran W, editors (1967) Statistical Methods, 6th ed, Iowa State College Press, Ames, IA.
- Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. (2004) Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology (Berl) 175:68–75 [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. (1999) Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol 371:123–135 [DOI] [PubMed] [Google Scholar]
- Tam SW. (1983) Naloxone-inaccessible sigma receptor in rat central nervous system. Proc Natl Acad Sci USA 80:6703–6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276:2048–2050 [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. (2005) Medication development for addictive disorders: the state of the science. Am J Psychiatry 162:1432–1440 [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Elkashef A. (2005) Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry 18:265–270 [DOI] [PubMed] [Google Scholar]
- Winger G, Skjoldager P, Woods JH. (1992) Effects of buprenorphine and other opioid agonists and antagonists on alfentanil- and cocaine-reinforced responding in rhesus monkeys. J Pharmacol Exp Ther 261:311–317 [PubMed] [Google Scholar]
- Witkin JM, Nichols DE, Terry P, Katz JL. (1991) Behavioral effects of selective dopaminergic compounds in rats discriminating cocaine injections. J Pharmacol Exp Ther 257:706–713 [PubMed] [Google Scholar]
- Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, Zhang J, Xu M. (2005) Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology 30:1443–1454 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.