Abstract
Many drugs used to treat anxiety are positive modulators of GABAA receptors, which mediate fast inhibitory neurotransmission. The GABAA receptors can be assembled from a combination of at least 16 different subunits. The receptor’s subunit composition determines its pharmacologic and functional properties, and subunit expression varies throughout the brain. A primary goal for new treatments targeting GABAA receptors is the production of subunit-selective modulators acting upon a discrete population of receptors. The anxiolytic 4-amino-7-hydroxy-2-methyl-5,6,7,8,-tetrahydrobenzo[b]thieno[2,3-b]pyridine-3-carboxylic acid, but-2-ynyl ester (SB-205384) is widely considered to be selective for α3-containing GABAA receptors. However, it has been tested only on α1-, α2-, and α3-containing receptors. We examined the activity of SB-205384 at recombinant receptors containing the six different α subunits and found that receptors containing the α3, α5, and α6 subunits were potentiated by SB-205384, with the α6 subunit conferring the greatest responsiveness. Properties associated with chimeric α1/α6 subunits suggested that multiple structural domains influence sensitivity to SB-205384. Point mutations of residues within the extracellular N-terminal domain identified a leucine residue located in loop E of the agonist binding site as an important determinant of high sensitivity to modulation. In the α6 subunit the identity of this residue is species-dependent, with the leucine found in rat subunits but not in human. Our results indicate that SB-205384 is not an α3-selective modulator, and instead acts at several GABAA receptor isoforms. These findings have implications for the side-effect profile of this anxiolytic as well as for its use in neuronal and animal studies as a marker for contribution from α3-containing receptors.
Introduction
Anxiety disorders are the most common mental illness in the United States, affecting approximately 40 million Americans over the age of 18, which represents nearly 18% of the adult population (Kessler et al., 2005). Many commonly used anxiolytics act by enhancing inhibitory neurotransmission through the GABAA receptors (Whiting, 2006; Mohler, 2012; Smith and Rudolph, 2012). These receptors are chloride-permeable ligand-gated ion channels composed of a pentameric combination of 16 different subunit subtypes (α1–6, β1–3, γ1–3, δ, ε, π, and θ). The expression of the different subunits varies both regionally and developmentally (Olsen and Sieghart, 2009). Therefore, drugs that act selectively on distinct receptor populations have the potential to produce targeted therapeutic benefit without unwanted side-effects (Basile et al., 2004; Rudolph and Knoflach, 2011).
The α3 subunit of the GABAA receptor is predominantly expressed in the developing nervous system, with more restricted expression in the adult brain (Laurie et al., 1992a). Although studies with genetically modified mice suggest that α2-, rather than α3-containing receptors mediate the anxiolytic effects of benzodiazepines (Rudolph and Mohler, 2004), drugs selectively targeting the α3-containing receptors have been investigated for treatment of anxiety and chronic pain (Dias et al., 2005; Knabl et al., 2008). One compound widely considered to be α3-selective is 4-amino-7-hydroxy-2-methyl-5,6,7,8,-tetrahydrobenzo[b]thieno[2,3-b]pyridine-3-carboxylic acid, but-2-ynyl ester (SB-205384) (Benham et al., 1994). SB-205384 has been shown to reduce anxiety in animal models through a site distinct from that used by benzodiazepines (Navarro et al., 2006, 2008). While this compound is commonly used in neuronal and animal studies with the assumption that it selectively modulates α3-containing GABAA receptors (i.e., see Ing and Poulter, 2007; Uusisaari and Knopfel, 2008; Belujon et al., 2009; Chun and Jo, 2010; Miller et al., 2010), only a limited number of subunit subtypes have actually been directly compared. SB-205384 was reported to slow the decay rate of the response to GABA when receptors contained α3 subunits, but not when they contained α1 or α2 subunits (Meadows et al., 1998). Properties of recombinant receptors containing the other α subtypes were not examined.
Because of the clinical and experimental importance of subunit-selective modulators, the goal of this work was to evaluate the effect of SB-205384 at receptors containing each of the six different α subunit-subtypes of the GABAA receptor. We used patch-clamp recordings from transiently transfected human embryonic kidney 293T cells to determine the modulatory effect of SB-205384 on the activity of recombinant GABAA receptors and to identify the structural basis for its subunit-selective activity.
Materials and Methods
Transfection of Mammalian Cells.
Full-length cDNAs for the rat or human GABAA receptor subunits (obtained from Dr. Robert Macdonald, Vanderbilt University) in mammalian expression vectors were transfected into the human embryonic kidney cell line 293T (GenHunter, Nashville, TN). Cells were maintained in Dulbecco’s modified Eagle’s medium plus 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells were passaged by a 2-minute incubation with 0.05% trypsin/0.02% EDTA solution in phosphate buffered saline (10 mM Na2HPO4, 150 mM NaCl, pH = 7.3).
The cells were transfected using calcium phosphate precipitation. Plasmids encoding GABAA receptor subunit cDNAs were added to the cells in 1:1:1 ratios (α:β:γ/δ) of 2 μg each. To allow isolation of positively transfected cells, 1 μg of the plasmid pHook-1 (Invitrogen/Life Technologies; Grand Island, NY) containing cDNA encoding the surface antibody sFv was also transfected into the cells (Chesnut et al., 1996). Following a 4–6-hour incubation at 3% CO2, the cells were treated with a 15% glycerol solution in BES-buffered saline (50 mM BES(N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid), 280 mM NaCl, 1.5 mM Na2HPO4) for 30 seconds. The selection procedure for pHook expression was performed 18–52 hours later. The cells were passaged and mixed for 30–60 minutes with 3–5 μl of magnetic beads coated with antigen for the pHook antibody (approximately 6 × 105 beads) (Chesnut et al., 1996). Bead-coated cells were isolated using a magnetic stand. The selected cells were resuspended into Dulbecco’s modified Eagle’s medium, plated onto glass coverslips treated with poly L-lysine, coated with collagen, and used for recordings 20–28 hours later.
Electrophysiological Recording Solutions and Techniques.
For all recordings the external solution consisted of (in mM): 142 NaCl, 8.1 KCl, 6 MgCl2, 1 CaCl2, and 10 HEPES with pH = 7.4 and osmolarity adjusted to 295–305 mOsm. Recording electrodes were filled with an internal solution of (in mM): 153 KCl, 1 MgCl2, 5 K-EGTA (ethylene glycol-bis (β-aminoethyl ether) N,N,N′N′-tetraacetate), and 10 HEPES with pH = 7.4 and osmolarity adjusted to 295–305 mOsm. GABA (Sigma-Aldrich, St. Louis, MO) was diluted into external solution from freshly made or frozen stocks in water. SB-205384 (Tocris Bioscience, Bristol, UK) and flumazenil (Tocris Bioscience) were dissolved in dimethylsulfoxide and diluted into external solution, with the highest dimethylsulfoxide level applied to cells of 0.1%. Patch pipettes were pulled from borosilicate glass with an internal filament (World Precision Instruments, Sarasota, FL) on a two-stage puller (Narishige, Tokyo, Japan) to a resistance of 5–10 MΩ. For whole-cell recordings GABA was applied to cells using a stepper solution exchanger with a complete exchange time of <50 milliseconds (open tip, SF-77B; Harvard Apparatus, Holliston, MA). For macropatch recordings the 3-barrel square glass was pulled to a final size near 200 μm. Rise times (10–90%) of the junction potential at the open tip were consistently faster than 400 microseconds and were tested using a diluted external solution. There was a continuous flow of external solution through the chamber. Currents were recorded with an Axon 200B (Axon Instruments, Foster City, CA) patch clamp amplifier.
Construction of Mutated Subunit cDNAs.
Point mutations were generated using the QuikChange procedure and products (Agilent Technologies, Santa Clara, CA). Oligonucleotide primers were synthesized by Integrated DNA Technologies (Coralville, IA), and DNA sequencing was performed by the University of South Carolina Environmental Genomics core facility (Columbia, SC).
Analysis of Whole-Cell and Macropatch Currents.
Whole-cell currents were analyzed using the programs Clampfit (pClamp9 suite; Axon Instruments) and Prism (GraphPad Software, San Diego, CA). Concentration-response data were fit with the four-parameter logistic equation: Current = (Minimum current + [Maximum current − Minimum current])/1 + (10^(log EC50−log [agonist])*n) where n represents the Hill number. All fits were made to normalized data with current expressed as a percentage of the maximum response for each cell. Macropatch currents from outside-out patches were digitized at 10 kHz and analyzed with the pClamp 9.0 suite of programs. The deactivation rate was determined by fitting the decay current with the Levenberg-Marquardt least squares method with two exponential functions. Student’s paired or unpaired t tests, analysis of variance, and Tukey-Kramer multiple comparisons tests were performed using the Instat program (GraphPad Software) with a significance level of P < 0.05.
Results
Effect of the α Subtype on Positive Modulation by SB-205384.
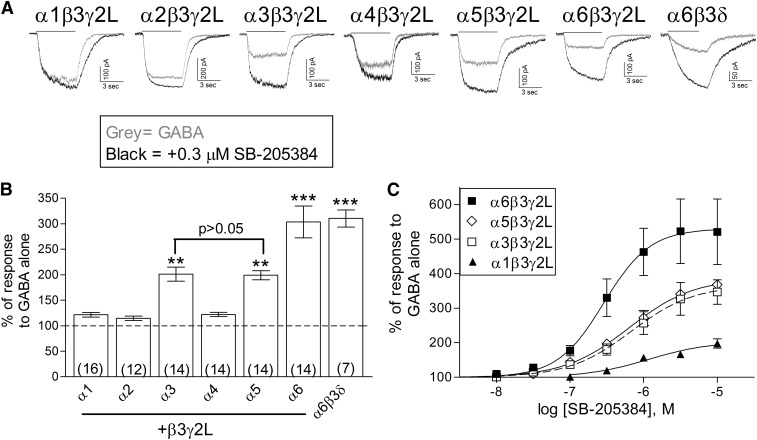
Cells were transfected with each of the six different α subunit subtypes along with the same β (β3) and γ (γ2L) subunits. To determine the sensitivity to modulation, a submaximal concentration of GABA (EC5–10) was coapplied with 0.3 μM SB-205384 for 5 seconds to cells voltage-clamped at −50 mV (Fig. 1, A and B). Three of the six isoforms, those containing the α3, α5, and α6 subunits, showed significantly greater enhancement of the current by this concentration of SB-205384. These findings are consistent with those of Meadows et al. (1998) in which only α1-, α2-, and α3-containing receptors were examined.
Fig. 1.
Effect of the α subtype on positive modulation by SB-205384. (A) Representative traces from cells transiently transfected with one of the α subtypes, as indicated, along with β3 and γ2L, showing the current response to GABA alone (gray) or GABA + 0.3 μM SB-205384 (black). Cells were voltage-clamped at −50 mV in the whole-cell recording configuration. GABA concentration was 0.03 μM (α6δ), 0.1 μM (α6γ2), 0.3 μM (α4, α5), 1 μM (α1, α2), or 3 μM (α3), representing an EC5-10 for each isoform (Saxena and Macdonald, 1996; Picton and Fisher, 2007). (B) The peak current amplitude was measured in response to GABA and GABA + 0.3 μM SB-205384. The response was normalized to the average response to GABA alone for each cell. Bars represent mean ± S.E.M., with the number of cells shown by the number in parentheses. **P ≤ 0.01; or ***P ≤ 0.001 indicate a significant difference compared with α1 using analysis of varience and Tukey-Kramer multiple comparisons tests. (C) Concentration-response relationships for SB-205384. The peak current amplitude was normalized to the response to GABA alone for each cell. Solid or dashed lines represent fits to averaged data with EC50 values (and maximum potentiation) of 288 nM (530.0%) for α6β3γ2L (n = 6), 635 nM (387.0%) for α5β3γ2L (n = 4), 680 nM (366.4%) for α3β3γ2L (n = 5), and 1.20 μM (203.5%) for α1β3γ2L (n = 4).
From full concentration-response relationships, we found that receptors with the α6-subunit had the greatest sensitivity to SB-205384 (Fig. 1C). The average EC50 (and peak response) for potentiation of the response to GABA was 280.4 ± 23.7 nM (531.9 ± 97.7%) for α6β3γ2L (n = 6). This compared with 695.5 ± 156.0 nM (359.6 ± 42.9%) for α3β3γ2L (n = 5) and 730.0 ± 200.0 nM (389.2 ± 11.0%) for α5β3γ2L (n = 4). The less sensitive isoform α1β3γ2L had an average EC50 (and maximum potentiation) of 1.73 ± 0.49 μM (209.4 ± 11.2%) (n = 4). The average log EC50 for α6 was significantly different from that of α1 (P ≤ 0.001), α3 (P ≤ 0.05), and α5 (P ≤ 0.05). The properties of α3- and α5-containing receptors were not different from one another (P > 0.05), but both were different from α1 (P ≤ 0.05).
Earlier studies showed that the γ subtype influenced sensitivity to modulation by SB-205384, with γ2-containing receptors showing a greater response than those with the γ1 subunit (Meadows et al., 1998). Since the α6 subunit commonly assembles with the δ subunit in extrasynaptic locations of cerebellar granule neurons (Farrant and Nusser, 2005) we examined the effect of 0.3 μM SB-205384 on the α6β3δ isoform (Fig. 1, A and B) and found that activity of these receptors was also enhanced by 0.3 μM SB-205384. A previous report suggested that SB-205384 was unlikely to act through the benzodiazepine binding site as it did not alter binding of flunitrazepam to neuronal GABAA receptors (Benham et al., 1994). Our findings that both α6-containing and γ-lacking receptors are sensitive to modulation also provide support for this conclusion. In addition, we found that the benzodiazepine site antagonist flumazenil (Ro15-1788) did not alter modulation of α3β3γ2L receptors, with an average enhancement of 202.9 ± 4.9% by 0.3 μM SB-205834 alone and 204.4 ± 8.5% with 10 μM flumazenil at the same cells (n = 3, P > 0.1, paired t test). We used α3-containing receptors for these experiments because flumazenil acts as an allosteric modulator of α6-containing receptors (Sigel and Baur, 2000). Together, these results demonstrate that SB-205384 acts through a site distinct from that of benzodiazepine agonists.
Effect of SB-205384 on Macroscopic Kinetic Properties of Recombinant GABAA Receptors.
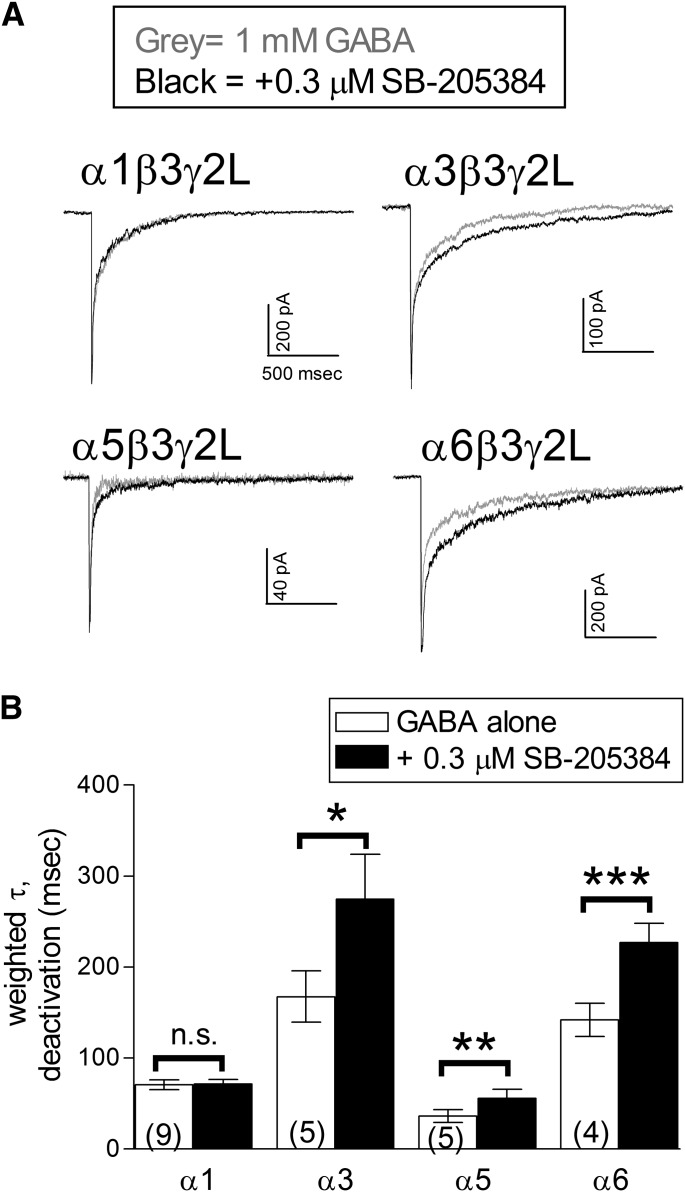
Previous studies using cultured cerebellar neurons (Meadows et al., 1997) or recombinant receptors expressed in oocytes (Meadows et al., 1998) found that the primary effect of SB-205384 was a slowing of the decay rate following agonist removal. We also observed a prolonged current decay in our whole-cell recordings (Fig. 1A). To quantify this effect and to better predict the impact of SB-205384 on postsynaptic responses to GABA, we examined its effect on the current deactivation rate following maximal receptor activation using rapid application recordings. GABA (1 mM) was applied for 5 milliseconds to excised, outside-out patches in combination with 0.3 μM SB-205384 (Fig. 2A). Consistent with our findings in whole-cell recordings, deactivation was significantly slowed for receptors containing the α3, α5, or α6 subunit, but not the α1 subunit (Fig. 2B).
Fig. 2.
Effect of SB-205384 on macroscopic deactivation. (A) Representative outside-out patch current traces from receptors containing the subunits indicated in response to a 5 ms application of 1 mM GABA (gray) or 1 mM GABA + 0.3 µM SB-205384 (black). Patches were voltage-clamped at −70 mV. (B) The current decay in response to a brief application of GABA was fit with the sum of two exponential components. Symbols and bars represent the weighted mean time constant ± S.E.M., and the number in parentheses indicates the number of patches examined. The average time constant for deactivation was significantly slowed by 0.3 μM SB-205384 for receptors containing the α3, α5, or α6 subunits. *P ≤ 0.05; **P ≤ 0.01; or ***P ≤ 0.001 indicate a significant difference compared with GABA alone applied to the same patch; n.s. indicates a nonsignificant difference (P > 0.05) (Student’s paired t test).
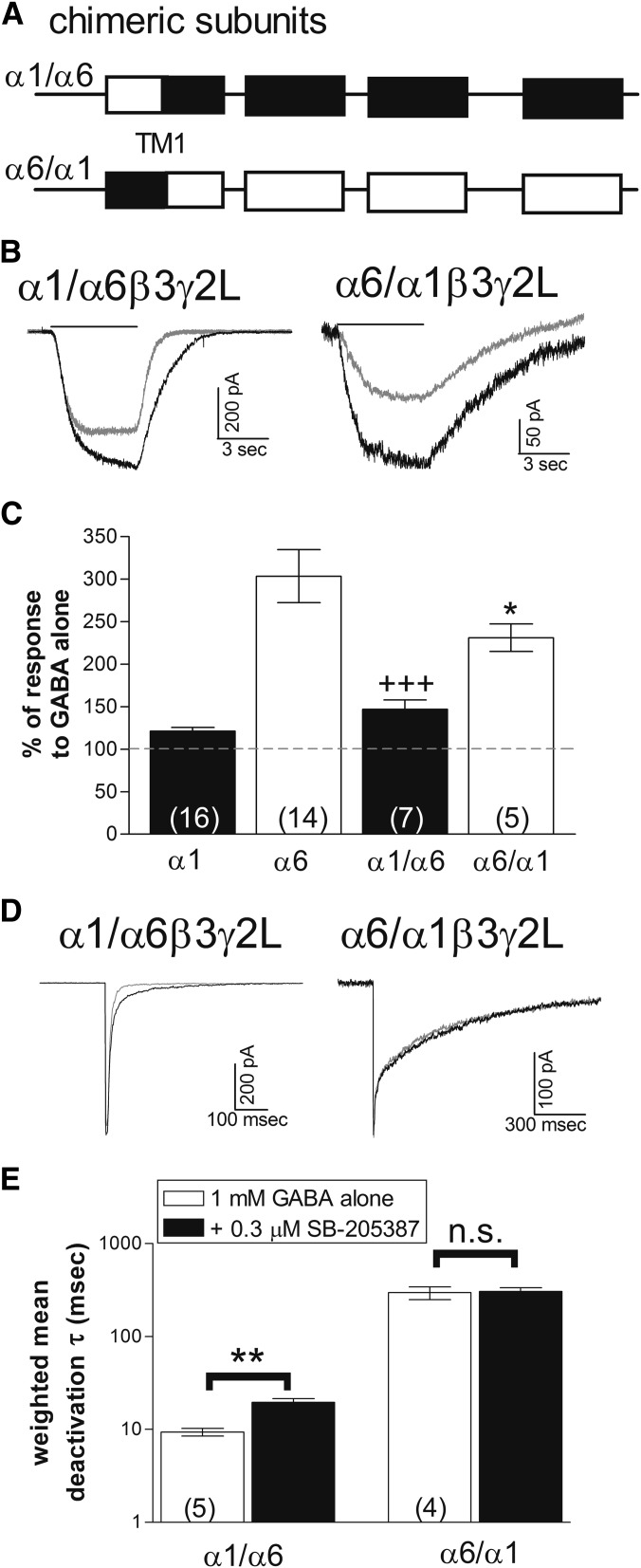
Multiple Domains of the α6 Subunit Influence Sensitivity to SB-205834.
To determine the structural basis for the subunit selective effects of SB-205834, we took advantage of chimeric subunits (Fig. 3A) that exchanged the extracellular N-terminal domains of the α1 and α6 subunits (Fisher et al., 1997). Previous studies showed that receptors containing the α1/α6 construct, which includes the N-terminal domain from the α1 subunit and the transmembrane domains from the α6 subunit, are characterized by a lower sensitivity to GABA and faster deactivation compared with α1-containing receptors. In contrast, receptors with the α6/α1 construct, which contain the extracellular N-terminal domain of the α6 subunit, have high GABA sensitivity and very slow deactivation (Fisher, 2004). We found that receptors containing either the α1/α6 or α6/α1 subunits showed some sensitivity to modulation by SB-205384, but that the effects on peak amplitude and slowing of deactivation appeared to be separated by these chimeric subunits. At the α1/α6-containing receptors, SB-205834 had little effect on the peak response (Fig. 3, B and C) but slowed the deactivation rate in outside-out recordings (Fig. 3, D and E). Conversely, at α6/α1-containing receptors, SB-205834 enhanced the peak response (Fig. 3, B and C) but did not substantially slow the deactivation rate (Fig. 3, D and E). This suggests that structures in multiple regions of the subunit contribute to the high sensitivity associated with the α6 subunit.
Fig. 3.
Different structural domains confer effects of SB-205384 on current amplitude and deactivation rate. (A) Schematic representation of chimeric subunits. Boxes indicate transmembrane domains. Chimeric splice site is within the first transmembrane domain (TM1) (Fisher et al., 1997). α1/α6 chimeras contain the extracellular N-terminal domain from the α1 subunit and transmembrane and intracellular domains from the α6 subunit, while α6/α1 chimeras contain the reverse. (B) Whole-cell recordings in response to 5-second applications of GABA alone (gray) or GABA + 0.3 μM SB-204384 (black). Cells were voltage-clamped at −50 mV. (C) The peak current amplitude was measured in response to GABA and GABA + 0.3 μM SB-205384. The response was normalized to the average response to GABA alone for each cell. Bars represent mean ± S.E.M. with the number of cells shown by the number in parentheses. Symbols indicate a significant difference of the chimeric subunits compared with α1 (*P ≤ 0.05) or to α6 (+++P ≤ 0.001) using analysis of variance and Tukey-Kramer multiple comparisons tests. (D) Representative outside-out patch recordings from receptors containing the chimeric subunits indicated in response to a 5-millisecond application of 1 mM GABA (gray) or 1 mM GABA + 0.3 µM SB-205384 (black). Patches were voltage-clamped at −70 mV. (E) The current decay was fit with the sum of two exponential components. Symbols and bars represent the weighted average ± S.E.M., and the number in parentheses indicates the number of patches. **P ≤ 0.01 indicates a significant difference compared with GABA alone, while n.s. indicates a nonsignificant difference (Student’s paired t test).
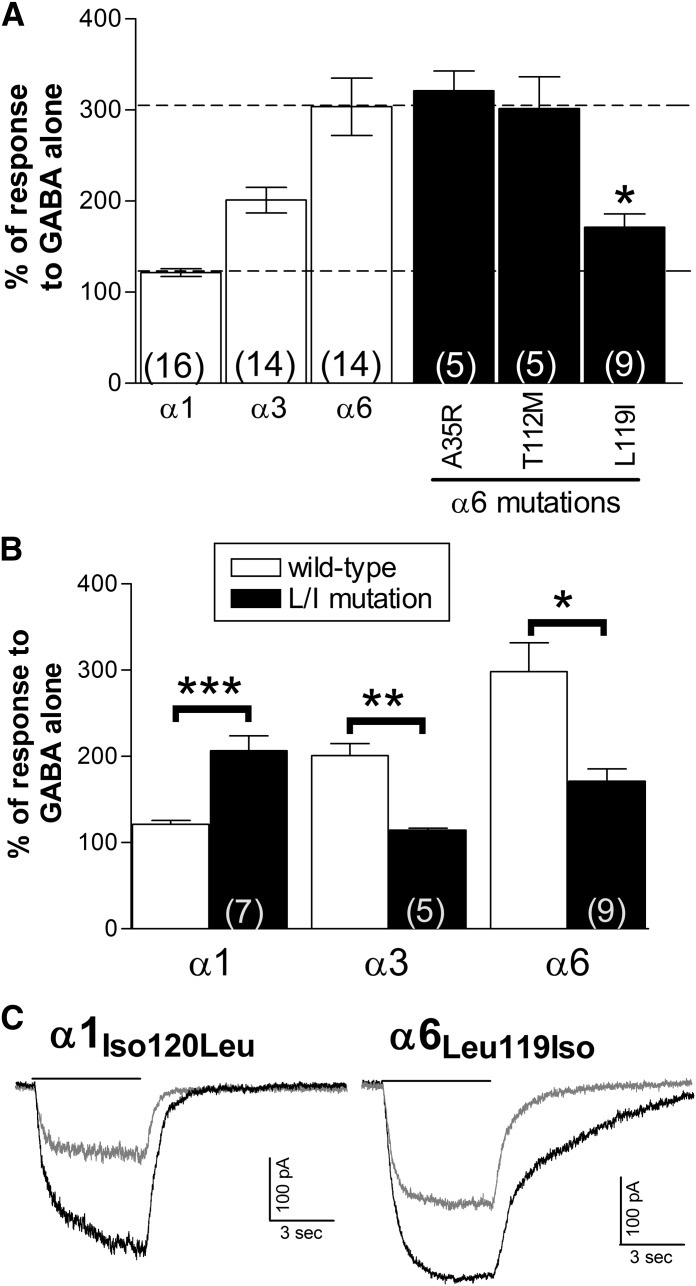
Residues within the Extracellular N-Terminal Domain Influence Subtype-Selectivity of SB-205384 Activity.
The results from the chimeric subunits showed that structures within the extracellular N-terminal domain of the α6 subunit contributed to the ability of SB-205384 to increase the amplitude of responses to submaximal GABA concentrations. This region contains nearly 200 amino acids but is fairly homologous among the α subunit subtypes. Comparing the sequences from rat subunits, we identified only three residues within this region that were unique to the α3, α5, and α6 subunits, and only two that were identical in α3, α5, and α6, but not shared by the other α subtypes. Each of the three sites was mutated in the α6 subunit to the homologous residue in the α1 subunit (Ala35Arg, Thr112Met, and Leu119Iso). None of these mutations altered GABA sensitivity of the receptors, with GABA EC50 values of 2.0 ± 0.3 μM (α6A35R, n = 3), 1.6 ± 0.3 μM (α6T112M, n = 3), and 1.2 ± 0.3 μM (α6L119I, n = 3), compared with 1.4 ± 0.3 μM (n = 3) for the wild-type α6β3γ2 receptor. Only the mutation of α6 leucine119 to isoleucine significantly reduced the potentiation by 0.3 µM SB-205384 (Fig. 4). The homologous mutation in the α3 subunit had a comparable effect, reducing responsiveness to modulation by SB-204384 (Fig. 4A), with no effect on GABA sensitivity [GABA EC50 values of 37.0 ± 2.7 (α3 wild-type, n = 4) and 33.4 ± 5.6 μM (α3L120I, n = 4)]. This leucine is shared among α3, α5, and α6 while the other α subunits all have an isoleucine at this location. The reverse mutation in the α1 subunit (Iso120Leu) significantly enhanced the potentiation by SB-205384 (Fig. 4, A and B) without changing GABA sensitivity: GABA EC50 values of 18.4 ± 1.6 (α1 wild-type, n = 4) and 21.0 ± 3.1 μM (α1I120L, n = 3). Consistent with the results from the chimeric subunits, the mutations at this site altered the ability of SB-205384 to enhance the current amplitude but did not appear to impact the slowing of the decay rate, which was still prominent in the α6(L119I)-containing receptors and not in the α1(I120L)-containing receptors (Fig. 4B).
Fig. 4.
A single residue within loop E of the extracellular N-terminal domain confers high sensitivity to modulation by SB-205384. (A) The peak current amplitude was measured in response to GABA and GABA + 0.3 μM SB-205384. The response was normalized to the average response to GABA alone for each cell. Bars represent mean ± S.E.M., with the number of cells shown by the number in parentheses. (B) Wild-type data are repeated from Fig. 1B. Symbols indicate a significant difference of the mutated subunits compared with their wild-type counterparts (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001) using analysis of variance and Tukey-Kramer multiple comparisons tests (α6) or unpaired Student’s t test (α1, α3). (C) Whole-cell recordings in response to 5 second applications of GABA alone (gray) or GABA + 0.3 μM SB-204384 (black). Cells were voltage-clamped at −50 mV.
While the α6(L119I) mutation significantly reduced the potentiation by SB-205384, it produced a receptor with characteristics intermediate to the wild-type response, and comparable to properties of the α1/α6 chimeric subunit. This suggests that other residues within the α6 subunit, likely found in regions beyond the first transmembrane domain, must also contribute to its higher sensitivity.
Discussion
The large degree of structural heterogeneity associated with the GABAA receptor has led to the search for subunit-selective modulators. We examined the effect of the α-subunit subtype on sensitivity to positive allosteric modulation by the benzothiophene compound SB-205384. We found that the activity of receptors containing α3, α5, or α6 subunits was enhanced by coapplication with this modulator, with an increase in current amplitude at submaximal GABA concentrations and a slowing of deactivation at saturating concentrations. We also found that the δ-containing receptors could be potentiated by SB-205384, demonstrating that a γ subunit is not necessary for modulation. Previous studies showed that the β2 and γ2 subunit subtypes conferred greater responsiveness than β1 or γ1 (Meadows et al., 1998). Altogether, this represents a unique subunit-selectivity profile compared with other widely used GABAA receptor modulators, and suggests that SB-205384 would be expected to slow the decay of postsynaptic responses and enhance inhibitory neurotransmission in brain regions where any of these three α subtypes are expressed. Lack of activity at α1-containing receptors, which are the most common isoforms in the adult brain, may reduce the occurrence of unwanted effects such as sedation (Rudolph and Knoflach, 2011).
Production of the α3 subunit is highly regulated by development, with widespread expression in the embryonic and neonatal brain (Laurie et al., 1992a). With maturation, the α3 subunit becomes more regionally restricted, and in the adult is found at high levels primarily in the cortex (Wisden et al., 1992). Several studies have suggested that receptors containing these subunits mediate at least part of the anxiolytic, analgesic, myorelaxant, and anesthetic effects of GABA-modulating drugs (Rudolph and Mohler, 2004; Dias et al., 2005; Knabl et al., 2008; Straub et al., 2013), and deficits in signaling through α3-containing receptors have been linked to disruption of sensorimotor gating (Yee et al., 2005). The anticonvulsant drug stiripentol preferentially enhances activity of α3-containing receptors (Fisher, 2009) and has been found to be effective in treatment of some childhood seizure disorders (Chiron, 2007). Modulation of α3-containing receptors by SB-205384 suggests that it may have anxiolytic effects and could have an age-dependent modulatory effect in reducing neuronal excitability. Expression of the α5 subunit is largely restricted to the hippocampus, where it contributes to the extrasynaptic receptor population responsible for the tonic inhibitory current in pyramidal neurons (Wisden et al., 1992; Caraiscos et al., 2004). Based on effects of α5-selective modulators as well as characteristics of mice lacking this subunit, positive modulation of these receptors by SB-205384 may be expected to decrease memory and cognitive performance (Atack et al, 2006; Cheng et al., 2006; Martin et al., 2009, 2010). The α6 subunits are found only in cerebellar granule cells, where they contribute to both synaptic and extrasynaptic populations of GABAA receptors (Laurie et al., 1992b; Nusser et al., 1998). We found that these subunits confer the highest sensitivity to modulation by SB-205384, consistent with the ability of this compound to modulate GABA-activated currents in cerebellar granule cells (Meadows et al., 1997), which primarily express the α1 and α6 subunits (Laurie et al., 1992b). The α6-containing receptors in the cerebellum are also targets for modulation by ethanol; positive modulation of both the synaptic (γ-containing) and extrasynaptic (δ-containing) populations would be expected to disrupt motor coordination (Hanchar et al., 2005).
The leucine/isoleucine residue identified as important for the α subtype–selective effect of SB-205384 is located within loop E in the extracellular domain, which forms part of the agonist binding site at the interface between β and α subunits (Kloda and Czajkowski, 2007; Bergmann et al., 2013). Interestingly, the identity of this residue in the α6 subunit is species-dependent. In humans (Hadingham et al., 1996) and most other mammals, an isoleucine is encoded, while the leucine residue is limited to rat, mouse, and hamster. The α3 and α5 subunits do not show this variation, and the leucine residue is encoded in rat and human sequences for both of these subunits (Wingrove et al., 1992; Hadingham et al., 1993). As a result, SB-205384 may be expected to have less effect on cerebellar function in humans than in rat or mouse models. Although we found that exchanging these residues in the α1, α3, or α6 subunit did not impact GABA sensitivity, consistent with our previous report (Drafts and Fisher, 2004), a less conservative mutation of α1-isoleucine120 to valine caused a 10-fold reduction in GABA EC50 (Westh-Hansen et al., 1997), showing that structural changes at this site can influence agonist sensitivity. Although our data cannot differentiate effects on binding from those on signal transduction we feel it is unlikely that α6-L119 forms part of the binding site for SB-205384 because of its location within the GABA binding pocket. If that were the case, SB-205384 might be expected to disrupt GABA binding and act either as an agonist or antagonist. Therefore, we consider it more likely that this residue contributes to the signal transduction pathway for allosteric modulation.
This leucine/isoleucine residue is adjacent to the highly conserved arginine residue (R119 in α1), which is critical for binding of agonists and antagonists (Westh-Hansen et al., 1999) and has been suggested to play an important role in the interaction between loop E of the α subunit and loop C of the β subunit (Cromer et al., 2002; Laha and Wagner, 2011; Bergmann et al., 2013). The conformation of loop C is considered to be an important regulator of the activation of cys-loop ion channels (Sine and Engel, 2006). Previous studies have demonstrated that long-distance conformational changes in loop E can occur in response to binding of allosteric modulators of GABAA receptors such as the benzodiazepines (Kloda and Czajkowski, 2007; Sancar and Czajkowski, 2011). This conformational change could then alter GABA affinity, thus providing a potential structural basis for positive allosteric modulation. Our results suggest that the substitution of isoleucine for leucine within this region reduces the modulatory effect of SB-205384, possibly by preventing its ability to effectively alter the agonist binding site. Our studies were not able to identify a potential binding site for SB-205384, although the ability to enhance activity of α6- and δ- containing receptors, along with the lack of inhibition by flumazenil, demonstrate that this compound does not act through the well-described high-affinity benzodiazepine site. The characteristics associated with the α1/α6 chimeric subunit suggest that its binding site may be located outside of the large N-terminal extracellular domain and could be associated with the transmembrane domains. Further studies will be needed to clarify the binding site(s) and mechanisms of action of this compound.
Our results demonstrate that SB-205384 is not an α3-selective modulator as previously believed. As a result, studies interpreted under this assumption should be reconsidered. However, its ability to also enhance activity of α5- and α6-containing receptors may increase its utility as a scientific tool to examine the roles of these GABAA receptor populations. In addition, our findings that structural variation within the GABA binding site can influence the subunit-selective effects of SB-205384 may identify a common signal transduction pathway used by a variety of allosteric modulators.
Acknowledgments
The authors thank Shana Dykema and Matt Fisher for technical assistance.
Abbreviations
- SB-205384
4-amino-7-hydroxy-2-methyl-5,6,7,8,-tetrahydrobenzo[b]thieno[2,3-b]pyridine-3-carboxylic acid, but-2-ynyl ester
Authorship Contributions
Participated in research design: Heidelberg, Fisher.
Conducted experiments: Heidelberg, Warren, Fisher.
Performed data analysis: Heidelberg, Warren, Fisher.
Wrote or contributed to the writing of the manuscript: Heidelberg, Fisher.
Footnotes
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant RO1-NS045950] (to J.L.F.); and the Office of Undergraduate Research at the University of South Carolina. Presentation of a portion of these results at the annual meeting of the Society for Neuroscience was supported by a travel award from the Faculty for Undergraduate Neuroscience (to L.S.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any other funding source.
Portions of this work were presented previously as follows: Heidelberg LS and Fisher JL (2009) Effect of the anxiolytic SB-205384 on recombinant GABAA receptors. Society for Neuroscience Annual Meeting; 2009 Oct 11–17; Chicago, IL.
References
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. (2006) L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAA receptors. Neuropharmacology 51:1023–1029 [DOI] [PubMed] [Google Scholar]
- Basile AS, Lippa AS, Skolnick P. (2004) Anxioselective anxiolytics: can less be more? Eur J Pharmacol 500:441–451 [DOI] [PubMed] [Google Scholar]
- Belujon P, Baufreton J, Grandoso L, Boué-Grabot E, Batten TFC, Ugedo L, Garret M, Taupignon AI. (2009) Inhibitory transmission in locus coeruleus neurons expressing GABAA receptor epsilon subunit has a number of unique properties. J Neurophysiol 102:2312–2325 [DOI] [PubMed] [Google Scholar]
- Benham CD, Meadows HJ, Thomas DR, and Wood MD (1994), inventors, SmithKline Beecham, assignee. BTP receptor modulation of the GABA-A/chloride channel complex for prolonging the duration of the GABA induced membrane current. Int Patent No. WO94/25027. 1994 Oct 11.
- Bergmann R, Kongsbak K, Sørensen PL, Sander T, Balle T. (2013) A unified model of the GABA(A) receptor comprising agonist and benzodiazepine binding sites. PLoS ONE 8:e52323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, et al. (2004) Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101:3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, et al. (2006) α5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci 26:3713–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut JD, Baytan AR, Russell M, Chang MP, Bernard A, Maxwell IH, Hoeffler JP. (1996) Selective isolation of transiently transfected cells from a mammalian cell population with vectors expressing a membrane anchored single-chain antibody. J Immunol Methods 193:17–27 [DOI] [PubMed] [Google Scholar]
- Chiron C. (2007) Stiripentol. Neurotherapeutics 4:123–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun SK, Jo YH. (2010) Loss of leptin receptors on hypothalamic POMC neurons alters synaptic inhibition. J Neurophysiol 104:2321–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer BA, Morton CJ, Parker MW. (2002) Anxiety over GABA(A) receptor structure relieved by AChBP. Trends Biochem Sci 27:280–287 [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WFA, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, et al. (2005) Evidence for a significant role of α 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci 25:10682–10688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drafts BC, Fisher JL. (2004) Structural determinants of the pharmacological properties of the GABAA receptor α6 subunit. J Pharmacol Exp Ther 309:1108–1115 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229 [DOI] [PubMed] [Google Scholar]
- Fisher JL. (2004) The α 1 and α 6 subunit subtypes of the mammalian GABA(A) receptor confer distinct channel gating kinetics. J Physiol 561:433–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL. (2009) The anti-convulsant stiripentol acts directly on the GABAA receptor as a positive allosteric modulator. Neuropharmacology 56:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Zhang J, Macdonald RL. (1997) The role of α1 and α6 subtype amino-terminal domains in allosteric regulation of γ-aminobutyric acida receptors. Mol Pharmacol 52:714–724 [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. (1993) Cloning of cDNA sequences encoding human α 2 and α 3 γ-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant α 1-, α 2-, α 3-, and α 5-containing human γ-aminobutyric acidA receptors. Mol Pharmacol 43:970–975 [PubMed] [Google Scholar]
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJS, Whiting PJ. (1996) Cloning of cDNAs encoding the human γ-aminobutyric acid type A receptor α 6 subunit and characterization of the pharmacology of α 6-containing receptors. Mol Pharmacol 49:253–259 [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. (2005) Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci 8:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing T, Poulter MO. (2007) Diversity of GABA(A) receptor synaptic currents on individual pyramidal cortical neurons. Eur J Neurosci 25:723–734 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloda JH, Czajkowski C. (2007) Agonist-, antagonist-, and benzodiazepine-induced structural changes in the α1 Met113-Leu132 region of the GABAA receptor. Mol Pharmacol 71:483–493 [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, et al. (2008) Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451:330–334 [DOI] [PubMed] [Google Scholar]
- Laha KT, Wagner DA. (2011) A state-dependent salt-bridge interaction exists across the β/α intersubunit interface of the GABAA receptor. Mol Pharmacol 79:662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. (1992a) The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci 12:4151–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. (1992b) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci 12:1063–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Oh G, Orser BA. (2009) Etomidate targets α5GABAA receptors to regulate synaptic plasticity and memory blockade. Anesthesiology 111:1025–1035 [DOI] [PubMed] [Google Scholar]
- Martin LJ, Zurek AA, MacDonald JF, Roder JC, Jackson MF, Orser BA. (2010) α5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J Neurosci 30:5269–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows HJ, Harries MH, Thompson M, Benham CD. (1997) Effect of SB-205384 on the decay of GABA-activated chloride currents in granule cells cultured from rat cerebellum. Br J Pharmacol 121:1334–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows HJ, Kumar CS, Pritchett DB, Blackburn TP, Benham CD. (1998) SB-205384: a GABA(A) receptor modulator with novel mechanism of action that shows subunit selectivity. Br J Pharmacol 123:1253–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Long BC, Pletcher MT. (2010) Quantitative trait locus analysis identifies Gabra3 as a regulator of behavioral despair in mice. Mamm Genome 21:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhler H. (2012) The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62:42–53 [DOI] [PubMed] [Google Scholar]
- Navarro JF, Burón E, Martín-López M. (2006) Anxiolytic-like activity of SB-205384 in the elevated plus-maze test in mice. Psicothema 18:100–104 [PubMed] [Google Scholar]
- Navarro JF, Burón E, Martín-López M. (2008) Effects of SB-205384, a positive modulator of α3-subunit-containing GABA-A receptors, on isolation-induced aggression in male mice. Psicothema 20:144–147 [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. (1998) Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18:1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. (2009) GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton AJ, Fisher JL. (2007) Effect of the α subunit subtype on the macroscopic kinetic properties of recombinant GABA(A) receptors. Brain Res 1165:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. (2011) Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 10:685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. (2004) Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol 44:475–498 [DOI] [PubMed] [Google Scholar]
- Sancar F, Czajkowski C. (2011) Allosteric modulators induce distinct movements at the GABA-binding site interface of the GABA-A receptor. Neuropharmacology 60:520–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. (1996) Properties of putative cerebellar γ-aminobutyric acid A receptor isoforms. Mol Pharmacol 49:567–579 [PubMed] [Google Scholar]
- Sigel E, Baur R. (2000) Electrophysiological evidence for the coexistence of α1 and α6 subunits in a single functional GABA(A) receptor. J Neurochem 74:2590–2596 [DOI] [PubMed] [Google Scholar]
- Sine SM, Engel AG. (2006) Recent advances in Cys-loop receptor structure and function. Nature 440:448–455 [DOI] [PubMed] [Google Scholar]
- Smith KS, Rudolph U. (2012) Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABAA receptor subtypes. Neuropharmacologt 62:54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub CJ, Lau HM, Parlato R, Schuetz G, Fritschy JM, Rudolph U. (2013) Bidirectional regulation of intravenous general anesthetic actions by α3-containing γ-aminobutyric acid A receptors. Anesthesiology 118:562–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusisaari M, Knöpfel T. (2008) GABAergic synaptic communication in the GABAergic and non-GABAergic cells in the deep cerebellar nuclei. Neuroscience 156:537–549 [DOI] [PubMed] [Google Scholar]
- Westh-Hansen SE, Rasmussen PB, Hastrup S, Nabekura J, Noguchi K, Akaike N, Witt M-R, Nielsen M. (1997) Decreased agonist sensitivity of human GABA(A) receptors by an amino acid variant, isoleucine to valine, in the α1 subunit. Eur J Pharmacol 329:253–257 [PubMed] [Google Scholar]
- Westh-Hansen SE, Witt MR, Dekermendjian K, Liljefors T, Rasmussen PB, Nielsen M. (1999) Arginine residue 120 of the human GABAA receptor alpha 1, subunit is essential for GABA binding and chloride ion current gating. Neuroreport 10:2417–2421 [DOI] [PubMed] [Google Scholar]
- Whiting PJ. (2006) GABA-A receptors: a viable target for novel anxiolytics? Curr Opin Pharmacol 6:24–29 [DOI] [PubMed] [Google Scholar]
- Wingrove P, Hadingham K, Wafford K, Kemp JA, Ragan CI, Whiting P. (1992) Cloning and expression of a cDNA encoding the human GABA-A receptor α 5 subunit. Biochem Soc Trans 20:18S. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12:1040–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Keist R, von Boehmer L, Studer R, Benke D, Hagenbuch N, Dong Y, Malenka RC, Fritschy J-M, Bluethmann H, et al. (2005) A schizophrenia-related sensorimotor deficit links α 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci USA 102:17154–17159 [DOI] [PMC free article] [PubMed] [Google Scholar]