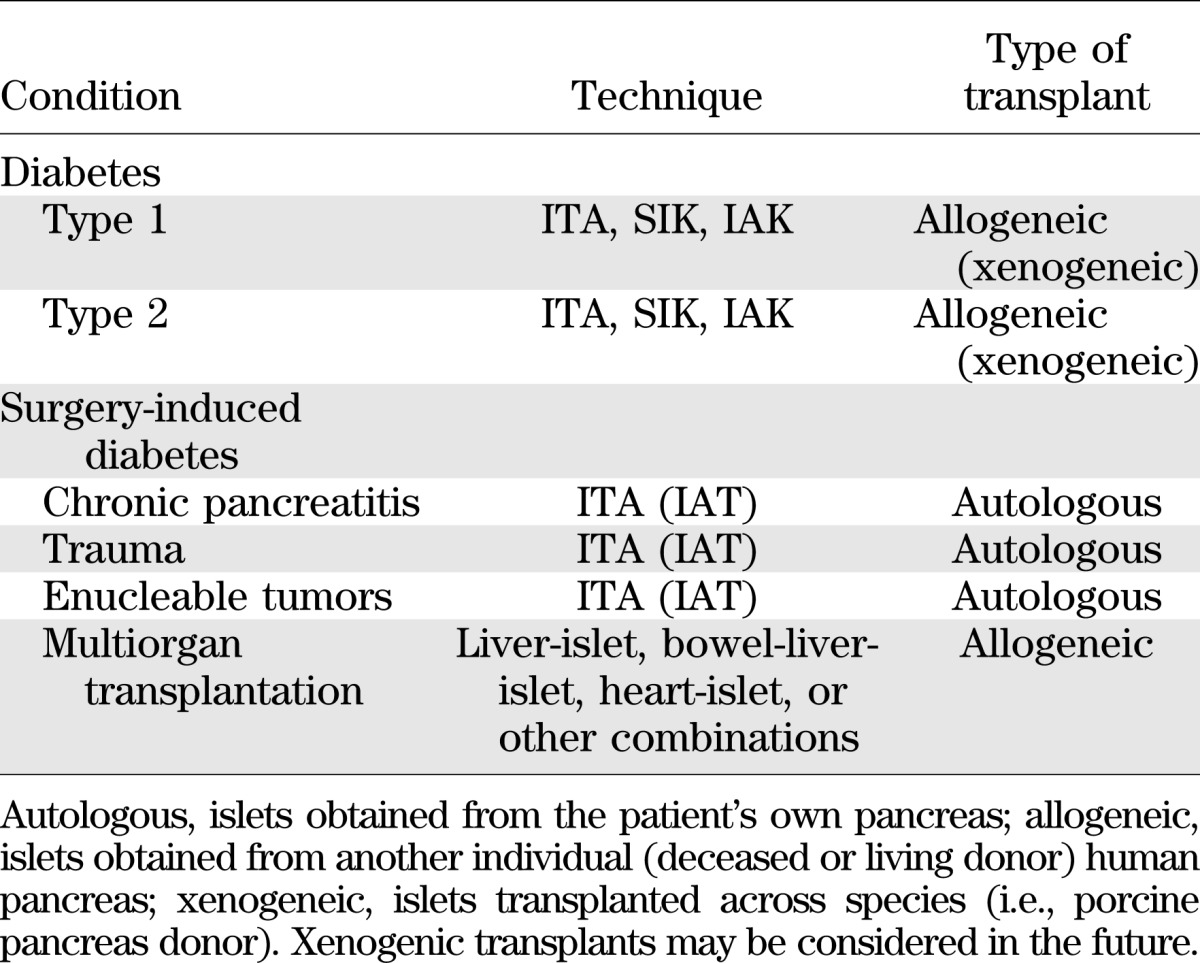
Transplantation of pancreatic islets represents a clinical therapeutic option to preserve and/or restore β-cell function in patients with diabetes (1,2). The source of the islets is the patient’s own pancreas (autologous, islet autotransplantation [IAT]) when the goal is preserving pancreatic endocrine function in pediatric and adult individuals undergoing total pancreatectomy due to pancreatitis (3,4) or trauma (5,6). Recently, IAT has been also proposed for enucleable benign (7) and malignant (8) pancreatic neoplasms. Transplantation of deceased-donor (allogeneic) islets is performed for patients with brittle type 1 diabetes and hypoglycemia unawareness as islet transplant alone (ITA) if nonuremic and as simultaneous islet–kidney (SIK) or sequential islet after kidney (IAK) transplantation procedures if uremic (end-stage renal disease) requiring kidney transplantation. Allogeneic islets can be part of cluster organ transplantation (Table 1). In recognition of the excellent metabolic control obtained after islet transplantation even when exogenous insulin treatment is required, reimbursement has been approved in several countries (e.g., Australia, Canada, France, Italy, Switzerland, U.K., Sweden, and the Nordic Network). In the U.S., only IAT is currently reimbursed, while biological licensure by the U.S. Food and Drug Administration should be imminent after recent completion of the Clinical Islet Transplant Consortium registration trials (www.citisletstudy.org).
TABLE 1.
Indications for islet cell transplantation
Since the 1970s, islets have been embolized into the hepatic portal system by a minimally invasive technique consisting of transhepatic cannulation of the portal vein under ultrasound and fluoroscopy guidance followed by sealing of the tract with thrombostatic treatment (2). Alternatively, in patients at risk for bleeding, the transplant is performed by cannulation of a tributary of the portal vein using open surgery (minilaparotomy) or laparoscopic approach.
An instant blood-mediated inflammatory reaction occurring after intraportal islet infusion may activate the coagulation cascade and the endothelium of the hepatic sinusoids, triggering adhesion of platelets and leukocytes and generation of thrombi and ischemia, contributing to the loss of a conspicuous mass of transplanted tissue. Nonspecific inflammation generated at the time of transplant may heighten the intensity of subsequent adaptive immune responses. In organ transplantation, these responses are responsible for higher incidence of acute and chronic rejection episodes, and in type 1 diabetes they also promote the recurrence of autoimmunity. Other disadvantages of the hepatic site include the relatively hyperglycemic environment and the elevated concentration of immunosuppressants (first-pass) that are toxic to islets.
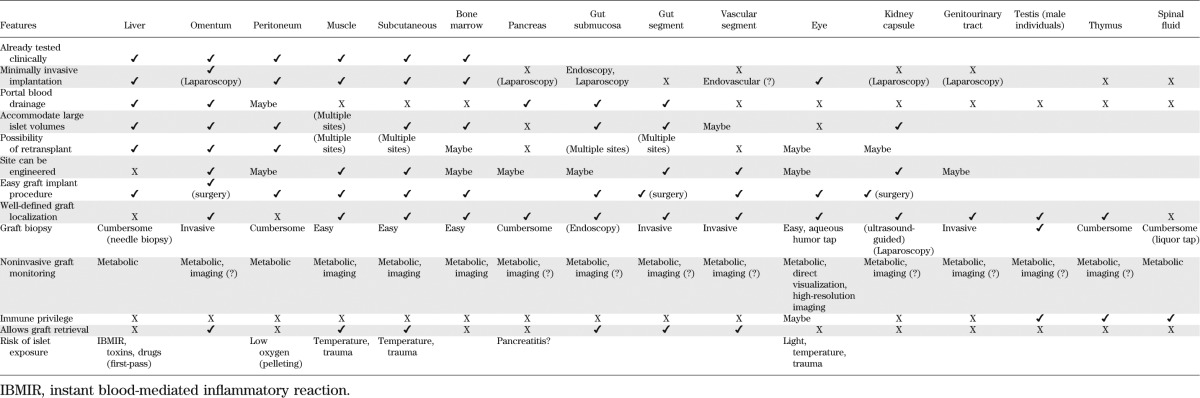
Definition of extrahepatic transplantation sites is recognized as a research priority. Ongoing investigations (Table 2) aimed at identifying a microenvironment that could provide prompt engraftment and minimize early inflammation and islet cell death while achieving sustained function are of particular interest. Engraftment of islet grafts in several extrahepatic sites with or without bioengineering strategies has been demonstrated in experimental models (2,9–11), although clinical translation for some remains arguable (Table 2). An ideal new “home” for islet grafts should accommodate relatively large volumes of tissue (e.g., low purity, or pooled donor islet preparations, and/or retransplantation), rely on minimally invasive transplant procedures, and allow for noninvasive longitudinal monitoring and easy access for biopsy. Portal blood drainage may be preferable to reproduce physiological metabolic responses. Confinement and retrievability of the graft is desirable, particularly for bioengineering approaches to optimize the site. Extrahepatic sites already tested in humans include muscle (12,13) and peritoneal cavity (to accommodate large microencapsulated islets) (14,15).
TABLE 2.
Implantation sites for islet cell transplantation
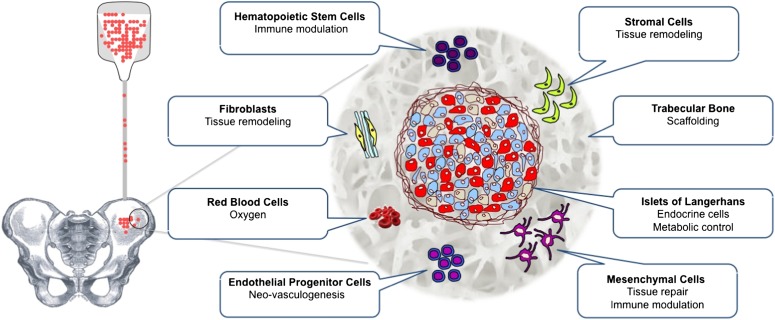
The new pilot study by Maffi et al. (16) in this issue supports the clinical feasibility and safety of intra-bone marrow (BM) islet transplantation. Four patients underwent total pancreatectomy and, as intrahepatic islet transplantation was contraindicated for anatomical or medical reasons, the autologous islet suspension (IAT) was injected via puncture of the iliac crest under local anesthesia (Fig. 1). Neither adverse events related to the transplant nor apparent alterations of hematopoiesis were observed. Successful intramarrow islet engraftment was documented in all patients as detectable fasting and simulated circulating C-peptide levels. Marrow biopsy and aspirate demonstrated physiologic microenvironment patterns. Well-preserved islet morphology, cytoarchitecture with normal distribution of endocrine cell subsets and vascular structures, and expression of transcription factors specific for endocrine precursors and mature β-cells were detected in collected specimens. Hypointense magnetic resonance imaging signal and calcifications detected at the transplant site possibly reflect microenvironment reactivity and remodeling worthy of further investigation.
FIG. 1.
Schematics of intra-BM islet transplantation and graft microenvironment.
The advantages emerging from this study of the marrow over the liver are easy graft implantation and monitoring by obtaining adequate biopsies (unlike the case for intrahepatic islets that are broadly dispersed in a large parenchyma). Appealing features of the marrow microenvironment include its richness in hematopoietic, mesenchymal (stromal), and endothelial cell precursors that could contribute to tissue repair/remodeling and, in turn, promote islet engraftment (Fig. 1). Immunomodulatory properties of BM cell subsets might assist in reducing early inflammation and improving the survival of allogeneic islet grafts in patients with type 1 diabetes (17–19). In previous rodent studies, engraftment and function of syngeneic islets had superior results in the femoral BM than in the liver (20). However, in the present clinical study, C-peptide levels appeared lower for intra-BM grafts than for comparable patients receiving intrahepatic IAT (16). Species differences may account for the discrepancy in outcomes between rodent and human transplants. Also, the limited number and heterogeneity of the clinical cases does not allow for generalizations at this stage.
The pilot trial is innovative as proof-of-concept that the BM is a viable clinical alternative to the intrahepatic site in cases in which the latter is contraindicated. However, application of the BM as gold standard for islet implantation is questionable at the present time. Besides lack of portal drainage and retrievability (limitations common to the liver), additional studies are needed to further the understanding of the features and potential of the BM site in humans, ascertaining the optimal islet mass needed to restore euglycemia, the possibility of infusing large islet volumes without compromising engraftment, the impact of immunotherapy on islet engraftment and function, and the long-term efficacy and safety of clinical intra-BM islet transplantation.
Identification of a clinically relevant new home for islet grafts will likely contribute to achieving reproducibly successful biological replacement of β-cell function in insulin-requiring diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (5U19AI050864-10, U01DK089538, 5U42RR016603-08S1, 1DP2DK083096-01, 1R01EB008009-02, 5R01DK059993-06, 1 R21 DK076098-01, 1 U01 DK70460-02, 5R01DK25802-24, 5R01DK56953-05), the Juvenile Diabetes Research Foundation International (17-2012-361, 17-2010-5, 4-2008-811, 6-39017G1, 4-2004-361, 4-2000-947), the American Diabetes Association (7-13-IN-32 to A.P.), The Leona M. and Harry B. Helmsley Charitable Trust, the University of Miami Interdisciplinary Research Development Initiative (to A.P.), the Diabetes Research Institute Foundation, and Converge Biotech. A.P. and C.R. are members of the scientific advisory board and stock option holders in Converge Biotech, licensee of some of the intellectual property that may be related to the topic discussed in this article. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 3523.
REFERENCES
- 1.Ricordi C. Islet transplantation: a brave new world. Diabetes 2003;52:1595–1603 [DOI] [PubMed] [Google Scholar]
- 2.Piemonti L, Pileggi A. 25 years of the Ricordi Automated Method for islet isolation. CellR4 2013;1:8–22 [PMC free article] [PubMed]
- 3.Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011;9:793–799 [DOI] [PMC free article] [PubMed]
- 4.Bellin MD, Balamurugan AN, Pruett TL, Sutherland DE. No islets left behind: islet autotransplantation for surgery-induced diabetes. Curr Diab Rep 2012;12:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garraway NR, Dean S, Buczkowski A, et al. Islet autotransplantation after distal pancreatectomy for pancreatic trauma. J Trauma 2009;67:E187–E189 [DOI] [PubMed] [Google Scholar]
- 6.Jindal RM, Ricordi C, Shriver CD. Autologous pancreatic islet transplantation for severe trauma (letter). N Engl J Med 2010;362:1550. [DOI] [PubMed] [Google Scholar]
- 7.Oberholzer J, Mathe Z, Bucher P, et al. Islet autotransplantation after left pancreatectomy for non-enucleable insulinoma. Am J Transplant 2003;3:1302–1307 [DOI] [PubMed]
- 8.Balzano G, Maffi P, Nano R, et al. Extending indications for islet autotransplantation in pancreatic surgery. Ann Surg 2013;258:210–218 [DOI] [PubMed] [Google Scholar]
- 9.Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011;11:364–374 [DOI] [PubMed] [Google Scholar]
- 10.Smink AM, Faas MM, de Vos P. Toward engineering a novel transplantation site for human pancreatic islets. Diabetes 2013;62:1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady AC, Martino MM, Pedraza E, et al. Pro-angiogenic hydrogels within macroporous scaffolds enhances islet engraftment in an extrahepatic site. Tissue Eng Part A. 22 June 2013 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafael E, Tibell A, Ryden M, et al. Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: a 2-year follow-up. Am J Transplant 2008;8:458–462 [DOI] [PubMed]
- 13.Dardenne S, Sterkers A, Leroy C, et al. Laparoscopic spleen-preserving distal pancreatectomy followed by intramuscular autologous islet transplantation for traumatic pancreatic transection in a young adult. JOP 2012;13:285–288 [PubMed]
- 14.Elliott RB, Escobar L, Tan PL, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 2007;14:157–161 [DOI] [PubMed] [Google Scholar]
- 15.Basta G, Montanucci P, Luca G, et al. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: four cases. Diabetes Care 2011;34:2406–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013;62:3523–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Y, Bushell A, Wood KJ. Mesenchymal stem-cell immunosuppressive capabilities: therapeutic implications in islet transplantation. Transplantation 2010;89:270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo JZ, Xiong F, Al-Homsi AS, Ricordi C, Luo L. Allogeneic bone marrow cocultured with human islets significantly improves islet survival and function in vivo. Transplantation 2013;95:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fotino C, Ricordi C, Lauriola V, Alejandro R, Pileggi A. Bone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetes. Rev Diabet Stud 2010;7:144–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantarelli E, Melzi R, Mercalli A, et al. Bone marrow as an alternative site for islet transplantation. Blood 2009;114:4566–4574 [DOI] [PubMed] [Google Scholar]