Abstract
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are incretin hormones that control the secretion of insulin, glucagon, and somatostatin to facilitate glucose disposal. The actions of incretin hormones are terminated via enzymatic cleavage by dipeptidyl peptidase-4 (DPP-4) and through renal clearance. GLP-1 and GIP promote β-cell proliferation and survival in rodents. DPP-4 inhibitors expand β-cell mass, reduce α-cell mass, and inhibit glucagon secretion in preclinical studies; however, whether incretin-based therapies sustain functional β-cell mass in human diabetic subjects remains unclear. GLP-1 and GIP exert their actions predominantly through unique G protein-coupled receptors expressed on β-cells and other pancreatic cell types. Accurate localization of incretin receptor expression in pancreatic ductal or acinar cells in normal or diabetic human pancreas is challenging because antisera used for detection of the GLP-1 receptor often are neither sufficiently sensitive nor specific to yield reliable data. This article reviews recent advances and controversies in incretin hormone action in the pancreas and contrasts established mechanisms with areas of uncertainty. Furthermore, methodological challenges and pitfalls are highlighted and key areas requiring additional scientific investigation are outlined.
Incretins are gut-derived circulating peptide hormones that potentiate glucose-dependent insulin secretion following ingestion of a meal. Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are the major incretin hormones. The insulinotropic actions of endogenously secreted GLP-1 and GIP are transient because both peptides are rapidly cleared by the kidney and inactivated by cleavage at the N-terminus by dipeptidyl peptidase-4 (DPP-4), a ubiquitous exopeptidase. Potentiation of incretin action underlines two therapeutic classes of glucose-lowering agents: GLP-1 receptor (GLP-1R) agonists and DPP-4 inhibitors (1). Original concepts of GIP and GLP-1 biology, which focused primarily on islet β-cells, have been expanded to include actions on other cell types within and outside the pancreas (2,3). There is now considerable interest in understanding how the potentiation of incretin action controls multiple facets of pancreatic biology, encompassing the regulation of glucose sensing, hormone secretion, and cell proliferation, differentiation, and survival. Recent studies have suggested that incretin therapies promote pancreatic inflammation as well as aberrant cell proliferation within the endocrine and exocrine pancreas (4,5); substantial technical and methodological issues, however, limit the generalizability of these findings. This Perspectives in Diabetes evaluates the science supporting existing dogma and discusses new concepts, controversies, and uncertainties in the biology of incretin action in the pancreas.
LOCALIZATION OF INCRETIN RECEPTOR EXPRESSION IN THE PANCREAS
Several dozen commercial antisera are available for detection of GLP-1R and GIP receptor expression by immunohistochemical techniques and Western blotting, and real-time PCR is widely used to quantify expression of incretin receptor genes in pancreatic exocrine and endocrine compartments. Most antisera used to detect GLP-1R expression (by immunohistochemistry or Western blot analysis) are neither sensitive nor specific (6,7). Important control experiments (absorption of the antibody with a peptide epitope, demonstration that the antibody recognizes only a single protein, and failure to generate a signal in cells that do not express a full-length receptor mRNA transcript or in tissues from Glp1r−/− mice) are usually absent. Furthermore, multiple studies describe GLP-1R protein expression in cells or tissues that do not express full-length Glp1r mRNA. The widespread use of tightly cropped bands in Western blot analysis precludes accurate assessment of whether a putative band/protein detected by Western blotting is the correct size, the only GLP-1R immunoreactive protein visualized, or one of several unrelated immunoreactive proteins detected by the same antisera.
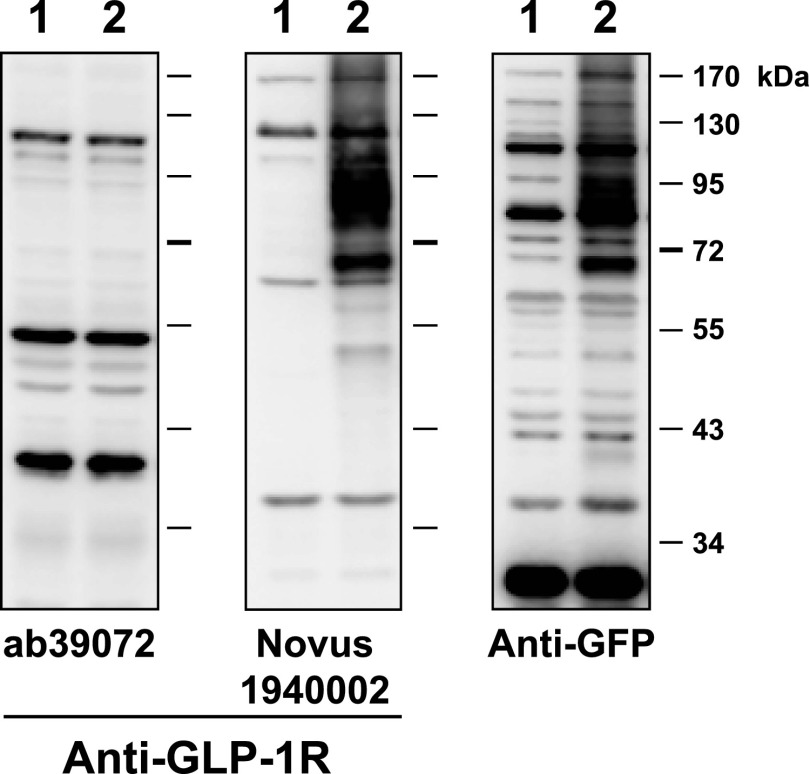
Scientists who are interested in the expression of incretin hormone receptors face the challenging task of assessing how much, if any, of the data published with these antisera is correct. For example, immunoreactive GLP-1R protein expression or Glp1r mRNA transcripts have been detected throughout the heart and ventricle; however, we and others determined that cardiac Glp1r expression was restricted to the atria and absent from the ventricles in mice (8) and rats (9). How do the limitations of available reagents affect our understanding of incretin action in the pancreas? The putative localization of incretin receptor expression in the exocrine pancreas provides an instructive example. Abundant immunohistochemical GLP-1R expression in ductal and acinar cells was reported in rodent and human pancreas, papillary thyroid cancer, and pancreatic adenocarcinoma (10,11). Characterization of multiple GLP-1R antisera, including Abcam39072 (11), one of the reagents used in these studies, revealed major problems with sensitivity and specificity. These antisera detected multiple spurious bands in Western blot analyses of fibroblasts that do not express the GLP-1R and in cellular extracts from Glp1r−/− mice (6). We now extend these analyses to detection of the human GLP-1R. Western blot analysis using fibroblasts transfected with human GLP-1R cDNA shows that Abcam39072 does not detect the human GLP-1R (Fig. 1). A second antiserum, distributed by Novus Biologicals (1940002), recognizes the human GLP-1R protein (Fig. 1) but also detects multiple spurious bands/proteins in control cells that do not express the Glp1r (Fig. 1). Similar problems with the sensitivity and specificity of GLP-1R antisera have been described by others (7). Hence the majority of published studies using multiple GLP-1R antisera must be discounted until the experimental data are independently verified with validated, highly sensitive, and highly specific antisera.
FIG. 1.
Characterization of the sensitivity and specificity of antisera against the human GLP-1R. Baby hamster kidney cells were transiently transfected with the vector pcDNA3.1 alone (lane 1) or with a human Glp1r cDNA tagged at the C-terminus with green fluorescent protein (GFP) cloned into pcDNA3.1 (lane 2). Whole-cell extracts were prepared 48 h after transfection and analyzed by immunoblotting with the indicated commercial GLP-1R antibodies or with a rabbit polyclonal antibody against GFP (Abcam ab6556). Molecular mass standards are indicated on the right. Both the anti-GLP-1R antibody Novus1940002 and the anti-GFP antibody detected similar immunoreactive proteins of ∼68 and ∼87 kDa, likely representing species of the GLP-1R-GFP fusion protein that were glycosylated differently.
Similar concerns relate to the interpretation of some experiments using regular PCR or real-time PCR to detect expression of the incretin receptor gene. Real-time PCR detects Glp1r mRNA transcripts by generating an amplicon of less than 100 base pairs, whereas regular PCR frequently uses primer pairs that generate Glp1r PCR products that are several hundred base pairs in length; both are far smaller than the entire full-length GLP-1R open reading frame. However, cells may generate noncoding mRNA transcripts detectable by regular or real-time PCR. Analysis of Gipr expression revealed ∼64 possible Gipr mRNA splice variants in RNA from human adipose tissue, only two of which were predicted to contain an open reading frame sufficient to give rise to a fully functional, membrane-spanning GIP receptor protein (12). Whether one or more of these variant Gipr RNA transcripts encodes a truncated GIP receptor protein that might exhibit dominant negative signaling activity, as described in mouse β-cells (13), requires further investigation. Furthermore, using a polyclonal antiserum, an immunoreactive GIP receptor protein was detected in human skeletal muscle (12), a tissue not previously reported to express full-length Gipr mRNA transcripts (14). Despite reports describing the detection of 1) partial Glp1r mRNA transcripts by PCR or 2) immunoreactive GLP-1R proteins by Western blotting or immunohistochemistry in murine liver, macrophages, or ventricular cardiomyocytes (2), we could not detect full-length Glp1r mRNA transcripts in the same cells and tissues (6,8).
Given the considerable limitations of commonly used reagents and techniques, how should we interpret available data reporting localization of GLP-1R expression in the endocrine and exocrine pancreas? The difficulty in isolating pure ductal, acinar, or islet cell RNA that is free from contamination by other cell types renders use of such cell fractions suboptimal for the analysis of cell-specific gene expression. Some groups have localized GLP-1R expression in islet α-cells (15); however, analysis of Glp1r mRNA transcripts in RNA from purified murine α-cells and β-cells that were sorted using a fluorescence-activated cell sorter failed to detect Glp1r mRNA transcripts in α-cells (K. Furuyama, P. Herrera, personal communication). Similarly, Glp1r and Gcgr mRNA transcripts were not detected by in situ hybridization in rat or mouse α-cells, respectively (16,17). Although Gipr mRNA transcripts were detected in rodent α-cells (18), less information is available regarding Glp1r or Gipr expression in human α-cells. GLP-1R activation stimulates secretion of islet somatostatin, but whether some, most, or few somatostatin-producing δ-cells express the GLP-1R has not been established. DPP-4 expression at the cell surface has been identified on murine α-cells and β-cells and even more strongly on ductal cells (19); however, whether DPP-4 activity locally regulates bioactive incretin activity within these pancreatic cell types has not been determined.
Glp1r mRNA transcripts have been detected in pancreatic ductal human pancreatic adenocarcinoma cell lines (20). However the GLP-1R agonist exendin-4 failed to stimulate growth or enhance cell survival in five different human pancreatic cancer cell lines that express an endogenous Glp1r mRNA transcript. Whether Glp1r mRNA transcripts are expressed in non-immortalized pancreatic ductal or acinar cells remains uncertain. Tornehave et al. (16) were unable to demonstrate Glp1r mRNA transcripts in pancreatic duct cells from mice and rats by in situ hybridization, despite detection of an immunoreactive protein in ducts using a GLP-1R antibody that was subsequently shown to exhibit suboptimal specificity (6). Transcriptome analysis of human pancreatic endocrine and exocrine cells detected glucagon receptor (Gcgr) expression in ductal cells, but Glp1r expression was not reported (21). Despite immunohistochemical depiction of robust GLP-1R immunopositivity in human pancreatic cancer cells (22), using transcriptome analysis of publicly available databases (oncomine.com, version 4.4.3, and Genome Expression Omnibus, http://www.ncbi.nlm.nih.gov/geo/) we have been unable to find evidence that Glp1r mRNA transcripts are overexpressed in these tumors. Similarly, using in situ ligand binding and autoradiography, Körner et al. (23) were unable to detect GLP-1 binding sites in pancreatic adenocarcinomas. New studies using individual endocrine or acinar cells purified by fluorescence-activated cell sorter analysis or isolation of single pancreatic cells using laser-capture microdissection followed by the use of validated antisera and/or PCR analysis using primers that span the full-length Glp1r open reading frame should refine our understanding of the direct cellular targets of GLP-1 action in the pancreas.
Incretin-mediated control of islet hormone secretion.
The increasing realization that β-cells exhibit considerable functional heterogeneity begs the question of whether there is a gradient of incretin receptor expression and action in different β-cells and whether these putative gradients vary among islets of different size and location across species. Although the insulin-stimulating properties of GLP-1R agonists are preserved in experimental models of diabetes and human subjects with type 2 diabetes (T2D), the actions of GIP on the diabetic β-cell likely are attenuated because of downregulation of Gipr expression and/or attenuation of signaling pathways coupling GIP receptor activation to insulin secretion (2). The loss of GIP action in the diabetic pancreas is reversible in animal and human studies. Reduction of glycemia with phlorizin restores islet GIP receptor expression and insulin secretion in response to GIP in diabetic rats (24,25), whereas treating human subjects with T2D with insulin for 4 weeks to reduce levels of glycated hemoglobin to ∼7% significantly improves the insulin secretory response to exogenous GIP (26).
GLP-1 and GIP exert different actions on islet α-cells. GLP-1R agonists (and DPP-4 inhibitors) inhibit glucagon secretion in normoglycemic and diabetic animals and humans (27), most likely via GLP-1R–dependent stimulation of islet somatostatin secretion. In turn, somatostatin inhibits glucagon secretion through expression of somatostatin receptor 2 on α-cells (28). Conversely, GIP stimulates glucagon secretion in humans under conditions of hyperglycemia (29,30), but whether these actions reflect direct activation of α-cell GIP receptors (29) remains unclear. Intriguingly, rodent and human α-cells express immunoreactive and bioactive GIP, hence an intraislet paracrine or autocrine GIP axis, with locally produced GIP acting through α-cell GIP receptors, cannot be excluded (31).
PANCREATIC INCRETIN RECEPTOR SIGNALING: CELL PROLIFERATION AND APOPTOSIS
Expansion of β-cell mass.
Multiple preclinical studies demonstrate proliferative and antiapoptotic actions of GLP-1, leading to the expansion of β-cell mass (32). Early experiments promoted the concept that GLP-1R agonists stimulated neogenesis of β-cells via activation of a ductal cell GLP-1R (2,32). However, the contribution of β-cell neogenesis from ductal precursors to a generation of new β-cells in adult mice has been elegantly disputed (33). Antiapoptotic actions of GLP-1R agonists have been demonstrated in rodent and human islets (2,32) and in preclinical studies of transplanted human islet cells. Results of clinical studies assessing whether GLP-1R agonists preserve β-cell function in subjects with type 1 diabetes (T1D) or T2D are more disappointing. There is little evidence that in subjects with T2D prolonged therapy with GLP-1R agonists modifies the progressive decline in β-cell function, an indirect surrogate of β-cell mass, independent of changes in weight loss (34). Similarly, treatment with exenatide for 6–9 months in subjects with long-standing T1D who are C-peptide-positive, with or without immunosuppression (daclizumab), did not enhance β-cell function or suppress meal-stimulated glucagon levels (35). The available evidence from randomized controlled trials does not support the contention that exenatide or liraglutide produce a sustained or progressive improvement in β-cell function in subjects with T1D following islet transplantation.
Why have we not seen clinical evidence of expansion of functional β-cell mass in diabetic subjects treated with GLP-1R agonists or DPP-4 inhibitors? The majority of positive preclinical experiments were carried out in younger animals (2), whereas older rodent β-cells exhibit a substantially diminished or absent proliferative response to multiple regenerative stimuli, including GLP-1R agonists (36,37). The diminution in the replicative capacity of β-cells in response to GLP-1R agonists has been attributed to loss of proteins that regulate the cell cycle, such as Skp2 (that controls p27), and sustained expression of p16Ink4a in older rodent and human β-cells (38). Human β-cells seem to be much less responsive to proliferative agents such as GLP-1 compared to rodent β-cells (39), and β-cell replication is substantially diminished in older human subjects (40). Hence more work is required to understand whether an older diabetic human β-cell retains a meaningful capacity to proliferate, resist cell death, or retain a functional differentiated state in response to GLP-1R agonists.
Control of α-cell mass.
Multiple studies demonstrate that GLP-1R agonists and DPP-4 inhibitors inhibit glucagon secretion (2,27). Surprisingly, hyperplasia of glucagon-producing α-cells was described in pancreata from diabetic human subjects who received sitagliptin (n = 7) or exenatide (n = 1) for at least 1 year, leading to speculation that exposure to DPP-4 inhibitors and/or GLP-1R agonists promotes α-cell hyperplasia via a reduction in glucagon secretion (5). Ki67+ proliferating α-cells were not detected in these pancreata; hence putative mechanisms linking incretin action to expansion of α-cell mass remain unknown. Remarkably, the diabetic controls and subjects treated with incretin were substantially mismatched with regard to age, duration of diabetes, sex, age at onset of diabetes, medication profile, and history of ketoacidosis, precluding any meaningful interpretation of the data. Furthermore, these observations are contradicted by extensive preclinical studies in rodents and nonhuman primates that failed to detect α-cell hyperplasia despite systemic multiples of exposures to GLP-1R agonists or DPP-4 inhibitors much greater than those achieved in human subjects (41–44). Because the majority (7/8) of human pancreata studied were from subjects taking sitagliptin (5), we reviewed preclinical studies of DPP-4 inhibitors that reported changes in α-cell numbers (Supplementary Table 1). One of 20 studies described an increase in α-cells, 6 studies reported no change in α-cells, and 13 articles described a reduction in α-cell number, decreased α-cell proliferation, or both. Hence a substantial body of independent scientific experimentation (Supplementary Table 1), taken together with extensive preclinical data spanning thousands of mice, rats, and monkeys (41–44), consistently reports α-cell findings diametrically opposed to those reported in a small human autopsy pancreas study (5).
Scientists reporting α-cell hyperplasia in pancreata from subjects treated with sitagliptin or exenatide envisioned a pathway linking GLP-1–mediated reduction of glucagon secretion to an expansion of α-cell mass independent of changes in α-cell proliferation (5). Complete genetic attenuation of Gcgr expression in all tissues or extinction of glucagon receptor signaling in the liver leads to compensatory expansion of α-cell mass in an attempt to restore glucagon action, which is achieved via mechanisms linked to increased α-cell proliferation (45,46) (Fig. 2). However, the robust expansion of α-cell mass secondary to elimination of Gcgr signaling is independent of GLP-1R signaling (47,48). Furthermore, heterozygous Gcgr+/− mice do not exhibit α-cell hyperplasia and less than complete blockade of the Gcgr using a Gcgr antagonist administered to mice fed a high-fat diet for 82 days did not result in α-cell hyperplasia (49). Complete elimination of glucagon production also leads to α-cell hyperplasia (50); however, DPP-4 inhibitors or GLP-1R agonists generally produce a 20–50% reduction in plasma glucagon levels (27,51), a scenario that has never been shown to trigger α-cell hyperplasia. Hence a large amount of independent experimentation refutes the existence of a speculative pathway (5) linking partial reduction of glucagon secretion to expansion of α-cell mass and neuroendocrine tumor formation independent of changes in α-cell proliferation.
FIG. 2.
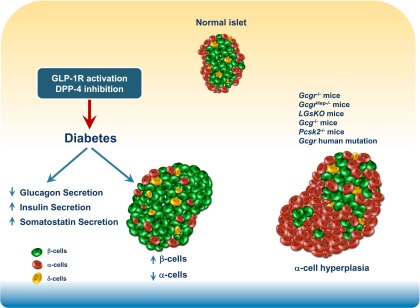
GLP-1 and DPP-4 inhibitor action in the endocrine pancreas. GLP-1R agonists and DPP-4 inhibitors enhance insulin and reduce glucagon secretion. In preclinical studies, these agents expand β-cell mass and reduce α-cell mass. Genetic mutations that disrupt glucagon receptor signaling or eliminate production of bioactive glucagon result in islet α-cell hyperplasia.
Acinar and ductal cells.
Notwithstanding the uncertainty about whether rodent or human acinar and ductal cells express a functional GLP-1R, older rodent pancreatic ductal cells retain the capacity to proliferate following GLP-1R activation. Indeed, a threefold increase in ductal proliferation was observed after a 7-day course of exendin-4 in three 7-month-old mice (38). Nevertheless, the hypothesis that sustained GLP-1R signaling and/or inhibition of Gcgr signaling (which also increases levels of GLP-1) will promote exocrine cell proliferation, leading to the expansion of exocrine mass (5), has not been consistently reproduced in nonsensitized preclinical models. Treatment with exendin-4 for 12 weeks in transgenic mice expressing an activated K-ras oncogene increased the expression of low-grade pancreatic intraepithelial neoplasia and enhanced ductal cell proliferation, but acinar cell proliferation was not reported (10). The assertion that Gcgr−/− mice or humans with a Gcgr null mutation exhibit enhanced exocrine proliferation (5) is not supported by the published data (52,53) cited by the same authors. Although Gcgr−/− mice exhibit pancreatic enlargement, increased acinar or ductal cell proliferation has not been detected by multiple independent groups that have studied these animals (45,46,48,52).
Histological analyses of the pancreas have been carried out after extensive chronic treatment (up to 2 years) with high doses of GLP-1R agonists in thousands of mice and rats and dozens of monkeys. None of the studies involving multiple doses of structurally distinct GLP-1R agonists has reported expansion of the ductal or exocrine compartments in rodents or nonhuman primates (41,42). Similarly, the DPP-4 inhibitors vildagliptin or sitagliptin, which were administered to hundreds of mice and rats continuously for 2 years (43,44) at doses producing high multiples of systemic drug exposure, did not result in acinar, ductal, or endocrine cell neoplasia. Although data from toxicology studies of diabetic animals is limited, a 3-month treatment regimen of exenatide twice daily at doses of 6, 40, and 250 µg/kg/day produced no changes in pancreatic exocrine structure or ductal proliferation (54). Similarly, no proliferative effects of exenatide or liraglutide were detected in the exocrine pancreas of diabetic Zucker diabetic fatty rats after 13 weeks of drug administration (55). Sitagliptin was administered for 3 months in monkeys, 12 months in dogs, and 24 months in mice and rats at doses producing levels of exposure considerably higher than those achieved clinically; no evidence of pancreatic abnormalities were detected on gross or histological analysis of the pancreas. However, precise details on the actual analyses carried out in these toxicology studies have not yet been published (44). Each pharmaceutical sponsor of a DPP-4 inhibitor or GLP-1R agonist is required to carry out 2-year carcinogenicity studies in 2 species; there have now been thousands of animals exposed to DPP-4 inhibitors and GLP-1R agonists in addition to the studies reported above. However, reports of ductal or acinar proliferation or pancreatic adenocarcinoma in preclinical studies, either in the form of toxicology reports submitted as part of new drug applications to regulatory authorities or as published manuscripts, have not yet been forthcoming.
Nevertheless, GLP-1R agonists increase the weight of the pancreas in some preclinical studies, most notably in young rodents (10,56), through mechanisms that are not completely understood. Selective restoration of the expression of human GLP-1R under the control of the Pdx1 promoter in β-cells and ducts normalized glucose homeostasis in Glp1r−/− mice but was not sufficient to mediate an increase in pancreatic weight in response to exogenous exendin-4 (57). Therefore, although insulin secretion is not sufficient for the increase in pancreatic mass observed secondary to GLP-1R activation, further studies are required to elucidate the precise cell types and mechanisms linking GLP-1R activation to changes in pancreatic weight.
GLP-1R signaling, DPP-4 inhibition, and pancreatic inflammation.
The glucose reduction achieved with DPP-4 inhibitors requires intact GLP-1R and GIP receptor signaling (58,59); however, non-glucoregulatory actions may be mediated by other substrates, including stromal-derived cell factor-1α (3,60). There are few data linking nonenzymatic signaling of DPP-4 to specific actions in the endocrine or exocrine pancreas. The widespread expression of GLP-1Rs on multiple immune cell populations (61), together with the expression and activity of DPP-4(CD26) in the immune system, provides a logical basis for exploring whether GLP-1R agonists and/or DPP-4 inhibitors modulate immune function. The majority of actions ascribed to DPP-4 in immune cells are attributable to nonenzymatic actions of the enzyme; hence DPP-4 signaling in immune cells proceeds independent of its catalytic enzyme activity (62). Accordingly, partial inhibition of the catalytic activity of DPP-4 using highly selective DPP-4 inhibitors would not be predicted to perturb immune function (60). Indeed, T-cell–dependent immune responses are preserved in Dpp4−/− mice and in mice treated with a highly selective DPP-4 inhibitor (63). Preclinical studies linking incretin action to enhanced pancreatic inflammation include the observation that one of eight human islet amyloid polypeptide (hIAPP) transgenic rats treated with sitagliptin for 12 weeks developed focal pancreatic inflammation (64).
In an attempt to reproduce abnormalities reported in the exocrine pancreas of hIAPP transgenic rats treated with sitagliptin for 12 weeks (64), Aston-Mourney et al. (65) fed hIAPP transgenic mice a high-fat diet and treated them with sitagliptin or metformin alone or in combination for 12 months. In contrast to findings observed in hIAPP transgenic rats (64), islet amyloid deposition, ductal cell proliferation, and pancreatic mass were not increased by sitagliptin in hIAPP transgenic mice; however, β-cell mass was increased, consistent with the known actions of sitagliptin in mice (2). Furthermore, sitagliptin treatment was not associated with pancreatic inflammation, necrosis, metaplasia, neoplasia, or periductal fibrosis; pancreatic mass was increased in mice treated with metformin but not in mice treated with sitagliptin (65).
Two reports describe nondiabetic rats treated with exenatide that developed pancreatic damage and inflammation (66,67). Notably, in both experiments, rats treated with exenatide experienced profound weight loss (25–30%); however, no pair-fed controls were included in these analyses, and mechanisms linking GLP-1R activation to increased pancreatic inflammation were not identified (66,67). Rapid, profound weight loss is frequently associated with a catabolic state, whereas more modest and gradual weight loss, particularly in the setting of preexisting obesity, is generally associated with reduced tissue and systemic markers of inflammation.
Increased pancreatic inflammation has not been detected in multiple preclinical studies examining chronic effects (up to 2 years) of administration of high doses of GLP-1R agonists or DPP-4 inhibitors in nondiabetic rodents or nonhuman primates (41–44). For example, treatment of diabetic rats with supratherapeutic doses of exenatide or liraglutide for 13 weeks was not associated with histological or biochemical evidence of pancreatic inflammation (54,55). Moreover, administration of GLP-1R agonists before or after the induction of experimental pancreatitis did not enhance pancreatic inflammation in normal or diabetic rats and mice (68,69); GLP-1R agonists unexpectedly induced an anti-inflammatory gene expression profile in the pancreas of insulin-resistant mice fed a high-fat diet (68).
Incretin-based therapies and inflammatory markers in humans.
Small increases in plasma levels of amylase and lipase have been reported in diabetic subjects treated with the DPP-4 inhibitors alogliptin and sitagliptin (70), and a separate observational study of diabetic subjects treated with sitagliptin, saxagliptin, or exenatide reported that 35.6% of subjects exhibited increases in plasma levels of amylase and/or lipase, with levels of lipase increasing to a relatively greater extent (71). Notably, elevated levels of amylase and lipase also were observed, albeit less frequently, in diabetic control subjects who did not receive a DPP-4 inhibitor or GLP-1R agonist. Further study is required to determine whether the increase in amylase and lipase reflects subclinical pancreatic inflammation or dysregulated synthesis, secretion, or clearance of these enzymes. Administration of GLP-1R agonists or DPP-4 inhibitors is associated with suppression of inflammation (72); however, many of these experiments do not control for concomitant reduction in glucose or body weight, which may also indirectly dampen inflammation. Exenatide administered twice daily for 12 weeks in subjects with T2D reduced circulating markers of inflammation in circulating mononuclear cells, independent of changes in body weight (73). A single, acute, 5-μg injection of exenatide significantly and rapidly reduced levels of reactive oxygen species, nuclear factor-κB binding activity, and expression of tumor necrosis factor-α, interleukin-1β, Jun NH2-terminal kinase-1, Toll-like receptor-4, and suppressor of cytokine signaling-3 mRNA transcripts in RNA isolated from circulating mononuclear cells (73). Similarly, administration of sitagliptin 100 mg once daily for 12 weeks to 12 subjects with T2D reduced expression of proinflammatory markers in circulating mononuclear cells, whereas acute administration of 100 mg sitagliptin to fasting diabetic subjects significantly reduced mononuclear cell expression of Toll-like receptor-2, IκB kinase β, chemokine receptor type 2, cluster of differentiation-26 mRNA transcripts and decreased nuclear factor-κB binding activity (74). Hence the available data indicate that GLP-1R agonists and DPP-4 inhibitors independently exert anti-inflammatory actions in tissues such as the exocrine and endocrine pancreas, as well as in circulating blood cells from diabetic subjects, although the mechanisms mediating these actions remain poorly understood.
SUMMARY AND PERSPECTIVE
The potential promise of incretin-based therapies has been partially realized in that we can now implement antidiabetic regimens associated with lower rates of hypoglycemia and weight gain. Although the first actions of GLP-1 on pancreatic islet cells were described more than 25 years ago, we still have much to learn about how GLP-1R signaling regulates β-cell function. For example, the molecular mechanisms underlying glucose-sensitive GLP-1R signaling have remained elusive. The precise cellular localization of the GLP-1R in islet and exocrine cells requires more careful study, not only in animals, but also in pancreata from human subjects over a broad range of ages and with and without preexisting diabetes or diseases of the pancreas.
Possible perils of incretin therapies include the development of complications, including pancreatitis and cancer. Although some studies combine groups of experimental subjects exposed to DPP-4 inhibitors and GLP-1R agonists for pooled analyses of adverse events (5,75), these two distinct drug classes exhibit mechanisms of action with multiple fundamental differences (2,60). Therefore it is not scientifically justifiable to pool subjects exposed to DPP-4 inhibitors and GLP-1R agonists. The hypothesis that activation of GLP-1R signaling might promote increased cell proliferation and increase the incidence or detection of specific neoplasms is reasonable. Indeed, rats and, to a lesser extent, mice exhibit C-cell hyperplasia and medullary thyroid cancer after prolonged, sustained exposure to GLP-1R agonists (76). Nevertheless, monkeys and humans exhibit major differences in GLP-1R expression in their thyroid C-cells, and in the vast majority of subjects calcitonin levels do not rise into the abnormal range following prolonged exposure to GLP-1R agonists (77). Studies assessing the pancreata of thousands and mice and rats have not shown dysplasia or tumor formation following treatment with GLP-1R agonists or DPP-4 inhibitors for periods up to 2 years. Furthermore, GLP-1 levels remain substantially elevated for years following many forms of bariatric surgery, yet rates of pancreatitis, medullary thyroid cancer, glucagonomas, and cancer of the pancreas are not increased in this patient population, despite more than 10 years of follow-up (78). Hence the hypothesis that GLP-1R agonists or DPP-4 inhibitors will promote tumor formation (4) is not supported by the available preclinical or clinical data.
Experimental evidence raising the possibility that incretin-based therapy may be associated with a predisposition to develop pancreatitis or pancreatic cancer generates important hypotheses that require testing in mechanistic preclinical studies and independent validation in large randomized, controlled clinical trials. Pathological pitfalls of incretin-based science include the use of nonspecific antisera and mismatched cases and controls and the generation of nonvalidated hypotheses and irreproducible data. As millions of patients with diabetes are being treated with incretin-based therapies, our collective responsibility to use higher-quality science has never been greater. Underpowered studies using poorly validated reagents or analysis of mismatched cases and controls (5) have a much greater certainty of not being reproducible and do not advance our understanding of incretin action in the pancreas. Emerging pharmacovigilance studies, such as the Safety Evaluation of Adverse Reactions in Diabetes (SAFEGUARD) study should shed additional clarity on the risk-to-benefit ratio of medications used to treat diabetes.
A great deal has been written about incretin action in the pancreas, including statements that are not substantiated or are contradicted by available data. For example, the claim that “production of exendin-4 causes rapid proliferation of intestinal tissue and a 50% increase in the size of the pancreas” (79) in Heloderma suspectum is simply incorrect and is clearly refuted by the actual experimental data cited (80). The ongoing debate surrounding the mechanisms of action and potential safety of incretin-based therapies reminds one of a quotation variably attributed to Daniel Patrick Moynihan, James Schlesinger, or Bernard Baruch: “Everyone is entitled to their own opinions, but they are not entitled to their own facts.” The beauty of science is that it is self-correcting, and provocative experiments and observations that are not highly reproducible are ultimately discarded. Over the next several years we will learn much more about the potential risks and benefits of incretin-based therapies from large, randomized, ongoing cardiovascular outcome studies, with rigorous independent adjudication of adverse events. Thoughtful scientists await the results of these and ongoing pharmacovigilance studies with great interest. The results of these trials will be extremely useful for increasing our understanding of incretin action in not only the cardiovascular system but also the diabetic pancreas.
ACKNOWLEDGMENTS
Pancreatic incretin research in the Drucker laboratory is supported by grants 123391 and 82700 from the Canadian Institute of Health Research.
D.J.D. is supported by appointments as a Canada Research Chair in Regulatory Peptides and the Banting and Best Diabetes Centre-Novo Nordisk Chair in Incretin Biology, and within the past 12 months has served as an advisor or consultant to Arisaph Pharmaceuticals Inc., Eli Lilly Inc., GlaxoSmithKline, Intarcia Therapeutics, Merck Research Laboratories, Novo Nordisk Inc., NPS Pharmaceuticals Inc., Sanofi, Takeda, and Transition Pharmaceuticals Inc. D.J.D. receives operating support for incretin research via grants to Mt. Sinai Hospital from GlaxoSmithKline, Merck, and Novo Nordisk. D.J.D. is party to a DPP-4 inhibitor license agreement, together with the University of Toronto, the University Health Network, Tufts University, and Arisaph Pharmaceuticals Inc. No other potential conflicts of interest relevant to this article were reported.
The author thanks Laurie Baggio, Jackie Koehler, and Bernardo Yusta (all from the Samuel Lunenfeld Research Institute, Mt. Sinai Hospital, Toronto, Ontario, Canada) for constructive comments. The author also thanks Jackie Koehler for her assessment of Glp1r expression in public databases, and Bernardo Yusta for performing Western blotting with the transfected GLP-1R cDNA and two GLP-1 receptor antisera.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0822/-/DC1.
REFERENCES
- 1.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 2.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013;17:819–837 [DOI] [PubMed] [Google Scholar]
- 3.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012;33:187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013;36:2118–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panjwani N, Mulvihill EE, Longuet C, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology 2013;154:127–139 [DOI] [PubMed] [Google Scholar]
- 7.Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor—or not? Endocrinology 2013;154:4–8 [DOI] [PubMed] [Google Scholar]
- 8.Kim M, Platt MJ, Shibasaki T, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 2013;19:567–575 [DOI] [PubMed] [Google Scholar]
- 9.Wohlfart P, Linz W, Hübschle T, et al. Cardioprotective effects of lixisenatide in rat myocardial ischemia-reperfusion injury studies. J Transl Med 2013;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model. Diabetes 2012;61:1250–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012;97:121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahlqvist E, Osmark P, Kuulasmaa T, et al. Link between GIP and osteopontin in adipose tissue and insulin resistance. Diabetes 2013;62:2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada N, Yamada Y, Tsukiyama K, et al. A novel GIP receptor splice variant influences GIP sensitivity of pancreatic beta-cells in obese mice. Am J Physiol Endocrinol Metab 2008;294:E61–E68 [DOI] [PubMed] [Google Scholar]
- 14.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993;133:2861–2870 [DOI] [PubMed] [Google Scholar]
- 15.Heller TB, Kieffer TJ, Habener JF. Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreas. Diabetes 1997;46:785–791 [DOI] [PubMed] [Google Scholar]
- 16.Tornehave D, Kristensen P, Rømer J, Knudsen LB, Heller RS. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J Histochem Cytochem 2008;56:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedees MH, Grigoryan M, Guz Y, Teitelman G. Differential expression of glucagon and glucagon-like peptide 1 receptors in mouse pancreatic alpha and beta cells in two models of alpha cell hyperplasia. Mol Cell Endocrinol 2009;311:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moens K, Heimberg H, Flamez D, et al. Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes 1996;45:257–261 [DOI] [PubMed] [Google Scholar]
- 19.Dorrell C, Grompe MT, Pan FC, et al. Isolation of mouse pancreatic alpha, beta, duct and acinar populations with cell surface markers. Mol Cell Endocrinol 2011;339:144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koehler JA, Drucker DJ. Activation of glucagon-like peptide-1 receptor signaling does not modify the growth or apoptosis of human pancreatic cancer cells. Diabetes 2006;55:1369–1379 [DOI] [PubMed] [Google Scholar]
- 21.Dorrell C, Schug J, Lin CF, et al. Transcriptomes of the major human pancreatic cell types. Diabetologia 2011;54:2832–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gier B, Butler PC. Glucagonlike peptide 1-based drugs and pancreatitis: clarity at last, but what about pancreatic cancer? JAMA Intern Med 2013;173:539–541 [DOI] [PubMed] [Google Scholar]
- 23.Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 2007;48:736–743 [DOI] [PubMed] [Google Scholar]
- 24.Xu G, Kaneto H, Laybutt DR, et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes 2007;56:1551–1558 [DOI] [PubMed] [Google Scholar]
- 25.Piteau S, Olver A, Kim SJ, et al. Reversal of islet GIP receptor down-regulation and resistance to GIP by reducing hyperglycemia in the Zucker rat. Biochem Biophys Res Commun 2007;362:1007–1012 [DOI] [PubMed] [Google Scholar]
- 26.Højberg PV, Vilsbøll T, Rabøl R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009;52:199–207 [DOI] [PubMed] [Google Scholar]
- 27.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 28.de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 2008;51:2263–2270 [DOI] [PubMed] [Google Scholar]
- 29.Chia CW, Carlson OD, Kim W, et al. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes 2009;58:1342–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund A, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am J Physiol Endocrinol Metab 2011;300:E1038–E1046 [DOI] [PubMed] [Google Scholar]
- 31.Fujita Y, Wideman RD, Asadi A, et al. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet alpha-cells and promotes insulin secretion. Gastroenterology 2010;138:1966–1975 [DOI] [PubMed] [Google Scholar]
- 32.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 2004;145:2653–2659 [DOI] [PubMed] [Google Scholar]
- 33.Xiao X, Chen Z, Shiota C, et al. No evidence for β cell neogenesis in murine adult pancreas. J Clin Invest 2013;123:2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rother KI, Spain LM, Wesley RA, et al. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care 2009;32:2251–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 2009;58:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 2009;58:1312–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tschen SI, Georgia S, Dhawan S, Bhushan A. Skp2 is required for incretin hormone-mediated β-cell proliferation. Mol Endocrinol 2011;25:2134–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parnaud G, Bosco D, Berney T, et al. Proliferation of sorted human and rat beta cells. Diabetologia 2008;51:91–100 [DOI] [PubMed] [Google Scholar]
- 40.Perl S, Kushner JA, Buchholz BA, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab 2010;95:E234–E239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkes DG, Mace KF, Trautmann ME. Discovery and development of exenatide: the first antidiabetic agent to leverage the multiple benefits of the incretin hormone, GLP-1. Expert Opin Drug Discov 2013;8:219–244 [DOI] [PubMed] [Google Scholar]
- 42.Nyborg NC, Mølck AM, Madsen LW, Knudsen LB. The human GLP-1 analog liraglutide and the pancreas: evidence for the absence of structural pancreatic changes in three species. Diabetes 2012;61:1243–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Busch SJ, Hoffmann P, Sahota P, et al. Studies in rodents with the dipeptidyl peptidase-4 inhibitor vildagliptin to evaluate possible drug-induced pancreatic histological changes that are predictive of pancreatitis and cancer development in man. Diabetes Obes Metab 2013;15:72–76 [DOI] [PubMed] [Google Scholar]
- 44.Engel SS, Williams-Herman DE, Golm GT, et al. Sitagliptin: review of preclinical and clinical data regarding incidence of pancreatitis. Int J Clin Pract 2010;64:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longuet C, Robledo AM, Dean ED, et al. Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: evidence for a circulating α-cell growth factor. Diabetes 2013;62:1196–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vuguin PM, Kedees MH, Cui L, et al. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology 2006;147:3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen M, Mema E, Kelleher J, et al. Absence of the glucagon-like peptide-1 receptor does not affect the metabolic phenotype of mice with liver-specific G(s)α deficiency. Endocrinology 2011;152:3343–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali S, Lamont BJ, Charron MJ, Drucker DJ. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J Clin Invest 2011;121:1917–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mu J, Jiang G, Brady E, et al. Chronic treatment with a glucagon receptor antagonist lowers glucose and moderately raises circulating glucagon and glucagon-like peptide 1 without severe alpha cell hypertrophy in diet-induced obese mice. Diabetologia 2011;54:2381–2391 [DOI] [PubMed] [Google Scholar]
- 50.Hayashi Y, Yamamoto M, Mizoguchi H, et al. Mice deficient for glucagon gene-derived peptides display normoglycemia and hyperplasia of islet alpha-cells but not of intestinal L-cells. Mol Endocrinol 2009;23:1990–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunning BE, Foley JE, Ahrén B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia 2005;48:1700–1713 [DOI] [PubMed] [Google Scholar]
- 52.Gelling RW, Du XQ, Dichmann DS, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A 2003;100:1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou C, Dhall D, Nissen NN, Chen CR, Yu R. Homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, alpha cell hyperplasia, and islet cell tumor. Pancreas 2009;38:941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tatarkiewicz K, Belanger P, Gu G, Parkes D, Roy D. No evidence of drug-induced pancreatitis in rats treated with exenatide for 13 weeks. Diabetes Obes Metab 2013;15:417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vrang N, Jelsing J, Simonsen L, et al. The effects of 13 wk of liraglutide treatment on endocrine and exocrine pancreas in male and female ZDF rats: a quantitative and qualitative analysis revealing no evidence of drug-induced pancreatitis. Am J Physiol Endocrinol Metab 2012;303:E253–E264 [DOI] [PubMed] [Google Scholar]
- 56.Baggio LL, Huang Q, Cao X, Drucker DJ. An albumin-exendin-4 conjugate engages central and peripheral circuits regulating murine energy and glucose homeostasis. Gastroenterology 2008;134:1137–1147 [DOI] [PubMed] [Google Scholar]
- 57.Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest 2012;122:388–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes 2007;56:3006–3013 [DOI] [PubMed] [Google Scholar]
- 59.Hansotia T, Baggio LL, Delmeire D, et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes 2004;53:1326–1335 [DOI] [PubMed] [Google Scholar]
- 60.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 2007;30:1335–1343 [DOI] [PubMed] [Google Scholar]
- 61.Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia 2010;53:730–740 [DOI] [PubMed] [Google Scholar]
- 62.Kirby M, Yu DM, O’Connor S, Gorrell MD. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin Sci (Lond) 2010;118:31–41 [DOI] [PubMed] [Google Scholar]
- 63.Vora KA, Porter G, Peng R, et al. Genetic ablation or pharmacological blockade of dipeptidyl peptidase IV does not impact T cell-dependent immune responses. BMC Immunol 2009;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matveyenko AV, Dry S, Cox HI, et al. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 2009;58:1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aston-Mourney K, Subramanian SL, Zraika S, et al. One year of sitagliptin treatment protects against islet amyloid-associated beta-cell loss and does not induce pancreatitis or pancreatic neoplasia in mice. Am J Physiol Endocrinol Metab 2013;305:E475–E484 [DOI] [PMC free article] [PubMed]
- 66.Yu X, Tang H, Huang L, Yang Y, Tian B, Yu C. Exenatide-induced chronic damage of pancreatic tissue in rats. Pancreas 2012;41:1235–1240 [DOI] [PubMed] [Google Scholar]
- 67.Nachnani JS, Bulchandani DG, Nookala A, et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia 2010;53:153–159 [DOI] [PubMed] [Google Scholar]
- 68.Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 2009;58:2148–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tatarkiewicz K, Smith PA, Sablan EJ, et al. Exenatide does not evoke pancreatitis and attenuates chemically induced pancreatitis in normal and diabetic rodents. Am J Physiol Endocrinol Metab 2010;299:E1076–E1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tokuyama H, Kawamura H, Fujimoto M, et al. A low-grade increase of serum pancreatic exocrine enzyme levels by dipeptidyl peptidase-4 inhibitor in patients with type 2 diabetes. Diabetes Res Clin Pract 2013;100:e66–e69 [DOI] [PubMed] [Google Scholar]
- 71.Lando HM, Alattar M, Dua AP. Elevated amylase and lipase levels in patients using glucagonlike peptide-1 receptor agonists or dipeptidyl-peptidase-4 inhibitors in the outpatient setting. Endocr Pract 2012;18:472–477 [DOI] [PubMed] [Google Scholar]
- 72.Drucker DJ, Rosen CF. Glucagon-like peptide-1 (GLP-1) receptor agonists, obesity and psoriasis: diabetes meets dermatology. Diabetologia 2011;54:2741–2744 [DOI] [PubMed] [Google Scholar]
- 73.Chaudhuri A, Ghanim H, Vora M, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab 2012;97:198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makdissi A, Ghanim H, Vora M, et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab 2012;97:3333–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med 2013;173:534–539 [DOI] [PubMed] [Google Scholar]
- 76.Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation [pulished correction appears in Endocrinology 2012;153:1000]. Endocrinology 2010;151:1473–1486 [DOI] [PubMed] [Google Scholar]
- 77.Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011;96:853–860 [DOI] [PubMed] [Google Scholar]
- 78.Sjöström L, Gummesson A, Sjöström CD, et al. Swedish Obese Subjects Study Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653–662 [DOI] [PubMed] [Google Scholar]
- 79.Gale E. Incretin therapy: should adverse consequences have been anticipated? BMJ 2013;346:f3617. [DOI] [PubMed] [Google Scholar]
- 80.Christel CM, DeNardo DF, Secor SM. Metabolic and digestive response to food ingestion in a binge-feeding lizard, the Gila monster (Heloderma suspectum). J Exp Biol 2007;210:3430–3439 [DOI] [PubMed] [Google Scholar]