In humans, there are a number of taste receptors on the tongue. These include G protein–coupled receptors (GPRs) for sweet, bitter, and umami (savory) tastes and ion channel–based receptors for salt and sour. In recent years, there has been increasing interest in the distribution and function of these receptors elsewhere in the body (1). This has led to interesting and occasionally surprising findings, some with potential therapeutic implications (e.g., activation of bitter taste receptors in the airway’s smooth muscle causes bronchodilation, suggesting a novel target for asthma therapy) (2). Of particular interest in the area of diabetes and obesity is the role of sweet taste receptors (STRs) in the intestine (3,4).
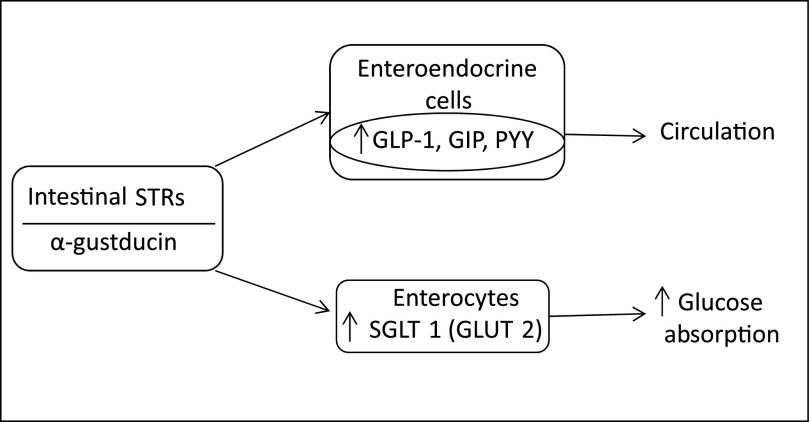
The upper gastrointestinal tract is well-endowed with taste and fat receptors, with sweet taste being detected, as elsewhere, by a heterodimer of the taste 1 receptor (T1R) family, T1R2/T1R3. These receptors have been localized to intestinal brush and enteroendocrine cells, and are coupled with α-gustducin as the α-subunit of the G protein (Fig. 1). They recognize sugars, d-amino acids, sweet proteins, and artificial sweeteners. Of importance to a possible role in the incretin response, these receptors are colocalized with glucagon-like peptide 1 (GLP-1) and L cells containing peptide YY (PYY) and K cells containing glucose-dependent insulinotropic polypeptide (GIP) (3). Incidentally, fatty acid responsive GPRs are also coupled to GLP-1 release, but are found predominantly in the colon (5).
FIG. 1.
Putative relationships between intestinal STRs, SGLT1 and GLUT2, glucose absorption, and incretin hormones GLP-1, GIP, and PYY.
The ability of intestinal STRs to activate the incretin response in humans without transport of sugars through the intestinal wall is not entirely clear. In vitro, nonnutritive sweeteners can release GLP-1 from L or GLUTag cells, a GLP-1–secreting cell line. Mice deficient in T1R3 or α-gustducin have reduced GLP-1 release in response to glucose (6), and lactisole, an STR blocker, reduces GLP-1 and PYY secretion and increases the glycemic response to intraduodenal (ID) or intragastric glucose in humans (7,8). On the other hand, the glucose transporters SGLT1 (luminal) and GLUT2 (apical, regarded as not rate limiting) are clearly important for incretin hormone release, and SGLT1-deficient mice have a loss of incretin responsiveness (9). In humans, there is equivocal evidence for an STR-mediated incretin response. Intragastric sucralose failed to stimulate GLP-1 or GIP release (10), but prior ingestion of sucralose-containing diet soda accentuated the GLP-1 response to oral glucose without significantly affecting insulin levels (11). It seems the role of the STRs may, in part, be to upregulate intestinal SGLT1, as this important intestinal transporter is increased by luminal sweeteners in normal, but not T1R3 or α-gustducin deficient, mice (12).
In this issue, Young et al. (13) add to what they and others have previously shown regarding STRs in type 2 diabetes. Prior evidence indicates that intestinal SGLT1 levels may be increased in type 2 diabetes and glucose transport in duodenal biopsies may be enhanced (14). Duodenal expression of STRs in the fasting state did not differ between diabetic and control subjects, but it was inversely related to glycemia in diabetic subjects (15). In rodents, intestinal STRs are rapidly downregulated by luminal glucose or nonnutritive sweeteners (16). In the new report, type 2 diabetic and control subjects were studied at euglycemia and hyperglycemia with a 30-min ID infusion of glucose containing 3-O-methyl-glucose (3-OMG) to assess glucose absorption. Duodenal biopsies were taken before and after the infusion. Basal intestinal mRNA copy number was substantially different for TRPM5 > α GD > TIR3 > TIR2, but did not differ between diabetic and control subjects. Further, 10–20% of TIR2 cells coexpressed GLP-1 or GIP, and hyperglycemia did not affect TIR2 levels in either group. However, ID glucose increased TIR2 in both groups at euglycemia, but at hyperglycemia, TIR2 was lower in control subjects but higher in diabetic subjects. Moreover, 3-OMG levels were significantly elevated at hyperglycemia in diabetic versus control subjects, but only at 60 min after initiation of ID glucose. The area under the curve for 3-OMG was greater with hyperglycemia, but not different between groups, an observation that may relate to competition for clearance between 3-OMG and glucose. Young et al. suggest the increased 60-min 3-OMG level was likely due to upregulation of SGLT1 (which was not directly measured in the study) by TIR2-related mechanisms. If correct, this would suggest that the STR response may act to enhance the relative rate of glucose absorption and accentuate postprandial hyperglycemia in type 2 diabetes.
It should be mentioned that there was, not unexpectedly, considerable variability in the STR component measurements. Thus, while the difference in the TIR2 response to ID glucose between diabetic and control subjects at hyperglycemia is convincing, other changes in STR components in response to ID glucose may have failed to reach statistical significance because of the relatively large variability in these measures.
Another salient point in this study relates to the incretin hormones GLP-1 and GIP, whose secretion in response to ID glucose corresponded with STR transcript levels. This was consistent with the previously mentioned role of the STRs in regulation of the incretin hormones. Interestingly, GLP-1 and GIP levels in diabetic subjects in response to ID glucose were as great or greater than control subjects, which contrasts with (inconsistent) previous data suggesting reduced GLP-1 secretion in type 2 diabetes (17,18). Young et al. suggest that the GLP-1 response to oral glucose in type 2 diabetes may be affected by well-described variability and delay in gastric emptying among these patients, whereas in this study the glucose was directly delivered to the intestine.
What are the implications of these and prior results? First, they are consistent with, but do not absolutely prove, an influence of intestinal STRs on incretin hormones in humans, thereby suggesting another possible route for pharmacological enhancement of the incretin response. Second, these findings suggest an unfavorable impact of disturbed STR regulation in type 2 diabetes—enhancement of glucose absorption during hyperglycemia, which could accentuate postprandial hyperglycemia and lessen the benefit of energy reduction from nonnutritive sweeteners. This raises the question as to whether these disturbances might occur in the prediabetic state. Although intake of low-calorie drinks might be expected to lessen adiposity and the risk of type 2 diabetes, it has nonetheless been associated epidemiologically with increased diabetes risk (19). Could the nonnutritive sweeteners in these drinks be accentuating postprandial glycemia via effects on STRs, thereby contributing to the progression of glucose intolerance?
Young et al. (13) add an extra chapter in the developing story of intestinal taste receptors, which clearly has a long way to go.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 3532.
REFERENCES
- 1.Yamamoto K, Ishimaru Y. Oral and extra-oral taste perception. Semin Cell Dev Biol 2013;24:240–246 [DOI] [PubMed] [Google Scholar]
- 2.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol 2013;11:e1001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol Metab 2013;24:92–100 [DOI] [PubMed] [Google Scholar]
- 4.Young RL. Sensing via intestinal sweet taste pathways. Front Neurosci 2011;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Kokrashvili Z, Mosinger B, Margolskee RF. Gustducin couples fatty acid receptors to GLP-1 release in colon. Am J Physiol Endocrinol Metab 2013;304:E651–E660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 2007;104:15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr 2011;30:524–532 [DOI] [PubMed] [Google Scholar]
- 8.Gerspach AC, Steinert RE, Schönenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab 2011;301:E317–E325 [DOI] [PubMed] [Google Scholar]
- 9.Gorboulev V, Schürmann A, Vallon V, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012;61:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J, Bellon M, Wishart JM, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 2009;296:G735–G739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care 2009;32:2184–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 2007;104:15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young RL, Chia B, Isaacs NJ, et al. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes 2013;62:3532–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 2002;282:G241–G248 [DOI] [PubMed] [Google Scholar]
- 15.Young RL, Sutherland K, Pezos N, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut 2009;58:337–346 [DOI] [PubMed] [Google Scholar]
- 16.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 2007;582:379–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001;86:3717–3723 [DOI] [PubMed] [Google Scholar]
- 18.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008;57:678–687 [DOI] [PubMed] [Google Scholar]
- 19.Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 2013;97:517–523 [DOI] [PubMed] [Google Scholar]