Abstract
We analyzed Sardinian registry data to assess time trends in incidence rates (IRs) of type 1 diabetes during the period 1989–2009 (2,371 case subjects 0–14 years of age). Poisson regression models were used to estimate the effects of sex, age, period of diagnosis, and birth cohorts. IR was 44.8 cases/100,000 person-years (95% CI 43.1–46.7). The annual increase was 2.12% (1.45–2.80; test for linear trend, P < 0.001). For boys, the increasing trend was evident up to 5 years of age and for girls up to 8 years of age. Compared with the 1989–1994 birth cohort, the relative risk increased from 0.78 (0.61–1.10) in 1974–1979 to 1.62 (1.18–2.23) in 2004–2009. The increase over period was less striking, with a tendency to regress in more recent years. The best-fitting model for boys included age and a linear time trend, and for girls age and nonlinear effects of calendar period and birth cohort. In conclusion, incidence increased over time, and the increase tended to level off in more recent years by calendar period but not by birth cohort, with some evidence of a stronger increase among girls than boys. Should the increase be attributable to the effects of some perinatal environmental factor, this would mean that such a factor has started affecting females before males.
The most striking feature of type 1 diabetes epidemiology is the evidence of large geographical variation in disease risk (1–3). Sardinia has the second-highest incidence rates (IRs) in the world after Finland, and this excess likely started around 1950, as shown by the analysis of incidence in male conscript cohorts in the period 1936–1979 (4).
An increasing trend of type 1 diabetes, ranging around 2–3%, has been shown in almost all areas covered by population-based registries (3). The increase involved both children and young adults in Turin, northern Italy (5), whereas it was limited to children in Sweden and interpreted as the anticipation of age at disease onset (6). Updated incidence data of registries included in the EURODIAB study have yielded the interesting finding that the lowest-risk areas of eastern Europe underwent the highest yearly increase up to year 2004, while in the highest-risk areas of northern Europe the increasing trend was lower (7).
The aims of this report from the high-risk Sardinia region were 1) to update the analysis of IRs of type 1 diabetes up to 2009 in children 0–14 years of age, 2) to assess temporal trends over the 21-year period 1989–2009, and 3) to assess whether it was possible to disentangle the effects of age, calendar period of diagnosis, and birth cohort and if such effects were heterogeneous by age and sex.
RESEARCH DESIGN AND METHODS
The study base of this report were all children 0–14 years of age and residents in Sardinia in the period 1989–2009. As previously described (8), the registration of incident cases was performed according to the EURODIAB criteria. Type 1 diabetes was defined on the basis of clinical diagnosis of insulin-dependent diabetes by a doctor, excluding cases occurring secondary to other conditions. The date of onset was taken as the date of first insulin injection. Diabetes clinics for children and adults and departments of internal medicine and endocrinology were the first source of ascertainment. The second independent data source was membership in the local patient associations and the personal National Health System identification cards needed by each patient to obtain medications and devices free of charge. The estimated completeness of ascertainment was 92% in the period 1989–1997, 90% in the period 1998–2003, and 91% in the period 2004–2009, with no differences among sexes and age-groups over the study period.
Population data by sex and year of birth were provided by the Italian National Institute of Statistics (http://www.demo.istat.it/). Age- and sex-specific IRs/100,000 person-years were calculated for calendar year of diagnosis and birth cohort (9). The χ2 test for trend in IRs used is the Mantel extension of the Armitage-Cochran trend test. The trend test is either stratified by sex or calculated separately for the two sexes. Poisson regression models were used to estimate the effects of sex, age (five 3-year age-groups: 0–2, 3–5, 6–8, 9–11, and 12–14 years), calendar time (seven 3-year periods: 1989–1991, 1992–1994, 1995–1997, 1998–2000, 2001–2003, 2004–2006, and 2007–2009), and birth cohorts (eleven 6-year birth cohorts: 1974–1979, 1977–1982, 1980–1985, 1983–1988, 1986–1991, 1989–1994, 1992–1997, 1995–2000, 1998–2003, 2001–2006, and 2004–2009). Six models were fitted to data: sex; sex and age; sex, age, and linear time trend (drift); sex, age, and cohort; sex, age, and period; and sex, age, period, and cohort (Supplementary Fig. 1). The latter five models were then fitted to data separately for the two sexes. The term “drift” denotes a linear temporal variation of rates that does not distinguish between the influences of two of the three temporal variables involved in the analysis. The hierarchically ordered models were compared through the likelihood ratio test. Further Poisson regression models were fitted to data including sex (or separate models were fitted to the two sexes), age, linear time trend (drift), and quadratic terms for period and cohort, after centering the two variables, in order to check if temporal variation was nonlinear. Statistical analyses were performed using STATA/SE, release 11.0.
RESULTS
In the period 1989–2009, 2,371 incident cases of type 1 diabetes were identified among children 0–14 years of age and residents of Sardinia. IRs by sex, age-group, and calendar period are shown in Table 1. The IR over the study period was 44.8 cases/100,000 person-years (95% CI 43.1–46.7), with significantly higher risk in boys (50.6 [95% CI 48.0–53.4]) than in girls (38.7 [36.4–41.2]). In the regression analysis, age was controlled for, the rate ratio (RR) for boys with respect to girls was 1.31 (95% CI 1.21–1.42). IRs in the age-groups 0–4, 5–9, and 10–14 years were 37.8 (34.9–41.0), 47.0 (43.9–50.3), and 48.4 (45.4–51.5), respectively; corresponding RRs (boys vs. girls) were 1.22 (1.04–1.44), 1.18 (1.03–1.36), and 1.49 (1.31–1.69).
TABLE 1.
IRs of type 1 diabetes among Sardinian children 0–14 years old in the years 1989–2009 by sex, age-group, and calendar period
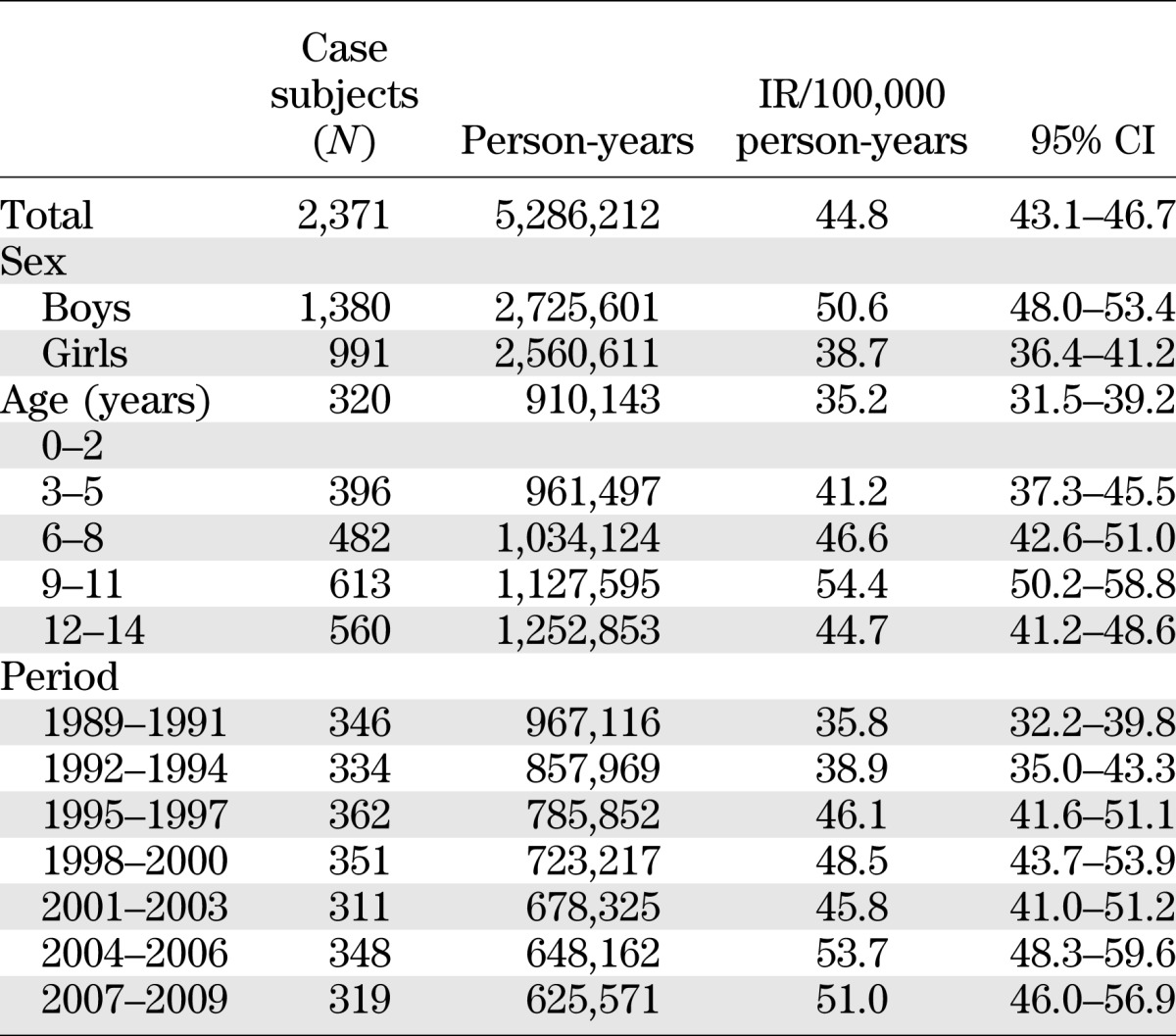
The IR steadily increased over the study period from 35.8 (95% CI 32.2–39.7) in 1989–1991 to 51.0 (45.7–56.9) in 2007–2009 (Table 1). With age and sex controlled for, the annual increase in the period 1989–2009 was 2.12% (1.45–2.80; test for linear trend: P < 0.001). With age controlled for, such an increase was higher in girls (2.67% [95% CI 1.63–3.73]) than in boys (1.73% [0.85–2.62]), although not significantly.
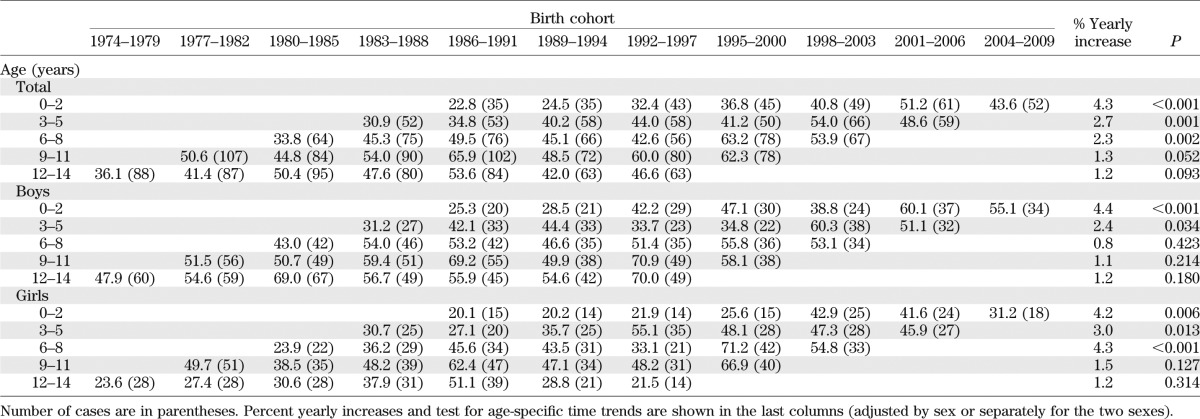
Table 2 shows age-specific IRs by birth cohort and calendar period (diagonals) for all children and for boys and girls separately. The table shows how IRs increased with each subsequent birth cohort in each age-group. The yearly increase, adjusted for age and sex, was highest in the age-group 0–2 years (4.3%, P < 0.001). Time trends were heterogeneous between sexes: in boys, large statistically significant increasing linear trends were evident in the age-group 0–2 years (4.4%, P < 0.001) and, to a smaller extent, 3–5 years of age (2.4%, P = 0.034), whereas in girls, a yearly increase of similar magnitude was evident not only in the age-groups 0–2 years (4.2%, P = 0.006) and 3–5 years (3.0%, P = 0.013), but also in the age-group 6–8 years (4.3%, P < 0.001).
TABLE 2.
Age-specific IRs (per 100,000 person-years) of type 1 diabetes among Sardinian children 0–14 years old in the years 1989–2009 by birth cohorts (1974–1979, …, 2004–2009) and by calendar periods (diagonals: 1989–1991, …, 2007–2009)
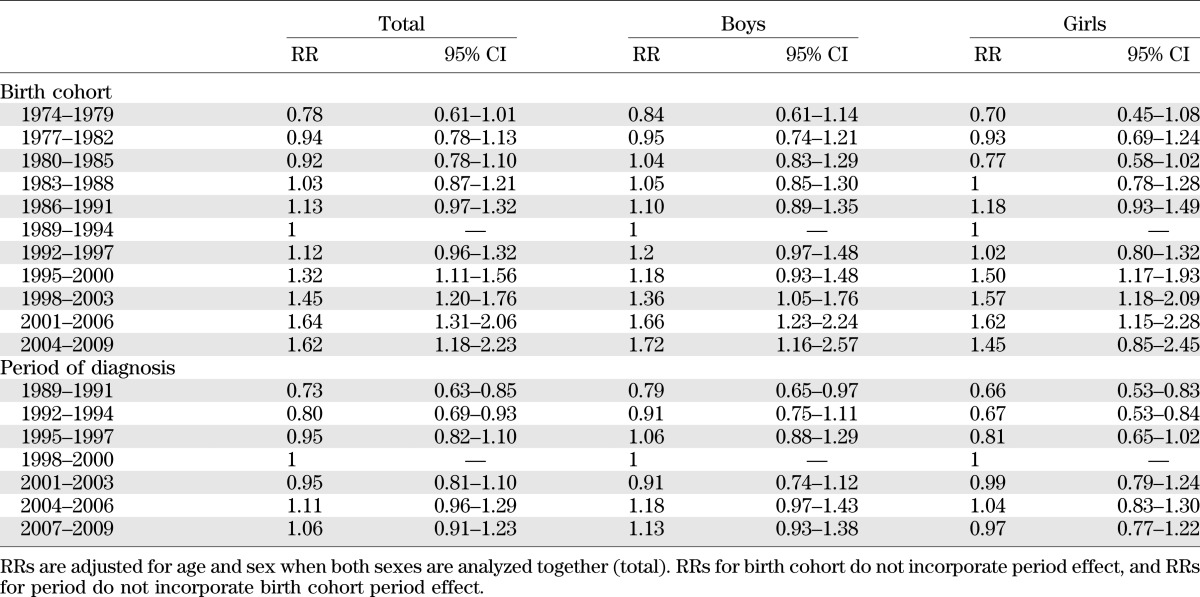
A different pattern of increase in time by birth cohorts and by calendar periods of diagnosis is evident analyzing age-adjusted RRs, which are shown in Table 3. With respect to the 1989–1994 birth cohort, the RR increased from 0.78 (95% CI 0.61–1.10) for children born in 1974–1979 to 1.62 (1.18–2.23) for those born in 2004–2009. In contrast, the pattern of increase of RRs by calendar period of diagnosis was less striking. Indeed, with respect to children diagnosed in the time period 1998–2000, the RR increased from 0.73 (0.63–0.85) for children diagnosed in 1989–1991 to 1.06 (0.91–1.23) for those diagnosed in 2007–2009.
TABLE 3.
RRs for each birth cohort taking those born in 1989–1994 as a reference and each calendar period of diagnosis taking those diagnosed in 1998–2000 as a reference
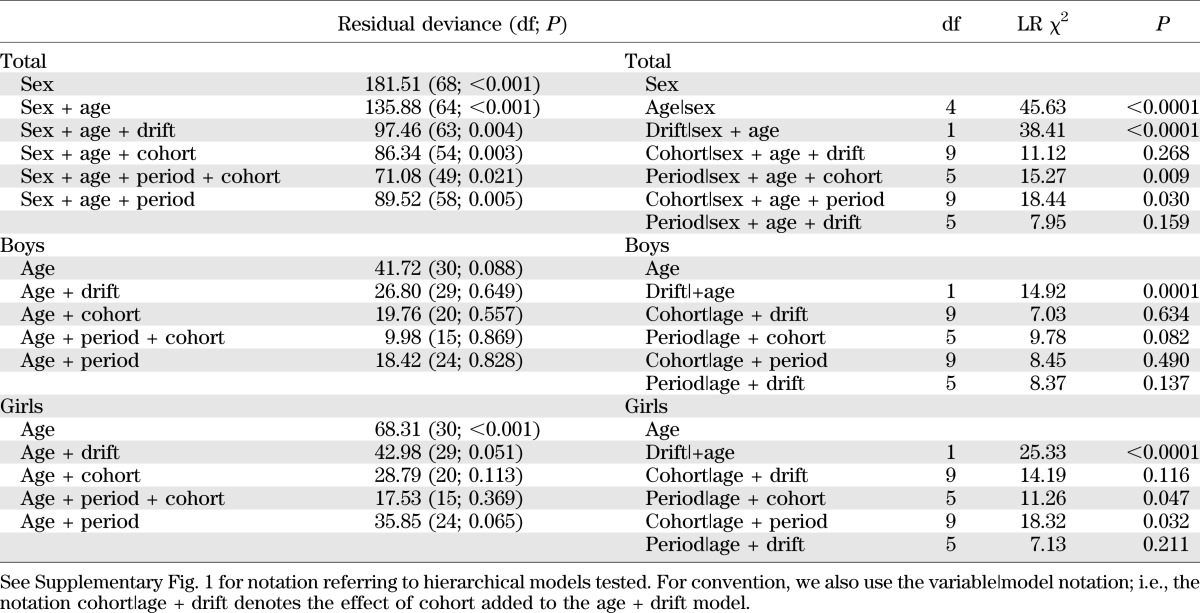
To try to disentangle the effect of age, calendar period, and birth cohort on the overall temporal trend of the disease in the period 1989–2009, we fitted hierarchically ordered Poisson regression models to the data, for all incident cases, and separately by sex, since there was evidence of interaction (sex × cohort, P = 0.018; sex × period, P = 0.143) (Table 4). For both boys and girls, with use of the Akaike information criteria the best model (model 2) was the one with age and a linear time trend (drift). This finding means that the variation over time has a linear component that cannot be ascribed to either the calendar period or the cohort. However, for girls as well as for all children analyzed together, nonlinear effects of both birth cohort (model 4) and period of diagnosis (model 5) are apparent. Fitting models including quadratic terms for period and cohort showed that the temporal variation was nonlinear. Indeed, this model fitted to the total (boys plus girls) data resulted in a slight improvement of the Akaike information criteria versus model 2. Opposite signs for the coefficients of the quadratic period and cohort terms indicated that, whereas there was a steady increase with birth cohort, in the most recent periods incidence started to show a tendency to decrease. This result was already apparent in Table 2, showing that IRs in children diagnosed in 2007–2009 (last diagonal from top right to bottom left, IRs 43.6 in 0–2 years, 48.6 in 3–5 years, …) were lower than those diagnosed in 2004–2006 in almost all age-groups (next diagonal on the left: 51.2 in 0–2 years, 54.0 in 3–5 years, …).
TABLE 4.
Summary of testing different age-period-cohort effects in relation to IRs of type 1 diabetes among Sardinian children 0–14 years old in the years 1989–2009
DISCUSSION
Our analysis of incidence data from the Sardinia registry of type 1 diabetes in the 21-year period 1989–2009 provides evidence of a strong increase of IRs among Sardinian children. Results of our age-period-cohort analysis of time trends are intriguing. Indeed, we provide evidence that the incidence of type 1 diabetes has increased nonlinearly with the birth cohort. Such a birth cohort effect seems to have started some 5–10 years earlier among girls, starting from those born in 1980–1985, than among boys, starting from those born in 1986–1991. This finding allows us to explain why annual percent increases are higher and statistically significant in girls up to 8 years of age but affect boys to a lesser extent and up to 5 years of age only. Should the increase be attributable to the effects of some perinatal environmental factor, this would mean that such a factor has started affecting females before males. In Sardinia, both a period and a cohort effect were evident, with heterogeneities among sexes. Indeed, the nonlinear temporal effects were more evident among girls, with larger increases with time than among boys. In more recent years, a tendency of time trends to level off was also evident, particularly in the youngest age-groups, which could merely reflect year-to-year variability in IRs and need to be confirmed over a longer time period.
Recently, Patterson et al. (10) pointed out that the potential of age-period-cohort analysis in disentangling the effects of time trends on different age-groups at different times is often hampered by the paucity of age classes and the limited length of time series. Indeed, IRs up to 14 years of age do not allow us to capture the total effect of time variations in the whole population. However, despite the small number of age-groups and the limited sample size, our analysis, taking advantage of >20 years of registration, shows that incidence has increased more than linearly with birth cohort, whereas the increase with calendar period has shown signs of regression in the most recent periods. Previous age-period-cohort analyses have found different and discordant results, including linear and nonlinear period effects (5,11–13) as well as cohort effects (14,15), often interpreted as a shift toward earlier age at onset (7,16–18).
Studies have proposed that the decreasing early life exposure to infectious diseases could be involved in the increasing temporal trends of immune-mediate disorders (19). Lower socioeconomic level and higher crowding in early life, proxy measures of early childhood infection, increase the risk of type 1 diabetes up to 3 years and decrease the risk in the following age-groups (20). Experimental models have shown a relationship between intestinal microbioma and type 1 diabetes (21). As regards Sardinia, elmintiasis, which was endemic up to the 1980s, has now almost completely disappeared. The role of Mycobacterium avium paratuberculosis infection has also been suggested to play a role, and a homology between its epitopes and β-cell antigen ZnT8 has been identified in Sardinian patients with type 1 diabetes (22).
Another intriguing and still unsolved issue in the epidemiology of type 1 diabetes is the heterogeneity of risk among sexes. Whereas in most of the countries incidence is higher in males than in females among young adults only, Sardinian boys have a statistically significant 31% higher risk than girls of the same age, which is not chromosome Y–linked (23), but until now no other hypothesis has been tested. Moreover, our data show heterogeneities among sexes in time trend patterns, with girls appearing to show the potential effects of hypothetical environmental factor exposures at least 5–10 years before boys. This finding is intriguing, and further experimental and epidemiological studies should explore sex differences in the interaction between environment and genetics in the pathogenesis of type 1 diabetes.
In conclusion, our study showed a strong increase of IRs of type 1 diabetes among Sardinian children in the period 1989–2009, which was nonlinear with the birth cohort, whereas the increase with calendar period showed signs of regression in the most recent periods. The birth cohort effect seems to have started earlier among girls than among boys. Should the increase be attributable to the effects of some perinatal environmental factor, this would mean that such a factor has started affecting females before males.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
G.B. and M.M. researched data and wrote the manuscript. A.B. researched data and contributed to the discussion. A.L. and C.M. researched data. F.M. contributed to the discussion and reviewed and edited the manuscript. M.S. researched data, contributed to the discussion, and reviewed and edited the manuscript. G.B. and M.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 48th European Association for the Study of Diabetes Annual Meeting, 1–5 October 2012, Berlin, Germany.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1771/-/DC1.
REFERENCES
- 1.Pundziūte-Lyckå A, Dahlquist G, Urbonaite B, Zalinkevicius R, Swedish Childhood Diabetes Study Group. Lithuanian Childhood Diabetes Study Group Time trend of childhood type 1 diabetes incidence in Lithuania and Sweden, 1983-2000. Acta Paediatr 2004;93:1519–1524 [DOI] [PubMed] [Google Scholar]
- 2.Bruno G, Maule M, Merletti F, et al. RIDI Study Group Age-period-cohort analysis of 1990-2003 incidence time trends of childhood diabetes in Italy: the RIDI study. Diabetes 2010;59:2281–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soltesz G, Patterson CC, Dahlquist G, EURODIAB Study Group Worldwide childhood type 1 diabetes incidence—what can we learn from epidemiology? Pediatr Diabetes 2007;8(Suppl. 6):6–14 [DOI] [PubMed] [Google Scholar]
- 4.Casu A, Pascutto C, Bernardinelli L, Songini M, Sardinian Conscript Type 1 Diabetes Registry Bayesian approach to study the temporal trend and the geographical variation in the risk of type 1 diabetes. The Sardinian Conscript Type 1 Diabetes Registry. Pediatr Diabetes 2004;5:32–38 [DOI] [PubMed] [Google Scholar]
- 5.Bruno G, Novelli G, Panero F, et al. Piedmont Study Group for Diabetes Epidemiology The incidence of type 1 diabetes is increasing in both children and young adults in Northern Italy: 1984-2004 temporal trends. Diabetologia 2009;52:2531–2535 [DOI] [PubMed] [Google Scholar]
- 6.Dahlquist GG, Nyström L, Patterson CC, Swedish Childhood Diabetes Study Group. Diabetes Incidence in Sweden Study Group Incidence of type 1 diabetes in Sweden among individuals aged 0-34 years, 1983-2007: an analysis of time trends. Diabetes Care 2011;34:1754–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 8.Casu A, Pascutto C, Bernardinelli L, Songini M. Type 1 diabetes among sardinian children is increasing: the Sardinian diabetes register for children aged 0-14 years (1989-1999). Diabetes Care 2004;27:1623–1629 [DOI] [PubMed] [Google Scholar]
- 9.Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med 2007;26:3018–3045 [DOI] [PubMed] [Google Scholar]
- 10.Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia 2012;55:2142–2147 [DOI] [PubMed] [Google Scholar]
- 11.Nyström L, Dahlquist G, Rewers M, Wall S. The Swedish childhood diabetes study. An analysis of the temporal variation in diabetes incidence 1978-1987. Int J Epidemiol 1990;19:141–146 [DOI] [PubMed] [Google Scholar]
- 12.Tuomilehto J, Rewers M, Reunanen A, et al. Increasing trend in type 1 (insulin-dependent) diabetes mellitus in childhood in Finland. Analysis of age, calendar time and birth cohort effects during 1965 to 1984. Diabetologia 1991;34:282–287 [DOI] [PubMed] [Google Scholar]
- 13.Aamodt G, Stene LC, Njølstad PR, Søvik O, Joner G, Norwegian Childhood Diabetes Study Group Spatiotemporal trends and age-period-cohort modeling of the incidence of type 1 diabetes among children aged <15 years in Norway 1973-1982 and 1989-2003. Diabetes Care 2007;30:884–889 [DOI] [PubMed] [Google Scholar]
- 14.Svensson J, Carstensen B, Mølbak A, et al. Increased risk of childhood type 1 diabetes in children born after 1985. Diabetes Care 2002;25:2197–2201 [DOI] [PubMed] [Google Scholar]
- 15.Berhan Y, Waernbaum I, Lind T, Möllsten A, Dahlquist G, Swedish Childhood Diabetes Study Group Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes 2011;60:577–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feltbower RG, McKinney PA, Parslow RC, Stephenson CR, Bodansky HJ. Type 1 diabetes in Yorkshire, UK: time trends in 0-14 and 15-29-year-olds, age at onset and age-period-cohort modelling. Diabet Med 2003;20:437–441 [DOI] [PubMed] [Google Scholar]
- 17.Dahlquist G, Mustonen L, Swedish Childhood Diabetes Study Group Analysis of 20 years of prospective registration of childhood onset diabetes time trends and birth cohort effects. Acta Paediatr 2000;89:1231–1237 [DOI] [PubMed] [Google Scholar]
- 18.Pundziute-Lyckå A, Dahlquist G, Nyström L, et al. Swedish Childhood Diabetes Study Group The incidence of Type I diabetes has not increased but shifted to a younger age at diagnosis in the 0-34 years group in Sweden 1983-1998. Diabetologia 2002;45:783–791 [DOI] [PubMed] [Google Scholar]
- 19.Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology 2009;126:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno G, Spadea T, Picariello R, et al. Piedmont Study Group for Diabetes Epidemiology Early life socioeconomic indicators and risk of type 1 diabetes in children and young adults. J Pediatr 2013;162:600–605, e1 [DOI] [PubMed] [Google Scholar]
- 21.Vaarala O. Is the origin of type 1 diabetes in the gut? Immunol Cell Biol 2012;90:271–276 [DOI] [PubMed] [Google Scholar]
- 22.Masala S, Paccagnini D, Cossu D, et al. Antibodies recognizing Mycobacterium avium paratuberculosis epitopes cross-react with the beta-cell antigen ZnT8 in Sardinian type 1 diabetic patients. PLoS ONE 2011;6:e26931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contu D, Morelli L, Zavattari P, et al. Sex-related bias and exclusion mapping of the nonrecombinant portion of chromosome Y in human type 1 diabetes in the isolated founder population of Sardinia. Diabetes 2002;51:3573–3576 [DOI] [PubMed] [Google Scholar]


