Abstract
We analyzed demographic and genetic differences between children with various diabetes-associated autoantibodies reflecting the autoimmune process. In a prospective birth cohort comprising children with HLA-conferred susceptibility to type 1 diabetes (T1D), the pattern of autoantibody appearance was analyzed in 520 children with advanced β-cell autoimmunity associated with high risk for disease. In 315 cases, a single biochemical autoantibody could be identified in the first positive sample as insulin (insulin autoantibody [IAA]) in 180, as GAD (GAD antibody [GADA]) in 107, and as IA-2 antigen (IA-2 antibody [IA-2A]) in 28. The age at seroconversion differed significantly between the three groups (P = 0.003). IAA as the first autoantibody showed a peak time of appearance during the second year of life, whereas GADA as the first autoantibody peaked later, between 3 and 5 years of age. The risk-associated insulin gene rs689 A/A genotypes were more frequent in children with IAA as the first autoantibody compared with the other children (P = 0.002). The primary autoantigen in the development of β-cell autoimmunity and T1D seems to strongly correlate with age and genetic factors, indicating heterogeneity in the initiation of the disease process.
Immune destruction of the insulin-producing β-cells leading to clinical type 1 diabetes (T1D) is often a long-lasting process. During this preclinical period, antibody responses to several autoantigens can be detected. The number of detectable autoantibodies usually increases with time and correlates with the probability of disease development (1). It is not clear whether a primary autoantigen does exist and immune responses against other molecules represent secondary antigen spreading, or whether multiple molecules can be primary targets (2). Heterogeneities in the preferential age of appearance and in HLA associations have been described for various autoantibodies (3–6). The early appearance and association with younger age at diagnosis of insulin autoantibodies (IAAs) together with the essential role of the insulin molecule in the NOD mouse model have implied (prepro)insulin as the primary antigen (7). Antibody responses to all major antigens nevertheless accumulate with increasing age during the follow-up.
Natural history studies of children at high risk who have undergone sequential blood sampling during short intervals provide a unique possibility for analyzing the appearance of autoimmunity in detail and for helping to resolve the question of whether one or multiple primary antigens exist. In the current study, we analyzed the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study children who developed advanced β-cell autoimmunity, indicating multiple autoantibodies and high risk of disease, and correlated the specificity of the primary autoantibody with demographic and genetic parameters.
RESEARCH DESIGN AND METHODS
The DIPP study protocol included a follow-up with sequential blood sampling and autoantibody measurements starting from birth and continuing at 3- to 6-month intervals until the age of 12 months, and then measurements at 6- to 12-month intervals thereafter in children with HLA-conferred susceptibility to T1D (Supplementary Table 1 and Supplementary Fig. 1) (8,9). Islet cell antibodies were measured using immunofluorescence and antibodies against insulin, GAD65 (GAD antibody [GADA]), and IA-2 antigen (IA-2A), with specific radiobinding assays using cutoff rates based on the 99th percentile for >350 nondiabetic subjects (10). Disease sensitivities and specificities of the assays in our laboratory according to the 2002–2010 Diabetes Antibody Standardization Program workshops were 44–50% and 96–99% for IAA, 82–92% and 94–97% for GADA, and 64–72% and 97–100% for IA-2A, respectively. For the current study, we selected children with “advanced autoimmunity,” defined as at least two consecutive samples positive for two biochemically defined autoantibodies (IAA, GADA, and/or IA-2A), two consecutive samples positive for one biochemical autoantibody, and development of clinical diabetes or repeated positivity for islet cell antibodies and IAA; the combination of these also has been associated with high risk of T1D in the DIPP study (10). This resulted in a cohort of 520 children; in this cohort, a single biochemically defined autoantibody was detected before the development of multiple autoantibodies in 315 children. Cases of islet cell antibodies appearing simultaneously with IAA as the first autoantibody were included as this group (n = 66) and were similar to those cases in the group with IAA alone regarding age and T1D progression. Accordingly, we distinguished three groups of children as follows: those with IAA (n = 180; 109 male) as the first biochemical autoantibody; those with GADA (n = 107; 55 male) as the first biochemical autoantibody; and those with IA-2A (n = 28; 18 male) as the first biochemical autoantibody.
Genotyping methods.
The major Caucasian HLA-DR/DQ haplotypes were defined using panels of sequence-specific oligonucleotide probes as described previously (11). INS rs689 and PTPN rs2476601 were genotyped using the Sequenom platform in the Genome Center of the University of Eastern Finland, Kuopio, or using the TaqMan SNP genotyping array.
Statistical analysis.
Data were analyzed using SPSS 19.0 software (IBM SPSS, Chicago, IL). Kaplan-Meier log-rank test was used for comparing progression to clinical disease, and Kruskal-Wallis test was used for comparing age distribution at the time of autoantibody seroconversion. Frequencies of genotypes were compared with Pearson χ2 test and Fisher exact test when appropriate.
RESULTS
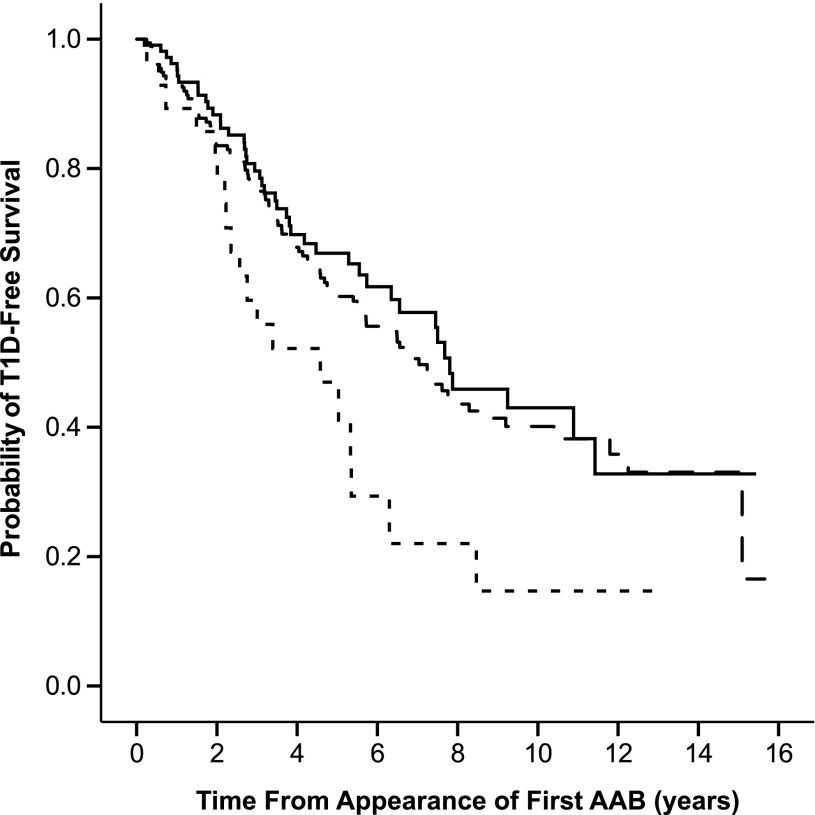
The high probability of progression to clinical diabetes among children with advanced β-cell autoimmunity regardless of the specificity of the autoantibody initiating the process is demonstrated in Fig. 1. The significant heterogeneity between the three groups (P = 0.021, Kaplan-Meier test) was attributable to the more rapid progression in children with IA-2A as their first autoantibody. No difference was seen between children with either IAA or GADA as the first autoantibody (P = 0.490, Kaplan-Meier test).
FIG. 1.
Progression to clinical T1D among children with advanced autoimmunity when divided according to the first biochemical autoantibody to appear (IAA, dashed line; GADA, solid line; and IA-2A, dotted line). The follow-up started when the first biochemical autoantibody appeared. AAB, autoantibody.
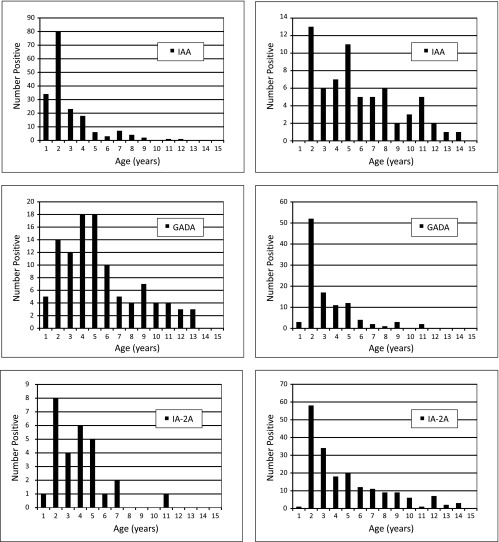
When analyzing the age at the appearance of the first biochemical autoantibody, the groups with various first autoantibodies clearly differed from each other (Fig. 2, left panels). IAA as the first autoantibody usually appeared very early, with a sharp peak between ages 1 and 2 years (median age, 1.49 years; interquartile range, 1.01–2.99 years), whereas the appearance of GADA as the first autoantibody was considerably more widespread, peaking between ages 3 and 5 years and slowly decreasing thereafter (median age, 4.04 years; interquartile range, 2.57–6.80 years). IA-2A as the first autoantibody was relatively rare, with an intermediate pattern compared with IAA and GADA (median age, 3.03 years; interquartile range, 1.52–4.28). The difference in the age distribution between the three groups was highly significant (P = 0.003, Kruskal-Wallis test). Secondary antibodies revealed totally opposite patterns, which mask the differences regarding primary autoantibodies if both are combined (Fig. 2, right panels). GADA as the secondary antibody usually occurs very rapidly when the primary antibody response is IAA, and the peak also occurs before the age of 2 years. Secondary IAA mostly occurs after GADA, and thus the age distribution is relatively even over a wide age range.
FIG. 2.
The age distribution for the appearance of the first autoantibody in the group of children with advanced β-cell autoimmunity (left panels) compared with the age distribution for the secondary autoantibodies appearing after the first autoantibody (right panels). The clear differences seen in the first autoantibody are masked by secondary autoantibodies if combined.
The three groups with different primary autoantibodies were compared for the frequency of HLA-DR/DQ genotypes and genotypes of the two most important risk-associated non-HLA loci, INS and PTPN22. Children positive for the HLA-DR3-DQ2 haplotype without HLA-DR4-DQ8 were rare in the study group because of the HLA screening criteria, and we could not find significant differences in HLA genotypes when all three groups were compared and the number of comparisons was taken into account. However, all three DR3-DQ2 homozygous children were in the group with GADA as the first autoantibody, whereas children with IA-2A as the first autoantibody were less often positive for the DR3-DQ2 haplotype (4/28; 14.3%) than were others (95/287; 33.1%). These differences reached nominal significance at P = 0.05.
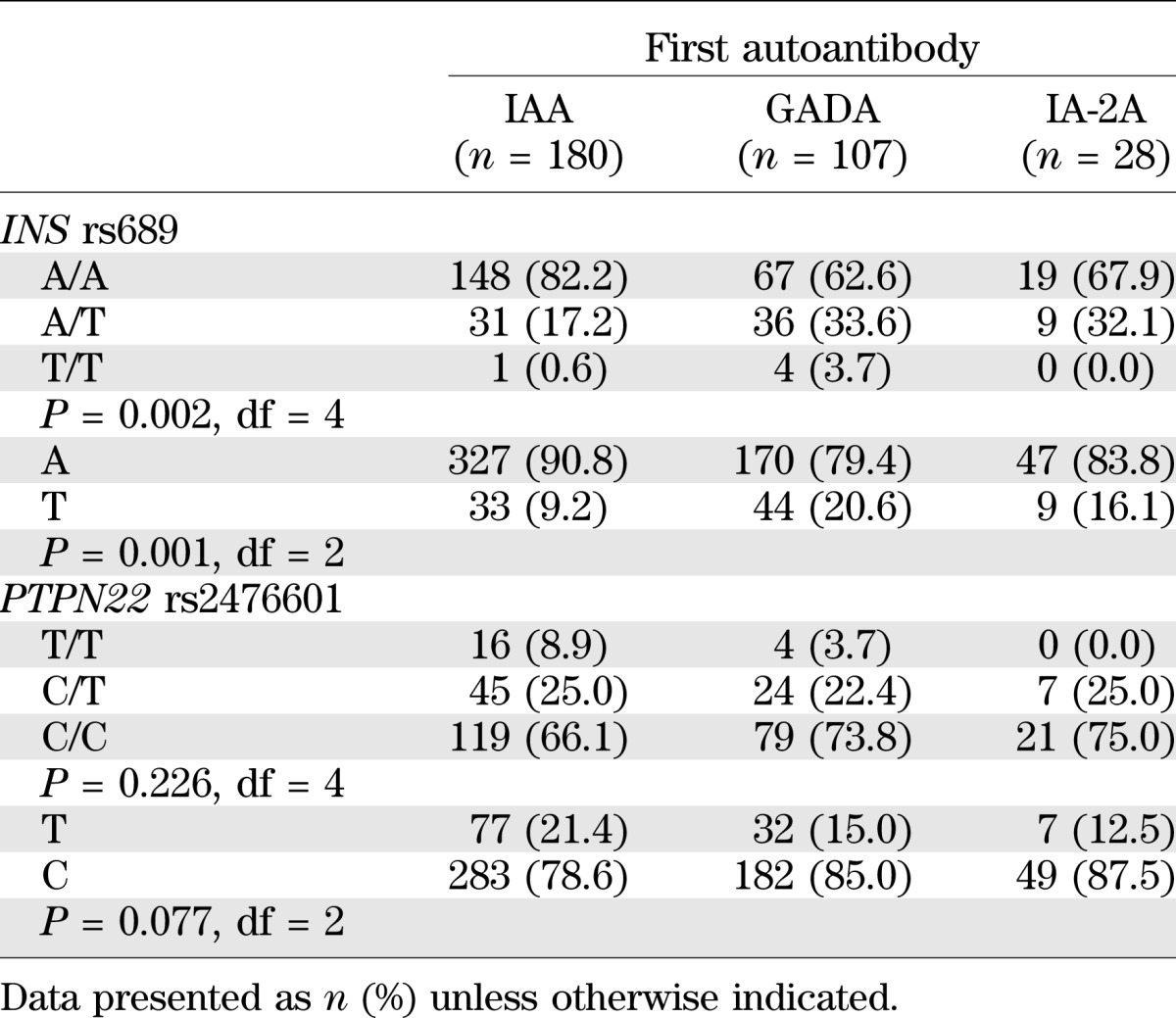
Table 1 demonstrates significant differences in the distribution of INS rs689 genotypes between groups with different first autoantibodies. The frequencies of INS rs689 risk genotype A/A and the A allele were increased among children with IAA as the first autoantibody. There were no significant differences among PTPN22 rs2476601 genotypes. The association with INS gene polymorphism was not observed for IAA as the secondary autoantibody. The risk-associated rs689 A/A genotype was seen in only 44 out of 67 (65.7%) children with IAA as the secondary autoantibody, which is significantly lower than the proportion seen in children with IAA as the primary autoantibody (82.2%) (P = 0.0054).
TABLE 1.
Genotypes of T1D risk–associated SNP polymorphisms in INS and PTPN22 genes according to the first autoantibody
DISCUSSION
The appearance of various diabetes-associated autoantibodies in children at genetic risk traditionally has been described by curves showing a continuously increasing prevalence of each autoantibody, with IAA starting to appear as the first and IA-2A appearing as the last (12,13). When the rate of appearance was assessed more closely, a peak in the appearance of all biochemical autoantibodies was demonstrated before age 2 years; thereafter, the rate of new seroconversions decreased (14,15).
In the current study, we focused on examining the specificity of the first autoantibody to appear and we analyzed secondary autoantibodies separately. Doing so revealed clear differences in the timing of autoantibody appearance, which remain unnoticed when combining primary and secondary autoantibody responses. We observed that the instance of IAA as the first autoantibody appears very early (median age, 1.5 years), but IAA emerges evenly over the course of many years as a secondary autoantibody. The development of GADA as the first autoantibody is distributed rather evenly over the course of the preschool ages (2–5 years of age); however, as a secondary antibody it has its peak early, similar to IAA. The kinetics of secondary autoantibodies are related to the rapid antigen spreading during the progression of the autoimmune response, and the conspicuous difference in the first autoantibody responses accordingly can be detected only during a follow-up program with short intervals during early childhood. The short interval of only 3 months between samples collected from those younger than age 2 years allowed us to distinguish the specificity of the primary autoantibody in most individuals who developed advanced autoimmunity.
The major groups of children with either IAA or GADA as the first autoantibody were extremely similar in the survival analysis; ∼60% of the children in both groups progressed to T1D after follow-up for <10 years, but the small group presenting with IA-2A as their first autoantibody showed an even more rapid progression. Remarkable differences in the peak age at the appearance of IAA or GADA as the first autoantibody despite similar progression rates after the appearance of the autoantibody indicate differences in the phase of initial induction of autoimmunity. The specificity of the first autoantibody could be determined by genetic factors. INS gene risk allele was associated with the appearance of IAA as the first autoantibody. The children with IAA had frequency similar to that observed in Finnish children with T1D (16), whereas children with GADA or IA-2A as the first autoantibody had frequencies that resembled those of background controls. This specific association of the INS gene polymorphism with IAA is in line with previous findings (17,18). However, INS gene risk allele was associated with only IAA as the first autoantibody, and not with IAA appearing as the secondary autoantibody. The genetic regulation of insulin-specific immune response seemed to differ depending on whether IAA was induced as the first or as the secondary autoantibody. INS gene polymorphism regulates the thymic expression of insulin (19), and thus its role is linked to the thymic immune regulation, i.e., central tolerance and induction of natural regulatory T-cells. These factors may be more important in the generation of insulin-specific immune response in the children with early induction of IAA, whereas the development of IAA as the secondary autoantibody, during antigen spreading, may be regulated by the mechanisms of peripheral tolerance. The specificity of the first autoantibody also was strongly dependent on age, which suggests that the specificity of the initial autoantibody also may reflect different environmental factors exerting their influence during early infancy and in later childhood.
Distinct HLA associations of various autoantibodies also are known. On diagnosis of the disease, the HLA-DR3-DQ2 haplotype is more often seen in patients positive for GADA, whereas DR4-DQ8 shows preferential association with IAA and IA-2A (3–6,20). As a result of the inclusion criteria in the DIPP study, there were few children positive for HLA-DR3-DQ2 without HLA-DR4-DQ8, but it is still conspicuous that all three homozygotes had GADA as the first autoantibody. HLA-DR3-DQ2 was less often present in the small group with IA-2A as the first autoantibody. These findings are in accordance with the observations seen in studies performed when T1D was first diagnosed.
The follow-up was most intensive, and thus the results were most representative for β-cell autoimmunity launched early during childhood. Some bias for higher numbers of cases showing seroconversion at an early age was caused by accumulation of dropouts during the follow-up, but this does not affect the validity of the comparisons between the groups. Expanded follow-up series will be needed to characterize autoantibody development in T1D diagnosed during adulthood, which in most countries includes a majority of the patients. The best-characterized autoantibodies, so far, were included in the analysis, but further information might be available by including additional autoantibody specificities, most obviously zinc transporter 8 antibodies and the recently described electrochemiluminescent assay for IAA. However, the contribution of zinc transporter 8 antibodies to the overall risk estimation seems to be small among children who are positive for multiple other autoantibodies, and zinc transporter 8 antibodies rarely present as the first autoantibody (21–23). Electrochemiluminescent assay for IAA was found to be more sensitive than radioimmunoassay to detect persistent IAA associated with T1D development, and 94% of children with progression to T1D in Diabetes Autoimmunity Study in the Young (DAISY) were positive at 18 months (24). Data from both DAISY and our study emphasize the importance of IAA as an early marker of β-cell autoimmunity in children.
In conclusion, these results show age-related, genetics-related, and antigen specificity–related heterogeneity during the initiation of β-cell autoimmunity. This might be informative in the search for the environmental triggers and ways of preventing the disease.
ACKNOWLEDGMENTS
The study was supported by the Medical Research Council of the Academy of Finland (J.I., O.S., and M.K., Centre of Excellence in Molecular Systems Immunology and Physiology Research 2012–2017, decision no. 250114), Sigrid Jusélius Foundation, and Juvenile Diabetes Research Foundation.
No potential conflicts of interest relevant to this article were reported.
J.I. acquired and reviewed the research data used in the analysis, performed statistical analysis, interpreted the results, drafted the manuscript, and designed the DIPP study. A.H. and A.-P.L. acquired the data. J.L. acquired data, interpreted the results, and critically reviewed the manuscript. O.V. interpreted the results and critically reviewed the manuscript. R.V. acquired the data, interpreted the results, critically reviewed the manuscript, and performed the autoantibody analyses. O.S. acquired the data, interpreted the results, critically reviewed the manuscript, and designed the DIPP study. M.K. acquired the data, performed the autoantibody analyses, and designed the DIPP study. J.I. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the Federation of Clinical Immunology Societies meeting, Boston, Massachusetts, 27–30 June 2013.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0300/-/DC1.
REFERENCES
- 1.Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity 2010;32:468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med 2012;2:a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler AG, Standl E, Albert E, Mehnert H. HLA-associated insulin autoantibody formation in newly diagnosed type I diabetic patients. Diabetes 1991;40:1146–1149 [DOI] [PubMed] [Google Scholar]
- 4.Sabbah E, Savola K, Ebeling T, et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care 2000;23:1326–1332 [DOI] [PubMed] [Google Scholar]
- 5.Vandewalle CL, Falorni A, Lernmark A, et al. Associations of GAD65- and IA-2- autoantibodies with genetic risk markers in new-onset IDDM patients and their siblings. The Belgian Diabetes Registry. Diabetes Care 1997;20:1547–1552 [DOI] [PubMed] [Google Scholar]
- 6.Graham J, Hagopian WA, Kockum I, et al. Diabetes Incidence in Sweden Study Group. Swedish Childhood Diabetes Study Group Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes 2002;51:1346–1355 [DOI] [PubMed] [Google Scholar]
- 7.Wegmann DR, Eisenbarth GS. It’s insulin. J Autoimmun 2000;15:286–291 [DOI] [PubMed] [Google Scholar]
- 8.Kupila A, Muona P, Simell T, et al. Juvenile Diabetes Research Foundation Centre for the Prevention of Type I Diabetes in Finland Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia 2001;44:290–297 [DOI] [PubMed] [Google Scholar]
- 9.Näntö-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008;372:1746–1755 [DOI] [PubMed] [Google Scholar]
- 10.Siljander HT, Simell S, Hekkala A, et al. Predictive characteristics of diabetes-associated autoantibodies among children with HLA-conferred disease susceptibility in the general population. Diabetes 2009;58:2835–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann R, Turpeinen H, Laine AP, et al. HLA DR-DQ-encoded genetic determinants of childhood-onset type 1 diabetes in Finland: an analysis of 622 nuclear families. Tissue Antigens 2003;62:162–169 [DOI] [PubMed] [Google Scholar]
- 12.Kimpimäki T, Kulmala P, Savola K, et al. Natural history of beta-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J Clin Endocrinol Metab 2002;87:4572–4579 [DOI] [PubMed] [Google Scholar]
- 13.Hummel M, Bonifacio E, Schmid S, Walter M, Knopff A, Ziegler AG. Brief communication: early appearance of islet autoantibodies predicts childhood type 1 diabetes in offspring of diabetic parents. Ann Intern Med 2004;140:882–886 [DOI] [PubMed] [Google Scholar]
- 14.Parikka V, Näntö-Salonen K, Saarinen M, et al. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 2012;55:1926–1936 [DOI] [PubMed] [Google Scholar]
- 15.Ziegler AG, Bonifacio E, BABYDIAB-BABYDIET Study Group Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia 2012;55:1937–1943 [DOI] [PubMed] [Google Scholar]
- 16.Laine AP, Holmberg H, Nilsson A, et al. Finnish Paediatric Diabetes Registry Two insulin gene single nucleotide polymorphisms associated with type 1 diabetes risk in the Finnish and Swedish populations. Dis Markers 2007;23:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter M, Albert E, Conrad M, et al. IDDM2/insulin VNTR modifies risk conferred by IDDM1/HLA for development of type 1 diabetes and associated autoimmunity. Diabetologia 2003;46:712–720 [DOI] [PubMed] [Google Scholar]
- 18.Hermann R, Laine AP, Veijola R, et al. The effect of HLA class II, insulin and CTLA4 gene regions on the development of humoral beta cell autoimmunity. Diabetologia 2005;48:1766–1775 [DOI] [PubMed] [Google Scholar]
- 19.Pugliese A. Central and peripheral autoantigen presentation in immune tolerance. Immunology 2004;111:138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mäkinen A, Härkönen T, Ilonen J, Knip M, Finnish Pediatric Diabetes Register Characterization of the humoral immune response to islet antigen 2 in children with newly diagnosed type 1 diabetes. Eur J Endocrinol 2008;159:19–26 [DOI] [PubMed] [Google Scholar]
- 21.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009;52:1881–1888 [DOI] [PubMed] [Google Scholar]
- 23.Long AE, Gooneratne AT, Rokni S, Williams AJ, Bingley PJ. The role of autoantibodies to zinc transporter 8 in prediction of type 1 diabetes in relatives: lessons from the European Nicotinamide Diabetes Intervention Trial (ENDIT) cohort. J Clin Endocrinol Metab 2012;97:632–637 [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Dong F, Miao D, Fouts AR, Wenzlau JM, Steck AK. Proinsulin/insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care 2013;36:2266–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]