Abstract
While sporadic human genetic studies have permitted some comparisons between rodent and human pancreatic development, the lack of a robust experimental system has not permitted detailed examination of human pancreatic development. We previously developed a xenograft model of immature human fetal pancreas grafted under the kidney capsule of immune-incompetent mice, which allowed the development of human pancreatic β-cells. Here, we compared the development of human and murine fetal pancreatic grafts either under skeletal muscle epimysium or under the renal capsule. We demonstrated that human pancreatic β-cell development occurs more slowly (weeks) than murine pancreas (days) both by differentiation of pancreatic progenitors and by proliferation of developing β-cells. The superficial location of the skeletal muscle graft and its easier access permitted in vivo lentivirus-mediated gene transfer with a green fluorescent protein-labeled construct under control of the insulin or elastase gene promoter, which targeted β-cells and nonendocrine cells, respectively. This model of engraftment under the skeletal muscle epimysium is a new approach for longitudinal studies, which allows localized manipulation to determine the regulation of human pancreatic development.
Understanding the mechanisms that control human pancreatic islet development remains a target for deciphering the pathophysiological mechanisms of disease and for developing innovative therapeutic approaches. In rodents, genomic gene disruption experiments have enabled inference of the transcriptional regulatory network and paracrine soluble factors that control pancreas specification and later endocrine and exocrine fate determinations. In humans, rare genetic deficiencies are currently the way that regulatory factors for pancreatic islet development are determined.
In rodents, the pancreas-committed endodermal region of the foregut first expresses the transcription factor pancreatic and duodenal homeobox 1 (PDX1); mice and humans deficient in PDX1 lack a pancreatic gland (1,2). PDX1 later becomes restricted to mature pancreatic Δ-cells and β-cells where it activates insulin gene expression (3). These undifferentiated PDX1+ pancreatic progenitors proliferate through the mesenchymal effector Fibroblast Growth Factor 10 (4) and differentiate into endocrine, ductal, and acinar cells. The endocrine specification rests upon the transient expression of the basic helix-loop-helix factor, neurogenin3 (NGN3), and NGN3-deficient mice lack pancreatic endocrine cells (5). NGN3-expressing cells are unipotent endocrine precursors (6) and differentiate into the four pancreatic endocrine cell types (α-, β-, Δ-, and PP cells, producing, respectively, glucagon, insulin, somatostatin, and pancreatic polypeptide). Subsequently, combinations of additional transcription factors will determine the specific fate and stability of each pancreatic endocrine cell type (7). As an example, NKX2.2 is first expressed in early mouse pancreatic PDX1+ progenitors. Afterward, it is maintained in early endocrine progenitors along with NGN3 (7,8) and later becomes restricted to endocrine cells (except Δ-cells) as islets develop (9). In rodent pancreas, NKX2.2 plays a major role in maintaining β-cell identity (10).
Less is known on pancreatic development in humans. This is at least in part due to the difficulty in accessing properly staged human fetal pancreatic tissues and to the frequently poor quality of such tissues. This paucity of information is also due to the absence of dynamic assays to follow human β-cell development from pancreatic progenitors and to the lack of assays to genetically modify pancreatic cells at different stages of their development. Many aspects seem conserved between rodent and human pancreatic development. For example, different arguments derived from human genetic studies strongly suggest that PDX1 and NGN3 play similar roles during rodent and human pancreatic development (2,11,12). But differences seem to exist on the pancreatic role of other transcription factors such as, for example, MAFB or GATA family members (13–17). This is not unexpected, since, while rodent and human adult pancreatic β-cells share a large number of similarities, a number of data also indicate marked differences between species (18,19).
In early studies, human fetal pancreases corresponding to late fetal stages (15–24 weeks of development) were grafted to immune-incompetent rodents (20–22). More recently, we developed and validated a model of xenograft of early human fetal pancreas (7–9 weeks of development) under the kidney capsule of immune-incompetent severe combined immunodeficiency (SCID) mice. We demonstrated that this grafting model was permissive for proper development of the fetal tissue into a functional human endocrine pancreas (23). However, grafting under the kidney capsule is time consuming, limiting the number of SCID mice to be grafted and the amount of data to be produced. Moreover, due to its deep anatomical localization, in close contact to the kidney parenchyma after grafting, the growing pancreas is difficult to genetically modify to examine lineage tracing or longitudinal transcription factor expression.
In this context, we have developed an alternative site by grafting human fetal pancreas under the epimysium of the thigh muscle. Using this model, we have examined in detail the major steps and transcription factor expression that takes place during human pancreatic development. Finally, we have demonstrated the feasibility of cell-type, specific virus-mediated gene transfer to allow lineage tracing to observe islet and nonendocrine cell development in human fetal pancreas.
RESEARCH DESIGN AND METHODS
Mouse and human pancreases.
Pregnant Swiss mice were purchased from the Janvier breeding center (Janvier, Le Genest, France). At 12.5 days of gestation (E12.5), mice were killed by CO2 asphyxiation, according to the guidelines of the French Animal Care Committee, and the embryos were harvested. The dorsal pancreas was isolated and preserved in Hank’s balanced salt solution until use.
Human fetal pancreases were extracted from tissue fragments (24) that were obtained immediately after elective termination of pregnancy between 7 and 9 weeks of gestation in compliance with the French bioethics legislation. Approval was obtained from Agence de Biomedecine, the French competent authority along with maternal written consent.
Animals and grafting into SCID mice.
SCID mice (Charles River, L’Arbresle, France) were kept in isolators supplied with sterile-filtered, temperature-controlled air. Cages, bedding, and drinking water were autoclaved. Food was sterilized by X-ray irradiation. The French animal ethics committee approved these studies. For grafting, 6- to 8-week-old SCID mice were anesthetized with a ketamine/xylazine mix. Fetal pancreases were implanted (1 pancreas per graft) without prior dissociation, using a dissecting microscope, either under the kidney capsule as previously described (23) or under the muscle epimysium of either the biceps femoris or the gluteus superficialis. At different time points after grafting, grafts were removed, fixed in formalin 3.7%, and embedded in paraffin.
Immunolabeling and quantification.
Graft sections (4–5 μm thick) were prepared and processed as previously described (25). The following primary antibodies were used for immunostaining: mouse anti-BrdU (Amersham, Courtaboeuf, France), rabbit anti-carboxypeptidase A (1:600; Biogenesis, Poole, U.K.), rabbit anti-green fluorescent protein (GFP) (1:1,000; Abcam, Paris, France), mouse anti-glucagon (1:2,000; Sigma, St. Louis, MO); mouse anti-insulin (1:1,000; Sigma), mouse anti-Ki67 (1:20; BD, Franklin Lakes, NJ), sheep anti-human NGN3 (1:400; R&D Systems, Lille, France), mouse anti-mouse NGN3 (1:1,000; Beta Cell Biology Consortium), mouse anti-NKX2.2 (1:50; Developmental Studies Hybridoma Bank), rabbit anti–pan-cytokeratin (1:500; Dako, Trappes, France), rabbit anti-PDX1 (1:1,000) (26), and mouse anti-somatostatin (1:500; Beta Cell Biology Consortium). The secondary antibodies were dyelight or fluorescein isothiocyanate–conjugated anti-rabbit antibodies (1:200; Beckman Coulter, Villepinte, France), Texas red or fluorescein isothiocyanate anti-mouse antibodies (1:200; Beckman Coulter); Alexa Fluor anti-rabbit antibodies (1:400; Biogenex, Fremont, CA). For NGN3, revelation was performed using the Vectastain ABC kit (Vector, Malakoff, France).
Cell proliferation.
For cell proliferation analyses, mice were injected with 50 mg/kg BrdU 4 h before being killed. To measure the proliferation of PDX1+ pancreatic progenitors, we counted the frequency of BrdU+ nuclei among PDX1+ cells. To measure the proliferation of β-cells, we counted the frequency of BrdU+ nuclei among insulin+ cells. At least 1,000 cells per graft were counted in each condition. The same tissues were also stained with Ki67 antibody, and quantification was performed as described above as a second readout for cell proliferation.
Electron microscopy.
For standard electron microscopy, human pancreatic grafts were removed, cut into 1-mm cubes, and fixed overnight in 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.2. For gold immunolabeling, the fixative solution was 2.5% paraformaldehyde plus 0.5% glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.2. All fixed specimens were stored at 4°C until processed into resin; Spurr’s resin (Elektron Technology U.K., Stansted, U.K.) was used for structural observations, and London Resin Gold (Elektron Technology U.K.) was used for immunolabeling. Ultrathin (70 nm) sections were cut onto nickel grids, contrasted with uranyl acetate and lead citrate, and viewed with a Joel 1010 electron microscope. London Resin Gold sections were immunogold labeled for insulin using guinea pig anti-insulin (Sigma, Dorset, U.K.) and protein A gold (British Biocell International, Cardiff, U.K.)
Induction of diabetes with alloxan.
For determination of the capacity of the human graft to regulate the glycemia of the mouse, grafted (3-month grafts) and nongrafted (control mice) SCID mice were injected intravenously with alloxan (90 mg/kg body wt; Sigma-Aldrich), which is known to destroy rodent, but not human, β-cells (27). Glucose concentrations were measured on blood collected from the tail vein, once a week during 4 weeks, using a portable glucose meter (OneTouch Vita; LifeScan France, Issy les Moulineaux, France). For confirmation of the contribution of the graft to the normalization of blood glucose values in the host, grafts were removed 30 days after the injection of alloxan and blood glucose concentrations were measured during one more week. Circulating insulin concentrations were measured at day 0 after alloxan injection by the ELISA method (Mercodia insulin ELISA, human; Mercodia, Uppsala, Sweden).
Lentivirus-mediated gene transfer.
The lentiviral construct pTRIP ΔU3.RIP405-GFP has previously been described (28). The lentiviral construct pTRIP ΔU3.ElastaseP-GFP was derived from the pTRIP ΔU3.RIP405-GFP. Briefly, the insulin promoter was removed by MluI and BamHI digestion and replaced by a 500-bp fragment of the Elastase promoter flanked with the same MluI and BamHI restriction sites. The Elastase PCR fragment was amplified using phusion high-fidelity polymerase (Finnzyme) from Elastase GFP pcDNA3 plasmid kindly provided by David Tosh (University of Bath, Bath, U.K.) using forward primer 5′ ACGCGTCAGATCAGCTTATCGTATGAA 3′ and reverse primer 5′ GGATCCCGAGACCACTGCCCCTTGC 3′. Lentiviral vectors were prepared and titrated as previously described (28). Grafted (3-month grafts) SCID mice were anesthetized. One hundred microliters of the lentiviral vector solutions (105 transduction units) were injected using 32-gauge needles into multiple sites of the developing human pancreas. Pancreases were harvested at 7, 14, and 30 days after injections and used for immunofluorescence analyses.
RESULTS
Development of mouse and human fetal pancreas upon grafting under the muscle epimysium or under the kidney capsule.
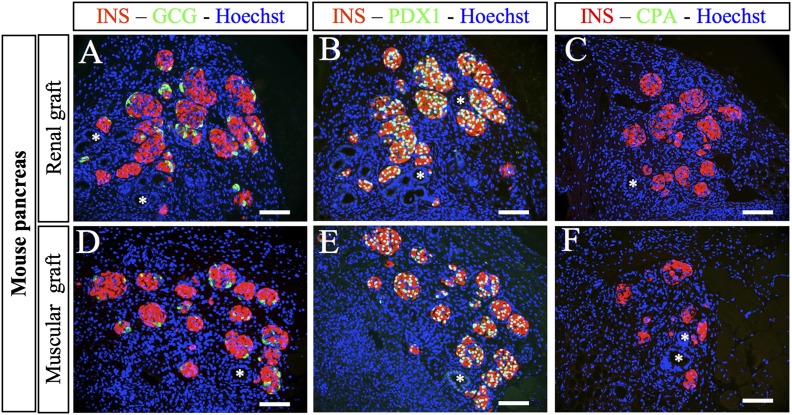
The renal capsule has been widely used as a grafting site for mature rodent and human islets and for fetal pancreas (23,29,30). We first compared the development of mouse fetal pancreas grafted either under the kidney capsule (renal graft) or at the level of the muscle epimysium (muscular graft). We found that undifferentiated E12.5 pancreases developed efficiently and in a similar fashion at both sites. Two weeks after grafting, insulin- and glucagon-expressing cells were present, forming islet-like structures with insulin+ clusters surrounded by glucagon-expressing cells (Fig. 1A and D). Insulin+ cells expressed PDX1 (Fig. 1B and E). Duct-like structures also developed similarly at both sites (Fig. 1A–F). Interestingly, acinar cells, visualized either after carboxypeptidase-A staining (Fig. 1C and F) or after amylase staining (data not shown), developed at none of the grafting sites.
FIG. 1.
Development of E12.5 mouse pancreases after grafting. Mouse E12.5 pancreases were grafted either under the kidney capsule (A–C) or under the muscle epimysium (D–F). Two weeks later, the grafts were removed, sectioned, and stained with anti-insulin, anti-glucagon, anti-PDX1, and anti-CPA antibodies. *Ducts. Scale bars: 100 µm. CPA, carboxypeptidase A; GCG, glucagon; INS, insulin.
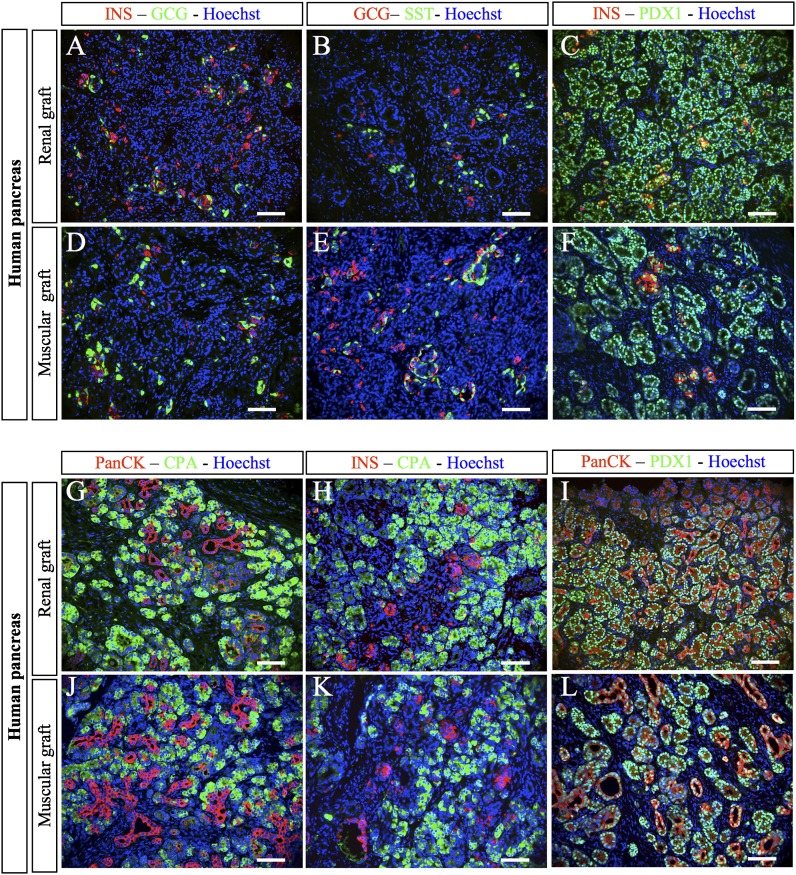
We next compared human fetal pancreas development after grafting under the muscle epimysium or the kidney capsule. Three months after grafting, insulin-, glucagon-, and somatostatin-expressing cells were observed on both sites (Fig. 2A, B, D, and E). β-Cells stained positive for PDX1 (Fig. 2C and F) and were never found positive for glucagon (Fig. 2A and D), supporting the differentiated status of such newly formed β-cells (31,32). Ductular structures that stained positive for pan-cytokeratin were observed on both sites (Fig. 2G, I, J, and L). Acinar cells that stained positive for carboxypeptidase-A developed from human fetal pancreas grafted on either site (Fig. 2G, H, J, and K). These cells also stained positive for trypsin, a second acinar marker (data not shown). This contrasts with the findings with grafted mouse pancreas (Fig. 1C and F).
FIG. 2.
Development of human fetal pancreases after grafting. Human fetal pancreases were grafted either under the kidney capsule (A–C and G–I) or under the muscle epimysium (D–F and J–L). Three months later, the grafts were removed, sectioned, and stained with anti-insulin, anti-glucagon, anti-somatostatin, anti-PDX1, anti-CPA, and anti-PanCK antibodies. Scale bars: 100 µm. CPA, carboxypeptidase A; GCG, glucagon; INS, insulin; SST, somatostatin.
We then characterized in more detail β-cells that developed from human fetal pancreases grafted under the muscle epimysium. For functional analysis, 3 months after grafting, we destroyed endogenous mouse β-cells with alloxan (27), which gave rise to increased glycemia in nongrafted mice (Supplementary Fig. 1). Among five grafted mice, one did not regulate its glycemia and had low circulating human insulin levels and poor β-cell differentiation (Supplementary Fig. 1). In four mice, glycemia was efficiently regulated, with circulating human insulin levels reaching 0.6 ng/mL and efficient β-cell differentiation (Supplementary Fig. 1). Of note, all four mice became hyperglycemic after removal of the grafts.
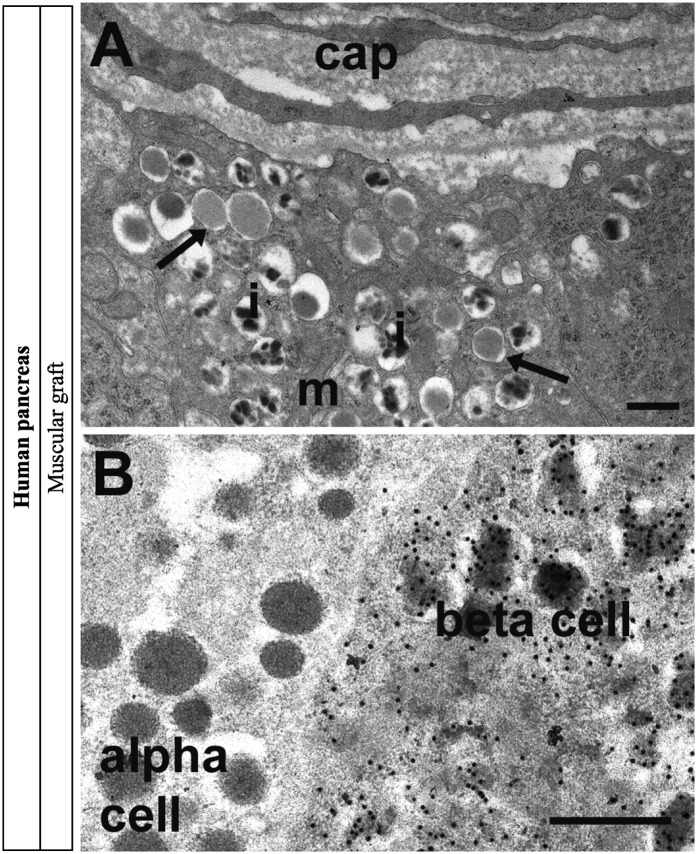
For morphological analysis, electron microscopy of the human fetal graft was performed 8 months after grafting. It indicated the presence of well-granulated α- and β-cells and a developed capillary network (Fig. 3A). Immunogold-electron microscopy indicated the presence of insulin within structurally mature β-cell granules (Fig. 3B).
FIG. 3.
Electron microscopy and gold immunolabeling for insulin in human pancreas 8 months postgrafting in skeletal muscle. A: The graft was well vascularized with capillaries (cap) adjacent to β-cells. β-Cells were well granulated and contained mitochondria (m), mature secretory granules with characteristic crystalline cores of human adult pancreas (i), and some immature granules (arrows). B: Gold immunolabeling for insulin was present over the secretory granules in β-cells but absent from adjacent α-cells. Scale bars: 500 nm.
These data demonstrated that pancreatic development of mouse or human pancreas was similar upon grafting under the muscle or the kidney capsule. Since grafting at the muscle site was technically easier and faster and could potentially allow perturbation experiments, we thus performed muscular grafting in the remaining part of this study.
PDX1+ pancreatic progenitors after grafting.
We analyzed the presence of PDX1+ pancreatic progenitors after grafting. In engrafted mouse fetal pancreas, all PDX1+ cells stained positive for insulin 2 weeks after grafting (Fig. 1E). This indicated that within 2 weeks, the pool of PDX1+/endocrine− pancreatic progenitors had been depleted. In contrast, many PDX1+/INS− cells were observed 3 months after grafting of human fetal pancreas (Fig. 2F).
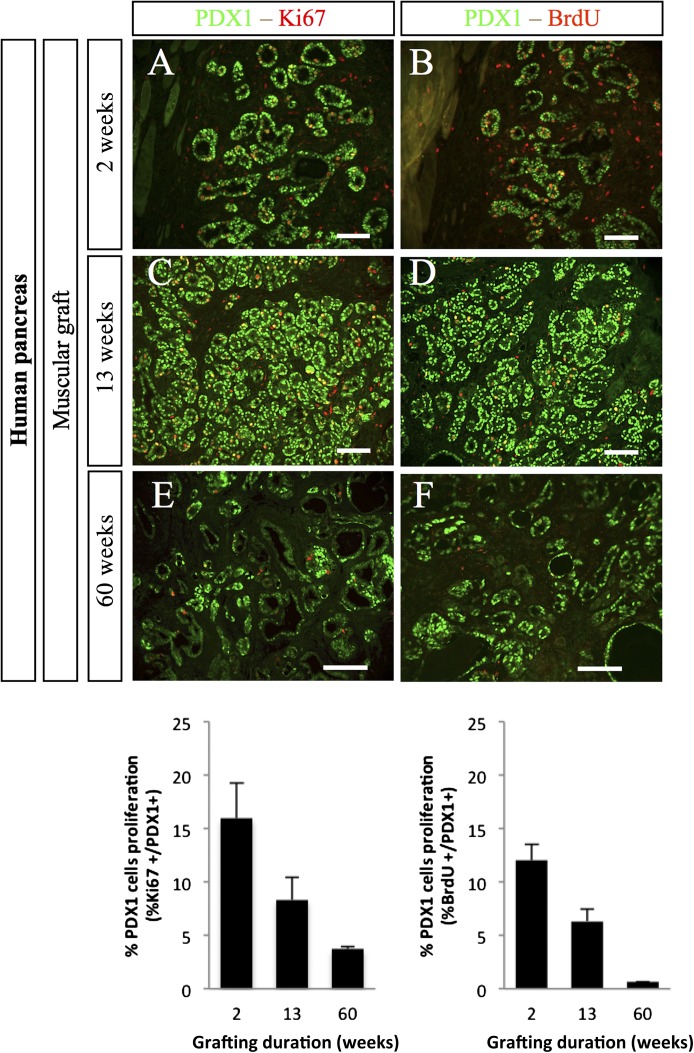
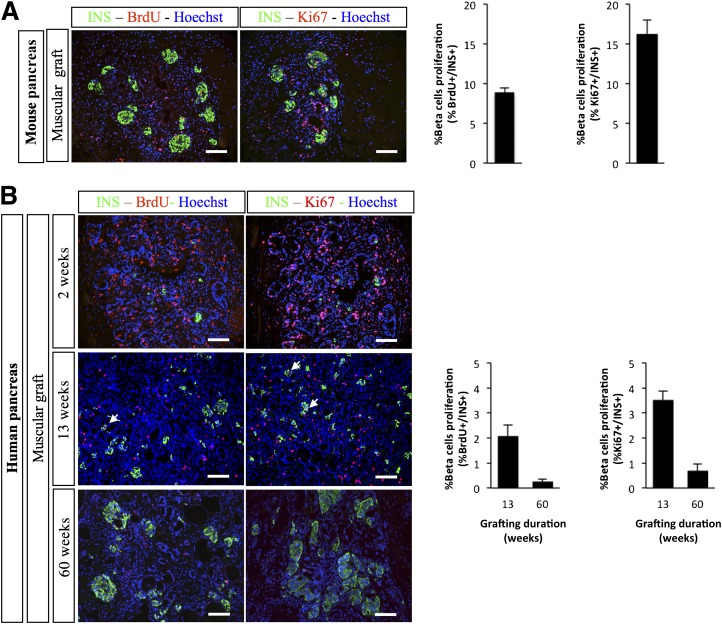
We next measured the proliferation of PDX1+ pancreatic progenitors at different time points after grafting of human fetal pancreas. Two weeks after grafting, 16% of PDX1+ cells stained positive for Ki67 and 12% of PDX+ cells stained positive for BrdU after a 4-h pulse (Fig. 4). These proportions decreased by nearly twofold 13 weeks after grafting and further decreased 60 weeks after grafting (Fig. 4).
FIG. 4.
Proliferation of PDX1+ cells after grafting of human fetal pancreases under the muscle epimysium. Human fetal pancreases were grafted under the muscle epimysium. At different time points (2, 13, and 60 weeks), mice were injected with BrdU and killed 4 h later. The grafts were removed, sectioned, and stained with anti-PDX1, anti-Ki67, and anti-BrdU antibodies. Top panel: Representative staining at three time points. Scale bars: 100 μm. Lower panel: Quantification of the proportions of PDX1+ cells that were also labeled for either Ki67 or BrdU. n = 4 grafts per group except for at 60 weeks, when n = 2.
Endocrine progenitors after grafting.
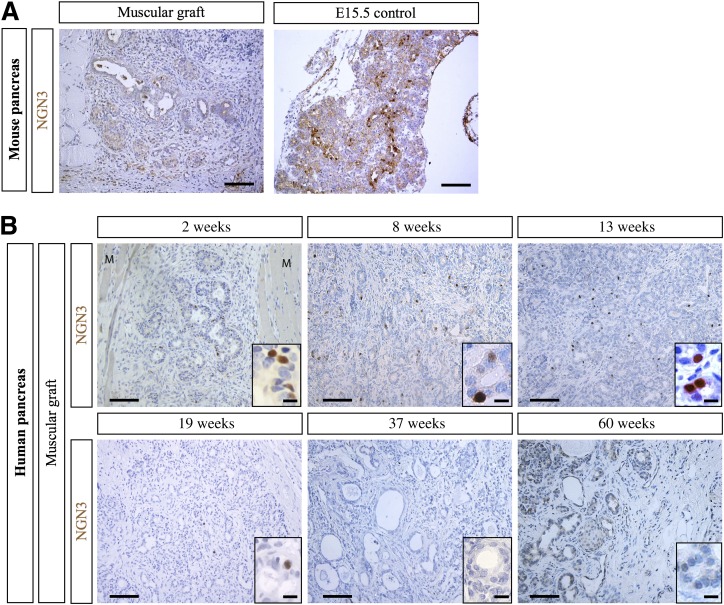
We analyzed the expression of NGN3, a transient marker of endocrine progenitors (5) after grafting. Two weeks after grafting of mouse fetal pancreas, NGN3+ endocrine progenitors were not observed (Fig. 5A). In grafted human pancreas, NGN3 was detected at weeks 2, 8, 13, and 19 postgrafting, its expression being undetectable at weeks 37 and 60 after grafting (Fig. 5B). Thus, NGN3 is expressed during at least 19 weeks in the grafted human fetal pancreas.
FIG. 5.
NGN3 expression in mouse and human muscular grafts. A: Mouse E12.5 pancreases were grafted under the muscle epimysium. Two weeks later, the grafts were removed, sectioned, and stained with anti-NGN3 antibodies. Ungrafted E15.5 mouse pancreas was used as a positive control. Scale bars: 100 μm. B: Human fetal pancreases were grafted under the muscle epimysium. At different time points (2, 8, 13, 19, 37, and 60 weeks), the grafts, surrounded by muscle fibers (M), were removed, sectioned, and stained with anti-NGN3 antibodies. Scale bars: 100 μm and 25 μm in the insets.
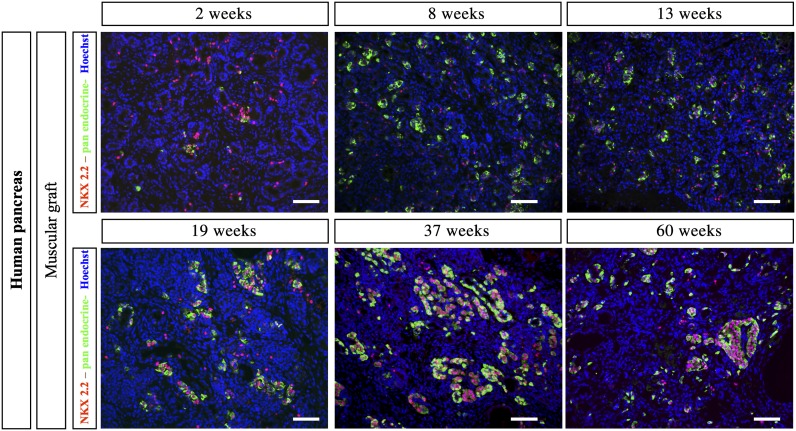
During mouse pancreatic development, NKX2.2 is expressed in early pancreatic progenitors and later becomes restricted to endocrine cells (except Δ-cells) as islets develop (9). Two weeks after grafting of mouse fetal pancreas, NKX2.2 expression was restricted to endocrine cells (data not shown). In grafted human fetal pancreas, 2 weeks after grafting, NKX2.2 was expressed in the first developing endocrine cells and also in many endocrine− cells (Fig. 6). Many NKX2.2+/endocrine− cells remained present until at least week 19 after grafting. At later postgrafting time points, the number of differentiated endocrine cells increased and such cells stained positive for NKX2.2. In parallel, the number of NKX2.2+/endocrine− cells decreased (Fig. 6).
FIG. 6.
NKX2.2 expression in human muscular grafts. Human fetal pancreases were grafted under the muscle epimysium. At different time points (2, 8, 13, 19, 37, and 60 weeks), the grafts were removed, sectioned, and stained with a cocktail of anti-insulin, anti-glucagon, anti-somatostatin, and anti–pancreatic polypeptide antibodies, revealed in green, and anti-NKX2.2 antibodies, revealed in red. Scale bars: 100 μm.
Taken together, such results indicate that in mouse, the pool of pancreatic and endocrine progenitors was depleted within a 2-week period, whereas in human pancreas pancreatic and endocrine progenitors remained present for at least 4 months after grafting, creating a pool of cells giving rise to endocrine cells.
β-Cell proliferation in grafted pancreases.
Two weeks after grafting, 8.9% of β-cells that developed from mouse fetal pancreas incorporated BrdU after a 4-h BrdU pulse and 16.15% of β-cells stained positive for Ki67 (Fig. 7A). In grafted human fetal pancreas, β-cell development increased during a 60-week time period with the development of larger islet-like structures (Supplementary Fig. 2). Thirteen weeks after grafting, 2% of β-cells incorporated BrdU after a 4-h pulse and 3.5% of β-cells stained positive for Ki67 (Fig. 7B). These proportions measured in grafted human fetal pancreases were lower than the ones measured in grafted mouse pancreas and further decreased by more than fivefold 60 weeks after grafting (Fig. 7B).
FIG. 7.
Proliferation of INS+ cells after grafting of fetal pancreases under the muscle epimysium. Mouse E12.5 (A) and human fetal pancreases (B) were grafted under the muscle epimysium. At different time points, mice were injected with BrdU and killed 4 h later. The grafts were removed, sectioned, and stained with anti-insulin, anti-Ki67, and anti-BrdU antibodies. Representative sections are presented. Arrows point to double-stained cells. Scale bars: 100 μm. Quantification of the proportion of INS+ cells that stained positive for either Ki67 or BrdU is also shown. Quantification was performed on three grafts for mouse pancreas, four grafts for human pancreas at week 13, and two grafts for human pancreas at week 60. INS, insulin.
Virus-mediated gene transfer into the developing human pancreatic cells.
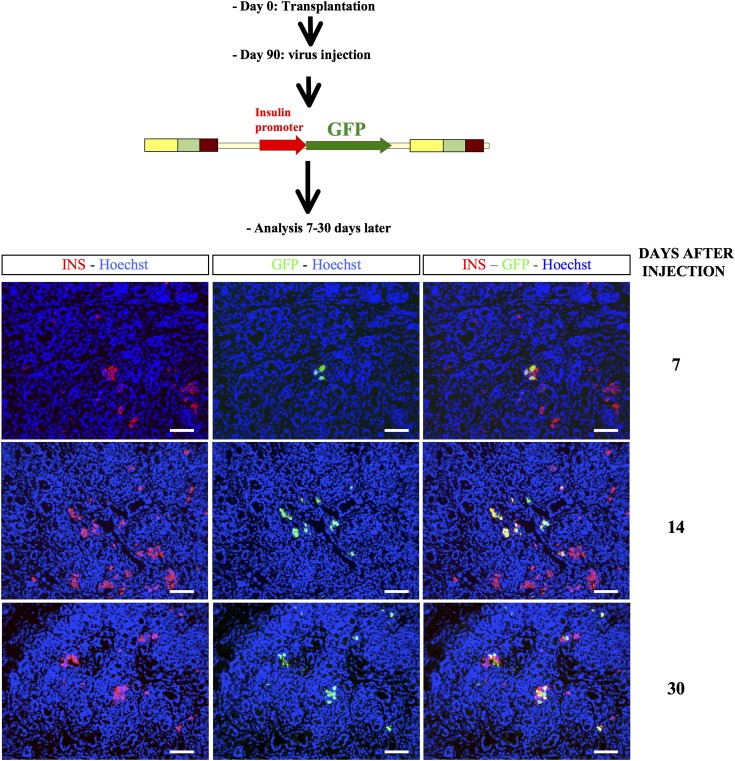
We finally asked whether it was feasible to specifically target human pancreatic cell types in the above-described model of human fetal pancreas that has developed under the muscle epimysium. Three months after grafting of human fetal pancreases under the muscle epimysium, we injected within the developing tissue lentiviral vectors that expressed GFP under the control of either the insulin promoter or the elastase promoter. Grafts were removed and analyzed 7–30 days later. When GFP was under the control of the insulin promoter, we labeled insulin+ cells with this approach (Fig. 8), while endocrine− cells that could represent nascent acinar cells were labeled when GFP was under the control of the elastase promoter (Supplementary Fig. 3).
FIG. 8.
Lentivirus-mediated gene transfer into the developing human pancreatic cells for β-cell–specific gene expression. Human fetal pancreases were grafted under the muscle epimysium. Three months later, lentiviral vectors expressing GFP under the control of the insulin promoter were injected into the developing grafts. Grafts were harvested 7, 14, and 30 days postinjection and stained with anti-insulin and anti-GFP antibodies. Scale bars: 100 µm. INS, insulin.
DISCUSSION
During the past few years, major progress has been made in understanding factors controlling mouse pancreatic islet-cell development. This is at least in part due to the development of a large number of innovative approaches, for example, transgenic mice for gain- and loss-of-function experiments or in vitro assays to screen for signals that modulate pancreatic development (33). On the other hand, information on human pancreatic islet development remained scarce. A first major limitation to efficient progress on human pancreas development is the inability to longitudinally track or impact on human pancreas development with models developed thus far. A second limitation is the difficult access to human fetal pancreases corresponding to specific stages of development in large enough quantity and quality.
In this work, we developed and validated a new grafting model for the study of human pancreatic islet development. Moreover, this model permitted us to perform virus-mediated gene transfer in specific human pancreatic cell types.
We previously developed a model of xenograft of human fetal pancreas under the kidney capsule of SCID mice. This model was permissive for pancreatic development and gave rise to functional human β-cells (23). However, grafting under the kidney capsule is technically challenging when a large number of grafts are performed and time-consuming. Moreover, our attempts to perform virus-mediated gene transfer into the developing human pancreas grafted under the kidney capsule failed because of difficulties accessing the graft and hemodynamic drastic variations when trying to mobilize the kidney (data not shown). In this context, we modified our initial model and used the muscular site as an alternative. The muscular site has been used in the past to graft mature islets. For example, the forearm muscle was used with success in humans for islet autotransplantation after total pancreatectomy for severe hereditary pancreatitis (34). In addition, a murine model of islets grafted within the cremaster muscle was developed and showed an efficient intraislet blood supply after 2 weeks of engraftment (35). Moreover, islet injections into biceps femoralis in the rat or into gracilis muscle in minipig induced a sustained reversal of diabetes (36,37). Finally, human islets grafted into the muscle of the forearm could be imaged, demonstrating their survival 1 year after implantation (38). On the other hand and to the best of our knowledge, the muscle has not been used as a grafting site for undifferentiated fetal pancreas and it was unknown whether this site was permissive for proper pancreatic cell differentiation. Here, we have demonstrated that rodent and human fetal pancreas properly develop upon grafting under the muscle epimysium. This site could thus represent a new site for grafting of human pancreatic progenitors. Indeed, protocols are now available to generate PDX1+ pancreatic progenitors from human embryonic stem cells (39). These progenitors need to be grafted into mice to differentiate into functional pancreatic endocrine cells (40). There is, however, a recurrent discussion on the best site for grafting of either mature islets or pancreatic progenitors that would develop into functional β-cells (41). In this context, the muscular epimysium represents an interesting site, as 1) functional β-cells develop from human fetal pancreatic progenitors; 2) the developing graft can easily be removed if necessary for malignant transformation of grafted cells; 3) it should be possible to image the developing β-cells, using approaches similar to the ones used for imaging β-cells grafted in muscle forearm (38); and 4) finally, such a model of grafting of human fetal pancreas where different steps in development can be studied should be useful to better understand how human pancreas develops, such a type of information being useful to efficiently generate functional β-cells from human embryonic stem cells.
In this model of muscular grafting, within 2 weeks after transplantation of a E12.5 mouse pancreas, differentiated cells developed, while the pool of PDX1+ pancreatic progenitors was depleted and all PDX1+ cells expressed insulin. This result perfectly fits with rodent data obtained either in vivo or in vitro (25,42). In contrast, 3 months after grafting of human fetal pancreas, many PDX1+/INS− cells were still observed. They were efficiently proliferating based on both Ki67 staining and BrdU incorporation. These proliferation indices decreased at later time points postgrafting. Taken together, such results suggest that pancreatic cell differentiation remains active during a long period in humans. It is, however, important to take into account that in humans, the presence of PDX1+/INS− cells represents an indirect sign of active differentiation as PDX1 remains expressed within pancreatic duct cells during adult life (43). But more direct arguments indicate that endocrine cell differentiation takes place during a long period after grafting of human fetal pancreas. Two weeks after grafting of mouse E12.5 pancreas, endocrine progenitors that expressed the transient NGN3 marker could not be observed, as is the case in vivo and in in vitro rodent models (5,25,42). On the other hand, NGN3+ cells could be observed within the grafted human fetal pancreas up to at least 19 weeks after engraftment. NGN3 expression was undetectable 37 and 60 weeks after grafting. This suggests that the process of endocrine differentiation occurs over periods of weeks. Similar results were obtained by looking at the expression of NKX2.2. In rodents, NKX2.2 is expressed in early pancreatic progenitors. Its expression is later maintained in mature endocrine cells (9). In the grafted human fetal pancreas, cells positive for NKX2.2 but negative for endocrine markers could be detected for at least 19 weeks after grafting.
Based on this longitudinal study of PDX1 and NGN3 and NKX2.2 expression patterns in the grafted human fetal pancreas, we propose that endocrine cell differentiation takes place during a long time window during development. This long time frame in humans should be useful to attempt to capture and amplify in vitro human pancreatic progenitors, as performed in the case of neural progenitors (44). Moreover, as previously suggested (14), such long-lasting developmental windows of human pancreatic development could increase the likelihood that deleterious effects due to haploinsuffficiency of a number of transcription factors such as GATA6, hepatocyte nuclear factor (HNF)1A, HNF1B, and HNF4 cause diabetes in humans.
β-Cell proliferation in this grafting model reproduced β-cell proliferation observed in vivo both for rodent and human pancreas. This is the case for mouse E12.5 pancreases engrafted during 2 weeks, where 8.9% of β-cells incorporated BrdU, as reported for newborn mouse pancreas (45). This is also the case for human fetal pancreases, where 2% of β-cells incorporated BrdU 3 months after grafting with 3.5% of β-cells positive for Ki67. Such levels decreased 1 year after grafting with 0.2% β-cells that incorporated BrdU and 0.7% that stained positive for Ki67. These data obtained with Ki67 antibodies are in accordance to those reported in fetal and neonatal pancreases at the same developmental stages (46,47). Of note, the above-described dynamic model of human pancreatic development permitted further support of data obtained with Ki67 antibodies with data obtained after BrdU injections. To our knowledge, such a type of experiment on human PDX1+ progenitors or human fetal β-cells entering the S-phase using BrdU incorporation had not been described in the past owing to the lack of proper experimental system. Such a dynamic model of β-cell proliferation should thus be useful to better dissect the mechanisms that regulate human β-cell proliferation during the perinatal period (48).
Overall, our results demonstrate the efficiency of the muscular fetal pancreatic graft model to recapitulate pancreatic development. The difference observed between mouse and human pancreatic islet development after grafting further emphasizes the specificity of each species regarding cell differentiation, maturation, and proliferation. The fact that we successfully performed cell type–specific in vivo lentiviral-mediated gene transfer in human pancreas grafted under the muscle epimysium represents a first step to the use of this model in a dynamic manner for gain- and loss-of-function experiments.
ACKNOWLEDGMENTS
C.C. was supported by Fondation pour la Recherche Médicale and by Association des Jeunes Diabétiques. This work was supported by grants to R.S. from the Beta Cell Biology Consortium (grant 1U01DK089571-01); from the 7th Framework Program of the European Union under grant agreements no. 241883; from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 155005, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations companies’ kind contribution; and from the bilateral program Bundesministerium fur Bildung und Forschung Agence Nationale de la Recherche, convention no. 2009 GENO10502. The R.S. laboratory belongs to the Laboratoire d’Excellence consortium Revive.
No potential conflicts of interest relevant to this article were reported.
C.C. conceived experiments, researched data, contributed to the discussion, and wrote the manuscript. M.-T.S. and V.A. researched data. A.C. performed the electron microscopy. Y.A. participated in the discussion and critically reviewed the manuscript. P.R. contributed tools. R.S. conceived experiments, contributed to the discussion, and also wrote the manuscript. R.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0554/-/DC1.
REFERENCES
- 1.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994;371:606–609 [DOI] [PubMed] [Google Scholar]
- 2.Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 1997;15:106–110 [DOI] [PubMed] [Google Scholar]
- 3.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 1993;12:4251–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhushan A, Itoh N, Kato S, et al. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 2001;128:5109–5117 [DOI] [PubMed] [Google Scholar]
- 5.Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA 2000;97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development 2009;136:3567–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev 2008;22:1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwitzgebel VM, Scheel DW, Conners JR, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 2000;127:3533–3542 [DOI] [PubMed] [Google Scholar]
- 9.Sussel L, Kalamaras J, Hartigan-O’Connor DJ, et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 1998;125:2213–2221 [DOI] [PubMed] [Google Scholar]
- 10.Papizan JB, Singer RA, Tschen SI, et al. Nkx2.2 repressor complex regulates islet β-cell specification and prevents β-to-α-cell reprogramming. Genes Dev 2011;25:2291–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinney SE, Oliver-Krasinski J, Ernst L, et al. Neonatal diabetes and congenital malabsorptive diarrhea attributable to a novel mutation in the human neurogenin-3 gene coding sequence. J Clin Endocrinol Metab 2011;96:1960–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubio-Cabezas O, Jensen JN, Hodgson MI, et al. Permanent neonatal diabetes and enteric anendocrinosis associated with biallelic mutations in NEUROG3. Diabetes 2011;60:1349–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorrell C, Schug J, Lin CF, et al. Transcriptomes of the major human pancreatic cell types. Diabetologia 2011;54:2832–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodríguez-Seguí S, Akerman I, Ferrer J. GATA believe it: new essential regulators of pancreas development. J Clin Invest 2012;122:3469–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrasco M, Delgado I, Soria B, Martín F, Rojas A. GATA4 and GATA6 control mouse pancreas organogenesis. J Clin Invest 2012;122:3504–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xuan S, Borok MJ, Decker KJ, et al. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest 2012;122:3516–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lango Allen H, Flanagan SE, Shaw-Smith C, et al. International Pancreatic Agenesis Consortium GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat Genet 2012;44:20–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 2013;75:155–179 [DOI] [PubMed] [Google Scholar]
- 19.Scharfmann R, Rachdi L, Ravassard P. Concise review: in search of unlimited sources of functional human pancreatic beta cells. Stem Cells Transl Med 2013;2:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuch BE, Ng AB, Jones A, Turtle JR. Histologic differentiation of human fetal pancreatic explants transplanted into nude mice. Diabetes 1984;33:1180–1187 [DOI] [PubMed] [Google Scholar]
- 21.Si Z, Tuch BE, Walsh DA. Development of human fetal pancreas after transplantation into SCID mice. Cells Tissues Organs 2001;168:147–157 [DOI] [PubMed] [Google Scholar]
- 22.Goldrath AW, Chen KE, Weide LG, Pour PM, Lebkowski JS, Alters SE. Retention of endocrine function in the SCID-Hu pancreas mouse—a model for the development of human fetal islet tissue. Transplantation 1995;59:1497–1500 [DOI] [PubMed] [Google Scholar]
- 23.Castaing M, Péault B, Basmaciogullari A, Casal I, Czernichow P, Scharfmann R. Blood glucose normalization upon transplantation of human embryonic pancreas into beta-cell-deficient SCID mice. Diabetologia 2001;44:2066–2076 [DOI] [PubMed] [Google Scholar]
- 24.Polak M, Bouchareb-Banaei L, Scharfmann R, Czernichow P. Early pattern of differentiation in the human pancreas. Diabetes 2000;49:225–232 [DOI] [PubMed] [Google Scholar]
- 25.Attali M, Stetsyuk V, Basmaciogullari A, et al. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes 2007;56:1248–1258 [DOI] [PubMed] [Google Scholar]
- 26.Duvillié B, Attali M, Bounacer A, Ravassard P, Basmaciogullari A, Scharfmann R. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes 2006;55:582–589 [DOI] [PubMed] [Google Scholar]
- 27.Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerström C, Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc Natl Acad Sci USA 1994;91:9253–9256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castaing M, Guerci A, Mallet J, Czernichow P, Ravassard P, Scharfmann R. Efficient restricted gene expression in beta cells by lentivirus-mediated gene transfer into pancreatic stem/progenitor cells. Diabetologia 2005;48:709–719 [DOI] [PubMed] [Google Scholar]
- 29.Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 1995;59:817–820 [PubMed] [Google Scholar]
- 30.Montaña E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest 1993;91:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Krijger RR, Aanstoot HJ, Kranenburg G, Reinhard M, Visser WJ, Bruining GJ. The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev Biol 1992;153:368–375 [DOI] [PubMed] [Google Scholar]
- 32.Riedel MJ, Asadi A, Wang R, Ao Z, Warnock GL, Kieffer TJ. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia 2012;55:372–381 [DOI] [PubMed] [Google Scholar]
- 33.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol 2009;326:4–35 [DOI] [PubMed] [Google Scholar]
- 34.Rafael E, Tibell A, Rydén M, et al. Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: a 2-year follow-up. Am J Transplant 2008;8:458–462 [DOI] [PubMed] [Google Scholar]
- 35.Christoffersson G, Henriksnäs J, Johansson L, et al. Clinical and experimental pancreatic islet transplantation to striated muscle: establishment of a vascular system similar to that in native islets. Diabetes 2010;59:2569–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund T, Korsgren O, Aursnes IA, Scholz H, Foss A. Sustained reversal of diabetes following islet transplantation to striated musculature in the rat. J Surg Res 2010;160:145–154 [DOI] [PubMed] [Google Scholar]
- 37.Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013;13:891–898 [DOI] [PubMed] [Google Scholar]
- 38.Pattou F, Kerr-Conte J, Wild D. GLP-1-receptor scanning for imaging of human beta cells transplanted in muscle. N Engl J Med 2010;363:1289–1290 [DOI] [PubMed] [Google Scholar]
- 39.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24:1392–1401 [DOI] [PubMed] [Google Scholar]
- 40.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 [DOI] [PubMed] [Google Scholar]
- 41.Jacobs-Tulleneers-Thevissen D, Bartholomeus K, Suenens K, et al. Human islet cell implants in a nude rat model of diabetes survive better in omentum than in liver with a positive influence of beta cell number and purity. Diabetologia 2010;53:1690–1699 [DOI] [PubMed] [Google Scholar]
- 42.Jensen J, Heller RS, Funder-Nielsen T, et al. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes 2000;49:163–176 [DOI] [PubMed] [Google Scholar]
- 43.Heimberg H, Bouwens L, Heremans Y, Van De Casteele M, Lefebvre V, Pipeleers D. Adult human pancreatic duct and islet cells exhibit similarities in expression and differences in phosphorylation and complex formation of the homeodomain protein Ipf-1. Diabetes 2000;49:571–579 [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Pollard S, Conti L, et al. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol Cell Neurosci 2008;38:245–258 [DOI] [PubMed] [Google Scholar]
- 45.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. Clin Invest 2004;114:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 2000;49:1325–1333 [DOI] [PubMed] [Google Scholar]
- 47.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Gu X, Liu Y, et al. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 2011;478:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]