Abstract
We previously established that the intestinal sweet taste receptors (STRs), T1R2 and T1R3, were expressed in distinct epithelial cells in the human proximal intestine and that their transcript levels varied with glycemic status in patients with type 2 diabetes. Here we determined whether STR expression was 1) acutely regulated by changes in luminal and systemic glucose levels, 2) disordered in type 2 diabetes, and 3) linked to glucose absorption. Fourteen healthy subjects and 13 patients with type 2 diabetes were studied twice, at euglycemia (5.2 ± 0.2 mmol/L) or hyperglycemia (12.3 ± 0.2 mmol/L). Endoscopic biopsy specimens were collected from the duodenum at baseline and after a 30-min intraduodenal glucose infusion of 30 g/150 mL water plus 3 g 3-O-methylglucose (3-OMG). STR transcripts were quantified by RT-PCR, and plasma was assayed for 3-OMG concentration. Intestinal STR transcript levels at baseline were unaffected by acute variations in glycemia in healthy subjects and in type 2 diabetic patients. T1R2 transcript levels increased after luminal glucose infusion in both groups during euglycemia (+5.8 × 104 and +5.8 × 104 copies, respectively) but decreased in healthy subjects during hyperglycemia (−1.4 × 104 copies). T1R2 levels increased significantly in type 2 diabetic patients under the same conditions (+6.9 × 105 copies). Plasma 3-OMG concentrations were significantly higher in type 2 diabetic patients than in healthy control subjects during acute hyperglycemia. Intestinal T1R2 expression is reciprocally regulated by luminal glucose in health according to glycemic status but is disordered in type 2 diabetes during acute hyperglycemia. This defect may enhance glucose absorption in type 2 diabetic patients and exacerbate postprandial hyperglycemia.
Glucose in the small intestinal lumen induces feedback that regulates gastric emptying, absorptive function, and energy intake (1–3), mediated both by vagal nerve pathways and secretion of gut peptides (4), including glucose-dependent insulinotropic polypeptide (GIP) from enteroendocrine K cells and glucagon-like peptide 1 (GLP-1) from L cells. These “incretins” substantially augment insulin secretion when glucose is given orally compared with an isoglycemic intravenous infusion (5). The rate of gastric emptying and the secretion and action of the incretin hormones are both key determinants of postprandial glycemia. However, the precise mechanism of glucose detection in the small intestine remains unclear.
Lingual sweet taste cells possess two G-protein–coupled receptors, T1R2 and T1R3, which form a heterodimeric sweet taste receptor (STR) for sugars, d-amino acids, sweet proteins, and artificial sweeteners (6,7). T1R2/R3 activation liberates the α-subunit of the G-protein gustducin (α-gustducin), leading to intracellular Ca2+ release, gating of a taste-specific transient receptor potential ion channel TRPM5 (8), cellular depolarization, and release of mediators that activate lingual afferent nerves.
We, and others, have shown that STRs, α-gustducin, and TRPM5 are also expressed with cellular and regional specificity in the animal and human intestine, where they may serve as glucose sensors (4,9–13). In addition to expression in intestinal sweet taste cells, some of these taste components are also expressed in separate intestinal cell populations that detect umami (T1R3, α-gustducin, TRPM5), bitter, and fats (α-gustducin, TRPM5) (4). STR activation may be linked to gut hormone secretion, because mice deficient in T1R3 or α-gustducin exhibit defective glucose-induced GLP-1 release (14), whereas the STR blocker, lactisole, decreases GLP-1 secretion and increases glycemic excursions after intragastric or intraduodenal glucose infusion in humans (15,16). Animal studies also indicate that STR activation increases the availability and function of the primary intestinal glucose transporter, sodium-glucose cotransporter-1 (SGLT-1) (17,18), although this link has not been assessed directly in humans.
Patients with type 2 diabetes frequently demonstrate disordered gastrointestinal responses to nutrients, with delayed gastric emptying in up to 30–50%, abnormally rapid emptying in a few (19,20), and a high prevalence of gastrointestinal symptoms (21). GLP-1 and GIP secretion has been inconsistently reported to be diminished in patients with type 2 diabetes (22,23), whereas intestinal levels of SGLT-1 and the capacity for glucose absorption may be increased (24). Any of these abnormalities could potentially relate to disordered intestinal sensing of glucose. We previously reported that duodenal expression of STRs during fasting was comparable in unselected patients with type 2 diabetes and nondiabetic control subjects but was inversely related to the blood glucose concentration at the time of biopsy in type 2 diabetic patients (13). In rodents, we, and others, have also shown that intestinal STR transcript and protein levels are rapidly downregulated upon acute luminal exposure to glucose or artificial sweeteners (13,25). Our current aims were, therefore, to evaluate the modulation of duodenal STR expression in response to acute changes in luminal and systemic glucose exposure in healthy humans and to determine whether STR regulation is disordered in type 2 diabetes and related to changes in glucose absorption and/or gut hormone secretion.
RESEARCH DESIGN AND METHODS
Subjects.
Fourteen healthy subjects and 13 patients with type 2 diabetes were studied in randomized, crossover fashion. The mean duration of known diabetes in the latter group was 5 ± 1 years, HbA1c was 6.3 ± 0.2% (45 ± 2 mmol/mol), and all were free of significant comorbidities and managed by diet alone. The protocol was approved by the Human Research Ethics Committee of the Royal Adelaide Hospital and conducted in accordance with the Declaration of Helsinki as revised in 2000. Each subject provided written informed consent.
Screening visit.
Each subject attended the laboratory at 0830 h after an overnight fast of 12 h for solids and 10 h for liquids. An intravenous cannula was inserted for blood sampling, and subjects consumed a glucose drink (75 g glucose dissolved in water to 300 mL, labeled with 150 mg 13C acetate) within 5 min (T = −5 to 0 min). Blood was sampled at T = −5, 30, 60, 120, and 180 min to measure blood glucose by a glucometer (Medisense Precision QID; Abbott Laboratories, Bedford, MA). Breath samples were collected before and every 5 min after oral glucose during the first hour, and every 15 min for a further 2 h to measure 13CO2 concentrations by isotope ratio mass spectrometer (ABCA 2020; Europa Scientific, Crewe, U.K.). The gastric half-emptying time was calculated using the formula of Ghoos et al. (26). Gastrointestinal symptoms were assessed by a standard questionnaire (maximum score, 27), as previously described (27). Autonomic nerve function was assessed in the type 2 diabetes patients using standardized cardiovascular reflex tests, with a score ≥3 (of a maximum of 6) indicating autonomic dysfunction (28).
Endoscopy protocol.
After the screening visit, each subject was studied twice, separated by at least a week, with female subjects studied exclusively during the follicular phrase of the menstrual cycle to limit variations in gut hormone concentrations (29). Subjects attended the laboratory at 0830 h after an overnight fast, and an insulin/glucose clamp was established to achieve euglycemia (∼5 mmol/L) or hyperglycemia (∼12 mmol/L) (30). A 50-mL intravenous bolus of 25% glucose (Baxter Healthcare, Old Toongabbie, NSW, Australia) was administered on the hyperglycemic day, and 0.9% saline (Baxter Healthcare) on the euglycemic day, over 1 min each, followed by continuous infusion of the same solution starting at 150 mL/h and adjusted according to blood glucose measurements every 5 min on the hyperglycemic day or remaining at 150 mL/h on the euglycemic day. On the euglycemic day, 25% dextrose was infused intravenously if the blood glucose concentration fell below 5 mmol/L. In addition, 100 IU of insulin (Actrapid; Novo Nordisk, Baulkham Hills, NSW, Australia), in 500 mL 4% succinylated gelatin solution (Gelofusine; B. Braun Australia, Bella Vista, NSW, Australia), was infused intravenously at a variable rate to maintain euglycemia. Once blood glucose concentrations were stable for 30 min (12.3 ± 0.2 mmol/L on the hyperglycemic day or 5.2 ± 0.2 mmol/L on the euglycemic day), a small diameter video endoscope (GIF-XP160; Olympus, Tokyo, Japan) was passed via an anesthetized nostril into the second part of the duodenum, from which mucosal biopsy specimens were collected using standard biopsy forceps and placed into RNAlater (Qiagen, Sydney, NSW, Australia) or 4% paraformaldehyde for 2 h. At T = 0 an intraduodenal infusion containing 30 g glucose and 3 g glucose absorption marker 3-O-methyglucose (3-OMG; Sigma-Aldrich, St. Louis, MO) was commenced via the biopsy channel of the endoscope, and continued for 30 min (1 g/min; 4 kcal/min). At T = 10 and T = 30 min, additional biopsy specimens were collected. Blood samples (20 mL) were taken every 10 min over 1 h to determine concentrations of 3-OMG, C-peptide, GLP-1, and GIP.
Assays.
Plasma total GLP-1 concentrations were measured by radioimmunoassay (GLPIT-36HK; Millipore, Billerica, MA) with sensitivity of 3 pmol/L and intra- and interassay coefficients of variation (CV) of 4.2% and 10.5%. Total plasma GIP was measured by radioimmunoassay as previously reported, with sensitivity of 2 pmol/L and intra- and interassay CV of 6.1% and 15.4%, respectively (31). Plasma C-peptide concentrations were measured by ELISA (10-1136-01; Mercodia, Uppsala, Sweden), with sensitivity of 15 pmol/L and intra- and interassay CV of 3.6% and 3.3%. Serum 3-OMG concentrations were measured by liquid chromatography and mass spectrometry, with sensitivity of 10 pmol/L (32).
Quantification of gene expression by real-time RT-PCR.
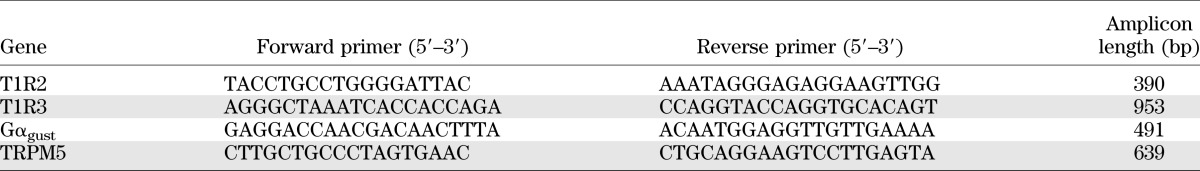
RNA was extracted from tissues using an RNeasy Mini kit (Qiagen), following the manufacturer’s instructions, and RNA yield and quality were determined using a NanoDrop (NanoDrop Technologies, Wilmington, DE). Quantitative real-time RT-PCR was then used to determine the absolute expression of sweet taste molecules. Validated human primers for T1R2, α-gustducin, and TRPM5 were used as primer assays (QuantiTect, Qiagen). T1R3 primers were designed using Primer 3.0 software (Applied Biosystems, Foster City, CA) based on target sequences obtained from the National Center for Biotechnology Information nucleotide database (Table 1). Absolute standard curves were generated by including known copy number standards in RT-PCR for each target (Table 2), as described (13). RT-PCR was performed on a Chromo4 (MJ Research, Waltham, MA) real-time instrument attached to a PTC-200 Peltierthermal cycler (MJ Research) using a QuantiTect SYBR Green one-step RT-PCR kit (Qiagen) according to the manufacturer’s specifications, as previously described (13). Each assay was performed in triplicate and included internal no-template and no-RT controls. All replicates were averaged for final mRNA copy number, which was expressed as copies/50 ng of total RNA.
TABLE 1.
Human primers used for absolute quantification of target genes in RT-PCR
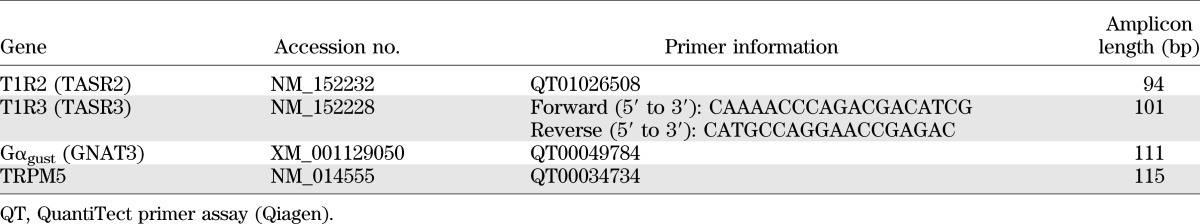
TABLE 2.
Human primers used to generate RT-PCR product containing target amplicon to create absolute standard curves
Immunohistochemistry.
Fixed tissues were cryoprotected (30% sucrose in PBS), embedded in cryomolds, and frozen before sectioning at 6–10 μm (Cryocut 1800; Leica Biosystems, Nussloch, Germany) and thaw mounting onto gelatin-coated slides. Immunoreactivity was detected using rabbit T1R2 primary (H90, 1:400, SC-50305; Santa Cruz Biotechnology, Santa Cruz, CA), goat GLP-1 primary (1:400, SC-7782; Santa Cruz Biotechnology), monoclonal 5-hydroxytryptamine (5-HT; 1:1000, M0758; Dako Australia, Victoria, Australia), and GIP primary antibodies (1:800, AB30679; Abcam). All were visualized using species-specific secondary antibodies conjugated to Alexa Fluor dyes (1:200 in PBS-Tween 20) as previously described (12,13). Antigen retrieval (S1700; Dako) was performed for T1R2 according to the manufacturer’s instructions. Nucleated epithelial cells immunopositive for individual targets were counted per square millimeter of high-power field and averaged over at least 10 intact transverse sections per subject.
Data analysis.
The incremental area under the curve (iAUC) for 3-OMG, GLP-1, and GIP concentrations was calculated using the trapezoidal rule (33) and analyzed by one-factor ANOVA using Prism software (version 6.0; GraphPad Software Inc., La Jolla, CA). These variables were also assessed using repeated-measures ANOVA, with treatment and time as factors. Post hoc comparisons, adjusted for multiple comparisons by Holm-Sidak’s correction, were performed if ANOVAs showed significant effects. One-way ANOVA, with Holm-Sidak’s post hoc test, was used to compare differences in duodenal levels of STR transcripts between healthy subjects and type 2 diabetic patients. Relationships between transcript expression and other factors were evaluated by the Pearson correlation coefficient (r). We calculated that 12 subjects had 80% power to detect a one-third difference in duodenal T1R2 expression in paired studies (α = 0.05), compared with control (13). P values ≤ 0.05 were considered statistically significant. Data are expressed as mean ± SEM.
RESULTS
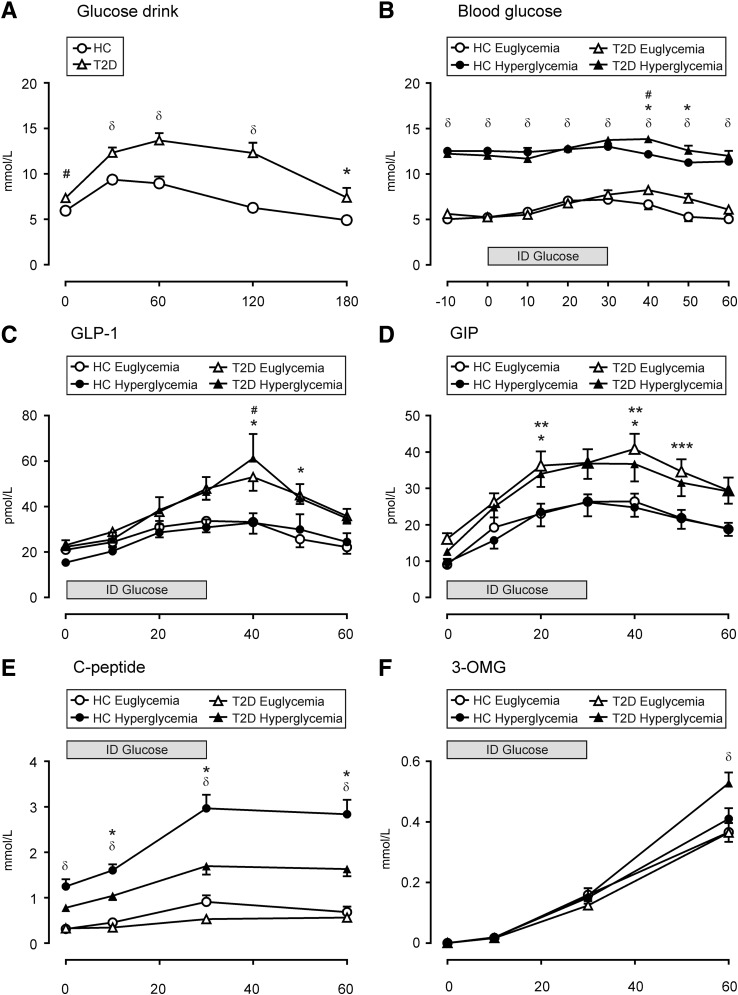
All subjects tolerated the study well. The patients with type 2 diabetes were older than the healthy subjects, but gastrointestinal symptom scores, BMI, and gastric emptying of glucose did not differ (Table 3). Five type 2 diabetic patients had autonomic dysfunction, but none had evidence of peripheral neuropathy, nephropathy, retinopathy, or macrovascular complications. As expected, blood glucose concentrations were higher in type 2 diabetic patients during fasting and after oral glucose (P < 0.05; Fig. 1A).
TABLE 3.
Demographic, anthropometric, metabolic, and gastrointestinal parameters of the study participants
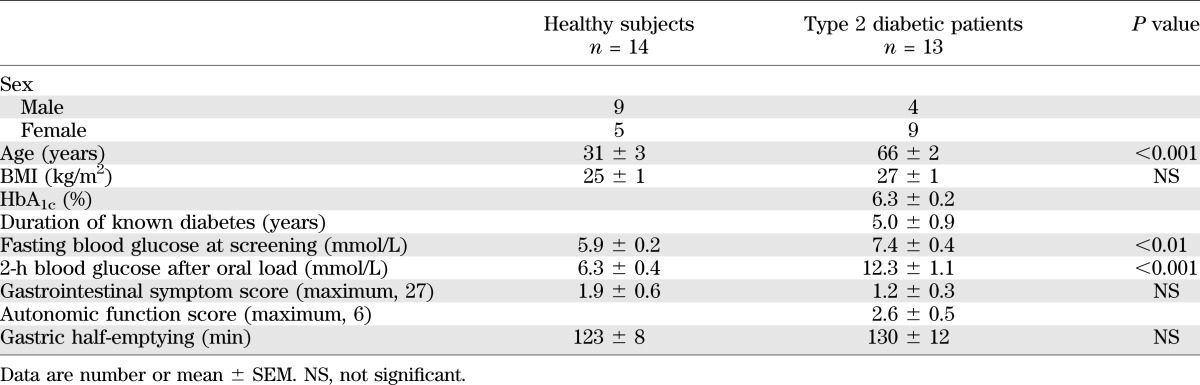
FIG. 1.
Effects of oral glucose or intraduodenal (ID) glucose infusion on blood glucose levels and plasma levels of hormones and the glucose absorption marker 3-OMG in healthy control (HC) subjects and type 2 diabetic (T2D) patients during euglycemia or hyperglycemia. (A) Blood glucose levels after a glucose drink in HC subjects and T2D patients. *P < 0.05, #P < 0.01, δP < 0.001 T2D compared with HC. (B) Blood glucose levels after ID glucose infusion during glycemic clamp. δP < 0.001 HC euglycemic compared with hyperglycemic groups and T2D euglycemic compared with T2D hyperglycemic; *P < 0.05 T2D euglycemic compared with HC euglycemic; #P < 0.05 T2D hyperglycemic compared with HC hyperglycemic. (C) Plasma GLP-1. *P < 0.05 T2D groups compared with HC euglycemic; #P < 0.01 T2D groups compared with HC hyperglycemic. (D) Plasma GIP. *P < 0.05 T2D groups compared with HC euglycemic; **P < 0.05 T2D groups compared with HC hyperglycemic; ***P < 0.05 T2D euglycemic compared with HC groups. (E) C-peptide. δP < 0.001 HC hyperglycemic compared with euglycemic groups; *P < 0.05 T2D hyperglycemic compared with other groups. (F) 3-OMG. δP < 0.001 T2D hyperglycemic compared with other groups. Data are mean ± SEM; significance represents treatment × time interactions.
Baseline STR expression.
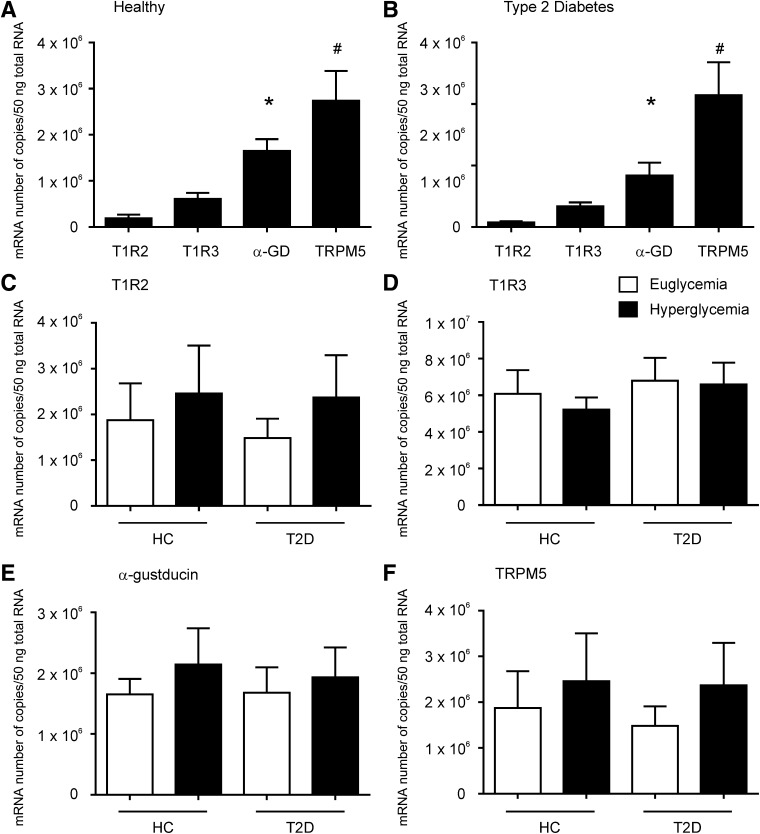
Transcripts for T1R2, T1R3, α-gustducin, and TRPM5 were readily detected in duodenal biopsy specimens by quantitative RT-PCR. TRPM5 was the most abundant STR transcript in all subjects, with lower levels of α-gustducin and much lower levels of T1R2 and T1R3; T1R2 was the least expressed transcript (Fig. 2A). TRPM5 transcript levels in healthy subjects during euglycemia were 34 ± 8-fold higher than those of T1R2 (P < 0.001), whereas α-gustducin levels were 22 ± 7-fold higher (P < 0.05) and T1R3 levels were 12 ± 5-fold higher.
FIG. 2.
Absolute transcript levels of STR in the duodenum of healthy subjects and type 2 diabetic patients at stable euglycemia and hyperglycemia. Absolute expression (copy number) of STR transcripts at baseline in the duodenum of healthy subjects (A) or patients with type 2 diabetes (B). (A) TRPM5 levels were 15-fold higher, α-gustducin 9-fold higher, and T1R3 3-fold higher than T1R2 levels in healthy subjects. (B) TRPM5 levels were 29-fold higher, α-gustducin 11-fold higher, and T1R3 5-fold higher than T1R2 levels in patients with type 2 diabetes. *P < 0.05 and #P < 0.01 compared with T1R2. Duodenal levels of T1R2 (C), T1R3 (D), α-gustducin (E), and TRPM5 (F) transcript in healthy control (HC) subjects and type 2 diabetic (T2D) patients at stable euglycemia or hyperglycemia. No significant differences in transcript levels were detected at stable baseline. Data are mean ± SEM. α-GD, α-gustducin
Effects of acute changes in glycemia on STR expression.
Fasting expression of STR transcripts was unaffected by the glycemic state in health or type 2 diabetes and did not differ between the groups (Fig. 2B–E).
Effects of luminal glucose on duodenal STR expression.
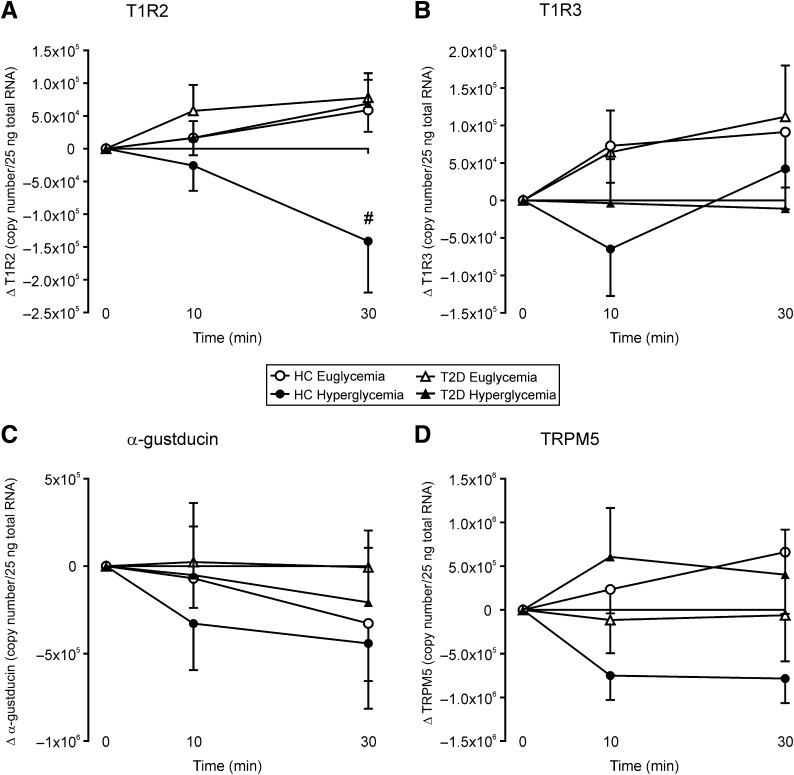
Owing to intersubject variability in STR expression, responses to luminal glucose were evaluated as changes from baseline. During euglycemia, T1R2 transcript levels increased in response to duodenal glucose infusion in health and in type 2 diabetes after 30 min (+5.9 × 104 and +5.8 × 104 copies; Fig. 3A). During hyperglycemia, T1R2 transcript levels decreased in healthy subjects after 30 min (−1.4 × 104 copies) but increased in type 2 diabetic patients (+6.9 × 105 copies), so that levels in health were lower at 30 min during hyperglycemia than euglycemia and lower than in type 2 diabetic patients during either glycemic state (subject × time interactions P < 0.01 for each). Levels of T1R3, α-gustducin, and TRPM5 transcript, in contrast, did not significantly change in response to luminal glucose under either glycemic condition (Fig. 3B–D).
FIG. 3.
Effects of intraduodenal glucose infusion on sweet taste molecule transcript levels in healthy control (HC) subjects and type 2 diabetic (T2D) patients during euglycemia or hyperglycemia. (A) Change in absolute expression of T1R2 in human duodenum during intraduodenal glucose infusion under euglycemic or hyperglycemic clamp. #P < 0.01 HC hyperglycemic compared with all other groups. T1R3 (B), α-gustducin (C), and TRPM5 (D). Data are mean ± SEM.
Plasma hormone concentrations.
Fasting plasma GLP-1 concentrations did not differ between health and type 2 diabetes and were not acutely affected by the glycemic state. Plasma GLP-1 increased in response to duodenal glucose infusion in all groups (P < 0.001; Fig. 1C), with higher concentrations evident in type 2 diabetic patients at 40 min irrespective of glycemic status (subject × time interactions P < 0.01) and at 50 min during euglycemia compared with healthy subjects (subject × time interactions P < 0.05). The iAUC for GLP-1 was higher in type 2 diabetic patients during euglycemia and hyperglycemia compared with healthy subjects (P < 0.05 each; Table 4).
TABLE 4.
iAUC for GLP-1, GIP, C-peptide, and 3-OMG in healthy subjects and type 2 diabetic patients
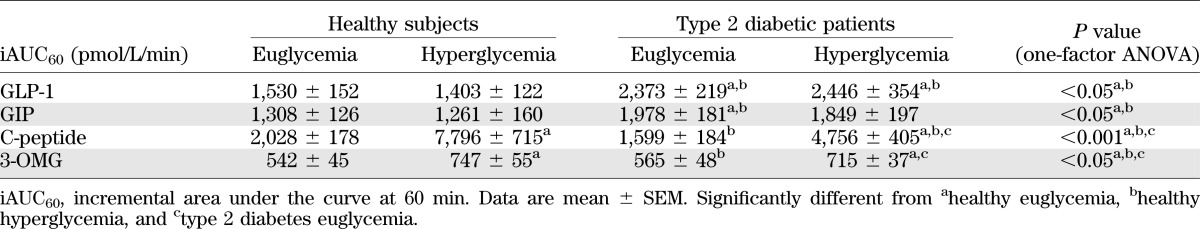
Fasting plasma GIP concentrations did not differ between healthy subjects and type 2 diabetic patients and were not acutely affected by the glycemic state. Plasma GIP increased in response to duodenal glucose infusion in both groups (P < 0.001; Fig. 1D), with higher GIP concentrations evident in type 2 diabetic patients at 40 min irrespective of glycemic status and higher concentrations during euglycemia at 20 and 50 min compared with healthy subjects (subject × time interaction P < 0.05). The iAUC was higher in type 2 diabetic patients during euglycemia compared with healthy subjects (P < 0.05; Table 4).
Fasting C-peptide concentrations were higher during hyperglycemia than during euglycemia in healthy subjects (P < 0.001; Fig. 1E) but not in type 2 diabetic patients. C-peptide concentrations increased in response to duodenal glucose infusion in both groups during hyperglycemia (subject × time interaction P < 0.05; iAUC P < 0.001) but not during euglycemia (Fig. 1E and Table 4). C-peptide concentrations during hyperglycemia were higher in healthy subjects than in type 2 diabetic patients throughout the glucose infusion (subject × time interaction P < 0.05; iAUC P < 0.001).
Serum 3-OMG concentrations.
Serum 3-OMG concentrations increased over time in all groups but were higher at 60 min in type 2 diabetic patients during hyperglycemia than in any other group (subject × time interaction P < 0.001; Fig. 1F). The iAUC for 3-OMG was higher in type 2 diabetic patients and in healthy subjects during hyperglycemia than during euglycemia (P < 0.05; Table 4).
Phenotype of human intestinal sweet taste cells.
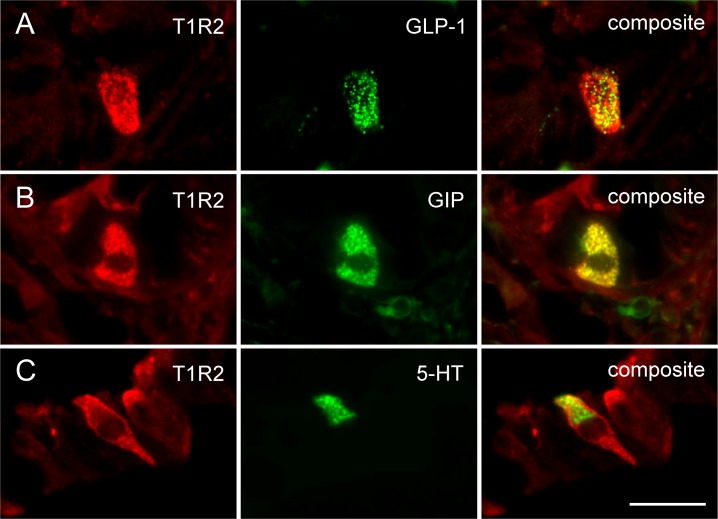
Immunolabeling for T1R2 was evident in single cells dispersed throughout the mucosal epithelium in healthy subjects and type 2 diabetic patients (Fig. 4). Immunopositive cells showed a homogenous distribution of the label throughout the cytoplasm, were largely open or “flask-shaped,” and were found with equal frequency within villi or crypts. In dual-labeling experiments in healthy subjects, 19 ± 11% of T1R2-labeled duodenal cells coexpressed GLP-1, whereas 13 ± 8% of L cells coexpressed T1R2 (Fig. 4A). In a similar manner, 15 ± 10% of T1R2-labeled duodenal cells coexpressed GIP, whereas 12 ± 8% of K cells coexpressed T1R2 (Fig. 4B). Separate populations of T1R2-labeled cells coexpressed 5-HT (31 ± 6%), whereas 5 ± 1% of enterochromaffin (EC) cells coexpressed T1R2 in healthy subjects (Fig. 4C). During fasting, an equivalent number of T1R2 immunopositive cells were evident in healthy subjects and in type 2 diabetic patients, under euglycemia or hyperglycemia, and the number did not change during the duodenal glucose infusion. Similarly, the proportion of cells immunopositive for GLP-1, GIP, and 5-HT did not differ between healthy subjects and type 2 diabetic patients or with glycemic state or exposure to luminal glucose, although a trend for increased L cells in fasting type 2 diabetic patients was evident (data not shown; P = 0.07).
FIG. 4.
Subsets of L cells, K cells, and EC cells express STR in healthy human duodenum. (A) Immunolabeling for GLP-1 was present in 19 ± 11% of T1R2-labeled duodenal cells in healthy control subjects at euglycemia, whereas 13 ± 8% of L cells coexpressed T1R2. (B) GIP was present in 15 ± 10% of T1R2-labeled cells in healthy control subjects at euglycemia, whereas 12 ± 8% of K cells coexpressed T1R2. (C) In a similar manner, separate populations of T1R2-labeled cells coexpressed 5-HT (31 ± 6%), whereas 5 ± 1% of EC cells coexpressed T1R2. (A–C) Scale bar = 20 µm.
Relationships between variables.
Absolute copy numbers of STR transcripts during fasting and after the 30-min glucose infusion did not correlate with age, sex, BMI, symptom score, or gastric half-emptying time in either group, and in type 2 diabetic patients, they were not related to duration of diabetes, HbA1c, autonomic dysfunction, or symptom score. In contrast, the change in T1R2 transcript level after luminal glucose exposure correlated with the iAUC for 3-OMG in healthy subjects during euglycemia (r = 0.73, P < 0.05), and the change in TRPM5 transcript level with plasma GLP-1 concentrations at 30 min (r = 0.62, P < 0.05) in the same group. Changes in T1R2 (r = 0.78, P < 0.01) and T1R3 transcript levels (r = 0.59, P < 0.05) in type 2 diabetic patients during hyperglycemia also correlated with plasma GIP concentrations at 30 min, and the change in T1R2 correlated with the iAUC for GIP (r = 0.69, P < 0.05).
DISCUSSION
This study is the first to define changes in expression of intestinal STR transcripts in healthy humans and patients with type 2 diabetes in response to acute changes in systemic and luminal glucose. We have shown that absolute levels of STR transcripts are unaffected by acute variations in glycemia during fasting in either group but that T1R2 expression increases upon exposure to luminal glucose during euglycemia. In contrast, T1R2 expression decreases markedly in response to luminal glucose during hyperglycemia in health but increases under the same conditions in type 2 diabetes. Type 2 diabetic patients also exhibit increased glucose absorption during acute hyperglycemia compared with healthy subjects, suggesting that dysregulated expression of intestinal STRs can perpetuate postprandial hyperglycemia in this group.
We confirmed our previous observation that fasting STR transcript levels are similar in health and in type 2 diabetes irrespective of age, sex, or BMI (13). Although we previously observed that levels of STR transcript were inversely related to fasting blood glucose concentrations in unselected type 2 diabetic patients presenting for endoscopy, we have now established unequivocally that acute changes in glycemia do not influence fasting intestinal STR expression in health or in “well-controlled” type 2 diabetes. The apparent discrepancy in these observations may reflect the effects of more longstanding hyperglycemia or differences in the duration of fasting in the earlier cross-sectional study. We have now shown that the intestinal STR system is, in contrast, highly responsive to the presence of luminal glucose, with rapid and reciprocal regulation of T1R2 transcripts in health, depending on the prevailing blood glucose concentration. Comparable changes were evident in T1R3 and TRPM5 transcript levels, although these were not statistically significant. Increased intersubject variability seen for T1R3 and TRPM5 transcript levels may be due to their expression in additional populations of intestinal cells tuned to detect other taste modalities and, therefore, unresponsive to luminal and/or systemic glucose.
Healthy subjects who displayed the largest glucose-induced increase in duodenal T1R2 transcript levels during euglycemia had the highest plasma concentrations of the glucose absorption marker 3-OMG. Because SGLT-1 is responsible for the active transport of luminal 3-OMG, our findings support a role of intestinal T1R2 signals in the regulation of glucose absorption via SGLT-1. Indeed, intestinal STR activation has been shown to upregulate SGLT-1 transcript, apical protein, and function in a number of species (17,18). Accordingly, reciprocal regulation of T1R2 in human health may increase SGLT-1 function at euglycemia to facilitate glucose absorption and reduce SGLT-1 function during hyperglycemia to limit postprandial glycemic excursion. However, despite a reduction in T1R2 transcript after luminal glucose exposure during hyperglycemia, our healthy subjects still displayed greater rates of glucose absorption than during euglycemia, which might be accounted for by changes in SGLT-1 lagging behind those in T1R2. Our finding that plasma 3-OMG concentrations were elevated in type 2 diabetic patients during hyperglycemia is in keeping with the concept that SGLT-1 transporter capacity was maintained, or increased, in the presence of luminal glucose under these conditions. In fact, even small changes in SGLT-1 may increase this risk, because type 2 diabetic patients are reported to have up to fourfold higher levels of transcript, protein, and function of this transporter at baseline compared with healthy control subjects (24). We note that an increased level of facilitated glucose transport via the basolateral glucose transporter GLUT2 may have contributed to plasma levels of 3-OMG in the current study; however, the role of STR signals to direct the apical insertion of GLUT2 in enterocytes appears to be limited to rodents (25,34).
The link between STR stimulation and incretin hormone release in healthy humans is not clear. Most in vivo studies indicate that acute administration of nonnutritive sweeteners does not trigger incretin secretion in humans or rodents (35–37). Nonetheless, we observed that subsets of duodenal L cells, K cells, and EC cells were immunopositive for T1R2, in accord with previous reports (4,12,14). Together with positive associations between luminal glucose-induced changes in some STR transcripts and measures of GLP-1 and GIP secretion in the current study, it remains possible that STRs do have a regulatory role in gut hormone release. The inhibition of glucose-induced GLP-1 secretion in healthy humans by the STR blocker, lactisole (15), supports this concept. We also recognize that STR signals may serve autocrine and/or paracrine functions within the intestinal mucosa that are not reflected in circulating gut hormone concentrations; the latter appear to be a blunt marker for local concentrations of GLP-1 (38). There is also a large body of evidence indicating that the intestinotrophic gut peptide, glucagon-like peptide 2 (GLP-2), coreleased from L cells with GLP-1, is a powerful local stimulus to increase intestinal glucose transport via SGLT-1 and GLUT2 in rodents and in patients with short bowel syndrome (39–41). Importantly, GLP-2 release has recently been revealed as STR-dependent in animals and in a human enteroendocrine cell line (42,43), highlighting an important link between STRs and GLP-2 in the regulation of intestinal glucose transport.
Reports concerning postprandial incretin hormone release in patients with type 2 diabetes have been inconsistent, with plasma GLP-1 concentrations after a mixed meal being either reduced (22) or intact (44), although such studies are potentially confounded by failure to control for differences in the rate of gastric emptying, which is frequently delayed in longstanding diabetes or during acute hyperglycemia (20). Our observation that GLP-1 and GIP responses to a standardized rate of duodenal glucose infusion were maintained, and indeed increased, in type 2 diabetic patients, supports our previous findings (45) and is in keeping with the trend for increased L-cell density in these patients in the current study and a report of an increased density of L cells and mixed L/K cells in the duodenum of well-controlled type 2 diabetic patients (46). There is now strong evidence that SGLT-1 transport is a key stimulus for release of GLP-1 and GIP, which occurs even after exposure to nonmetabolized SGLT-1 substrates and is inhibited by pharmacological blockade or genetic ablation of SGLT-1 in rodents (47–49). Therefore, increased SGLT-1 capacity could explain enhanced glucose-induced GLP-1 and GIP responses in our type 2 diabetic patients. Any deficiency in the incretin effect in type 2 diabetes is likely to be explained by impaired β-cell function rather than by deficient incretin hormone secretion (45,50), and indeed, defective C-peptide responses in our type 2 diabetic patients during hyperglycemia support this assertion. Acute hyperglycemia had no effect on GLP-1 or GIP secretion, as noted previously (51,52). Although SGLT-1 transport appears a major determinant of GLP-1 and GIP release, other transporters (49,53) or signaling pathways (54) may also be involved, so increased glucose absorptive capacity during hyperglycemia may not necessarily result in enhanced GLP-1 or GIP concentrations.
Our study had a number of limitations. Transcriptional regulation of intestinal T1R2 occurred rapidly in humans, but we did not quantify changes in STR protein in parallel due to ethical considerations on the additional biopsy specimens required. However, similarly rapid changes in these proteins after glucose or sucralose exposure are known to occur in apical membrane vesicles of rat jejunum (25). We did not assess effects on SGLT-1 transcript or protein here, although measures of glucose absorption with 3-OMG reflect, in large part, SGLT-1 function as the primary intestinal glucose transporter in humans. There was considerable interindividual variability in baseline expression of intestinal STR transcripts, so that our study was insufficiently powered to detect relationships between absolute transcript levels and concentrations of gut hormones and 3-OMG. Our 3-OMG measurements were limited to 60 min, and differences between groups or glycemic states might have become more marked after this point. The duodenal glucose infusion was also relatively brief, being limited by the tolerability of unsedated endoscopy. Our type 2 diabetic patients had relatively good glycemic control, and more marked differences from health might be observed in patients with a higher HbA1c. The type 2 diabetic patients were older than the healthy control subjects, although we have not previously shown any age-related differences in postprandial GLP-1 responses (55).
In conclusion, we have shown that the intestinal STR system is reciprocally regulated in the presence of luminal glucose according to glycemic status in health but not in type 2 diabetes. In the latter, T1R2 dysregulation potentially increases the risk of postprandial hyperglycemia, but the intestinal STR system appears unlikely to be a major determinant of circulating GLP-1 or GIP concentrations in humans.
ACKNOWLEDGMENTS
This study was supported by grants awarded to R.L.Y. by the National Health and Medical Research Council (NHMRC) of Australia (grant no. 627127) and to R.L.Y., M.H., and C.K.R. from Diabetes Australia. T.W. was supported by an NHMRC Overseas Clinical Postdoctoral Training Fellowship (grant no. 519349)
No potential conflicts of interest relevant to this article were reported.
R.L.Y. and C.K.R. conceived, designed, and supervised the study, obtained funding, acquired data, undertook statistical analyses and interpreted data, and drafted and critically reviewed the manuscript. B.C. and N.J.I. acquired data and provided technical support. J.M., J.K., and T.W. assisted in study design, acquired data, and critically reviewed the manuscript. M.H. designed the study, interpreted data, and critically reviewed the manuscript. R.L.Y. and C.K.R. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the Endoscopy Unit staff, Department of Gastroenterology and Hepatology, Royal Adelaide Hospital, for their assistance with the study; Dr. Kate Sutherland, University of Sydney, for contributing to the molecular assays in this study; and Kylie Lange, National Health and NHMRC Centre of Research Excellence in Translating Nutritional Science to Good Health, University of Adelaide, for professional biostatistical support.
Preliminary accounts of this study were presented at the Digestive Disease Week meeting of the American Gastroenterological Association, Chicago, IL, 7–11 May 2011; and at the Joint International Neurogastroenterology & Motility Meeting, Bologna, Italy, 6–8 September 2012.
Footnotes
See accompanying commentary, p. 3336.
REFERENCES
- 1.Raybould HE, Hölzer H. Dual capsaicin-sensitive afferent pathways mediate inhibition of gastric emptying in rat induced by intestinal carbohydrate. Neurosci Lett 1992;141:236–238 [DOI] [PubMed] [Google Scholar]
- 2.Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993;36:857–862 [DOI] [PubMed] [Google Scholar]
- 3.Pilichiewicz AN, Chaikomin R, Brennan IM, et al. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab 2007;293:E743–E753 [DOI] [PubMed] [Google Scholar]
- 4.Young RL. Sensing via intestinal sweet taste pathways. Front Neurosci 2011;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 2004;287:E199–E206 [DOI] [PubMed] [Google Scholar]
- 6.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 2001;106:381–390 [DOI] [PubMed] [Google Scholar]
- 7.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A 2002;99:4692–4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez CA, Huang L, Rong M, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 2002;5:1169–1176 [DOI] [PubMed] [Google Scholar]
- 9.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A 1996;93:6631–6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 2005;33:302–305 [DOI] [PubMed] [Google Scholar]
- 11.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 2007;32:41–49 [DOI] [PubMed] [Google Scholar]
- 12.Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol 2007;292:G1420–G1428 [DOI] [PubMed] [Google Scholar]
- 13.Young RL, Sutherland K, Pezos N, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut 2009;58:337–346 [DOI] [PubMed] [Google Scholar]
- 14.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A 2007;104:15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr 2011;30:524–532 [DOI] [PubMed] [Google Scholar]
- 16.Gerspach AC, Steinert RE, Schönenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab 2011;301:E317–E325 [DOI] [PubMed] [Google Scholar]
- 17.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A 2007;104:15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg 2010;251:865–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rayner CK, Schwartz MP, van Dam PS, et al. Small intestinal glucose absorption and duodenal motility in type 1 diabetes mellitus. Am J Gastroenterol 2002;97:3123–3130 [DOI] [PubMed] [Google Scholar]
- 20.Horowitz M, O’Donovan D, Jones KL, Feinle C, Rayner CK, Samsom M. Gastric emptying in diabetes: clinical significance and treatment. Diabet Med 2002;19:177–194 [DOI] [PubMed] [Google Scholar]
- 21.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med 2001;161:1989–1996 [DOI] [PubMed] [Google Scholar]
- 22.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001;86:3717–3723 [DOI] [PubMed] [Google Scholar]
- 23.Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 2001;50:609–613 [DOI] [PubMed] [Google Scholar]
- 24.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 2002;282:G241–G248 [DOI] [PubMed] [Google Scholar]
- 25.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 2007;582:379–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 1993;104:1640–1647 [DOI] [PubMed] [Google Scholar]
- 27.Horowitz M, Maddox AF, Wishart JM, Harding PE, Chatterton BE, Shearman DJ. Relationships between oesophageal transit and solid and liquid gastric emptying in diabetes mellitus. Eur J Nucl Med 1991;18:229–234 [DOI] [PubMed] [Google Scholar]
- 28.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1982;285:916–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan IM, Feltrin KL, Nair NS, et al. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol 2009;297:G602–G610 [DOI] [PubMed] [Google Scholar]
- 30.Rayner CK, Verhagen MA, Hebbard GS, DiMatteo AC, Doran SM, Horowitz M. Proximal gastric compliance and perception of distension in type 1 diabetes mellitus: effects of hyperglycemia. Am J Gastroenterol 2000;95:1175–1183 [DOI] [PubMed] [Google Scholar]
- 31.Vanis L, Gentilcore D, Rayner CK, et al. Effects of small intestinal glucose load on blood pressure, splanchnic blood flow, glycemia, and GLP-1 release in healthy older subjects. Am J Physiol Regul Integr Comp Physiol 2011;300:R1524–R1531 [DOI] [PubMed] [Google Scholar]
- 32.Deane AM, Summers MJ, Zaknic AV, et al. Glucose absorption and small intestinal transit in critical illness. Crit Care Med 2011;39:1282–1288 [DOI] [PubMed] [Google Scholar]
- 33.Wolever TM. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br J Nutr 2004;91:295–301 [DOI] [PubMed] [Google Scholar]
- 34.Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc 2011;70:185–193 [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Bellon M, Wishart JM, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 2009;296:G735–G739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu T, Zhao BR, Bound MJ, et al. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am J Clin Nutr 2012;95:78–83 [DOI] [PubMed] [Google Scholar]
- 37.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care 2009;32:2184–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Alessio D, Lu WJ, Sun W, et al. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol 2007;293:R2163–R2169 [DOI] [PubMed] [Google Scholar]
- 39.Cheeseman CI. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol 1997;273:R1965–R1971 [DOI] [PubMed] [Google Scholar]
- 40.Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J 2002;367:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 2001;120:806–815 [DOI] [PubMed] [Google Scholar]
- 42.Daly K, Al-Rammahi M, Arora DK, et al. Expression of sweet receptor components in equine small intestine: relevance to intestinal glucose transport. Am J Physiol Regul Integr Comp Physiol 2012;303:R199–R208 [DOI] [PubMed] [Google Scholar]
- 43.Sato S, Hokari R, Kurihara C, et al. Dietary lipids and sweeteners regulate glucagon-like peptide-2 secretion. Am J Physiol Gastrointest Liver Physiol 2013;304:G708–G714 [DOI] [PubMed] [Google Scholar]
- 44.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008;57:678–687 [DOI] [PubMed] [Google Scholar]
- 45.Ma J, Pilichiewicz AN, Feinle-Bisset C, et al. Effects of variations in duodenal glucose load on glycaemic, insulin, and incretin responses in type 2 diabetes. Diabet Med 2012;29:604–608 [DOI] [PubMed] [Google Scholar]
- 46.Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 2006;290:E550–E559 [DOI] [PubMed] [Google Scholar]
- 47.Gorboulev V, Schürmann A, Vallon V, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012;61:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med 2010;12:e1. [DOI] [PubMed] [Google Scholar]
- 49.Parker HE, Adriaenssens A, Rogers G, et al. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 2012;55:2445–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woerle HJ, Carneiro L, Derani A, Göke B, Schirra J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes 2012;61:2349–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilsbøll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 2003;88:2706–2713 [DOI] [PubMed] [Google Scholar]
- 52.Kuo P, Wishart JM, Bellon M, et al. Effects of physiological hyperglycemia on duodenal motility and flow events, glucose absorption, and incretin secretion in healthy humans. J Clin Endocrinol Metab 2010;95:3893–3900 [DOI] [PubMed] [Google Scholar]
- 53.Cani PD, Holst JJ, Drucker DJ, et al. GLUT2 and the incretin receptors are involved in glucose-induced incretin secretion. Mol Cell Endocrinol 2007;276:18–23 [DOI] [PubMed] [Google Scholar]
- 54.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 2008;8:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trahair LG, Horowitz M, Rayner CK, et al. Comparative effects of variations in duodenal glucose load on glycemic, insulinemic, and incretin responses in healthy young and older subjects. J Clin Endocrinol Metab 2012;97:844–851 [DOI] [PubMed] [Google Scholar]