Abstract
Endogenous hyperinsulinemia and insulin receptor (IR)/IGF-I receptor (IGF-IR) phosphorylation in tumors are associated with a worse prognosis in women with breast cancer. In vitro, insulin stimulation of the IR increases proliferation of breast cancer cells. However, in vivo studies demonstrating that IR activation increases tumor growth, independently of IGF-IR activation, are lacking. We hypothesized that endogenous hyperinsulinemia increases mammary tumor growth by directly activating the IR rather than the IGF-IR or hybrid receptors. We aimed to determine whether stimulating the IR with the insulin analog AspB10 could increase tumor growth independently of IGF-IR signaling. We induced orthotopic mammary tumors in control FVB/n and hyperinsulinemic MKR mice, and treated them with the insulin analog AspB10, recombinant human IGF-I, or vehicle. Tumors from mice with endogenous hyperinsulinemia were larger and had greater IR phosphorylation, but not IGF-IR phosphorylation, than those from control mice. Chronic AspB10 administration also increased tumor growth and IR (but not IGF-IR) phosphorylation in tumors. IGF-I led to activation of both the IGF-IR and IR and probably hybrid receptors. Our results demonstrate that IR phosphorylation increases tumor growth, independently of IGF-IR/hybrid receptor phosphorylation, and warrant consideration when developing therapeutics targeting the IGF-IR, but not the IR.
Individuals with obesity, the metabolic syndrome (MetS), and type 2 diabetes (T2D) have increased breast cancer incidence and mortality (1–3). Endogenous hyperinsulinemia appears to be an important factor linking obesity, T2D, MetS, and breast cancer (4–6). The association between endogenous insulin concentration and breast cancer risk seems to be independent of obesity (6,7). In women without diabetes, with early-stage breast cancer, hyperinsulinemia is associated with a lower disease-free and overall survival (8).
It is hypothesized that hyperinsulinemia may increase tumor growth by direct and/or indirect mechanisms. Direct mechanisms involve insulin acting on the insulin receptor (IR) or IGF-I receptor (IGF-IR) on tumor cells, activating signaling pathways and tumor growth (9,10). Indirect mechanisms include hyperinsulinemia stimulating hepatic IGF-I synthesis, decreasing IGF binding protein-1 synthesis, and thus increasing local IGF-I concentrations to act on the tumor (10,11). In vitro studies are unable to distinguish these potential direct and indirect effects. Studies have reported that increased IR expression in breast cancers is associated with decreased survival (12). The presence of phosphorylated IR/IGF-IR in the primary tumor is also associated with a worse prognosis (12). However, these studies have not been able to discriminate between IR and IGF-IR phosphorylation. Additionally, human studies provide associations, but not mechanistic links between hyperinsulinemia and breast cancer growth.
In vivo studies demonstrating that hyperinsulinemia increases tumor growth by acting directly on the tumor IR are lacking. We previously reported that, in an animal model, endogenous hyperinsulinemia increases mammary tumor growth by increasing phosphorylation of the IR/IGF-IR (9). We have shown that decreasing endogenous insulin levels and blocking the IR/IGF-IR using a tyrosine kinase inhibitor decreased tumor growth and metastases (9,13,14). However, we have not previously demonstrated that the greater tumor growth in these mice is a result of insulin acting directly on the IR, rather than through the IGF-IR (9). Previous studies of exogenous human insulin administration have not demonstrated an increase in mammary tumor growth in rodents (15,16). However, the insulin analog AspB10, a rapid-acting insulin analog, has been shown to increase mammary tumor development in rats (17,18). AspB10 binds the IR with greater affinity than human insulin and has a slower rate of dissociation from the IR in vitro, raising the possibility that activation of the IR is mediating its tumor-promoting effects (19–25).
We hypothesized that hyperinsulinemia increases mammary tumor growth through the direct effects on the IR. We also hypothesized that chronic activation of the IR in vivo is capable of promoting tumor growth independently of IGF-IR activation. For this study, we used the female MKR mouse, a nonobese mouse model of endogenous hyperinsulinemia (9). The female MKR mice demonstrate no hyperglycemia or dyslipidemia; have normal circulating levels of cytokines and IGF-I; and have no increase in leptin or decrease in adiponectin (9,13). Therefore, this animal model has allowed us to determine the effects of hyperinsulinemia in isolation from many of the other factors reported to contribute to breast cancer growth with obesity, T2D, and the MetS (10).
In this study, we found that in mice with endogenous hyperinsulinemia orthotopic mammary tumors had IR phosphorylation, but not IGF-IR phosphorylation. Additionally, we report that chronic stimulation of IR phosphorylation, without increased IGF-IR phosphorylation, enhanced mammary tumor growth in these models. Our findings indicate that, in the setting of endogenous hyperinsulinemia, insulin is directly driving tumor growth by acting on the IR, rather than through indirect effects mediated by IGF-I or the IGF-IR.
RESEARCH DESIGN AND METHODS
Animals.
Animal study protocols were approved by the Mount Sinai School of Medicine Institutional Animal Care and Use Committee. Mice were housed in The Mount Sinai School of Medicine Center for Comparative Medicine and Surgery, an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility. Mice had a 12-h light/dark cycle, free access to mouse chow (Picolab rodent diet #5053; LabDiet, St. Louis, MO) and water. All mice were female, on the FVB/N background, and 8–12 weeks old. The MKR mice express a human dominant-negative IGF-IR expressed in skeletal muscle only that forms hybrids with the IR, leading to skeletal muscle insulin resistance, with subsequent whole-body insulin resistance (26). The generation and characteristics of the MKR mice have been previously described (9,26).
In vitro studies.
The MVT1 murine mammary carcinoma cell line was derived from an explant tumor culture from MMTV-c-Myc/Vegf transgenic mice (27). Met1 murine mammary tumor cells were derived from MMTV-Polyoma virus middle T antigen (PyVmT) transgenic mice (28). Met1 and MVT1 cells were cultured as previously described (9,14). Cells were stimulated with PBS, 10 nmol/L insulin (Humulin R; Eli Lilly, Indianapolis, IN), AspB10 (provided by Sanofi-Aventis, Frankfurt am Main, Germany), or recombinant human IGF-I (rhIGF-I) (Ipsen, Brisbane, CA) for 10 min. After treatment, cells were lysed for protein extraction.
Orthotopic tumor models.
Met-1 and MVT1 cells were prepared for injection as described previously (9,14). A total of 250,000 Met-1 cells or 100,000 MVT1 cells were inoculated into the fourth mammary fat pad of 8–10-week-old female virgin MKR and WT mice. Mice were treated with rhIGF-I (Ipsen, Brisbane, CA) (1 mg/kg, twice daily i.p.), AspB10 (12.5 IU/kg, twice daily s.c.; provided by Sanofi-Aventis, Frankfurt am Main, Germany), or PBS (vehicle). Tumor growth was measured in three dimensions using calipers. Tumor volume was calculated as follows: 4/3 × π × r1 × r2 × r3 (r = radius). At the end of the study, mice were killed; tumors were removed and flash frozen in liquid nitrogen. Lungs were inflated and fixed with formalin; the number of surface macrometastases was quantified.
Body weights, composition, blood glucose, insulin, and IGF-I measurement.
Body weights were measured weekly. Body composition analysis was performed using the EchoMRI 3-in-1 nuclear magnetic resonance system (Echo Medical Systems, Houston, TX), before tumor cell injection and at the end of treatment. Fed blood glucose measurements were performed on tail vein blood during tumor studies using a Bayer Contour Glucometer (Bayer Healthcare, Mishawaka, IN), prior to commencing treatment and weekly thereafter. Plasma insulin levels were measured at the end of the studies using the Sensitive Rat Insulin RIA kit (Millipore, St. Charles, MO). Serum IGF-I levels were measured by radioimmunoassay (ALPCO, Salem, NH).
Insulin tolerance test.
MKR mice were fasted for 2 h prior to the insulin tolerance test, blood glucose was measured from the tail vein using a Bayer Contour Glucometer at time 0, immediately before PBS, human insulin (Humulin R; Eli Lilly, Indianapolis, IN), or AspB10 injection, and at 0.5, 1, 2, 4, 7, and 10 h after injection, at which time the mice were refed.
Protein extraction, Western blot, and immunoprecipitation.
Protein extraction and Western blot analysis were performed as previously described (9,13). The following antibodies were used: anti-phospho-IGF-IRβ(Tyr1135/1136)/IRβ(Tyr1150/1151), total IGF-IRβ, phospho(Ser473) and total Akt, phospho(Thr202/Tyr204), and total p44/42 mitogen-activated protein kinase (Erk1/2) (Cell Signaling Technology, Danvers, MA), total IRβ and total IGF-IRβ (Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin (Sigma-Aldrich, St. Louis, MO). Densitometric analysis was performed using ImageJ V1.44 software (National Institutes of Health). Immunoprecipitation of the IRβ and IGF-IRβ were performed using magnetic Dynabeads Protein G (Invitrogen Dynal, Oslo, Norway) as per the manufacturer’s protocol, with the following modifications: 10 μg anti-IRβ antibody or anti-IGF-IRβ was added to 1,000 μg protein lysate, incubated with rotation overnight at 4°C; samples were incubated with the magnetic beads with rotation for 4 h at 4°C, after which they were washed with ice-cold Tris buffer (pH 7.4); and antigens were eluted using 3× loading buffer supplemented with dithiothreitol, boiled at 96°C for 5 min, separated from the beads on a magnet (DynaMag; Invitrogen Dynal, Oslo, Norway), and loaded on an 8% Tris-Glycine gel (Novex; Life Technologies, Carlsbad, CA). The gel was probed for phospho-IGF-IRβ(Tyr1135/1136)/IRβ(Tyr1150/1151), total IRβ, and total IGF-IRβ.
Statistical analysis.
Differences between groups were calculated by the two-tailed Student t test when comparing two groups with equal variance and a one-way ANOVA with Holm-Sidak post hoc test when comparing more than two groups, using the statistics software package SPSS Statistics (IBM, Armonk, NY). P values <0.05 were considered statistically significant.
RESULTS
Insulin and AspB10 led to IR phosphorylation, while IGF-I led to IGF-IR/hybrid receptor activation in vitro.
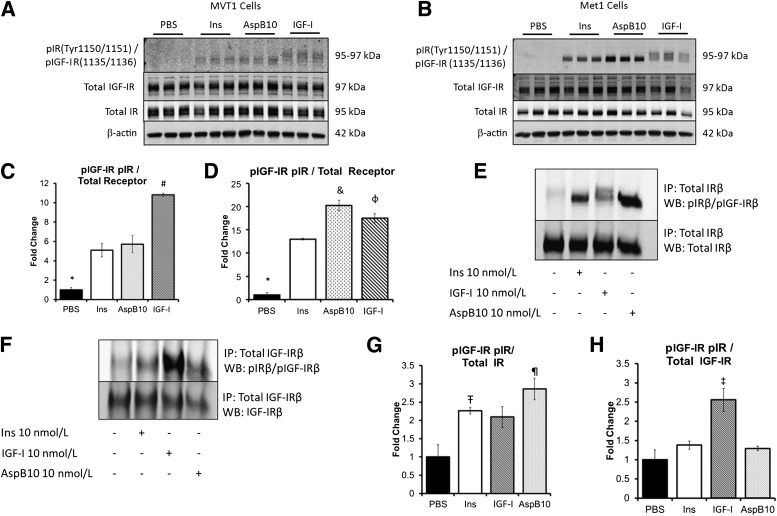
We first aimed to determine whether differences in IR and IGF-IR phosphorylation could be detected in two different murine tumor cell lines, with different oncogenes, in response to insulin, rhIGF-I, and the insulin analog AspB10. MVT1 and Met1 cells were stimulated with PBS or 10 nmol/L insulin, AspB10, or IGF-I for 10 min (Fig. 1A–D). Western blot analysis using the primary pIGF-IRβ(Tyr1135/1136)/pIRβ(Tyr1150/1151) antibody suggested that insulin and AspB10 stimulation led to IRβ phosphorylation, observed as a distinct band on the membrane at 95 kDa (Fig. 1A and B) (29). IGF-I stimulation led to phosphorylation of the IGF-IRβ at 97 kDa, as well as the IRβ at 95 kDa (Fig. 1A and B) (29). These findings suggested that in both MVT1 and Met1 cells, IGF-I–mediated phosphorylation of the IGF-IRβ and the IRβ, and probably the IR/IGF-IR hybrids. To confirm the Western blot findings, we performed immunoprecipitation of the IRβ and IGF-IRβ from the Met1 cell lysates. We found that insulin and AspB10 were indeed phosphorylating the IRβ, but not the IGF-IRβ (Fig. 1E–H), whereas IGF-I stimulation led to phosphorylation of the IGF-IRβ and probable IGF-IR/IR hybrids (Fig. 1E–H). Incidentally, we noted that AspB10 stimulation led to greater IRβ phosphorylation than insulin in Met1 cells (Fig. 1D). These results show that it is possible to distinguish differences in IRβ and IGF-IRβ phosphorylation in response to insulin, IGF-I, and AspB10 by Western blot as confirmed by immunoprecipitation in Met1 and MVT1 cell lines in vitro.
FIG. 1.
Insulin and AspB10 led to IR phosphorylation but not IGF-IR phosphorylation in vitro. MVT1 and Met1 cells were stimulated with 10 nmol/L insulin (Ins), the insulin analog AspB10 (AspB10), rhIGF-I (IGF-I), or PBS. Western blot (WB) analysis of protein lysates (from MVT1 cells, A; from Met1 cells, B) and densitometry (from MVT1 cells, C; and from Met1 cells, D) demonstrated that insulin and AspB10 led to phosphorylation of the IRβ(Tyr 1150/1151) at 95 kDa, whereas IGF-I stimulation led to phosphorylation of the IGF-IRβ(Tyr 1135/1136) at 97 kDa and the IRβ(Tyr 1150/1151) at 95 kDa, most likely through hybrid receptor phosphorylation (A and B). Immunoprecipitation (IP) of the IR in Met1 cell lysates after in vitro stimulation with PBS, 10 nmol/L Ins, IGF-I, or AspB10 confirmed that insulin and AspB10 caused phosphorylation of the IRβ(Tyr 1150/1151), whereas IGF-I led to IGF-IRβ(Tyr 1135/1136) and IRβ(Tyr 1150/1151) receptor phosphorylation, the latter likely through IR and hybrid receptor (E and F) with densitometry (G and H). Representative blots from experiments are shown. All experiments were performed two to three times. Bar graphs display means and SEM. *PBS group significantly lower than all other groups, P < 0.05; #IGF-I group significantly higher than all other groups, P < 0.05; &AspB10 group significantly higher than human insulin group, P < 0.05; ΦIGF-I group significantly higher than human insulin group, P < 0.05; Ŧhuman insulin group significantly greater than PBS, P < 0.05; ¶AspB10 significantly greater than PBS, P < 0.05; ‡IGF-I group greater than PBS, P = 0.05. p, phosphorylated.
Endogenous hyperinsulinemia and exogenous rhIGF-I increase the growth of Met1 and MVT1 tumors.
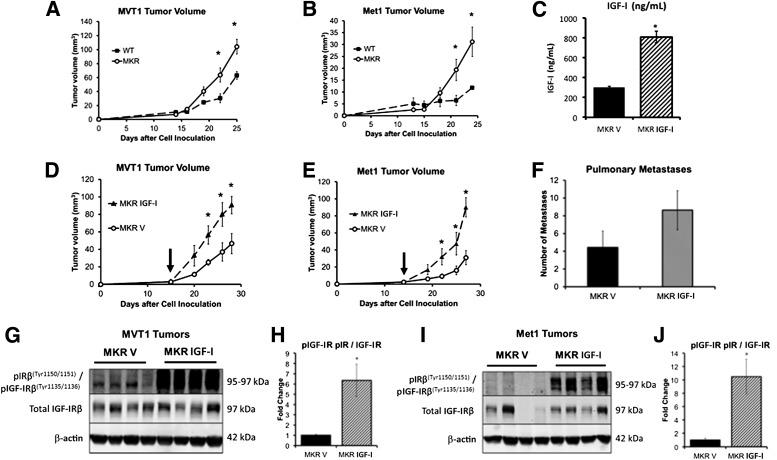
We have previously shown that MKR mice with endogenous hyperinsulinemia develop larger primary tumors and more metastases than WT mice using a variety of orthotopic and transgenic tumor models with different oncogenes (9,14). We aimed to determine whether endogenous hyperinsulinemia stimulated Met1 and MVT1 tumor growth by acting directly on the IR in vivo or by indirect mechanisms, namely through phosphorylation of the IGF-IRβ. To assess the effect of endogenous hyperinsulinemia on tumor growth and receptor phosphorylation, compared with rhIGF-I in vivo, we studied Met1 and MVT1 tumors from WT mice and MKR mice treated with rhIGF-I or vehicle. WT and MKR mice were orthotopically injected with 100,000 MVT1 or 250,000 Met1 tumor cells. Consistent with our previous studies, MKR mice developed larger MVT1 and Met1 tumors than WT mice (Fig. 2A and B). MVT1 and Met1 tumors were then induced in MKR mice, and when tumors were measurable, the MKR mice were divided into two groups with equal mean tumor size. From that time, rhIGF-I (1 mg/kg, twice daily i.p.) or vehicle was administered for 2 weeks. Serum IGF-I levels were measured 2 h after injection at the end of the study and were 2.6 times higher in the rhIGF-I–treated mice (806 ± 59.05 ng/mL) than vehicle-treated mice (302 ± 8.75 ng/mL, P < 0.05) (Fig. 2C). rhIGF-I–treated MKR mice developed significantly larger tumors than the vehicle-treated MKR mice (Fig. 2D and E). Repeated studies demonstrated a nonsignificant increase in the number of MVT1-derived pulmonary macrometastases in the rhIGF-I–treated group compared with the MKR vehicle-treated group (Fig. 2F). Western blot analysis of tumor lysates demonstrated that rhIGF-I treatment led to phosphorylation of the IGF-IRβ at 97 kDa, and of IRβ at 95 kDa, in both Met1 and MVT1 tumors (Fig. 2G and I). In contrast, tumors from vehicle-treated MKR mice had only IRβ phosphorylation at 95 kDa (Fig. 2G–I). These results suggest that endogenous hyperinsulinemia increases mammary tumor growth by directly acting on the IR of the tumors and not by indirectly increasing IGF-IR phosphorylation. IGF-I in contrast leads to phosphorylation of the IGF-IRβ and IRβ, most likely through IGF-IR/IR hybrid receptors.
FIG. 2.
IGF-I increased orthotopic MVT1 and Met1 tumor growth in the hyperinsulinemic MKR mice by increasing IGF-IR phosphorylation. WT and MKR mice were injected with tumor cells on Day 0. MKR mice developed larger MVT1 and Met1 tumors than WT mice (A and B). MVT1 and Met1 tumor cells were orthotopically injected into MKR mice, mice were divided into two groups with equal mean tumor size, and mice were administered either rhIGF-I or vehicle (vertical arrow indicates time when treatment began). Administration of rhIGF-I led to a further stimulation in tumor growth, over endogenous hyperinsulinemia (D and E). Serum IGF-I concentration in the rhIGF-I treatment group was 2.6 times that of the control group (C). The greater mean number of pulmonary macrometastases in the mice treated with rhIGF-I did not reach statistical significance, compared with vehicle-treated MKR mice (F). Western blot analysis of tumor lysates demonstrated that MKR mice with endogenous hyperinsulinemia (MKR V) show IRβ phosphorylation at 95 kDa, and rhIGF-I treatment led to increased IGF-IRβ and IRβ or hybrid receptor phosphorylation in MVT1 and Met1 tumors (G–J). Representative images from three repeated experiments of tumor volume and Western blots are displayed. The graphs represent the average for each group; error bars indicate SEM (A–F, H, and I). Statistical analysis was performed using a two-tailed t test; *indicates statistically significant differences (P < 0.05) between the groups. n = 8–11 mice per group. p, phosphorylated.
Chronic administration of the insulin analog AspB10 increased Met1 and MVT1 tumor growth.
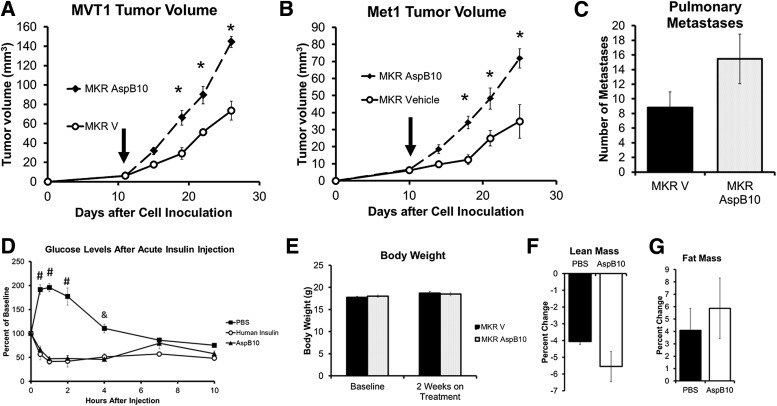
Our in vitro studies had shown that AspB10 increased IRβ phosphorylation in both Met1 and MVT1 cells, without increasing IGF-IRβ phosphorylation (Fig. 1A–H). Therefore, to investigate whether IR activation could truly increase tumor growth independently of IGF-IR activation, we used the insulin analog AspB10 to chronically activate the IR of the tumors in vivo. After orthotopic injection with MVT1 or Met1 tumor cells, mice were treated with AspB10 (12.5 IU/kg, twice daily s.c.) or vehicle for ∼2 weeks after the tumors became measurable. AspB10 treatment led to a significant increase in the size of both MVT1 and Met1 tumors (Fig. 3A and B). The increased average number of surface pulmonary macrometastases in the AspB10-treated group did not reach statistical significance (Fig. 3C). AspB10 treatment significantly increased plasma insulin concentrations to 70.2 ± 21.2 µg/L, compared with vehicle-treated mice (1.08 ± 0.3 µg/L, P < 0.05), when measured 2 h after insulin or vehicle injection. An insulin tolerance test revealed that AspB10 (12.5 units/kg s.c.) led to a reduction in blood glucose levels, similar to those for human insulin, from 30 min to 4 h after injection that reached a nadir (43.9 ± 6.1% baseline) 2 h after injection (Fig. 3D). No differences in body weight were observed between the AspB10- and vehicle-treated groups before or after 2 weeks of treatment (Fig. 3E). Although overall body weight was not different between the treatment groups, both vehicle- and AspB10-treated mice showed a relative loss of lean mass and a relative gain in fat mass; however, the change in lean and fat mass did not differ between the vehicle- and AspB10-treated groups (Fig. 3F and G).
FIG. 3.
Chronic activation of the IR by the insulin analog AspB10 increased orthotopic Met1 and MVT1 tumor growth. MKR mice were injected with MVT1 or Met1 tumor cells on Day 0. Treatment was started with AspB10 (12.5 IU/kg, twice daily s.c.) or vehicle, indicated by vertical arrow (A and B). AspB10 led to increased growth of both MVT1 and Met1 tumors (A and B). The number of pulmonary macrometastases showed a nonsignificant increase in the AspB10-treated group (C). An insulin tolerance test was performed with AspB10 (12.5 IU/kg s.c.), regular human insulin (12.5 IU/kg s.c.), and PBS (vehicle). Blood glucose was measured at 0.5, 1, 2, 4, 7, and 10 h after injection (D). Statistical analysis was performed using a one-way ANOVA for comparing more than two groups: #P < 0.05 between PBS and AspB10 and human insulin groups; &P < 0.05 between PBS- and AspB10-treated groups; n = 4 per group. #PBS group was significantly greater than other groups, P < 0.05; &PBS group was significantly higher than AspB10 group, P < 0.05. No change in body weight (E) or difference in relative lean or fat mass (F and G) was observed after 2 weeks of AspB10 administration. Graphs are representative of two studies. All graphs show the mean for each group, and error bars represent the SEM. Statistical analysis was performed using two-tailed t test. *P < 0.05 between groups. n = 9–11 mice per group. MKR V, MKR mice with endogenous hyperinsulinemia.
Endogenous hyperinsulinemia and AspB10 increased mammary tumor growth by directly activating the IR, but not the IGF-IR.
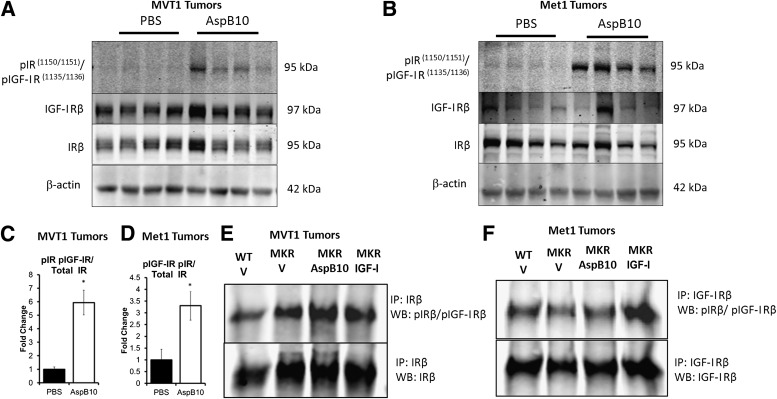
To ascertain whether AspB10 stimulated tumor growth in vivo by acting directly on the IR, we examined IRβ and IGF-IRβ phosphorylation in Met1 and MVT1 tumors after 2 weeks of AspB10 or vehicle treatment. Western blot analysis of the tumor lysates revealed that AspB10 treatment led to phosphorylation of the IRβ, but not the IGF-IRβ, in both MVT1 and Met1 tumors (Fig. 4A–D). To confirm these findings, the tumor lysates of MVT1 and Met1 tumors from WT mice, vehicle-treated MKR mice, IGF-I–treated MKR mice, and AspB10-treated MKR mice were subjected to immunoprecipitation of the IRβ and the IGF-IRβ, and were immunoblotted for the phosphorylated IGF-IRβ/IRβ. Tumors from vehicle-treated MKR mice demonstrated 1.38 ± 0.17-fold increased phosphorylation of the IRβ compared with tumors from WT mice (Fig. 4E), but no increase in IGF-IRβ phosphorylation was seen in the vehicle-treated MKR mice (Fig. 4F). Similarly, by immunoprecipitation AspB10 treatment led to 1.81 ± 0.17-fold increased phosphorylation of the IRβ compared with WT mice (Fig. 4E), but not the IGF-IRβ (Fig. 4F). In contrast, rhIGF-I administration led to phosphorylation of both the IGF-IRβ and IRβ (Fig. 4E and F). Taken together, these data demonstrate that endogenous hyperinsulinemia directly increases tumor growth by acting on the IR of the tumors, but not the IGF-IR and that stimulating the IR, independently of the IGF-IR, further exacerbates tumor growth.
FIG. 4.
Endogenous hyperinsulinemia and AspB10 treatment led to increased IR phosphorylation in MVT1 and Met1 tumors. Western blot (WB) analysis (representative blots, A and B) revealed that chronic AspB10 treatment led to increased IRβ phosphorylation at 95 kDa (C and D). Immunoprecipitation (IP) of the IRβ (representative blot, E) and IGF-IRβ (representative blot, F) was performed on MVT1 and Met1 tumor protein lysates. They were immunoblotted for the phosphorylated IGF-IRβ/IRβ. Endogenous hyperinsulinemia (MKR V) and chronic AspB10 administration (MKR AspB10) led to increased IR phosphorylation (E). rhIGF-I administration led to increased IGF-IRβ and IRβ phosphorylation (E and F). Graphs are representative of two studies. All graphs show the mean for each group, and error bars represent the SEM. Statistical analysis was performed using two-tailed t test. *P value < 0.05 between groups. n = 9–11 mice per group. p, phosphorylated; MKR V, MKR mice with endogenous hyperinsulinemia.
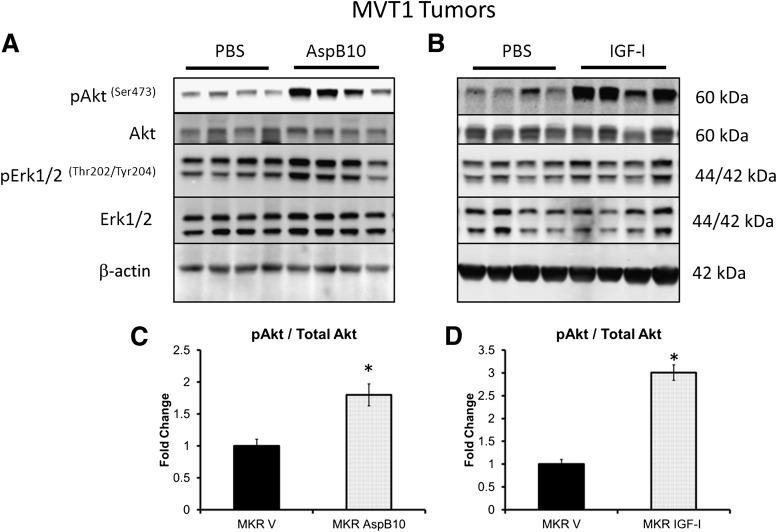
AspB10-induced IR phosphorylation and rhIGF-I-induced IGF-IR/hybrid receptor phosphorylation led to increased Akt phosphorylation in tumors.
In order to examine whether differences in receptor activation in vivo led to differences in downstream signaling, we examined Akt and Erk1/2 phosphorylation in the two tumor types from mice treated with AspB10 or IGF-I. Despite the differences in receptor phosphorylation resulting from AspB10 and rhIGF-I administration, increased Akt phosphorylation was observed in MVT1 tumors after treatment with both AspB10 and rhIGF-I (Fig. 5A–D), although a greater fold change was observed in the rhIGF-I–treated group. In Met1 tumors, a 2.5-fold increase in Akt phosphorylation after rhIGF-I treatment (P < 0.05) and a 15% increase after AspB10 treatment, which did not reach statistical significance, were observed. No increase in Erk1/2 phosphorylation was found in MVT1 or Met1 tumors after either rhIGF-I or AspB10 treatment (Fig. 5A and B). These results show that, in tumors with certain oncogenes, despite differences in IR and IGF-IR activation, both rhIGF-I and AspB10 led to activation of the Akt signaling, rather than mitogen-activated protein kinase signaling.
FIG. 5.
Chronic administration of IGF-I and AspB10 led to increased phosphorylation of Akt, but no change in Erk1/2 phosphorylation. Western blot analysis of MVT1 tumor lysates at the end of the treatment periods with rhIGF-I and AspB10 revealed that rhIGF-I and AspB10 treatment led to increased Akt phosphorylation and no change in Erk1/2 phosphorylation (A–D). Graphs are representative of two studies for AspB10 treatment and three studies for rhIGF-I treatment. All graphs show the mean for each group, and error bars represent the SEM. Statistical analysis was performed using two-tailed t test. *P < 0.05 between groups. n = 9–11 mice per group. p, phosphorylated; MKR V, MKR mice with endogenous hyperinsulinemia.
DISCUSSION
In this study, we aimed to establish whether endogenous hyperinsulinemia increases mammary tumor growth by directly acting on the IR, rather than through direct or indirect activation of the IGF-IR (9). Additionally, we aimed to determine whether chronic stimulation of the IR increased mammary tumor growth in the absence of IGF-IR phosphorylation. Our results demonstrate that endogenous hyperinsulinemia increases mammary tumor growth by acting directly on the IR. We observed no increase in IGF-IR phosphorylation in tumors from mice with endogenous hyperinsulinemia. Furthermore, we demonstrate that chronic stimulation of the IR, without IGF-IR activation, is capable of driving orthotopic mammary tumor growth in vivo.
Our findings add to the understanding of the link among obesity, T2D, the MetS, and breast cancer. Chronic endogenous hyperinsulinemia has been reported as a major factor linking these conditions (4,6–8,10). Our previous studies have demonstrated that MKR mice with endogenous hyperinsulinemia develop increased transgenic and orthotopic mammary tumor growth and metastases, with increased IGF-IRβ/IRβ phosphorylation, compared with tumors from WT mice (9,14). Lowering insulin levels or blocking IGF-IRβ/IRβ phosphorylation reduced tumor growth (9,13,14). It has been a matter of debate whether endogenous hyperinsulinemia in vivo has direct effects on the breast cancer IR, or whether endogenous hyperinsulinemia increases tumor growth by increasing the availability of local IGF-I to bind to and activate the IGF-IR. Our findings are important, because in women with hyperinsulinemia, increased activation of the IR, rather than the IGF-IR, may be responsible for promoting tumor growth. Increased IR signaling may also be responsible for the increase in breast cancer risk and mortality in women with T2D, the MetS, and obesity, conditions associated with insulin resistance and hyperinsulinemia.
Our study is the first study demonstrating that IR activation, independent of IGF-IR activation, increases tumor growth in vivo.
Previous in vitro studies on breast cancer cell lines have shown that insulin stimulation increases IR phosphorylation and cell proliferation (14,30,31), suggesting that a direct effect of insulin on the IR is responsible for increasing tumor growth. Studies in human cancers have not been able to distinguish between IR and IGF-IR phosphorylation in breast cancer specimens (12). Although there are correlations between high levels of IR expression in human breast cancer specimens and poor prognosis, these studies have not shown that IR signaling increases breast cancer growth (12). Our findings are consistent with the studies by Zhang et al. (32), who reported that knocking down the IR in tumor cell lines reduced tumor growth and metastasis in vivo. It has been reported that IR signaling compensates when IGF-IR is downregulated in breast cancer cell lines (33). Establishing that IR activation is capable of driving tumor growth is important, because cancer therapies targeting the IGF-IR have been less effective than expected, possibly because of compensatory IR signaling (34). Additionally, we found that rhIGF-I administration led to phosphorylation of the IGF-IR, IR, and probably hybrid receptors, as previously reported in in vitro studies (30). Therefore, it appears plausible that the IR is capable of driving tumor growth if the IGF-IR is selectively inhibited.
We used the insulin analog AspB10 to determine whether increased activation of the IR is capable of driving tumor growth, because it has previously been shown to induce spontaneous mammary tumors in rats (17) and our in vitro studies demonstrated increased IR phosphorylation after AspB10 stimulation. Some in vitro studies have reported increased IR phosphorylation after AspB10 stimulation (22), although others have reported that AspB10 stimulates cell proliferation by acting through the IR and IGF-IR (20). These in vitro studies found significantly greater IGF-IR phosphorylation compared with human insulin when using concentrations of AspB10 that were 10 times higher than those used in our studies (20). At concentrations of 10 nmol/L, IGF-IR phosphorylation was comparable to that seen in response to human insulin in MCF7 cells (20), findings consistent those from 5 nmol/L insulin or AspB10 stimulation in mouse embryonic fibroblasts (19). Differences between in vitro and in vivo potency of AspB10 have been previously described (35) and highlight the need for caution when interpreting the results of in vitro studies. In vivo studies have not previously demonstrated whether AspB10 exerts its mitogenic effects through the IR or IGF-IR (21,25). Understanding the mechanism through which AspB10 promotes tumor growth is important for the development of new insulin analogs to ensure they are not mitogenic. While AspB10 increases mammary tumor growth in the dose range used in our study, no increase in rodent tumor growth with similar doses of human insulin has been found; this may be due to differences in IR affinity or dissociation rates between human insulin and AspB10, as previously described by others in in vitro studies (22).
Consistent with the findings of previous studies (19,21), we found increased phosphorylation of Akt in the MVT1 and Met1 tumors from hyperinsulinemic MKR mice after chronic treatment with AspB10 and rhIGF-I. Erk1/2 phosphorylation was not increased after treatment with AspB10 or IGF-I in either Met1 or MVT1 tumors. Previous studies have reported increased Erk1/2 phosphorylation in myoblasts and cardiomyocytes in response to AspB10 (36), and in multiple cell lines in response to rhIGF-I. Our previous studies found no increase in Erk1/2 phosphorylation in MVT1 tumors in the setting of endogenous hyperinsulinemia, and no increase in Erk phosphorylation in response to insulin in vitro (14). Our in vitro studies found that MVT1 cells (expressing the c-myc/vegf oncogene) have constitutively active Erk. While Erk phosphorylation is known to lead to increased c-myc expression, some recent studies report feedback interactions between c-myc and Erk in different cell types, although the mechanism through which this feedback may occur in MVT1 cells has not been elucidated (37–40).
Our previous studies have demonstrated that the MKR mice develop more numerous pulmonary metastases after orthotopic and intravenous tumor cell injection (14). Additionally, rhIGF-I increased metastases from colon cancer orthografts in our previous studies (41).
In this study, the difference in the number of metastases seen after treatment with rhIGF-I or AspB10 showed an increase that did not reach statistical significance; however, our study was underpowered to detect a difference in the number of pulmonary metastases.
We did not address the issue of which IR isoform is driving tumor growth in the setting of hyperinsulinemia or AspB10 administration. Two isoforms of the IR exist: IR-A, in which exon 11 is spliced; and IR-B, which contains exon 11 (42). IR-A is preferentially expressed in fetal tissues and certain tumors (42). Different signaling pathways may be activated by insulin stimulation of IR-A and IR-B in different tissue (43–45). Furthermore, activation of IR-A by different ligands (insulin or IGF-II) leads to the differential signaling effects (42). Many breast cancers express higher levels of IR-A than IR-B, and therefore increased binding of insulin to IR-A is hypothesized to drive tumor growth and resistance to chemotherapeutic agents (44,46). It was hypothesized that AspB10 has a higher affinity for IR-A than IR-B, but recent studies demonstrate that AspB10 has a similar affinity for IR-A and IR-B (19). In the same study, in cells expressing the IR-A isoform, AspB10 led to more cell transformation than human insulin (19). Therefore, the transformational effects of AspB10 may be more pronounced in mammary tumors where the IR-A:IR-B ratio is increased. Future in vivo studies should establish whether activation of IR-A or IR-B signaling is responsible for increasing tumor growth.
Overall, the MKR mouse has provided us a unique opportunity to study the effects of endogenous hyperinsulinemia on mammary tumor growth in the absence of other confounding factors. The results of our study show that in the setting of endogenous hyperinsulinemia, insulin causes IR, but not IGF-IR, phosphorylation. Our study demonstrates for the first time, that direct stimulation of the IR, without IGF-IR activation, increases orthotopic mammary tumor growth. Therefore, in women with endogenous hyperinsulinemia, stimulation of the IR in breast tumors may increase tumor growth and metastases. This finding is an important consideration for the development of tailored treatments for women with obesity, T2D, the MetS, or endogenous hyperinsulinemia who develop breast cancer.
ACKNOWLEDGMENTS
This research was funded by a grant from Sanofi-Aventis, and supported by American Diabetes Association Grant 1-13-BS-108 to D.L.R. N.T. and U.W. are employees of Sanofi-Aventis. D.L.R is a consultant for Sanofi. No other potential conflicts of interest relevant to this article were reported.
E.J.G. contributed to experimental design and the writing of the manuscript. N.A. performed the in vivo experiments. A.T.-H., J.B., N.J.B., and Z.Z. performed in vivo and in vitro experiments. N.T. and U.W. contributed to experimental design and writing the manuscript. D.L.R. contributed to experimental design, manuscript writing, and editing. E.J.G. and D.L.R. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–1638 [DOI] [PubMed] [Google Scholar]
- 2.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care 2012;35:1835–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 2012;35:2402–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. Increased prevalence of prior breast cancer in women with newly diagnosed diabetes. Breast Cancer Res Treat 2006;98:303–309 [DOI] [PubMed] [Google Scholar]
- 5.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 2009;101:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Giudice ME, Fantus IG, Ezzat S, McKeown-Eyssen G, Page D, Goodwin PJ. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat 1998;47:111–120 [DOI] [PubMed] [Google Scholar]
- 7.Kabat GC, Kim M, Caan BJ, et al. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int J Cancer 2009;125:2704–2710 [DOI] [PubMed] [Google Scholar]
- 8.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 2002;20:42–51 [DOI] [PubMed] [Google Scholar]
- 9.Novosyadlyy R, Lann DE, Vijayakumar A, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res 2010;70:741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose DP, Vona-Davis L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr Relat Cancer 2012;19:R225–R241 [DOI] [PubMed] [Google Scholar]
- 11.Gonullu G, Ersoy C, Ersoy A, et al. Relation between insulin resistance and serum concentrations of IL-6 and TNF-alpha in overweight or obese women with early stage breast cancer. Cytokine 2005;31:264–269 [DOI] [PubMed] [Google Scholar]
- 12.Law JH, Habibi G, Hu K, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res 2008;68:10238–10246 [DOI] [PubMed] [Google Scholar]
- 13.Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, LeRoith D. Insulin-sensitizing therapy attenuates type 2 diabetes-mediated mammary tumor progression. Diabetes 2010;59:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson RD, Novosyadlyy R, Fierz Y, et al. Hyperinsulinemia enhances c-Myc-mediated mammary tumor development and advances metastatic progression to the lung in a mouse model of type 2 diabetes. Breast Cancer Res 2012;14:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu S, Lee WM, Archer MC. Insulin does not promote rat mammary carcinogenesis. Carcinogenesis 1998;19:699–702 [DOI] [PubMed] [Google Scholar]
- 16.Stammberger I, Bube A, Durchfeld-Meyer B, Donaubauer H, Troschau G. Evaluation of the carcinogenic potential of insulin glargine (LANTUS) in rats and mice. Int J Toxicol 2002;21:171–179 [DOI] [PubMed] [Google Scholar]
- 17.Dideriksen L, Jorgensen L, Drejer K. Carcinogenic effect of the human insulin analogue B10 Asp in female rats (Abstract). Diabetologia 1992;35:A3 [Google Scholar]
- 18.Hansen BF, Kurtzhals P, Jensen AB, Dejgaard A, Russell-Jones D. Insulin X10 revisited: a super-mitogenic insulin analogue. Diabetologia 2011;54:2226–2231 [DOI] [PubMed] [Google Scholar]
- 19.Sciacca L, Cassarino MF, Genua M, et al. Insulin analogues differently activate insulin receptor isoforms and post-receptor signalling. Diabetologia 2010;53:1743–1753 [DOI] [PubMed] [Google Scholar]
- 20.Milazzo G, Sciacca L, Papa V, Goldfine ID, Vigneri R. ASPB10 insulin induction of increased mitogenic responses and phenotypic changes in human breast epithelial cells: evidence for enhanced interactions with the insulin-like growth factor-I receptor. Mol Carcinog 1997;18:19–25 [DOI] [PubMed] [Google Scholar]
- 21.Oleksiewicz MB, Bonnesen C, Hegelund AC, et al. Comparison of intracellular signalling by insulin and the hypermitogenic AspB10 analogue in MCF-7 breast adenocarcinoma cells. J Appl Toxicol 2011;31:329–341 [DOI] [PubMed] [Google Scholar]
- 22.Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000;49:999–1005 [DOI] [PubMed] [Google Scholar]
- 23.Sommerfeld MR, Müller G, Tschank G, et al. In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PLoS One 2010;5:e9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glendorf T, Knudsen L, Stidsen CE, et al. Systematic evaluation of the metabolic to mitogenic potency ratio for B10-substituted insulin analogues. PLoS One 2012;7:e29198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamel FG, Siford GL, Fawcett J, Chance RE, Frank BH, Duckworth WC. Differences in the cellular processing of AspB10 human insulin compared with human insulin and LysB28ProB29 human insulin. Metabolism 1999;48:611–617 [DOI] [PubMed] [Google Scholar]
- 26.Fernández AM, Kim JK, Yakar S, et al. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev 2001;15:1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei XF, Noble MS, Davoli MA, et al. Explant-cell culture of primary mammary tumors from MMTV-c-Myc transgenic mice. In Vitro Cell Dev Biol Anim 2004;40:14–21 [DOI] [PubMed] [Google Scholar]
- 28.Borowsky AD, Namba R, Young LJ, et al. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin Exp Metastasis 2005;22:47–59 [DOI] [PubMed] [Google Scholar]
- 29.Johansson GS, Arnqvist HJ. Insulin and IGF-I action on insulin receptors, IGF-I receptors, and hybrid insulin/IGF-I receptors in vascular smooth muscle cells. Am J Physiol Endocrinol Metab 2006;291:E1124–E1130 [DOI] [PubMed] [Google Scholar]
- 30.Pandini G, Vigneri R, Costantino A, et al. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin Cancer Res 1999;5:1935–1944 [PubMed] [Google Scholar]
- 31.Osborne CK, Bolan G, Monaco ME, Lippman ME. Hormone responsive human breast cancer in long-term tissue culture: effect of insulin. Proc Natl Acad Sci USA 1976;73:4536–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Fagan DH, Zeng X, Freeman KT, Sachdev D, Yee D. Inhibition of cancer cell proliferation and metastasis by insulin receptor downregulation. Oncogene 2010;29:2517–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Pelzer AM, Kiang DT, Yee D. Down-regulation of type I insulin-like growth factor receptor increases sensitivity of breast cancer cells to insulin. Cancer Res 2007;67:391–397 [DOI] [PubMed] [Google Scholar]
- 34.Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater? J Natl Cancer Inst 2012;104:975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vølund A, Brange J, Drejer K, et al. In vitro and in vivo potency of insulin analogues designed for clinical use. Diabet Med 1991;8:839–847 [DOI] [PubMed] [Google Scholar]
- 36.Rakatzi I, Ramrath S, Ledwig D, et al. A novel insulin analog with unique properties: LysB3,GluB29 insulin induces prominent activation of insulin receptor substrate 2, but marginal phosphorylation of insulin receptor substrate 1. Diabetes 2003;52:2227–2238 [DOI] [PubMed] [Google Scholar]
- 37.Qiao D, Meyer K, Friedl A. Glypican-1 stimulates Skp2 autoinduction loop and G1/S transition in endothelial cells. J Biol Chem 2012;287:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Velpula KK, Dasari VR, Tsung AJ, Dinh DH, Rao JS. Transcriptional repression of Mad-Max complex by human umbilical cord blood stem cells downregulates extracellular signal-regulated kinase in glioblastoma. Stem Cells Dev 2012;21:1779–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall GM, Liu PY, Gherardi S, et al. SIRT1 promotes N-Myc oncogenesis through a positive feedback loop involving the effects of MKP3 and ERK on N-Myc protein stability. PLoS Genet 2011;7:e1002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin CJ, Cencic R, Mills JR, Robert F, Pelletier J. c-Myc and eIF4F are components of a feedforward loop that links transcription and translation. Cancer Res 2008;68:5326–5334 [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res 2002;62:1030–1035 [PubMed] [Google Scholar]
- 42.Frasca F, Pandini G, Scalia P, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 1999;19:3278–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sciacca L, Prisco M, Wu A, Belfiore A, Vigneri R, Baserga R. Signaling differences from the A and B isoforms of the insulin receptor (IR) in 32D cells in the presence or absence of IR substrate-1. Endocrinology 2003;144:2650–2658 [DOI] [PubMed] [Google Scholar]
- 44.Kalla Singh S, Brito C, Tan QW, De León M, De León D. Differential expression and signaling activation of insulin receptor isoforms A and B: a link between breast cancer and diabetes. Growth Factors 2011;29:278–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leibiger B, Leibiger IB, Moede T, et al. Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol Cell 2001;7:559–570 [DOI] [PubMed] [Google Scholar]
- 46.Harrington SC, Weroha SJ, Reynolds C, Suman VJ, Lingle WL, Haluska P. Quantifying insulin receptor isoform expression in FFPE breast tumors. Growth Horm IGF Res 2012;22:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]