Abstract
The derangement of endoplasmic reticulum (ER) homeostasis triggers β-cell apoptosis, leading to diabetes. Glucokinase upregulates insulin receptor substrate 2 (IRS-2) expression in β-cells, but the role of glucokinase and IRS-2 in ER stress has been unclear. In this study, we investigated the impact of glucokinase activation by glucokinase activator (GKA) on ER stress in β-cells. GKA administration improved β-cell apoptosis in Akita mice, a model of ER stress–mediated diabetes. GKA increased the expression of IRS-2 in β-cells, even under ER stress. Both glucokinase-deficient Akita mice and IRS-2–deficient Akita mice exhibited an increase in β-cell apoptosis, compared with Akita mice. β-cell–specific IRS-2–overexpressing (βIRS-2-Tg) Akita mice showed less β-cell apoptosis than Akita mice. IRS-2–deficient islets were vulnerable, but βIRS-2-Tg islets were resistant to ER stress–induced apoptosis. Meanwhile, GKA regulated the expressions of C/EBP homologous protein (CHOP) and other ER stress–related genes in an IRS-2–independent fashion in islets. GKA suppressed the expressions of CHOP and Bcl2-associated X protein (Bax) and protected against β-cell apoptosis under ER stress in an ERK1/2-dependent, IRS-2–independent manner. Taken together, GKA ameliorated ER stress–mediated apoptosis by harmonizing IRS-2 upregulation and the IRS-2–independent control of apoptosis in β-cells.
The decline in β-cell mass as a result of increased apoptosis is an important property of type 2 diabetes (1,2). Endoplasmic reticulum (ER) stress is a key mediator of β-cell apoptosis (3,4). Hence, the development of therapeutic strategies to safeguard residual β-cells against ER stress–induced apoptosis is needed for the adequate care and cure of type 2 diabetes.
Glucokinase, a member of the hexokinase family, is mainly expressed in hepatocytes, pancreatic β-cells, and certain subgroups of hypothalamic neurons, forming a key component of the main glucose sensor in β-cells (5–8). Glucokinase also mediates the glucose signal–induced upregulation of insulin receptor substrate 2 (IRS-2) expression in β-cells through calcineurin or CREB (9–12). IRS-2 is required for the maintenance of the β-cell mass and plays an important role in compensatory β-cell expansion against peripheral insulin resistance and in β-cell survival, preventing diabetes (9,13–15). Mice that were heterozygous for β-cell glucokinase (βGck+/−) and that were fed a diet rich in linoleic acid and sucrose exhibited increased ER stress and apoptosis in β-cells, compared with wild-type (WT) mice (16). Consequently, we speculated that glucokinase is also involved in the regulation of ER stress–induced apoptosis in β-cells.
Glucokinase activators (GKAs) have been shown to reduce blood glucose levels in several diabetic animal models and type 2 diabetic patients (10,11,17–19). GKAs promote β-cell proliferation, which is driven by the increased expression of IRS-2 and the activation of its downstream signaling pathway (11,20,21). However, the physiological advantage of GKA-mediated signaling during β-cell apoptosis has been obscure (22,23), and the effect of GKAs on ER stress in β-cells remains unknown.
These conditions inspired us to undertake a detailed investigation of the impact of GKA on ER stress and apoptosis in β-cells. Here, we report the protective effects of GKA against ER stress–induced apoptosis in β-cells, the nature of this mechanism, and the significance of glucokinase and IRS-2 in the regulation of ER stress in β-cells.
RESEARCH DESIGN AND METHODS
Animals and animal care.
Akita mice with a C57BL/6J background were obtained from Japan SLC. We backcrossed βGck+/− (7), IRS-2−/− (15), or β-cell–specific IRS-2–overexpressing (βIRS-2-Tg) mice (9) with C57BL/6J mice >10 times. Akita mice were crossed with βGck+/−, IRS-2−/−, or βIRS-2-Tg mice to obtain βGck+/−;Akita, IRS-2−/−;Akita, or βIRS-2-Tg;Akita offspring. db/db (Lepr+/−) mice were also obtained from CLEA Japan. High fat–fed WT and βGck+/− mice were generated as described previously (9). All the experiments were conducted on male littermates. All the animal procedures were performed in accordance with the institutional animal care guidelines and the guidelines of the Animal Care Committee of the Yokohama City University. The animal housing rooms were maintained at a constant room temperature (25°C) and on a 12-h light (7:00 a.m.)/dark (7:00 p.m.) cycle.
Drugs.
GKA Cpd A [2-amino-5-(4-methyl-4H-(1,2,4)-triazole-3-yl-sulfanyl)-N-(4-methyl-thiazole-2-yl)benzamide] (24), was purchased from Calbiochem, Ro-28-1675 [(R)-3-cyclopentyl-2-(4-methanesulfonyl-phenyl)-N-thiazol-2-yl-propionamide] (17) was purchased from Axon Medchem, and Cpd B (3-[(1S)-2-hydroxy-1-methylethoxy]-5-[4-(methylsulfonyl)phenoxy]-N-1,3-thiazol-2-ylbenzamide) (25) was provided by Merck Sharp & Dohme. The neonatal WT and Akita mice were weaned at 19 days after birth and were fed a standard diet (MF; Oriental Yeast, Tokyo, Japan) or a standard diet containing 0.01% GKA Cpd A (Calbiochem), 0.4% sitagliptin (STG) (Januvia; Suzuken Co., Nagoya, Japan), or 0.5% phloridzin (PHZ) (Sigma-Aldrich). βGck+/−;Akita and IRS-2−/−;Akita mice were fed a standard diet or a standard diet containing 0.01% GKA until 33 days of age or 0.5% PHZ until 8 weeks of age.
Biochemical parameters and glucose tolerance test.
The plasma glucose levels, blood insulin levels, and glycogen content in the liver were determined using a Glutest Neo Super (Sanwa Chemical Co.), an insulin kit (Morinaga), and a Determiner-GL-E Kit (Wako Pure Chemical Industries), respectively. The plasma alanine aminotransferase, free fatty acid, total cholesterol, and triglyceride levels were assayed using enzymatic methods (Wako Pure Chemical Industries). All the mice were denied access to food for 20–24 h before the oral glucose tolerance test (OGTT) and then were orally loaded with glucose at 1.5 mg/g body weight. For single administration experiments, 33-day-old mice received either the vehicle (Solutol HS-15; BASF) or GKA (30 mg/kg orally) before oral glucose loading (1.5 mg/g).
Histological analysis.
More than five pancreatic tissue sections from each animal were analyzed after fixation and paraffin embedding. The sections were immunostained with antibodies to insulin (Santa Cruz Biotechnology) or glucagon (Abcam). Biotinylated secondary antibodies, a VECTASTAIN Elite ABC Kit, and a DAB Substrate Kit (Vector Laboratories) were used to examine the sections using bright-field microscopy to determine the β-cell mass, and Alexa Fluor 488–, 555–, and 647–conjugated secondary antibodies (Invitrogen) were used for fluorescence microscopy. All the images were acquired using a BZ-9000 microscope (Keyence) or FluoView FV1000-D confocal laser scanning microscope (Olympus). The proportion of the area of pancreatic tissue occupied by the β-cells was calculated using BIOREVO software (Keyence), as described previously (16). TUNEL staining was performed in vivo or in vitro using the ApopTag In Situ Detection Kit (Chemicon). For TUNEL staining, at least 100 islets per mouse (in vivo) or 30 islets per isolated islet group (in vitro) attached to poly-l-lysine–coated coverslips (Falcon) were analyzed using the FluoView FV1000-D confocal laser scanning microscope to assess the proportion of immunostained nuclei among the insulin-positive cells.
Islet culture.
Islets were isolated from mice as described elsewhere (16). Isolated islets were cultured overnight in RPMI 1640 medium (Wako Pure Chemical Industries) containing 2.8, 5.6, 11.1, or 22.2 mmol/L glucose supplemented with 10% FCS, 100 units/mL penicillin, and 100 μg/mL streptomycin. Islets were treated with 1 μmol/L thapsigargin, 10 μmol/L tunicamycin, 50 μmol/L nifedipine, 10 μmol/L FK506, 20 μmol/L U0126, 10 μmol/L UK14304 (Sigma-Aldrich), 30 μmol/L GKA Cpd A, 0.5 μmol/L GSK-3 inhibitor (BIO), 10 μmol/L Akti-1/2 (Calbiochem), 10 mmol/L d-mannoheptulose (Toronto Research Chemicals), 200 μmol/L diazoxide (Wako Pure Chemical Industries), 200 nmol/L OSI-906 (Selleck Chemicals), 10 μmol/L GKA Ro-28-1675 (Axon Medchem), or 2 μmol/L GKA Cpd B (provided by Merck Sharp & Dohme). All the reagents were added concomitantly to the medium in each experiment.
Real-time PCR.
Total RNA was isolated from pancreatic islets using an RNase-free DNase and RNeasy Kit (Qiagen, Valencia, CA). cDNA was prepared using the High Capacity cDNA Reverse Transcription Kits (Applied Biosystems) and was subjected to quantitative PCR using TaqMan Gene Expression Assays (7900 Real-Time PCR System; Applied Biosystems) with THUNDERBIRD qPCR Master Mix (TOYOBO). All the probes were purchased from Applied Biosystems. Each quantitative reaction was performed in duplicate. Data were normalized according to the β-actin level.
Immunoblotting.
For immunoblotting, >100 isolated islets were lysed in ice-cold RIPA buffer (Cell Signaling Technology) with complete protease inhibitor cocktail (Roche Diagnostics). After centrifugation, the extracts were subjected to immunoblotting with antibodies to C/EBP homologous protein (CHOP) (GADD153), ATF4, GRP78, phosphor-eIF-2α, ATF3, Bcl2-associated X protein (Bax), X-box binding protein 1 (XBP1), ATF6α (Santa Cruz Biotechnology), GSK-3β, phospho-GSK-3β (Ser9), extracellular signal–related kinase 1/2 (ERK1/2), phospho-ERK1/2 (Thr202/Tyr204), IRS-2, phospho-eIF2α (Ser51), eIF2α, IRE1α, phospho-PERK, PERK (Cell Signaling Technology), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phospho-IRE1 (Ser724) (Abcam), and β-actin (Sigma-Aldrich). Densitometry was performed using Image J software.
cDNA microarray analysis.
Islets were isolated from mice as described elsewhere (16). Islets of 8-week-old C57BL/6J mice were treated for 24 h with 30 μmol/L GKA (Cpd A) or vehicle (DMSO) at 5.6 mmol/L glucose RPMI-1640 medium containing 5.6 mmol/L glucose supplemented with 10% FCS. The cDNA microarray analysis was performed using the Agilent-026655 whole mouse genome array (GPL10333) (Agilent). Replicate (n = 2) microarray studies were performed for each treatment. The data were analyzed using Genespring GX software (Agilent). The data were normalized in a per-chip and per-spot intensity-dependent manner. The data files have been deposited in the NCBI GEO database GSE41248 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE41248).
Flow cytometry.
To assay apoptosis based on cleaved caspase-3 expression, the islets were fixed with 2% formaldehyde, perforated with 0.01% Triton X-100, stained with phycoerythrin-conjugated anticleaved caspase-3 antibody (Asp175; Cell Signaling Technology), and analyzed using a FACS Canto II (BD Biosciences). The mean fluorescence of normal-sized (small populations of forward scatter were excluded) and propidium iodide-negative survival cell fractions was calculated using FACS Diva software (BD Biosciences).
Statistical analyses.
All the data were reported as the means ± SE and were analyzed using the Student t test or ANOVA. Differences were considered significant if the P value was <0.05 (*) or <0.01 (**).
RESULTS
GKA ameliorated β-cell apoptosis in Akita mice.
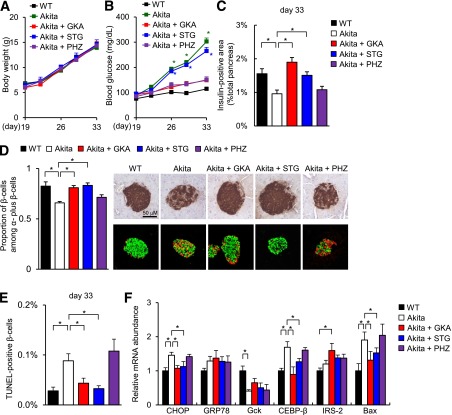
To address whether GKA ameliorates ER stress and apoptosis in β-cells, we used Akita mice, which carry a heterozygous conformation-altering mutation (Cys96Tyr) in the Ins2 gene and manifest enhanced ER stress and the apoptosis of β-cells (26,27). WT or Akita mice were fed a standard diet or a diet containing GKA, the DPP-4 inhibitor STG, or the SGLT1 and SGLT2 inhibitor PHZ (Supplementary Fig. 1A). Because DPP-4 inhibitor reportedly protected β-cell ER stress and apoptosis in diabetic mice and PHZ could lower blood glucose levels via a reduction of renal glucose transport, we assessed the impacts of these drugs on β-cell apoptosis as controls (16). Prior to the administration of these diets, the Akita mice already exhibited impaired glucose tolerance and deteriorated insulin secretion after glucose loading but retained similar body weights, fed blood glucose levels, β-cell areas, islet diameters, and β-cell proportions, compared with the WT mice (Supplementary Fig. 1B–H). Body weight gain and serum lipid profiles were not significantly different among the five groups (Fig. 1A and Supplementary Fig. 2A). GKA and PHZ, but not STG, improved the plasma glucose levels in Akita mice (Fig. 1B). The OGTT after a 20–24-h fast revealed that Akita mice had abnormal glucose tolerance and severely exacerbated insulin secretion, compared with WT mice (Supplementary Fig. 2B and C). Of note, GKA, STG, and PHZ did not restore insulin secretion during the OGTT, suggesting that these agents were insufficient to improve β-cell function in Akita mice (Supplementary Fig. 2B and C).
FIG. 1.
GKA ameliorated β-cell apoptosis in Akita mice. The experiments were performed in Akita mice after 14 days on a standard diet or a diet containing 0.01% GKA, 0.4% STG, or 0.5% PHZ. Details are described in Supplementary Fig. 1A. A: Body weight gain (n = 8). B: Fed blood glucose levels (n = 8). *P < 0.05 vs. WT mice. C: β-cell mass (n = 6–8). The β-cell area is shown as a proportion of the area of the entire pancreas (aged 33 days). D, left: Quantification of β-cell mass as a proportion of the total α-cell plus β-cell mass in the islet (aged 33 days, n = 6). D, right: Pancreatic sections were stained with antibodies to insulin (green) and glucagon (red). The scale bar represents 50 μm. E: The proportion of TUNEL-positive cells is shown as a percentage of the total number of insulin-positive cells in the sections (aged 33 days, n = 6). F: The mRNA expression levels of the molecules indicated in the islets (aged 33 days, n = 5). *P < 0.05.
Subsequently, Akita and WT mice were treated with GKA or a vehicle 30 min before an OGTT. GKA significantly reduced the blood glucose levels before and after glucose administration in both WT and Akita mice (Supplementary Fig. 2D). GKA facilitated insulin secretion in WT mice, but it failed to improve impaired insulin secretion in Akita mice (Supplementary Fig. 2E). On the other hand, in Akita mice, the liver glycogen content was increased by GKA (Supplementary Fig. 2F). Accordingly, the glucose-lowering effect of GKA in Akita mice was partly attributed to the facilitation of glucose utilization in the liver.
In Akita mice, the β-cell mass and the β-cell ratio in the islets were significantly decreased, the islet morphology was abnormal, and the number of TUNEL-positive apoptotic β-cells was significantly greater than that in WT mice (Fig. 1C–E). GKA and STG, but not PHZ, restored the β-cell mass, normalized the islet morphology, and significantly decreased the number of TUNEL-positive apoptotic β-cells in Akita mice (Fig. 1C–E and Supplementary Fig. 2G). GKA significantly decreased the mRNA expression of CHOP, CEBP-β, and Bax, and it significantly increased the mRNA expression of IRS-2 in islets, compared with the expression levels in untreated Akita mice (Fig. 1F).
Role of glucokinase in ER stress–induced β-cell apoptosis.
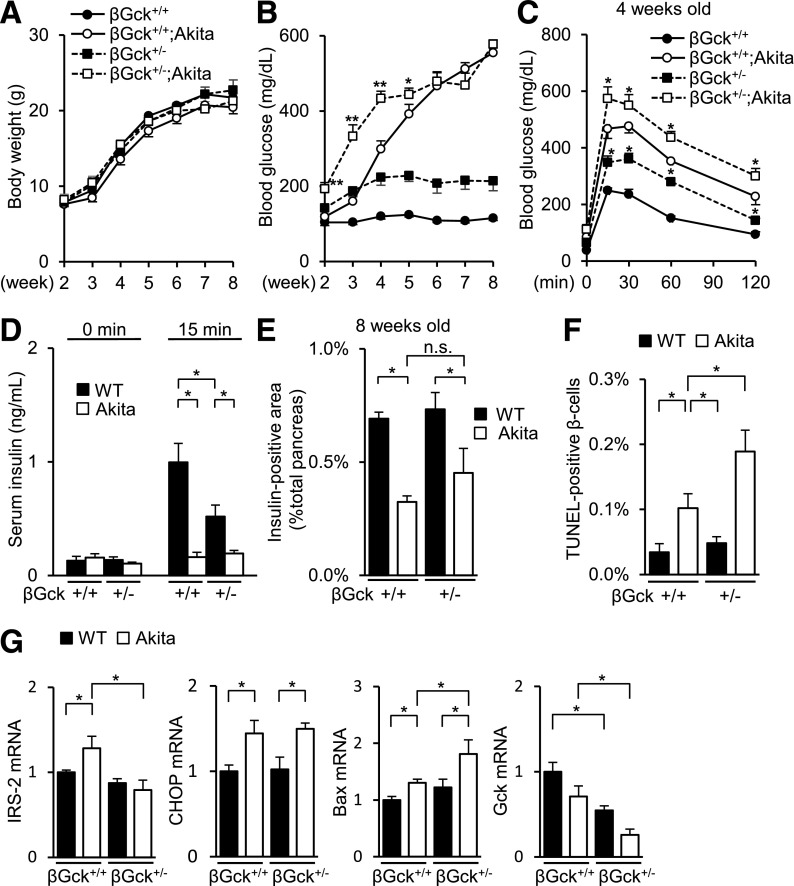
To assess the role of glucokinase in ER stress, we generated Akita mice with the β-cell–specific heterozygous ablation of glucokinase (βGck+/−;Akita). The βGck+/−;Akita mice exhibited an equivalent body weight gain and lipid levels but had aggravated blood glucose levels and glucose tolerance (Fig. 2A–D and Supplementary Fig. 3A–D). However, glucokinase deficiency no longer produced any impairment in insulin secretion in insulin-defective Akita mice (Fig. 2D and Supplementary Fig. 3C). Although the βGck+/−;Akita mice depicted a similar β-cell mass (Fig. 2E), the number of TUNEL-positive β-cells was significantly higher in the βGck+/−;Akita mice than in WT mice (Fig. 2F and Supplementary Fig. 3E). Of note, the reversal of hyperglycemia by PHZ in βGck+/−;Akita mice did not improve β-cell apoptosis (Supplementary Fig. 3F–J). A gene expression analysis of isolated islets demonstrated a decreased expression of IRS-2 and an increased expression of Bax in βGck+/−;Akita mice, compared with Akita mice (Fig. 2G). These results suggested that the insufficiency of glucokinase in β-cells was vulnerable to ER stress by the absence of the induced expression of IRS-2.
FIG. 2.
β-Cell–specific heterozygous ablation of glucokinase (βGck+/−) worsened β-cell apoptosis in Akita mice. A: Body weight gain (n = 8–14). B: Fed blood glucose levels (n = 8–14). *P < 0.05 and **P < 0.01 vs. Akita mice. C and D: Glucose tolerance test (aged 4 weeks, n = 6–8). C: Plasma glucose levels. *P < 0.05 vs. Akita mice. D: Serum insulin levels. E: β-Cell mass (n = 6). The β-cell area is shown as a proportion of the area of the entire pancreas (aged 8 weeks). F: The proportion of TUNEL-positive cells is shown as a percentage of the total number of insulin-positive cells in the sections (aged 8 weeks, n = 6). G: The mRNA expression levels of the molecules indicated in the islets (aged 8 weeks, n = 6). *P < 0.05. n.s., not significant.
Role of IRS-2 in ER stress–induced β-cell apoptosis.
Next, we generated IRS-2–deficient Akita (IRS-2+/−;Akita and IRS-2−/−;Akita) mice. IRS-2+/−;Akita mice exhibited a modest decrease in body weight gain and deterioration in their blood glucose levels, compared with Akita mice (Fig. 3A and B). As we reported (11), IRS-2−/− mice remained euglycemic with normal β-cell mass in the C57BL/6J background, while these mice demonstrated diabetes and decreased β-cell mass in other genetic backgrounds. IRS-2−/−;Akita mice showed a more severe impairment of body weight gain, overt hyperglycemia, increased mortality, a further decrease in β-cell mass, and the enhancement of β-cell apoptosis compared with Akita mice (Fig. 3A–D and Supplementary Fig. 4A and B). The serum lipid levels in the IRS-2−/−;Akita mice were similar to those in the Akita mice (Supplementary Fig. 4C). The administration of PHZ partly reduced the blood glucose levels and lethality but did not affect the β-cell mass or β-cell apoptosis in IRS-2−/−;Akita mice (data not shown).
FIG. 3.
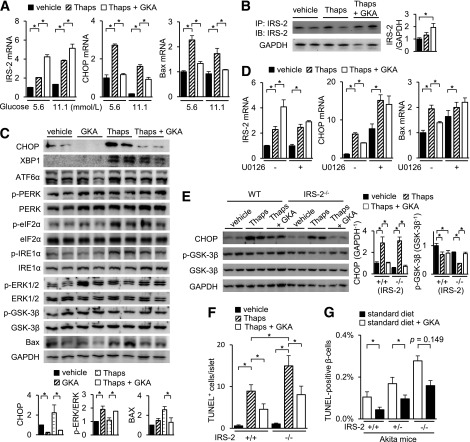
IRS-2–deficient Akita (IRS-2−/−;Akita) mice demonstrated a decreased β-cell mass and the enhancement of β-cell apoptosis. A: Body weight gain (n = 8–18). *P < 0.05 vs. Akita mice. B: Fed blood glucose levels (n = 8–18). *P < 0.05 and **P < 0.01 vs. Akita mice. C: β-Cell mass (n = 8). The β-cell area is shown as a proportion of the area of the entire pancreas (aged 8 weeks). D: The proportion of TUNEL-positive cells is shown as a percentage of the total number of insulin-positive cells in the sections (aged 8 weeks, n = 8). E–H: Isolated islets of WT (IRS-2+/+) or IRS-2−/− mice were incubated with 1 μmol/L thapsigargin (Thaps) or vehicle for 24 h at 2.8, 5.6, or 11.1 mmol/L glucose (E) or at 5.6 mmol/L glucose (F–H). E: mRNA expression levels in the islets (n = 8). F, left: The total cell extracts from the islets were subjected to immunoblotting as indicated. F, right: Intensity of the signals quantified by densitometry (n = 4). G: Flow-cytometric analysis of cleaved caspase-3 levels in islets. Values shown represent the mean fluorescence of normal-sized cells (upper populations of forward scatter [FSC], i.e., viable cells) in the indicated population. The results of one of three independent experiments are shown. H: Number of TUNEL-positive β-cells in the islets (at least 30 islets per indicated group). A GSK-3 inhibitor, 0.5 μmol/L BIO, was added concomitantly with thapsigargin. *P < 0.05. n.s., not significant.
We investigated changes in gene expressions in isolated islets of IRS-2–deficient (IRS-2−/−) mice under ER stress induced by treatment with thapsigargin. The expression levels of CHOP or Bax were increased in IRS-2−/− islets under ER stress, compared with WT islets, at 5.6 or 11.1 mmol/L glucose but not at 2.8 mmol/L glucose (Fig. 3E). Under basal unstimulated conditions, elevated expression levels of GPR78, ATF6α, PERK, eIF2α, and IRE1α were observed in IRS-2−/− islets (Fig. 3F and Supplementary Fig. 4D). IRS-2−/− islets also showed reduced phosphorylation levels of GSK-3β (Fig. 3F), increased expression of cleaved caspase-3 (Fig. 3G), and augmentation of β-cell apoptosis (Fig. 3H and Supplementary Fig. 4E) in the presence of ER stress at 5.6 mmol/L glucose. Correspondingly, a GSK-3 inhibitor (BIO) recuperated this apoptosis in IRS-2−/− islets (Fig. 3H).
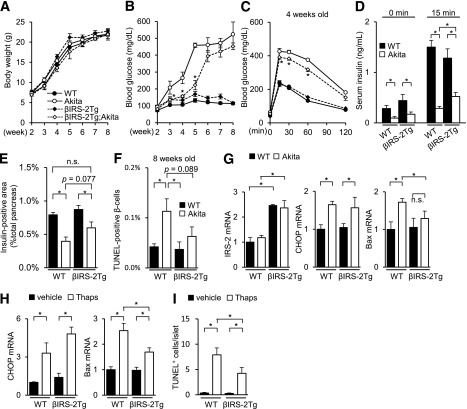
Akita mice with the β-cell–specific transgenic overexpression of IRS-2 (βIRS-2-Tg;Akita) were also generated. Although the body weight gain and β-cell mass showed no significant changes, βIRS-2-Tg;Akita mice exhibited an improved glucose tolerance, enhanced glucose-induced insulin secretion, and the amelioration of β-cell apoptosis, compared with Akita mice (Fig. 4A–F and Supplementary Fig. 5A–C). The β-cell mass of βIRS-2-Tg;Akita mice tended to be higher than that of Akita mice, with a value similar to that in WT mice (Fig. 4E). Consistent with the results of the apoptotic analysis (Fig. 4F), the expression level of Bax in islets was significantly lower in the βIRS-2-Tg;Akita mice than in Akita mice (Fig. 4G). Isolated βIRS-2-Tg islets also showed reduced expression levels of Bax (Fig. 4H) and the restoration of β-cell apoptosis (Fig. 4I and Supplementary Fig. 5D) in the presence of ER stress.
FIG. 4.
Akita mice with β-cell–specific transgenic overexpression of IRS-2 (βIRS-2-Tg;Akita) were resistant to ER stress–induced apoptosis. A: Body weight gain (n = 10–14). B: Fed blood glucose levels (n = 10–14). *P < 0.05 vs. Akita mice. C and D: Glucose tolerance test (aged 4 weeks, n = 7–9). C: Plasma glucose levels. *P < 0.05 vs. Akita mice. D: Serum insulin levels. E: β-Cell mass (n = 7). The β-cell area is shown as a proportion of the area of the entire pancreas (aged 8 weeks). F: The proportion of TUNEL-positive cells is shown as a percentage of the total number of insulin-positive cells in the pancreas sections (aged 8 weeks, n = 6). G: mRNA expression levels in the islets (aged 8 weeks, n = 6). H and I: Isolated islets of WT or βIRS-2-Tg mice were incubated with 1 μmol/L thapsigargin (Thaps) or vehicle for 24 h at 5.6 mmol/L glucose. H: mRNA expression levels in the islets (n = 6). I: Number of TUNEL-positive β-cells in the islets (at least 30 islets per indicated group). *P < 0.05. n.s., not significant.
IRS-2–independent regulation of ER stress–related genes by GKA.
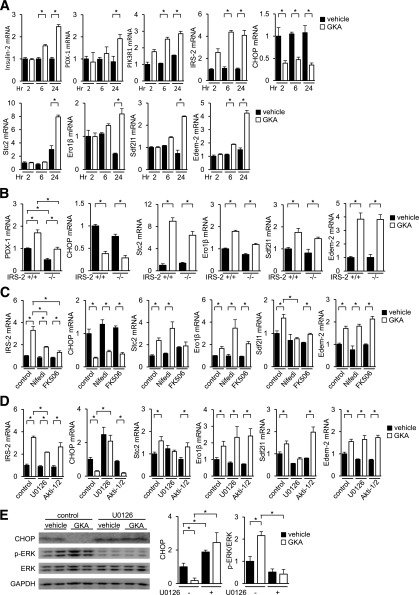
We compared the gene expression profiles between GKA-treated and vehicle-treated isolated islets to identify the target genes of GKA. Genes for which the expression levels were increased by greater than twofold or reduced by <0.5-fold by GKA treatment, relative to the expression levels in control islets, were identified (NCBI GEO database GSE41248) (Supplementary Table 1). We confirmed that insulin gene and insulin signal molecules, such as IRS-2, PDX-1, and PIK3R1, were upregulated by GKA (Fig. 5A). Interestingly, GKA decreased the expression of CHOP and increased the expression of Stc2, Ero-1β, Sdf2l1, and Edem-2, which are reportedly ER stress–related genes (Fig. 5A). However, the expressions of the other ER stress–related genes, such as Bip, IRE-1α, PERK, ATF6, Xbp1, ATF3, ATF4, or 4EBP1, were unaffected by GKA (data not shown). High glucose evoked identical changes in the gene expressions of ER stress–related genes (Supplementary Fig. 6A), and mannoheptulose, a glucokinase inhibitor, completely abolished these changes (Supplementary Fig. 6B). In the presence of diazoxide, a KATP channel opener, the changes in IRS-2, Stc2, and Sdf2l1 were abrogated (Supplementary Fig. 6C). Treatment with the IGF-1R/insulin receptor inhibitor OSI-906 did not affect GKA-induced changes in ER stress–related gene expressions (Supplementary Fig. 6D). GKA decreased the expression of CHOP also in MIN6 cells (Supplementary Fig. 6H).
FIG. 5.
IRS-2–independent and ERK-dependent regulation of ER stress–related genes by GKA in islets. A–D: The mRNA expression levels in the islets (aged 8 weeks, n = 6). A: Islets of C57BL/6J mice were incubated with 30 μmol/L GKA (Cpd A) at 5.6 mmol/L glucose. B: Islets of WT (IRS-2+/+) or IRS-2−/− mice were incubated with 30 μmol/L GKA at 5.6 mmol/L glucose for 24 h. C: Islets of C57BL/6J mice were incubated with 30 μmol/L GKA at 5.6 mmol/L glucose for 24 h, in the presence of 50 μmol/L nifedipine (Nifedi, calcium channel blocker) or 10 μmol/L FK506 (tacrolimus, calcineurin inhibitor). D: Islets of C57BL/6J mice were incubated with 30 μmol/L GKA at 5.6 mmol/L glucose for 24 h, in the presence of 20 μmol/L U0126 (MEK inhibitor) or 10 μmol/L Akti-1/2 (Akt/PKB inhibitor). E: Islets of C57BL/6J mice were incubated with 30 μmol/L GKA at 5.6 mmol/L glucose for 24 h in the presence of 20 μmol/L U0126. E, left: Total cell extracts from islets were subjected to immunoblotting as indicated. E, right: Intensity of the signals quantified by densitometry (ImageJ) (n = 4). *P < 0.05.
Because ER stress in β-cells reportedly occurs in db/db mice, the effects of GKA on ER stress–related genes were assessed in islets from 9-week-old db/db mice. Interestingly, the upregulation of IRS-2, PDX1, Stc2, Sdf2l1, and Ero1-β was attenuated, but the induction of Edem-2 was retained in db/db islets after GKA treatment (Supplementary Fig. 6E). βGck+/− mice failed to exhibit an elevated expression of IRS-2 in a diet-induced obesity (DIO) model (9). Although the upregulation of IRS-2 by GKA was greater in islets from DIO mice than from lean mice with a βGck+/+ background, the βGck+/− islets from the DIO and lean mice exhibited similar IRS-2 expression levels (Supplementary Figure 6F). βGck+/− islets also exhibited the increased expression of CHOP under unstimulated conditions and a reduction in Stc2 induction after GKA treatment (Supplementary Figure 6F).
Whether IRS-2 is required for the regulation of ER stress–related genes by GKA is uncertain. Accordingly, we used IRS-2−/− islets to address this question. In IRS-2−/− islets, the GKA-induced changes in the expression levels of genes related to ER stress were essentially preserved, not impaired (Fig. 5B). Hence, GKA regulates the expressions of ER stress–related genes, regardless of IRS-2 deficiency. Blocking L-type Ca2+ channels with nifedipine or calcineurin with FK506 (tacrolimus) prevented the GKA-induced increase in the expression of IRS-2 but had little effect on the regulation of ER stress–related gene expressions by GKA (Fig. 5C). In contrast, the changes in CHOP, Stc2, and Sdf2l1 expressions by GKA were completely blunted by the blockade of MEK1/2 with U0126 but not by the Akt inhibitor (Akti-1/2) (Fig. 5D). The upregulation of IRS-2 by GKA was retained despite its significant attenuation by U0126 (Fig. 5D). GKA phosphorylated ERK1/2 and reduced CHOP protein in islets, and these actions induced by GKA were restricted in the presence of U0126 (Fig. 5E). The selective α2-adrenergic agonist UK14304 reduced glucose-stimulated ERK1/2 activation (28). UK14304 suppressed the GKA-mediated modifications in the expression levels of IRS-2, CHOP, Stc2, and Sdf2l1 (Supplementary Fig. 6G). Thus, the regulation of CHOP and other ER stress–related genes by GKA was IRS-2 independent and partly ERK1/2 dependent.
IRS-2–independent amelioration of ER stress–induced apoptosis by GKA.
CHOP triggers ER stress–induced apoptosis in β-cells in several diabetic mouse models (27,29). Since GKA reduced the expression of CHOP via an ERK-dependent, IRS-2–independent manner, we assessed the effects of GKA on isolated islets under ER stress. GKA increased the gene expression of IRS-2 and decreased the expressions of CHOP and Bax in WT islets under ER stress induced by thapsigargin, an inhibitor of sarco/endoplasmic reticulum Ca2+ ATPase (Fig. 6A). Interestingly, a transient increase in IRS-2 expression was observed in islets and MIN6 insulinoma cells after treatment with either thapsigargin or tunicamycin, a blocker of the synthesis of all N-linked glycoproteins (N-glycans) (Fig. 6A and Supplementary Fig. 7I). This transient IRS-2 induction is also displayed in Akita mice in vivo (Supplementary Fig. 2H). The protein level of IRS-2 was increased by GKA but not by thapsigargin alone (Fig. 6B). The expressions of ATF3 and ATF4 were not affected by GKA (Supplementary Fig. 7A). GKA induced a similar outcome in WT islets under both palmitate-induced ER stress (Supplementary Fig. 7B) and tunicamycin-induced ER stress (data not shown). Two other classes of GKAs, Ro-28-1675 and Cpd-B, also produced parallel changes in gene expression in the islets (Supplementary Fig. 7C). In islets under ER stress, GKA significantly increased the phosphorylation level of ERK and decreased the protein levels of CHOP and Bax (Fig. 6C). But GKA did not influence the protein levels of XBP1, ATF6α, PERK, eIF2α, and IRE1α and those phosphorylation levels (Fig. 6C and Supplementary Fig. 7D). In the presence of the MEK inhibitor U0126 or selective α2-adrenergic agonist UK14304, the GKA-induced suppression of CHOP and Bax under ER stress was inhibited (Fig. 6D and Supplementary Fig. 7E and F), but OSI906 had no effect on them (Supplementary Fig. 7G). A high glucose level also increased IRS-2 and decreased CHOP expression but did not affect Bax levels under ER stress (Supplementary Fig. 7H). GKA reduced the expression of cleaved caspase-3 in β-cells (Supplementary Fig. 7J) and TUNEL-positive β-cells (Fig. 6F) under ER stress. Thus, GKA directly protected ER stress–induced apoptosis in islets. In accordance with the IRS-2–independent regulation of ER stress–related genes by GKA, GKA suppressed the expressions of CHOP and Bax even in IRS-2−/− islets under ER stress (Fig. 6E). Furthermore, GKA increased the phosphorylation level of GSK-3β, decreased the CHOP protein level, and protected against β-cell apoptosis in IRS-2−/− islets under ER stress (Fig. 6E and F and Supplementary Fig. 7K). To assess this IRS-2–independent potency of GKA in vivo, we fed IRS-2+/−;Akita and IRS-2−/−;Akita mice diets containing GKA for 14 days and evaluated the levels of β-cell apoptosis. GKA partially reversed the increase in β-cell apoptosis in IRS-2+/−;Akita and IRS-2−/−;Akita mice (Fig. 6G and Supplementary Fig. 7L).
FIG. 6.
GKA ameliorates ER stress–induced apoptosis via IRS-2–independent pathway. Islets from C57BL/6J mice (aged 8 weeks) were incubated with 1 μmol/L thapsigargin (Thaps) and 30 μmol/L GKA (Cpd A) at 5.6 (A–C) or 11.1 mmol/L (A) glucose for 24 h. A: mRNA expression levels in the islets (n = 6). B, left: The immunoprecipitated or total cell extracts from the islets were subjected to immunoblotting as indicated. B, right: Intensity of the signals quantified by densitometry (n = 4). C, top: The total cell extracts from the islets were subjected to immunoblotting as indicated. C, bottom: Intensity of the signals quantified by densitometry (n = 4). D: Islets of C57BL/6J mice (aged 8 weeks) were incubated with 30 μmol/L GKA or 1 μmol/L Thaps at 5.6 mmol/L glucose for 24 h in the presence of 20 μmol/L U0126 (MEK inhibitor). The mRNA expression levels (n = 6). E and F: Islets of WT (IRS-2+/+) or IRS-2−/− mice were incubated with 1 μmol/L Thaps and 30 μmol/L GKA for 24 h at 5.6 mmol/L glucose. E: mRNA expression levels in the islets (n = 6). F: Number of TUNEL-positive β-cells in the islets (at least 30 islets per indicated group). G: IRS-2+/+;Akita, IRS-2+/−;Akita, or IRS-2−/−;Akita mice were fed standard diet containing GKA from day 19 to 33 after birth. The proportion of TUNEL-positive cells is shown as a percentage of the total number of insulin-positive cells in the pancreas sections (aged 33 days, n = 4–7). *P < 0.05. IB, immunoblotting; IP, immunoprecipitation.
DISCUSSION
The present report describes a previously unidentified function of the activation of glucokinase in governing ER stress–induced β-cell apoptosis (Fig. 7). Consistent with the established antiapoptotic effects of IRS-2–mediated signaling in β-cells (13), genetic modification of this signaling pathway altered the levels of β-cell apoptosis induced by ER stress in Akita mice. Notably, GKA reduced the expression of CHOP and Bax and directly ameliorated ER stress–induced apoptosis in β-cells through an ERK-dependent, IRS-2–independent pathway.
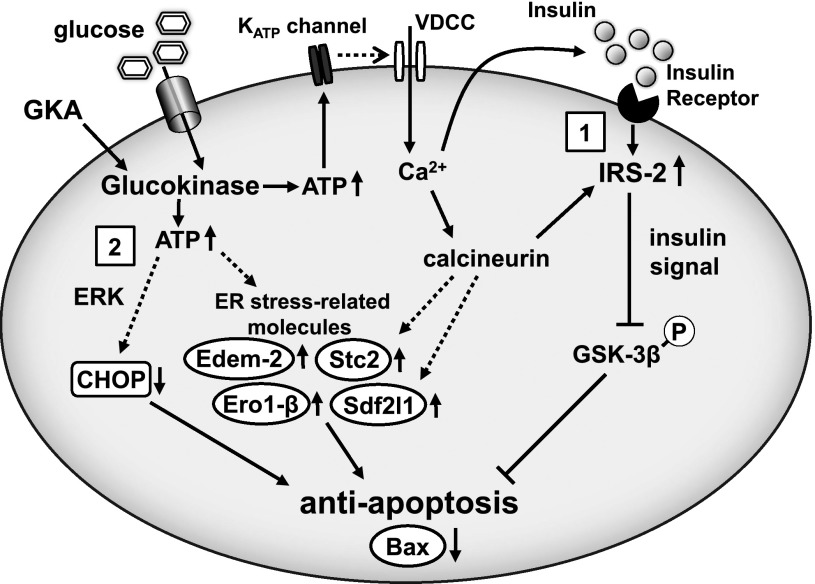
FIG. 7.
An illustrative model of amelioration of ER stress–induced β-cell apoptosis by GKA. Activation of glucokinase in β-cells induces insulin secretion and the upregulation of IRS-2, which results in the activation of insulin signaling and β-cell proliferation. Under ER stress, GKA correspondingly increases IRS-2 and inactivates GSK-3β by its phosphorylation (1). GKA also decreases CHOP expression via ERK-dependent pathway and increases the expression of ER stress–related molecules, such as Stc2, Ero-1, Sdf2l1, and Edem-2 (2). These multiple pathways in β-cells coordinately suppress the proapoptotic gene Bax, allowing adaptation and survival against ER stress. VDCC, voltage-dependent Ca2+ channel; KATP channel, ATP-sensitive potassium channel.
In Akita mice, GKA improved β-cell apoptosis in a glycemic control–independent manner, since the improvement of hyperglycemia by PHZ did not affect β-cell apoptosis. The antihyperglycemic action of PHZ was thought to be caused by the inhibition of intestinal glucose uptake and renal glucose reabsorption, but these mechanisms might not be sufficient for the preservation of β-cell mass in Akita mice. This concept was also supported by the results that the increase in β-cell apoptosis in βGck+/−;Akita mice was not reversed by glycemic control with PHZ. GKA retained the normoglycemia in Akita mice, possibly by safeguarding the β-cell mass.
Since ER stress led to the reactive induction of IRS-2 expression in β-cells, we hypothesized that a reduction in IRS-2 expression would lead to an increase in β-cell apoptosis in βGck+/−;Akita mice. Predictably, the loss of IRS-2 aggravated glycemic control and β-cell apoptosis in Akita mice. IRS-2 deficiency in islets also caused a reduction in the inhibition of GSK-3β by its phosphorylation under ER stress, and an inhibitor of GSK-3 restored ER stress–induced apoptosis in IRS-2−/− islets. Thus, the glucokinase/IRS-2/Akt/GSK-3β signaling pathway is thought to be involved in the antiapoptotic effect of GKA against ER stress. Our results are consistent with previous observations that GSK-3β haploinsufficiency corrected β-cell apoptosis in IRS-2−/− mice (30), and the attenuation of the regulation of GSK-3β by insulin signaling partly caused ER stress–induced apoptosis (31). Considering the report that PDX1 deficiency induced β-cell susceptibility to ER stress (32), the GKA-induced upregulation of PDX1 expression might be somewhat associated with the regulation of apoptosis.
A major question to be addressed in future studies is the pathophysiological significance of the modulation of ER stress–related genes, such as the downregulation of CHOP and the upregulation of Stc2, Ero-1β, Sdf2l1, or Edem-2 that are triggered by GKA. The suppression of CHOP evidently averts apoptosis induced by ER stress (27,29,33). The overexpression of Stc2 also reduces pancreatic tissue apoptosis in cerulein-induced pancreatitis model mice through the alteration of PERK signaling (34). Ero-1β, an oxidoreductase, plays a role in the efficient oxidative maturation of proinsulin and glycemic control in mice (35). Edem-2 assists folding-incompetent glycoprotein degradation from the ER via ER-associated degradation (36). Thus, increases in the expressions of Stc2, Ero-1β, and Edem-2 in response to GKA make physiological sense with respect to the prevention of ER stress–induced apoptosis. Sdf2l1 is also known to be an ER stress–inducible gene (37), but its function in ER stress remains obscure.
Some studies have shown that CREB or the Ca2+/calcineurin signal mediates the glucose signal–induced augmentation of IRS-2 expression in β-cells (9,12,38), and ATF3 evokes the repression of IRS-2 expression, resulting in β-cell apoptosis (39). We demonstrated that GKA upregulated IRS-2 via Ca2+-calcineurin but did not affect ATF3 levels in β-cells. The insulin receptor plays a crucial role in β-cell proliferation in response to insulin resistance (40). However, we noted that even if signals from insulin and IGF-1 receptors were canceled by OSI-906, GKA satisfactorily increased IRS-2 and regulated ER stress–related genes. Meanwhile, ERK1/2 mediated the modification of ER stress–related gene expressions by GKA. As the suppression of CHOP by glucose signals has been implicated in ERK1/2 and MafA (41), MafA might influence other GKA-induced ER stress regulators. The cooperative nature of insulin signaling and ERK for cell survival against ER stress has been previously reported (42). We advocate this concept as being a valid model for β-cells.
Numerous studies have shown that chronic exposure to high glucose induces β-cell ER stress and reactive oxygen species production, resulting in cell death (43,44). Does this mean that the hyperactivation of glucokinase in response to GKA is toxic to β-cells? Some reports had suggested that β-cell apoptosis induced by exposure to chronic high glucose levels arises from a reduction in glucokinase expression and a reduction in the interactions between glucokinase and mitochondria (45–47). Furthermore, the glucokinase-binding domain of BAD controls insulin secretion and β-cell apoptosis at the mitochondria (48). Glucose signaling may have two-sided effects, providing crucial tasks for β-cell adaptation or apoptosis depending on the unique metabolic niche. Therefore, a balance between β-cell adaptation and β-cell apoptosis is presumably scheduled by the combination of diversity, magnitude, and timing of glucose signaling. Based on our results, glucokinase activation could provide plural points for the regulation of ER stress–induced apoptosis in β-cells (Fig. 7). These multiple pathways in β-cells may allow a timely adaptation and survival against ER stress. In this study, successful treatment was portrayed by early intervention with GKA. One proposal is that glucokinase activation should be promoted during the early stage of diabetes or prediabetes, but not in overt diabetes, since we and others have previously reported that GKA failed to restore serious β-cell damage under oxidative stress (11,49).
In this study, ER stresses were assessed in insulin gene–mutated Akita mice and were induced by chemicals such as thapsigargin. Under conditions related to obesity and type 2 diabetes, ER stress is activated in various tissues such as hypothalamus, liver, muscle, adipose tissue, and β-cells in both human and mouse (50,51). In the context of insulin resistance, ER stress in liver serves as a key homeostatic regulator of protein, lipid, and glucose metabolism (52,53). Therefore, more physiological or pathophysiological models ought to be examined for possible effects of GKA on ER stress. Lastly, but perhaps most importantly, further analyses of the efficacy and feasibility of glucokinase activation in the clinical management of diabetes are needed to open new avenues for β-cell–protective interventions.
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aid for Scientific Research (B) 21390282 and (B) 24390235 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a Medical Award from the Japan Medical Association, a Grant-in-Aid from the Japan Diabetes Foundation, a Grant-in-Aid from the Uehara Memorial Foundation, a Grant-in-Aid from the Naito Foundation, a Grant-in-Aid from the Takeda Life Foundation (to Y.Te.), a Grant-in-Aid for JSPS fellows, a Grant-in-Aid from Yokohama General Promotion Foundation, a Grant-in-Aid from the Novo Nordisk Insulin Research Foundation, a Grant-in-Aid from Japan Foundation for Applied Enzymology, a Grant-in-Aid from Kanae Memorial Foundation, and a Grant-in-Aid from Banyu Life Science Foundation International (to J.S.). No other potential conflicts of interest relevant to this article were reported.
J.S. designed and performed experiments, analyzed the data, and wrote the manuscript. Y.To., K.T., K.O., and H.I. contributed to discussions. E.S. and M.K. performed experiments. N.K. and T.K. provided mice for this study, contributed to discussions, and reviewed the manuscript. Y.Te. wrote the manuscript, reviewed and edited the manuscript, and contributed to discussions. Y.Te. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011, and the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Misa Katayama (Yokohama City University) for secretarial assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0052/-/DC1.
REFERENCES
- 1.Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia 2004;47:581–589 [DOI] [PubMed] [Google Scholar]
- 2.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 3.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008;29:42–61 [DOI] [PubMed] [Google Scholar]
- 4.Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 2007;50:752–763 [DOI] [PubMed] [Google Scholar]
- 5.Matschinsky F, Liang Y, Kesavan P, et al. Glucokinase as pancreatic beta cell glucose sensor and diabetes gene. J Clin Invest 1993;92:2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grupe A, Hultgren B, Ryan A, Ma YH, Bauer M, Stewart TA. Transgenic knockouts reveal a critical requirement for pancreatic beta cell glucokinase in maintaining glucose homeostasis. Cell 1995;83:69–78 [DOI] [PubMed] [Google Scholar]
- 7.Terauchi Y, Sakura H, Yasuda K, et al. Pancreatic beta-cell-specific targeted disruption of glucokinase gene. Diabetes mellitus due to defective insulin secretion to glucose. J Biol Chem 1995;270:30253–30256 [DOI] [PubMed] [Google Scholar]
- 8.Shirakawa J, Tanami R, Togashi Y, et al. Effects of liraglutide on β-cell-specific glucokinase-deficient neonatal mice. Endocrinology 2012;153:3066–3075 [DOI] [PubMed] [Google Scholar]
- 9.Terauchi Y, Takamoto I, Kubota N, et al. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 2007;117:246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura A, Terauchi Y, Ohyama S, et al. Impact of small-molecule glucokinase activator on glucose metabolism and beta-cell mass. Endocrinology 2009;150:1147–1154 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura A, Togashi Y, Orime K, et al. Control of beta cell function and proliferation in mice stimulated by small-molecule glucokinase activator under various conditions. Diabetologia 2012;55:1745–1754 [DOI] [PubMed] [Google Scholar]
- 12.Demozay D, Tsunekawa S, Briaud I, Shah R, Rhodes CJ. Specific glucose-induced control of insulin receptor substrate-2 expression is mediated via Ca2+-dependent calcineurin/NFAT signaling in primary pancreatic islet β-cells. Diabetes 2011;60:2892–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennige AM, Burks DJ, Ozcan U, et al. Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J Clin Invest 2003;112:1521–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet 1999;23:32–40 [DOI] [PubMed] [Google Scholar]
- 15.Kubota N, Tobe K, Terauchi Y, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 2000;49:1880–1889 [DOI] [PubMed] [Google Scholar]
- 16.Shirakawa J, Amo K, Ohminami H, et al. Protective effects of dipeptidyl peptidase-4 (DPP-4) inhibitor against increased β cell apoptosis induced by dietary sucrose and linoleic acid in mice with diabetes. J Biol Chem 2011;286:25467–25476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimsby J, Sarabu R, Corbett WL, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science 2003;301:370–373 [DOI] [PubMed] [Google Scholar]
- 18.Efanov AM, Barrett DG, Brenner MB, et al. A novel glucokinase activator modulates pancreatic islet and hepatocyte function. Endocrinology 2005;146:3696–3701 [DOI] [PubMed] [Google Scholar]
- 19.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov 2009;8:399–416 [DOI] [PubMed] [Google Scholar]
- 20.Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development 2010;137:3205–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Futamura M, Yao J, Li X, et al. Chronic treatment with a glucokinase activator delays the onset of hyperglycaemia and preserves beta cell mass in the Zucker diabetic fatty rat. Diabetologia 2012;55:1071–1080 [DOI] [PubMed] [Google Scholar]
- 23.Wei P, Shi M, Barnum S, Cho H, Carlson T, Fraser JD. Effects of glucokinase activators GKA50 and LY2121260 on proliferation and apoptosis in pancreatic INS-1 beta cells. Diabetologia 2009;52:2142–2150 [DOI] [PubMed] [Google Scholar]
- 24.Futamura M, Hosaka H, Kadotani A, et al. An allosteric activator of glucokinase impairs the interaction of glucokinase and glucokinase regulatory protein and regulates glucose metabolism. J Biol Chem 2006;281:37668–37674 [DOI] [PubMed] [Google Scholar]
- 25.Iino T, Hashimoto N, Sasaki K, et al. Structure-activity relationships of 3,5-disubstituted benzamides as glucokinase activators with potent in vivo efficacy. Bioorg Med Chem 2009;17:3800–3809 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Takeuchi T, Tanaka S, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 1999;103:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 2002;109:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson TB, Lawrence MC, Gibson CJ, et al. Inhibition of glucose-stimulated activation of extracellular signal-regulated protein kinases 1 and 2 by epinephrine in pancreatic beta-cells. Diabetes 2006;55:1066–1073 [DOI] [PubMed] [Google Scholar]
- 29.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 2008;118:3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanabe K, Liu Z, Patel S, et al. Genetic deficiency of glycogen synthase kinase-3beta corrects diabetes in mouse models of insulin resistance. PLoS Biol 2008;6:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivasan S, Ohsugi M, Liu Z, Fatrai S, Bernal-Mizrachi E, Permutt MA. Endoplasmic reticulum stress-induced apoptosis is partly mediated by reduced insulin signaling through phosphatidylinositol 3-kinase/Akt and increased glycogen synthase kinase-3beta in mouse insulinoma cells. Diabetes 2005;54:968–975 [DOI] [PubMed] [Google Scholar]
- 32.Sachdeva MM, Claiborn KC, Khoo C, et al. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc Natl Acad Sci USA 2009;106:19090–19095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 2004;18:3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fazio EN, Dimattia GE, Chadi SA, Kernohan KD, Pin CL. Stanniocalcin 2 alters PERK signalling and reduces cellular injury during cerulein induced pancreatitis in mice. BMC Cell Biol 2011;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zito E, Chin KT, Blais J, Harding HP, Ron D. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cell Biol 2010;188:821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olivari S, Molinari M. Glycoprotein folding and the role of EDEM1, EDEM2 and EDEM3 in degradation of folding-defective glycoproteins. FEBS Lett 2007;581:3658–3664 [DOI] [PubMed] [Google Scholar]
- 37.Fukuda S, Sumii M, Masuda Y, et al. Murine and human SDF2L1 is an endoplasmic reticulum stress-inducible gene and encodes a new member of the Pmt/rt protein family. Biochem Biophys Res Commun 2001;280:407–414 [DOI] [PubMed] [Google Scholar]
- 38.Jhala US, Canettieri G, Screaton RA, et al. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev 2003;17:1575–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D, Yin X, Zmuda EJ, et al. The repression of IRS2 gene by ATF3, a stress-inducible gene, contributes to pancreatic beta-cell apoptosis. Diabetes 2008;57:635–644 [DOI] [PubMed] [Google Scholar]
- 40.Okada T, Liew CW, Hu J, et al. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA 2007;104:8977–8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawrence MC, McGlynn K, Naziruddin B, Levy MF, Cobb MH. Differential regulation of CHOP-10/GADD153 gene expression by MAPK signaling in pancreatic beta-cells. Proc Natl Acad Sci USA 2007;104:11518–11525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem 2004;279:49420–49429 [DOI] [PubMed] [Google Scholar]
- 43.Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 2012;81:767–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang C, Han P, Oprescu AI, et al. Evidence for a role of superoxide generation in glucose-induced beta-cell dysfunction in vivo. Diabetes 2007;56:2722–2731 [DOI] [PubMed] [Google Scholar]
- 45.Kooptiwut S, Kebede M, Zraika S, et al. High glucose-induced impairment in insulin secretion is associated with reduction in islet glucokinase in a mouse model of susceptibility to islet dysfunction. J Mol Endocrinol 2005;35:39–48 [DOI] [PubMed] [Google Scholar]
- 46.Kim WH, Lee JW, Suh YH, et al. Exposure to chronic high glucose induces beta-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in pancreatic beta-cells. Diabetes 2005;54:2602–2611 [DOI] [PubMed] [Google Scholar]
- 47.Joe MK, Lee HJ, Suh YH, et al. Crucial roles of neuronatin in insulin secretion and high glucose-induced apoptosis in pancreatic beta-cells. Cell Signal 2008;20:907–915 [DOI] [PubMed] [Google Scholar]
- 48.Danial NN, Walensky LD, Zhang CY, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med 2008;14:144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meininger GE, Scott R, Alba M, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2560–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flamment M, Hajduch E, Ferré P, Foufelle F. New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab 2012;23:381–390 [DOI] [PubMed] [Google Scholar]
- 51.Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med 2012;18:59–68 [DOI] [PubMed] [Google Scholar]
- 52.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab 2012;15:623–634 [DOI] [PubMed] [Google Scholar]
- 53.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 2012;56:952–964 [DOI] [PubMed] [Google Scholar]