The concept of a “diabetic cardiomyopathy” has been invoked to explain the higher than expected occurrence of congestive heart failure (CHF) in subjects with diabetes (1,2). However, the evidence supporting the existence of such a condition in humans is mostly inferential. Cardiomyopathies are chronic diseases of the myocardium in which the heart is abnormally enlarged, thickened, and/or stiffened. The weakened heart muscle has a reduced ability to pump blood effectively. Diabetic cardiomyopathy is frequently considered to be present when there is any abnormality of myocardial diastolic or systolic function, even when very mild, in a diabetic subject (or animal) without known hypertension or coronary artery disease. Isolated metabolic or biochemical abnormalities in the heart have also been taken as evidence of this entity. It has been referred to as a “specific” cardiomyopathy that may include features such as left ventricular (LV) hypertrophy, myocardial fibrosis, altered myocardial energetics, and variable degrees of myocardial mechanical dysfunction.
There is some controversy about the existence and/or nature of a diabetic cardiomyopathy because there are inconsistencies in the definition of the syndrome, there is a high reliance on findings in small animal models, there are problems with referral bias in many clinical studies, and there is a lack of prospective or longitudinal human studies (3,4). It may be argued that the simple criteria frequently used to characterize this condition are not adequate for defining a cardiomyopathy. The structural, mechanical, histological, and biochemical features mentioned above also are not specific to one disease state. Rather they are common features of nearly all myocardial diseases. Inclusion of type 2 diabetic patients in studies of diabetic cardiomyopathy is problematic because it is very difficult to separate the cardiovascular effects of obesity and diabetes. If we draw analogy to clinical guidelines, the level of evidence supporting the existence of a specific diabetic cardiomyopathy in humans would likely be “C,” based on expert opinion rather than on results of large, randomized clinical trials.
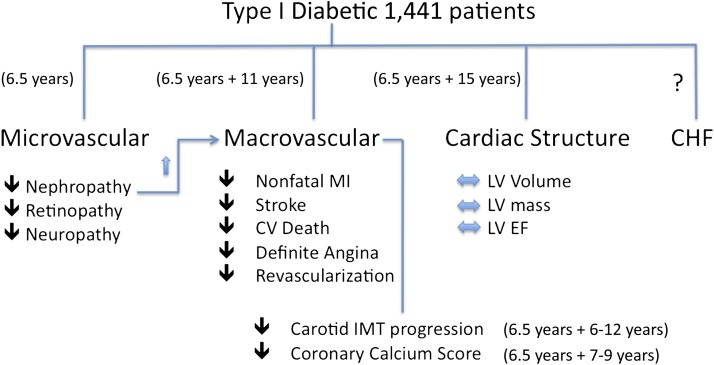
In this issue, we are treated to an article by Genuth et al. (5) showing findings after 21 years of total follow-up in a large cohort of type 1 diabetic patients who were enrolled in the Diabetes Control and Complications Trial (DCCT) (6) and subsequently the Epidemiology of Diabetes Intervention and Complications (EDIC) study (7). Between 1983 and 1989, DCCT randomized 1,441 subjects with type 1 diabetes to intensive versus conventional treatment for a mean of 6.5 years (8). Patients were followed for an additional 15 years in EDIC. In Genuth et al., 1,017 members (74%) of the original DCCT cohort underwent cardiac MRI for measurement of LV volumes, mass, ejection fraction, and aortic distensibility at ∼21 years after initial enrollment. The DCCT/EDIC study showed that intensive compared with conventional treatment during the DCCT was associated with a 57% reduction in a composite outcome of nonfatal myocardial infarction, stroke, or cardiovascular death from baseline DCCT through 11 years of EDIC (9). A summary of results from DCCT and EDIC relevant to the cardiovascular system is shown in Fig. 1. A history of microalbuminuria or albuminuria increased the risk of cardiovascular disease by a factor of 2.5 (9). At the time of the cardiac MRI in Genuth et al., mean age of the subjects was 49 years and mean duration of diabetes was 27 years. The main findings of the current study were that after 6.5 years of randomized treatment and 15 years of additional follow-up, there were no differences between the subjects in the DCCT intensive treatment versus the conventional treatment group in terms of LV end-diastolic volume (EDV), end-systolic volume, stroke volume, cardiac output, LV mass, LV ejection fraction, LV mass/EDV, or aortic distensibility.
FIG. 1.
Overview of the various cardiovascular and related end points from the DCCT and the EDIC study. Arrows indicate the direction of change in frequency of each complication (increased, decreased, or no change) in the DCCT group receiving intensive treatment relative to the conventional treatment group during the 6.5 years of the DCCT. The treatment during DCCT + duration of EDIC follow-up is indicated for each category. The presence of proteinuria significantly increased the incidence of cardiovascular events. Heart failure events have not been reported in these trials. CV, cardiovascular; EF, ejection fraction; IMT, intima-media thickness; MI, myocardial infarction.
Surprisingly, in this large population with long-standing diabetes and only fair long-term diabetes control (mean HbA1c over the course of the study was 7.7–8.3%), LV size, geometry, and function were remarkably normal and not different from reported values in nondiabetic subjects. The mean LV EDV in the study was ∼137 mL, stroke volume ∼84 mL, ejection fraction ∼61%, and LV mass ∼137 g. None of these parameters are very suggestive of cardiomyopathy, a condition in which we would anticipate finding dilated chambers, increased LV mass, and reduced ejection fraction. It may be argued that subtle diastolic and systolic parameters, such as those derived from myocardial strain analysis or tissue Doppler recordings, were not measured in this study and, thus, evidence of a diabetic cardiomyopathy was missed. However, subclinical myocardial dysfunction that is not progressive over time and does not result in chamber enlargement, hypertrophy, or clinical heart failure is not necessarily indicative of a cardiomyopathy. Subclinical systolic and diastolic dysfunction that can only be detected with strain imaging is often seen in healthy aging, obesity, hypertension, and many other conditions. Therefore, such findings, even when present, are not specific to diabetes.
Although there were no differences in cardiac geometry or function between the intensively and conventionally treated DCCT groups, the current study did show statistically significant relationships between mean HbA1c over the entire DCCT/ECIC study period and various MRI measurements of cardiac geometry. It should be noted that the relationships were very modest. For example, EDV decreased by 2.61 mL and LV mass increased by 2.68 g per 1% increase in HbA1c. An absolute increase in HbA1c of 1% is relatively large and almost certainly clinically significant, whereas a change in LV volume or mass of 2 mL or 2 g is small and clinically insignificant. There was no relationship between DCCT, EDIC, or long-term HbA1c and LV ejection fraction.
If poor glucose control produces functionally relevant, detrimental changes in the myocardium, we should expect to find a clearly increased incidence of CHF in patients with long-standing type 1 diabetes, particularly those with worse control. Incident CHF has not been previously reported for DCCT/EDIC patients. Although not the focus of the article, the investigators briefly mention in the discussion that only one case of CHF had occurred after year 13 of EDIC with another five cases since then. This would appear to represent a low incidence of CHF given the very long duration of diabetes. A large Swedish registry of type 1 diabetic patients reported the incidence of CHF to be approximately three per 1,000 patient-years (10). In that study, the risk of CHF increased with age and duration of diabetes. A large cohort study using a Kaiser Permanente database showed a linear relationship between HbA1c levels and CHF hospitalizations (four to nine per 1,000 patient-years) in patients with diabetes; however, only 2% of the heart failure events occurred in patients with type 1 diabetes (11). Unfortunately, the current study did not include biomarkers relevant to the diagnosis of cardiomyopathy and CHF; in particular, measurement of natriuretic peptides. Such data might be helpful in determining the functional significance of the small cardiac structural differences seen in patients with higher averaged HbA1c.
In summary, the long-term, prospective study in type 1 diabetic patients by Genuth et al. showed that 1) intensive treatment for 6.5 years did not affect cardiac geometry or function at a later time point, 2) cardiac geometry, size, and function were all normal despite an average duration of 27 years of diabetes, and 3) there was a very modest relationship between the mean HbA1c over the preceding 21 years and MRI-derived measures of LV geometry, but not ejection fraction. The article presents some of the strongest data in humans published to date on the long-term effects of diabetes on the heart. Although it is certainly true that type 1 diabetes is a major risk factor for complications of coronary artery disease, the findings raise questions about the extent to which type 1 diabetes has direct effects on the myocardium.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants R01 DK055006 and M01 RR000064.
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 3561.
REFERENCES
- 1.Schilling JD, Mann DL. Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin 2012;8:619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 2006;98:596–605 [DOI] [PubMed] [Google Scholar]
- 4.Konduracka E, Gackowski A, Rostoff P, Galicka-Latala D, Frasik W, Piwowarska W. Diabetes-specific cardiomyopathy in type 1 diabetes mellitus: no evidence for its occurrence in the era of intensive insulin therapy. Eur Heart J 2007;28:2465–2471 [DOI] [PubMed] [Google Scholar]
- 5.Genuth SM, Backlund JC, Bayless M, et al. for the DCCT/EDIC Research Group Effects of prior intensive versus conventional therapy and history of glycemia on cardiac function in type 1 diabetes in the DCCT/EDIC. Diabetes 2013;62:3561–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 7.Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 9.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lind M, Bounias I, Olsson M, Gudbjörnsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011;378:140–146 [DOI] [PubMed] [Google Scholar]
- 11.Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation 2001;103:2668–2673 [DOI] [PubMed] [Google Scholar]