Glucocorticoids commonly used in the posttransplant period have been demonstrated to reduce insulin sensitivity, impair α-cell function, and more recently, impair β-cell function and the incretin effect (1). Liraglutide is an incretin mimetic approved for the treatment of type 2 diabetes. One major concern about the use of liraglutide after transplantation is that it delays gastric emptying, which could potentially affect absorption of coadministered oral medications, such as tacrolimus, which has a narrow therapeutic index (2). This case series is the first to report on the safety of this drug combination in kidney transplant recipients (KTRs).
Nonpregnant, adult (≥18 years of age), and clinically stable (estimated glomerular filtration rate [eGFR] ≥60 mL/min/1.73 m2 and not experiencing allograft rejection or two serum creatinine values >30% of each other obtained at least 1 week apart within 4 weeks prior to enrollment) KTRs receiving unchanged tacrolimus doses for ≥4 weeks (goal trough concentration 5–15 ng/mL) were included. Patients with a history of preexisting diabetes or receiving antihyperglycemic agents or varying doses of glucocorticoids were excluded. The study was approved by the Henry Ford Hospital and the Wayne State University Institutional Review Boards. All participants provided written informed consent.
Measurements were performed before and after self-administration of a 21-day course of liraglutide (0.6 mg for 1 week, 1.2 mg during week 2, and 1.8 mg during week 3). Tacrolimus area under the curve (AUC)0–12h was measured with a previously validated multiple regression–derived limited sampling strategy (3). Fasting and postprandial (60 and 120 min after completion of a standardized test meal) blood glucose levels were measured. Body weight and safety and tolerability were captured. Descriptive statistics were performed.
Five patients had been exposed to concomitant liraglutide and tacrolimus therapy at our institution (age 55.4 ± 8.2 years, 3 male, 4 African American, BMI 30.1 ± 6.2 kg/m2, eGFR 93.0 ± 21.3 mL/min/1.73 m2). Two had prediabetes, and four were on chronic glucocorticoid therapy.
Primary and secondary outcomes assessed are included in Table 1. Compared with baseline, tacrolimus AUC0–12h appeared reduced after coadministration with liraglutide; however, trough concentrations were unaltered. Tacrolimus and maintenance corticosteroid doses remained unchanged, and there was no biopsy-proven evidence of acute rejection in any KTR. No patients experienced stage 1 acute kidney injury as defined by the Acute Kidney Injury Network criteria (≥26.5 μmol/L or ≥1.5- to 2.0-fold increase in serum creatinine from baseline). No differences were noted in fasting blood glucose (5.0 ± 1.2 vs. 5.3 ± 0.5 mmol/L). Liraglutide appeared to reduce blood glucose levels at 60 (7.3 ± 1.2 vs. 5.9 ± 0.5 mmol/L) and 120 min (7.1 ± 0.8 vs. 6.0 ± 0.4 mmol/L). Liraglutide administration was also associated with a reduction in body weight after 21 days of therapy (−2.1 ± 1.3 kg). No hypoglycemia or serious adverse events related to liraglutide therapy were reported. Nausea, reduced appetite, headache, injection site pain, and weakness were each reported by two KTRs. Indigestion was experienced by one KTR.
Table 1.
Primary and secondary outcomes during coadministration of liraglutide with tacrolimus in KTRs
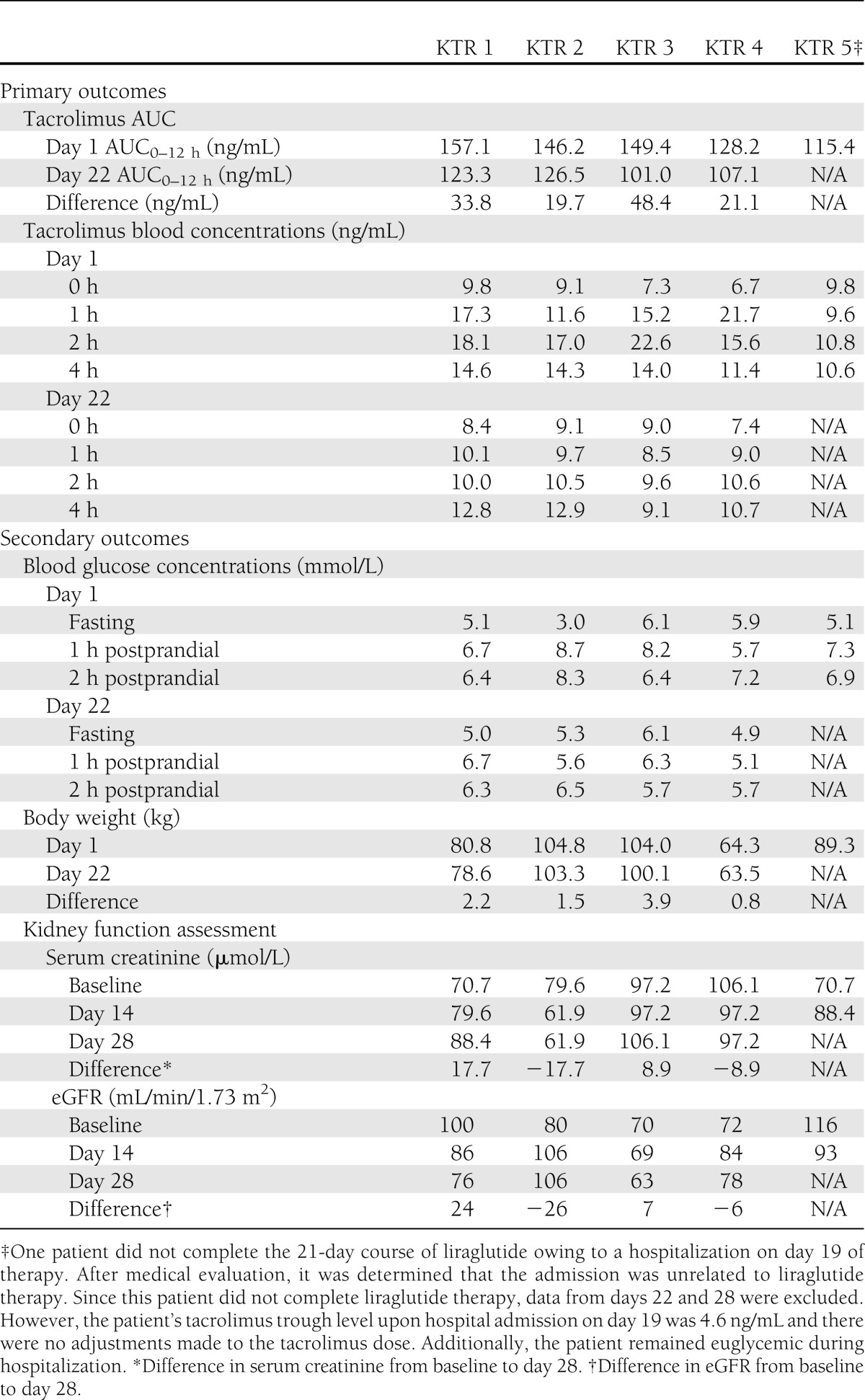
Evidence for the use of liraglutide in patients with chronic kidney disease is limited (4,5). These preliminary data suggest coadministration of liraglutide with tacrolimus does not clinically alter trough tacrolimus concentrations in stable KTRs with mild renal impairment. Clinical trials examining the efficacy and safety of liraglutide use in KTRs should be the focus of future research.
Acknowledgments
This work was funded by the 2011–2012 Eugene Applebaum College of Pharmacy & Health Sciences Faculty Research Award Program (to N.R.P.).
No potential conflicts of interest relevant to this article were reported.
N.R.P. researched data, contributed to discussion, and wrote the manuscript. A.P. and F.D.S. researched data, contributed to discussion, and reviewed and edited the manuscript. N.R.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
The authors acknowledge the dedication of Sabrina Grandi, Katelyn Payter, Genni Lipari, Matthew Morelli, and Ellen Chackunkal from Wayne State University, and Steven Gray, Andrea Dwyer, and Glenda Porter from the Henry Ford Hospital Transplant Institute for their valuable contributions to the success of the project.
References
- 1.van Raalte DH, van Genugten RE, Linssen MM, Ouwens DM, Diamant M. Glucagon-like peptide-1 receptor agonist treatment prevents glucocorticoid-induced glucose intolerance and islet-cell dysfunction in humans. Diabetes Care 2011;34:412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinelli NR, Moore CL, Tomasello S. Incretin-based therapy in chronic kidney disease. Adv Chronic Kidney Dis 2010;17:439–449 [DOI] [PubMed] [Google Scholar]
- 3.Barraclough KA, Isbel NM, Kirkpatrick CM, et al. Evaluation of limited sampling methods for estimation of tacrolimus exposure in adult kidney transplant recipients. Br J Clin Pharmacol 2011;71:207–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobsen LV, Hindsberger C, Robson R, Zdravkovic M. Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br J Clin Pharmacol 2009;68:898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson JA, Brett J, Falahati A, Scott D. Mild renal impairment and the efficacy and safety of liraglutide. Endocr Pract 2011;17:345–355 [DOI] [PubMed] [Google Scholar]


