Abstract
OBJECTIVE
Vascular dysfunction is a major contributor to diabetes complications. It is also the primary physiologic cause of erectile dysfunction and considered an independent predictor of cardiovascular disease (CVD) in males over age 40 years. A cohort of individuals with 50 or more years of type 1 diabetes, Joslin Medalists, have low rates of small but not large vessel complications. This study aims to identify the prevalence and longitudinal association of sexual dysfunction (SD) with CVD in Joslin Medalists.
RESEARCH DESIGN AND METHODS
Description and association of self-assessment of SD in males of the Medalist cohort by self-reported sexual problems with CVD. SD is validated through the use of the abbreviated International Index of Erectile Dysfunction (IIEF).
RESULTS
Of 301 males in the Medalist Study, 69.8% reported a history of SD. Unadjusted risk factors included elevated glycated hemoglobin (HbA1c) (P = 0.02), elevated BMI (P = 0.03), higher total cholesterol (P = 0.02), lower HDL (P < 0.01), and increased levels of interleukin-6 (P = 0.03). SD was independently associated with CVD (age-, HbA1c-, and BMI-adjusted OR 1.9 [95% CI 1.0–3.5]). In adjusted analyses, retinal, neural, and renal complications were not associated (P > 0.05) with SD. Current report of SD (IIEF score ≤17) in a subset of Medalists was significantly correlated with self-reported longitudinal SD.
CONCLUSIONS
SD in those with extreme-duration type 1 diabetes is independently associated with CVD, representing a large-vessel pattern. The findings suggest that SD may predict CVD in those with type 1 diabetes of long duration. These individuals have also been found to be relatively free of microvascular complications.
An increasing number of individuals are surviving to extreme durations of type 1 diabetes; therefore, more individuals will be at risk for developing related complications (1–4). The Joslin 50-Year Medalists, individuals with an average duration of 55 years of type 1 diabetes, have been characterized with low levels of microvascular complications, although levels of macrovascular disease may not follow the same pattern (3,4). Cardiovascular disease (CVD) is a leading cause of morbidity and mortality in those with diabetes. Multiple studies have shown that diabetes independently increases the risk for CVD up to 1.4-fold (5–7). The primary risk factors for heart disease associated with diabetes include dyslipidemia, elevated BMI, poor glycemic control, hypertension, insulin resistance, and history of smoking (8). However, individuals with type 1 diabetes are often leaner and have elevated HDL levels compared to those with type 2 diabetes, which, by standard risk scores, would place them at lower risk for CVD (9,10). For these individuals, a primary screening mechanism could be helpful for early intervention in the development of CVD. One proposed mechanism is screening for sexual dysfunction (SD) and its associated symptoms (5,11).
In this study, the relationship of reporting “lifetime sexual problems” with CVD in a large group of men with extreme duration of type 1 diabetes is examined. Identifying an early marker of CVD that a patient may be more likely to report, such as SD, could be helpful in interventions to alter the natural history of this disease.
RESEARCH DESIGN AND METHODS
Details of the Medalist Study have been described extensively elsewhere (3,4,12). Individuals who had documented 50 or more years of insulin use for type 1 diabetes were invited to participate in the study. Informed consent was obtained from all subjects prior to participation in the study. Individuals traveled to Joslin Diabetes Center (JDC) (Boston, MA) for physical and ophthalmic examination and biospecimen collection of urine and blood. Participants completed questionnaires regarding medical history, lifestyle, diet, and physical activity.
From 2005 to the time of analysis, 1,121 medals were awarded to residents of the United States who demonstrated 50 or more years of insulin-dependent type 1 diabetes. Of these Medalists, 800 participated in the study. Most Medalists (88%) received routine endocrine care outside JDC. The 12% who declined participation cited illness, time commitment, or financial issues. Glycated hemoglobin (HbA1c) was determined by high-performance liquid chromatography (Tosoh G7 and 2.2, Tokyo, Japan). Lipid profiles were determined by standard enzymatic methods (kits from Roche Diagnostics, Indianapolis, IN; Denka Seiken, Tokyo, Japan; and AsahiKasei, Tokyo, Japan). Inflammatory markers interleukin-6 (IL-6), plasminogen activator inhibitor 1 (PAI-1), and vascular cell adhesion molecule (VCAM) and testosterone and sex hormone–binding globulin (SHBG) levels were assayed by human serum ELISA assays at the JDC Specialized Assay Core (R&D Systems, Minneapolis, MN; ALPCO Diagnostics, Salem, NH). C-reactive protein (CRP) was determined by nephelometric methods, and urine albumin-creatinine ratios (ACRs) were determined by turbidimetric methods (Quest Diagnostics, Wallingford, CT).
CVD status was based on self-reported history of coronary artery disease, angina, heart attack, prior cardiac or leg angioplasty, or bypass graft surgery. Renal status was defined as those with nephropathy (Chronic Kidney Disease Epidemiology Collaboration formula [CKD-EPI] estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) versus those without (eGFR ≥60 mL/min/1.73 m2). A dilated eye examination was performed and retinopathy status was graded using guidelines from the Early Treatment Diabetic Retinopathy Study (ETDRS). Proliferative diabetic retinopathy (PDR) was defined as an ETDRS ≥60 (13). The Michigan Neuropathy Screening Instrument was used to assess neuropathy; scores >2 were considered positive (14).
Lifetime SD was defined as an affirmative response to the question, “Please indicate if you have ever had any sexual problems” on a self-administered medical history questionnaire. The five-item International Index of Erectile Dysfunction (IIEF) questionnaire was used to assess current SD in a subset of the males. The IIEF was mailed, marked only with personal study identification number to ensure anonymity (15). An IIEF score ≤17 was considered significant erectile dysfunction (ED).
All variables were visually inspected and analyzed for distribution to determine the appropriate statistical methods. Comparisons were used depending on variable distribution (Kruskal-Wallis test, Student t test, and χ2 [Fisher exact] test). SD was the outcome variable used in logistic regression models examining the relationship of both CVD and inflammatory markers. Logistic regression was used to estimate odds ratios (ORs) and 95% CIs and adjust for potential confounders, including comorbid conditions and sociodemographic and lifestyle factors.
All multivariate models were adjusted for age, HbA1c, and BMI. A type 1 error ≤0.05 was considered significant. As SD was the primary outcome, a multiple comparison correction was not applied. STATA (v12 SE, College Station, TX) and SAS (v9.2, Cary, NC) were used.
RESULTS
Of all Medalists, 48.7% (n = 320) were male and 301 answered the question regarding a history of sexual problems; 69.8% of males reported experiencing SD over their lifetime. These individuals had a mean age, age at diagnosis, and duration of diabetes of 71.7 ± 8.5 years, 12.4 ± 7.0 years, and 59.3 ± 7.3 years, respectively. Mean BMI was 25.8 ± 3.6 kg/m2, with mean insulin dose 0.49 ± 0.2 units/kg2. Mean HbA1c was 7.03% ± 0.89 (53 ± 6 mmol/mol). The lipid profile included a mean cholesterol of 152.0 ± 31.7 mg/dL, with HDL 57.3 ± 17.2 mg/dL and LDL 79.5 ± 22.8 mg/dL. Mean ACR was 28.3 ± 68.3 μg/mg, with 49.3% having PDR and 51.8% of males having CVD (Table 1).
Table 1.
Clinical characteristics of male Medalists by SD status
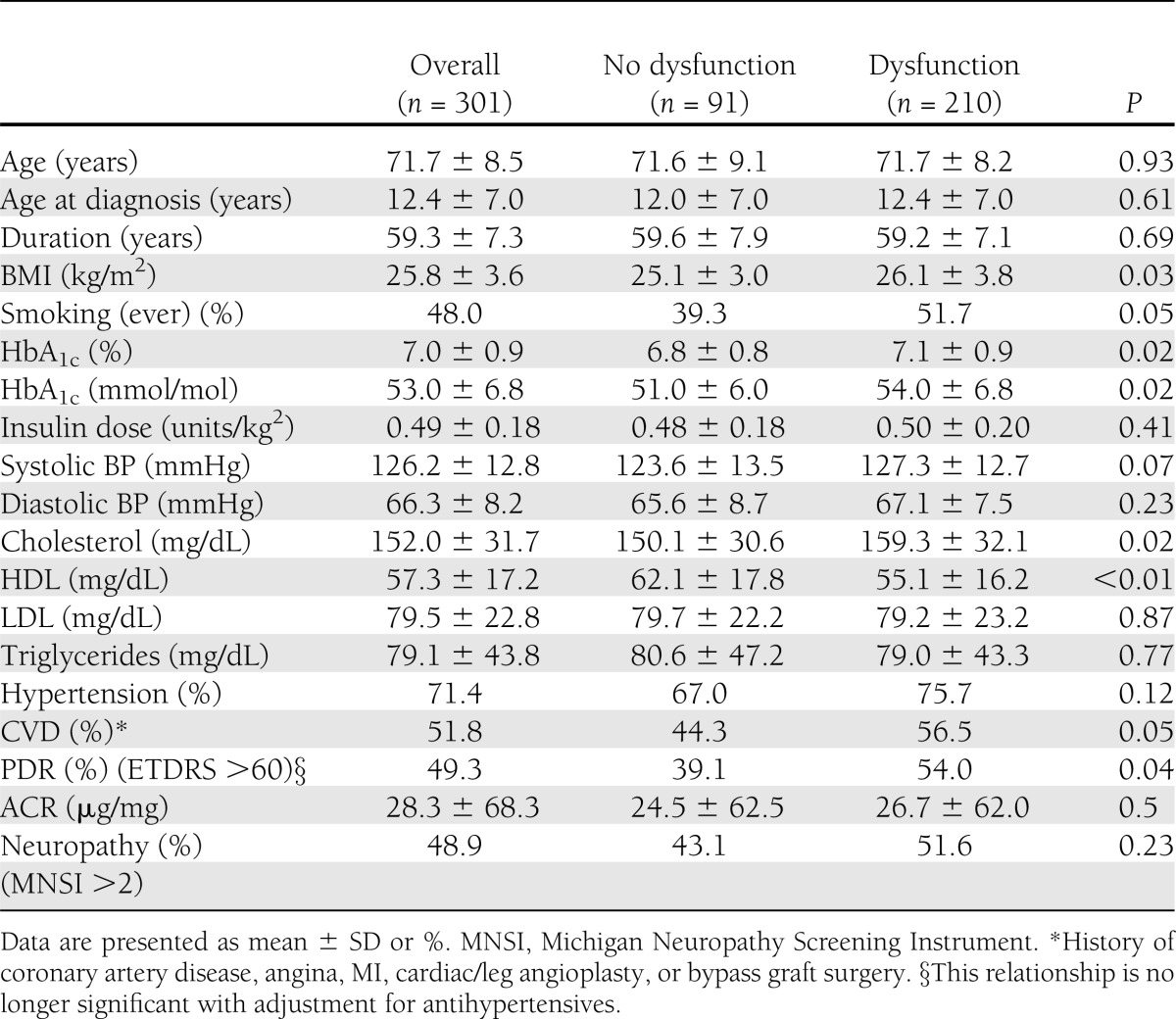
Among those with and without SD, age, age at diagnosis, and duration of type 1 diabetes were not significantly different (P = 0.93, P = 0.61, and P = 0.69, respectively) (Table 1). Mean BMI (26.1 ± 3.8 vs. 25.1 ± 3.0 kg/m2) was higher in those reporting SD (P = 0.03), as were ever smoking (51.7 vs. 39.3%, P = 0.05) and HbA1c (P = 0.02). Total cholesterol levels were significantly higher (159.3 ± 32.1 vs. 150.1 ± 30.6 mg/dL, P = 0.02), and HDL levels (55.1 ± 16.2 vs. 62.1 ± 17.8 mg/dL, P < 0.01) were significantly lower in those without SD. The percentage of those with PDR was slightly higher in Medalists reporting SD (54.0 vs. 39.1%, P = 0.04); however, no relationship was found with neuropathy (P = 0.23). Of those males without SD, 44.3% had CVD, compared with 56.5% with SD (P = 0.05) (Table 1).
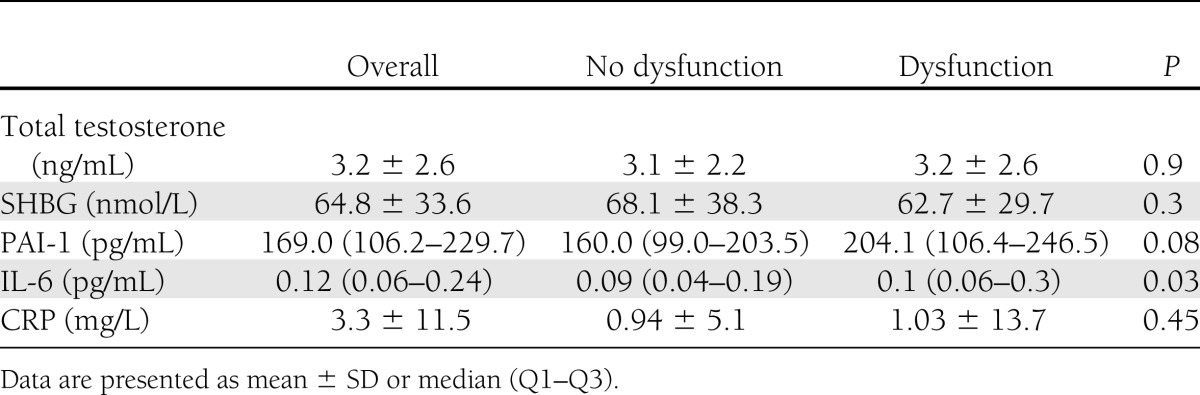
Levels of IL-6 (median [Q1–Q3]: 0.09 pg/mL [0.04–0.19] vs. 0.1 pg/mL [0.06–0.3], P = 0.03) were significantly higher in those reporting SD. Other inflammatory markers, including PAI-1 and CRP, were not significantly different between groups (P = 0.08 and P = 0.45). Total testosterone and SHBG did not vary between those with and without SD (P = 0.9 and P = 0.3) (Table 2).
Table 2.
Laboratory characteristics of male Medalists by SD status
There was a significant difference in the use of lipid-lowering agents (57.1% no SD vs. 74.3% SD, P = 0.003) and platelet medications (23.1% no SD vs. 35.2% SD, P = 0.04) but not in the use of blood pressure medication or adrenergic, β, or calcium channel blockers (Supplementary Table 1). Use of phosphodiesterase type 5 (PDE5) inhibitors was reported as 1.8% tadalafil, 2.5% sildenafil, and 0.7% vardenafil; penile implants were reported by 3.6% (Supplementary Table 2). There was no relationship between insulin pump use or current self-rated blood glucose control (P > 0.05) and SD. There was also no relationship with the number of times a day blood sugars were checked, level of education, or self-rated quality of life (P > 0.05) (Supplementary Table 3).
The five-item IIEF questionnaire was completed and returned by a subset of 63% (n = 197) of males, with 85.3% reporting a significant degree of ED (IIEF score ≥17), in agreement with the original self-report question (χ2 = 256.0, P < 0.001) (15). Demographic and basic clinical characteristics did not vary significantly between the subsets of men who did and did not complete the IIEF.
Male Medalists with CVD (51.8%) were older and had a longer type 1 diabetes duration than males without CVD (73.3 ± 8.5 vs. 70.0 ± 8.0 years [P = 0.005] and 60.5 ± 7.8 vs. 57.9 ± 6.4 years [P = 0.003], respectively), as well as higher HbA1c (7.2 ± 0.9 vs. 6.9 ± 0.8%; 55 ± 6.8 vs. 52 ± 6 mmol/mol [P = 0.002]). Individuals with CVD had higher total cholesterol, higher LDL, and higher HDL than those without CVD (P < 0.01). Males with CVD had a lower mean eGFR (61.7 ± 19.7 vs. 72.7 ± 18.7 mL/min/1.73 m2 [P < 0.001]) and higher rate of PDR (61.3 vs. 37.2%, P < 0.001). There was no difference in inflammatory markers, including CRP, IL-6, PAI-1, or VCAM, between those with and without CVD (P > 0.05) (Table 3). There was no significant difference in the use of PDE5 inhibitors by CVD disease; however, the frequency of men with a penile implant who have CVD (80 vs. 20%, P = 0.04) is higher than those without CVD.
Table 3.
Clinical characteristics of male Medalists with and without CVD

The association of lifetime SD and CVD remained with adjustment for age, BMI, cholesterol, HDL, smoking, IL-6, antihypertensive medication, and HbA1c (OR 3.7 [95% CI 1.5–9.0]). Additionally, lower inflammatory levels of IL-6 are associated with protection from reporting SD (0.4 [0.2–0.95]) when adjusted for age, BMI, HDL, smoking, and HbA1c.
CONCLUSIONS
Several studies have established the connection between ED and CVD in men starting the fourth decade of life (11,16–18). The hypothesized etiology is that vessels feeding the penis are smaller than those feeding the heart and therefore show clinical symptoms earlier. The physiologic mechanism is endothelial dysfunction resulting from the inhibition of the nitric oxide cascade, thus preventing dilation of the arteries impairing the blood flow imperative for rigidity (19). The etiology of endothelial dysfunction may be different, or synergistic, depending on endogenous risk between those with and without diabetes due to the inherent damaging effects of the hyperglycemic exposure. Importantly for type 1 diabetic patients, this relationship may be independent of previously identified risk factors for CVD, as used in the Framingham Index (20). This is supported by the independent relationship of CVD and SD from other risk factors, including age and BMI, among this group of extreme-duration type 1 diabetic patients.
The 50-Year Medalists are a group of individuals who have had type 1 diabetes for 50 or more years and resultant prolonged hyperglycemic exposure. An onset of diabetes in the early to middle part of the last century meant that blood glucose management consisted of weekly testing with once-daily injections, resulting in frequent bouts of diabetic ketoacidosis or hypoglycemia and the potential for significant endothelial damage. Previous literature on Medalists documented a lower than expected prevalence of microvascular complications, including PDR (50.6%), neuropathy (60.6%), and nephropathy (13.1%) (3,4,12,21–24). As reported, the prevalence of CVD among male Medalists is 51.8%. At an index age of 75 years, in the Framingham Health Study, the adjusted lifetime (up to 95 years of age) risk estimate for men is 54.5% (95% CI 52.2–56.9), demonstrating that there is no increased prevalence of CVD among Medalists (10). This is in contrast to the ancillary study of the Epidemiology of Diabetes Interventions and Control (uroEDIC) Study on urologic symptoms, which assessed the prevalence of SD among their participants who had a mean type 1 diabetes duration of 22.1 years, average age of 44.6 ± 6.6 years, and time-weighted average HbA1c of 8.07%, finding an overall prevalence of 58% ED (IIEF 0–20); they did not assess correlation with CVD (25). Our finding of no difference in testosterone levels in those with SD is consistent with the findings of Van Den Edeen et al. (25).
Klein et al. (26) in the Wisconsin Epidemiology Study of Diabetic Retinopathy examined markers of SD and found a cumulative incidence of 25% in men 21 years of age or older with 10 or more years of type 1 diabetes (mean age 34.4 ± 8.4 years and duration 20.5 ± 7.0 years) and mean HbA1c of 9.7%. Those 40 years of age and older had the highest overall incidence at 48.6%. Primary risk factors other than age in this population included untreated hypertension (OR 5.0 [95% CI 2.05–12.3]) and smoking status (current OR 2.4 [1.09–5.30])—established risk factors for CVD. No significant relationship was found with microvascular complications with adjustment for age, smoking, and untreated hypertension. No contemporary association was found with CVD; however, total cholesterol was associated with SD but not HDL (26). In similarly aged nondiabetic men, the Massachusetts Male Aging Study documented a complete impotence rate of 67% by 70 years, and the National Health and Nutrition Examination Survey (NHANES) reported a prevalence of 77.5% for those 75 years of age and older (27,28).
In this study, we examined prevalence of SD and its relationship to CVD. A limitation of the self-reporting of “lifetime sexual problems” is that it may capture those with a history of SD due to social, economic, or lifestyle factors instead of progressive endothelial pathology, which may precede larger-vessel disease. Close agreement of SD with IIEF scores suggests that the SD question may be capturing ED in our sample. Additionally, the IIEF cutoff was associated with cardiovascular risk factors, as well as CVD, the outcome of interest. Neurogenic, pharmacologic, and quality of life factors did not confound or influence the observed association of IIEF scores and CVD.
Duration, glycemic control, age, and lipid profile did not correlate with microvascular complications; however, CVD showed a significant relationship with HbA1c, age, duration, lipid profile, and inflammatory markers. In studies of those with type 2 diabetes, the relationship of ED and CVD is thought to begin with the damage caused by the milieu of metabolic changes associated with the metabolic syndrome, established as a direct predecessor of CVD (29–33). However, as the Medalists do not have the same lipid profile (total cholesterol levels <200 mg/dL, HDL levels >35 mg/dL, and a triglyceride level <70 mg/dL), increased weight, and insulin resistance characteristic of type 2 diabetes (insulin dose, 0.5 units/kg [0.37–0.57]), the same early warning signs for CVD are not present. Of the Medalists who have died, 58.6% died of CVD, demonstrating a significant mortality from this disease risk that necessitates early detection (29–33). Findings of this analysis indicate that history of SD provides an important screening tool in the absence of the typical risk profile of glycemic control, BMI, and dyslipidemia in patients with type 1 or type 2 diabetes.
Acknowledgments
This research was funded by the Juvenile Diabetes Research Foundation (8-2005-358, nPOD 25-2008-383, and 18-2008-363), the Beatson Foundation, the Brehm Foundation, the National Institutes of Health (1R24-DK-090961-01 and DP3-DK-094333-01), and the Peabody Foundation.
J.K.S. provided research support to Boston Micromachines and Genentech, was on the scientific advisory board for Abbott Laboratories, and was a consultant for Novartis. G.L.K. provided research support for Sanofi. No other potential conflicts of interest relevant to this article were reported.
S.J.T., S.M.H., and H.A.K. researched, collected, and analyzed data and wrote and reviewed the manuscript. J.K.S. collected and analyzed data and reviewed the manuscript. G.L.K. reviewed and edited the manuscript. S.J.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Varant Kupelian, PhD, for his insight and advice in the preparation of the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0294/-/DC1.
References
- 1.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010;53:2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain SC, Gill GV, Dyer PH, et al. Characteristics of type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 2003;20:808–811 [DOI] [PubMed] [Google Scholar]
- 3.Keenan HA, Costacou T, Sun JK, et al. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-Year Medalist Study. Diabetes Care 2007;30:1995–1997 [DOI] [PubMed] [Google Scholar]
- 4.Sun JK, Keenan HA, Cavallerano JD, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the Joslin 50-Year Medalist Study. Diabetes Care 2011;34:968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med 2003;139:161–168 [DOI] [PubMed] [Google Scholar]
- 6.Enzlin P, Mathieu C, Van Den Bruel A, Vanderschueren D, Demyttenaere K. Prevalence and predictors of sexual dysfunction in patients with type 1 diabetes. Diabetes Care 2003;26:409–414 [DOI] [PubMed] [Google Scholar]
- 7.Gandaglia G, Salonia A, Passoni N, Montorsi P, Briganti A, Montorsi F. Erectile dysfunction as a cardiovascular risk factor in patients with diabetes. Endocrine 2013;43:285–292 [DOI] [PubMed] [Google Scholar]
- 8.Böhm M, Baumhäkel M, Probstfield JL, et al. ONTARGET/TRANSCEND ED-Investigators Sexual function, satisfaction, and association of erectile dysfunction with cardiovascular disease and risk factors in cardiovascular high-risk patients: substudy of the ONgoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNT Study in ACE-INtolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND). Am Heart J 2007;154:94–101 [DOI] [PubMed] [Google Scholar]
- 9.Brown JS, Wessells H, Chancellor MB, et al. Urologic complications of diabetes. Diabetes Care 2005;28:177–185 [DOI] [PubMed] [Google Scholar]
- 10.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012;308:1795–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miner M, Seftel AD, Nehra A, et al. Prognostic utility of erectile dysfunction for cardiovascular disease in younger men and those with diabetes. Am Heart J 2012;164:21–28 [DOI] [PubMed] [Google Scholar]
- 12.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl.):823–833 [PubMed] [Google Scholar]
- 14.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 15.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–326 [DOI] [PubMed] [Google Scholar]
- 16.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA 2005;294:2996–3002 [DOI] [PubMed] [Google Scholar]
- 17.Araujo AB, Mohr BA, McKinlay JB. Changes in sexual function in middle-aged and older men: longitudinal data from the Massachusetts Male Aging Study. J Am Geriatr Soc 2004;52:1502–1509 [DOI] [PubMed] [Google Scholar]
- 18.Batty GD, Li Q, Czernichow S, et al. ADVANCE Collaborative Group Erectile dysfunction and later cardiovascular disease in men with type 2 diabetes: prospective cohort study based on the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation) trial. J Am Coll Cardiol 2010;56:1908–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponholzer A, Stopfer J, Bayer G, et al. Is penile atherosclerosis the link between erectile dysfunction and cardiovascular risk? An autopsy study. Int J Impot Res 2012;24:137–140 [DOI] [PubMed] [Google Scholar]
- 20.Araujo AB, Hall SA, Ganz P, et al. Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the Framingham risk score? J Am Coll Cardiol 2010;55:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785–794 [DOI] [PubMed] [Google Scholar]
- 22.Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol 1987;59:750–755 [DOI] [PubMed] [Google Scholar]
- 23.Krolewski AS, Warram JH, Rand LI, Christlieb AR, Busick EJ, Kahn CR. Risk of proliferative diabetic retinopathy in juvenile-onset type I diabetes: a 40-yr follow-up study. Diabetes Care 1986;9:443–452 [DOI] [PubMed] [Google Scholar]
- 24.Krolewski AS, Warram JH, Valsania P, Martin BC, Laffel LM, Christlieb AR. Evolving natural history of coronary artery disease in diabetes mellitus. Am J Med 1991;90(2A):56S–61S [DOI] [PubMed] [Google Scholar]
- 25.Van Den Eeden SK, Sarma AV, Rutledge BN, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Research Group Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 2009;32:664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE, Moss SE. Ten-year incidence of self-reported erectile dysfunction in people with long-term type 1 diabetes. J Diabetes Complications 2005;19:35–41 [DOI] [PubMed] [Google Scholar]
- 27.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54–61 [DOI] [PubMed] [Google Scholar]
- 28.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ, Urologic Diseases in America Project Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med 2006;166:207–212 [DOI] [PubMed] [Google Scholar]
- 29.Araña Rosaínz MdeJ, Ojeda MO, Acosta JR, et al. Imbalanced low-grade inflammation and endothelial activation in patients with type 2 diabetes mellitus and erectile dysfunction. J Sex Med 2011;8:2017–2030 [DOI] [PubMed] [Google Scholar]
- 30.Fedele D, Bortolotti A, Coscelli C, et al. Erectile dysfunction in type 1 and type 2 diabetics in Italy. On behalf of Gruppo Italiano Studio Deficit Erettile nei Diabetici. Int J Epidemiol 2000;29:524–531 [PubMed] [Google Scholar]
- 31.Ryan JG, Gajraj J. Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus. J Diabetes Complications 2012;26:141–147 [DOI] [PubMed] [Google Scholar]
- 32.De Berardis G, Franciosi M, Belfiglio M, et al. Quality of Care and Outcomes in Type 2 Diabetes (QuED) Study Group Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care 2002;25:284–291 [DOI] [PubMed] [Google Scholar]
- 33.Meldrum DR, Gambone JC, Morris MA, Meldrum DA, Esposito K, Ignarro LJ. The link between erectile and cardiovascular health: the canary in the coal mine. Am J Cardiol 2011;108:599–606 [DOI] [PubMed] [Google Scholar]


