Abstract
OBJECTIVE
Metformin has been associated with a reduction in breast cancer risk and may improve survival after cancer through direct and indirect tumor-suppressing mechanisms. The purpose of this study was to evaluate the effect of metformin therapy on survival in women with breast cancer using methods that accounted for the duration of treatment with glucose-lowering therapies.
RESEARCH DESIGN AND METHODS
This population-based study, using Ontario health care databases, recruited women aged 66 years or older diagnosed with diabetes and breast cancer between 1 April 1997 and 31 March 2008. Using Cox regression analyses, we explored the association between cumulative duration of past metformin use and all-cause and breast cancer–specific mortality. We modeled cumulative duration of past metformin use as a time-varying exposure.
RESULTS
Of 2,361 breast cancer patients identified, mean (± SD) age at cancer diagnosis was 77.4 ± 6.3 years, and mean follow-up was 4.5 ± 3.0 years. There were 1,101 deaths(46.6%), among which 386 (16.3%) were breast cancer–specific deaths. No significant association was found between cumulative duration of past metformin use and all-cause mortality (adjusted hazard ratio 0.97 [95% CI 0.92–1.02]) or breast cancer–specific mortality (0.91 [0.81–1.03]) per additional year of cumulative use.
CONCLUSIONS
Our findings failed to show an association between improved survival and increased cumulative metformin duration in older breast cancer patients who had recent-onset diabetes. Further research is needed to clarify this association, accounting for effects of cancer stage and BMI in younger populations or those with differing stages of diabetes as well as in nondiabetic populations.
Pre-existing diabetes may increase the risk of death by as much as 40% in cancer patients (1). Up to 16% of patients with breast cancer have pre-existing diabetes and are thus at risk for worse outcomes (2,3). Metformin, an insulin sensitizer, is the most commonly prescribed diabetes treatment and is currently recommended as first-line therapy for patients with type 2 diabetes (4,5). If glycemic targets are not met with metformin alone, other glucose-lowering medications are added to or substituted for metformin. Recent evidence suggests that metformin may have antitumor effects (6). Several studies have evaluated the effect of metformin on cancer incidence, and meta-analyses suggest that metformin is associated with a 20–30% reduction in new cancers (6–8). However, of greater interest is the potential therapeutic role of metformin in patients with pre-existing cancer.
There is mounting evidence that metformin may affect the prognosis of breast cancer. Metformin use has been associated with higher rates of pathologic complete response after chemotherapy in breast cancer patients with diabetes (9), and clinical trials have shown a reduction in tumor proliferation markers in nondiabetic breast cancer patients treated with metformin (10–12). However, observational studies evaluating the effect of metformin on survival after breast cancer have been inconsistent. One study of women with HER2+ breast cancer found metformin exposure was associated with a 48% reduction in overall mortality compared with other glucose-lowering medications (13). However, another study of women with triple-negative receptor breast cancer did not show a significant association between metformin and cancer mortality (hazard ratio [HR] 1.63 [95% CI 0.87–3.06]) (13,14). Interpretation of these previous studies is hampered by small sample sizes, heterogeneity of disease subtypes, inclusion of diabetic populations with varying disease severity and duration, and inconsistent definitions of metformin exposure. The objective of this study was to evaluate the relationship between cumulative metformin use and mortality in patients with breast cancer and recently diagnosed diabetes.
RESEARCH DESIGN AND METHODS
Data sources and population
Data for this study were obtained from administrative health care databases in Ontario, Canada, which include records for all individuals eligible for coverage under the universal health plan. Patient records are individually linked across databases using a unique patient anonymized identifier.
The study population was identified from a cohort of women with incident diabetes, aged 66 years or older, diagnosed with breast cancer between 1 April 1997 and March 31 2008. Our cohort was restricted to women age 66 years or older to capture prescription drug records in the year prior and throughout the study period, which are available through the Ontario Drug Benefit (ODB) program for all individuals aged 65 years or older.
We only included women diagnosed with diabetes after age 66 years for two reasons. First, this allowed for a homogenous cohort who had similar and measurable diabetes duration, thereby reducing biases due to diabetes severity. Second, we could account for all glucose-lowering drug exposure throughout the diabetes history for each individual, because all were diagnosed after becoming eligible for the provincial drug plan. Diabetes status was determined by linking subjects to the validated Ontario Diabetes Database, an administrative database registry of diabetes patients. This registry has been validated against primary care records and has a high sensitivity (86%) and specificity (97%) for identifying individuals clinically diagnosed with diabetes (15).
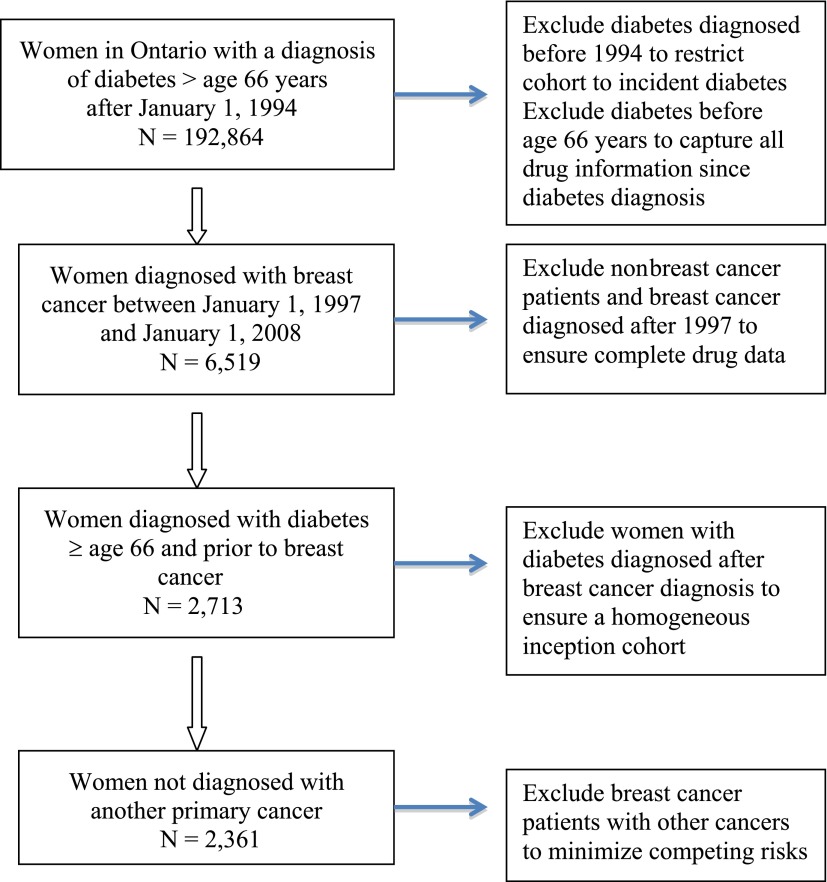
We then limited our cohort to women with a new diagnosis of invasive breast cancer after their diabetes diagnosis, determined through ICD-9 codes (174) in the Ontario Cancer Registry (OCR; Fig. 1). The OCR maintains records on all cancers (excluding nonmelanoma skin cancers) diagnosed in Ontario since 1964 and has a completeness rate of 95% for capturing all cancers (excluding nonmelanoma skin cancers) in Ontario (16). The date of breast cancer diagnosis served as the index date for women in our cohort.
Figure 1.
Flowchart describing study cohort with side boxes explaining the reasons for exclusion.
Follow-up and outcome
Women were monitored from their date of breast cancer diagnosis until death (the outcome of interest) or 30 March 2010, whichever came first. The primary outcome was all-cause mortality, based on death certificate records from the Registered Persons Database, which contains demographic and residential data on all Ontario residents but does not include information on cause of death. The secondary outcome was breast cancer–specific mortality, which was based on death records from the OCR. A validation study found that the OCR had high sensitivity (95%) and high specificity (88%) for defining breast cancer–specific mortality (17). The OCR cause-of-death databases are updated yearly but have a 3-year lag from the present. Given this, our cohort was monitored for breast cancer–specific mortality until 31 December 2008.
Drug exposure
We used the ODB database to identify drug prescriptions. Glucose-lowering drug exposure was captured from the time of diabetes diagnosis until the end of follow-up. These drugs were classified into the following categories: metformin, sulfonylureas (e.g., glyburide, gliclazide, repaglinide), thiazolidinediones (TZDs, e.g., rosiglitazone, pioglitazone), and insulins (e.g., glargine, detemir, Humalog, lispro, NPH). Patients who were not exposed to any of these glucose-lowering agents were classified as not receiving any pharmacological treatment for their diabetes.
Glucose-lowering drug use before the cancer diagnosis was recorded at baseline as any prior exposure to each category described above. To explore effects of ongoing use after cancer diagnosis, we modeled cumulative duration of drug exposure from the cancer diagnosis date until the end of follow-up. The cumulative duration of exposure was derived from dates and number of days supplied for each prescription filled. The variable denoting cumulative duration of exposure was updated after each day of follow-up, thereby accurately reflecting the cumulative duration of past use of a glucose-lowering medication on any given day of follow-up. We used a time-varying approach whereby a subject’s exposure classification was allowed to vary over time if prescriptions were filled during follow-up. This approach allows initial unexposed periods in subjects who later become exposed to contribute to the ‘unexposed’ category, thereby avoiding immortal time bias (18). Immortal time bias has been a concern in prior studies of the effect of metformin on cancer survival or progression (13,19).
Other covariates
Surgery for breast cancer was identified based on procedure codes from the Canadian Institute for Health Information Discharge Abstraction Database related to breast surgery (20). We captured the following procedures: total and partial mastectomy, lumpectomy, segmental wedge resection, quadrantectomy, excisional biopsy, and lymph node dissection. Information regarding radiotherapy and chemotherapy was derived from physician billing claims submitted to the Ontario Health Insurance Program. Exposure to hormonal therapy (aromatase inhibitors and tamoxifen) within the first year after the breast cancer diagnosis was obtained from prescriptions in the ODB. We also accounted for any exposure to glucose-lowering therapy from the time of diabetes diagnosis to cancer diagnosis. This exposure was classified as ever/never based on at least one prescription for metformin, sulfonylurea, insulin, or a TZD.
Income status was based on neighborhood income quintiles derived from census data linked to postal codes in the Registered Persons Database. Specific comorbidities were determined at baseline and derived from Discharge Abstraction Database and Ontario Health Insurance Program databases. Comorbidity score was estimated using the John Hopkins Adjusted Clinical Group (ACG) case-mix. We used the ACG weighted case-mix score to assess comorbidity because it has been shown to predict mortality in an ambulatory setting of patients with diabetes in Ontario (21).
We did not include data on cancer stage and subtype because these are only available for breast cancers diagnosed after 2007. By limiting our cohort to the 432 women with cancers with stage data after that date, we would have had insufficient power and follow-up time (maximum 3 years) to evaluate the effects of metformin use on cancer survival. The Ontario health care databases do not contain clinical data; thus, we were not able to adjust for BMI in our analyses.
Statistical analysis
Baseline statistics are described using summary statistics. Cox proportional hazard regression models were used to explore the association between metformin and other glucose-lowering drug use and all-cause and breast cancer–specific mortality. To account for time-varying covariates, the data were set up in a counting process format where each record in the dataset represented 1 day of follow-up for each individual (22). Time since breast cancer diagnosis was used as the underlying time scale for the Cox proportional hazard model, and patients were censored at the time of death or the end of follow-up. Two separate datasets were set up to model each outcome, given the different follow-up times.
Univariate analyses were performed for the association between mortality and metformin, sulfonylurea, insulin, and TZD use as fixed and as time-dependent covariates. Covariates that changed the HR by 10% or more, or that were considered clinically relevant, were included in the final multivariable model. We adjusted for the following variables in the final model: sulfonylurea (cumulative use), insulin (cumulative use), TZD (cumulative use), age at breast cancer diagnosis, duration of diabetes (years) before breast cancer, comorbidity score based on adjusted ACG score at time of cohort entry, breast cancer treatments (yes/no) received within 1 year of diagnosis (surgery, radiotherapy, chemotherapy, aromatase inhibitor, tamoxifen), and exposure to glucose-lowering drugs before breast cancer diagnosis (yes/no). We tested for effect modification using interaction terms in the fixed never/ever model. Stratified analyses were performed when these interaction terms were significant.
Secondary analyses
In secondary analyses, we assessed the effect of any exposure to metformin and other diabetic drugs after cancer diagnosis by modeling ever/never exposure to glucose-lowering therapies as a time-varying exposure. For example, a subject became “ever-exposed” to metformin on the date on which a prescription was filled and remained “ever-exposed” until the end of the follow-up period. This analysis appropriately categorizes an exposure as “ever” at the time that the exposure occurs. We also calculated the number of events, time in person-years, and event rates (per 1,000 person-years) from the date of first prescription until the end of follow-up for each drug category.
Sensitivity analyses
Several sensitivity analyses were also performed to explore the robustness of our results. First, in an attempt to exclude women with advanced-stage disease or those too ill to be appropriately treated for their breast cancer, we limited our cohort to only include women who had surgery as a baseline treatment. Second, given the potentially harmful effects of insulin as well as its role as an effect modifier, we repeated our analyses excluding insulin users. Third, because there is controversy about whether sulfonylureas and insulin may worsen cancer prognosis and that the effect of metformin may only be protective in comparison to these agents that raise insulin levels, we limited our cohort to metformin monotherapy users and patients who received no drug therapy. Finally, because of the high mortality rate in our cohort, we restricted analyses to women who survived at least the first 365 days of follow-up in an attempt to exclude women with more advanced disease at presentation.
Ethics
This project was approved by the Sunnybrook Health Sciences Centre Institutional Review Board, Toronto, Ontario, Canada.
RESULTS
Study population
The study population consisted of 2,361 women with incident diabetes and breast cancer (Table 1). Mean (± SD) age at breast cancer diagnosis was 77.4 ± 6.3 years, and women had a mean duration of diabetes of 3.6 ± 3.0 years at the time of the breast cancer diagnosis. Mean follow-up was 4.5 ± 3.0 years for all-cause mortality and 3.7 ± 2.8 years for breast cancer–specific mortality. During the follow-up period, 1,094 patients (46.3%) were prescribed metformin, 284 (12.0%) were prescribed glucose-lowering medications other than metformin (i.e., insulin, sulfonylureas, or TZDs), and 983 (41.6%) were not prescribed any glucose-lowering medications at any time during the follow-up. Among metformin users, 818 (34.7%) were also exposed to sulfonylureas, 142 (6.0%) to TZDs, and 261 (11.1%) to insulin. Overall, there were 1,101 deaths (46.6%) and 386 breast cancer–specific deaths (15.1%) in the cohort.
Table 1.
Baseline characteristics of study population according to metformin use during the follow-up period
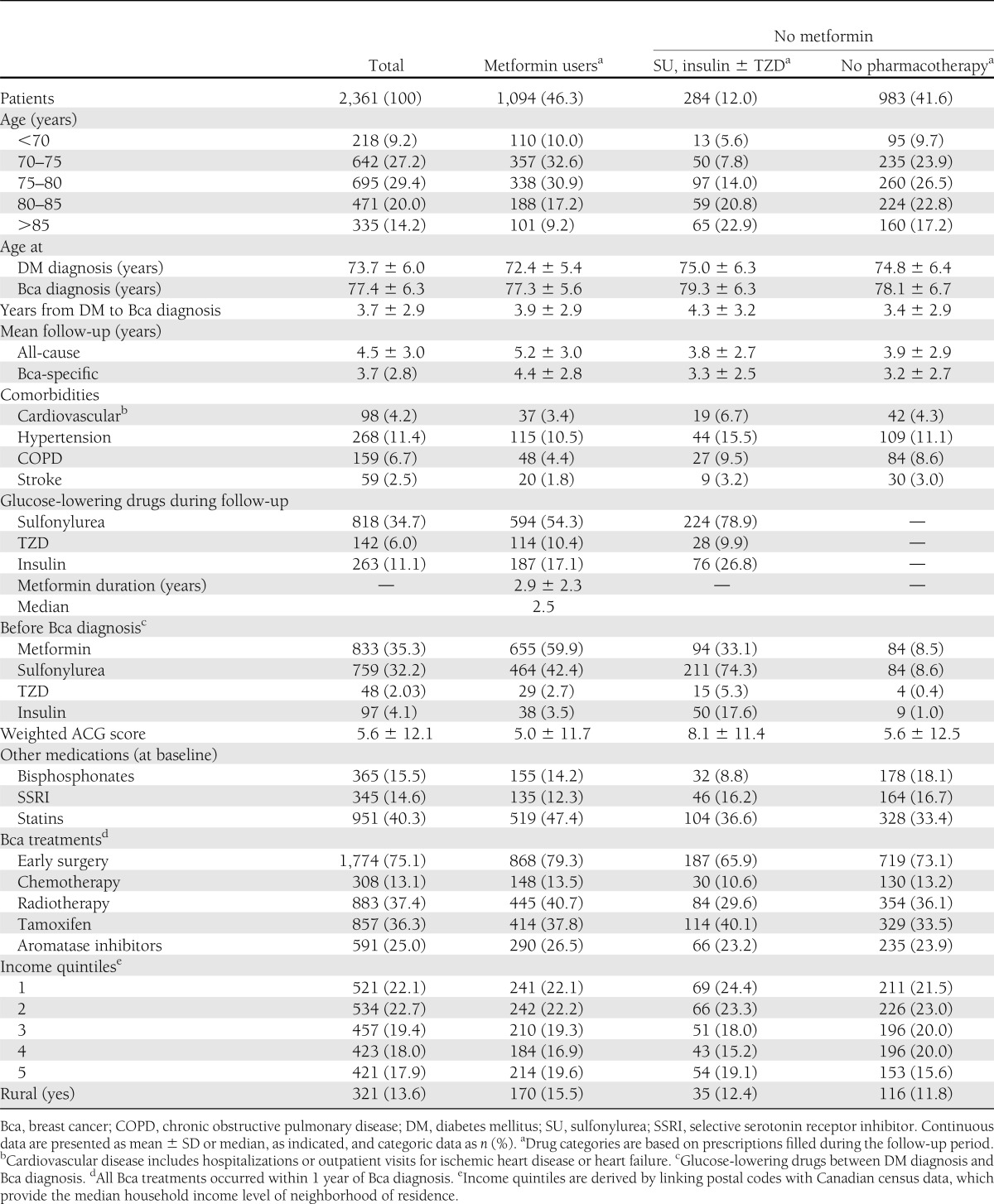
Table 1 describes baseline variables by drug exposure based on at least one prescription filled during follow-up. Metformin users were younger at their breast cancer diagnosis and had longer duration of follow-up compared with both nonmetformin groups. Mean duration of metformin therapy after cancer diagnosis was 2.9 ± 2.3 years. Metformin users were less likely to have comorbidities compared with the nonmetformin group receiving other glucose-lowering medications, but the baseline prevalence of comorbidities was similar between metformin users and nontreated diabetic patients. Metformin users were more likely to have surgery and radiation as primary treatments of their breast cancer, whereas subjects treated with other glucose-lowering drugs were the least likely to receive these treatments. Aromatase inhibitors were more likely to be prescribed to metformin users, whereas tamoxifen use was most common among the group prescribed glucose-lowering therapies other than metformin. Other baseline characteristics were similar among treatment groups (Table 1).
Associations with overall and breast cancer–specific survival
In our primary analyses, when modeling cumulative duration of past metformin exposure as a time-varying covariate, there was no association between duration of metformin exposure and all-cause or breast cancer–specific survival (HR 0.97 [95% CI 0.92–1.02] and 0.91 [0.81–1.03]) for each additional year of cumulative metformin exposure (Table 2). For other glucose-lowering medications, TZD use was associated with a significant increase in breast cancer–specific mortality (1.52 [1.02–2.26]) per additional year of exposure but not all-cause mortality (1.06 [0.75–1.27]). Conversely, exposure to sulfonylureas was associated with a significant reduction in all-cause mortality (0.95 [0.89–0.99]) per additional year of exposure, but there was no association with breast cancer–specific mortality (0.91 [0.80–1.02]). No association was found between insulin use and all-cause or breast cancer–specific mortality.
Table 2.
HRs from multivariable models modeling glucose-lowering drug use as a cumulative time-varying exposure
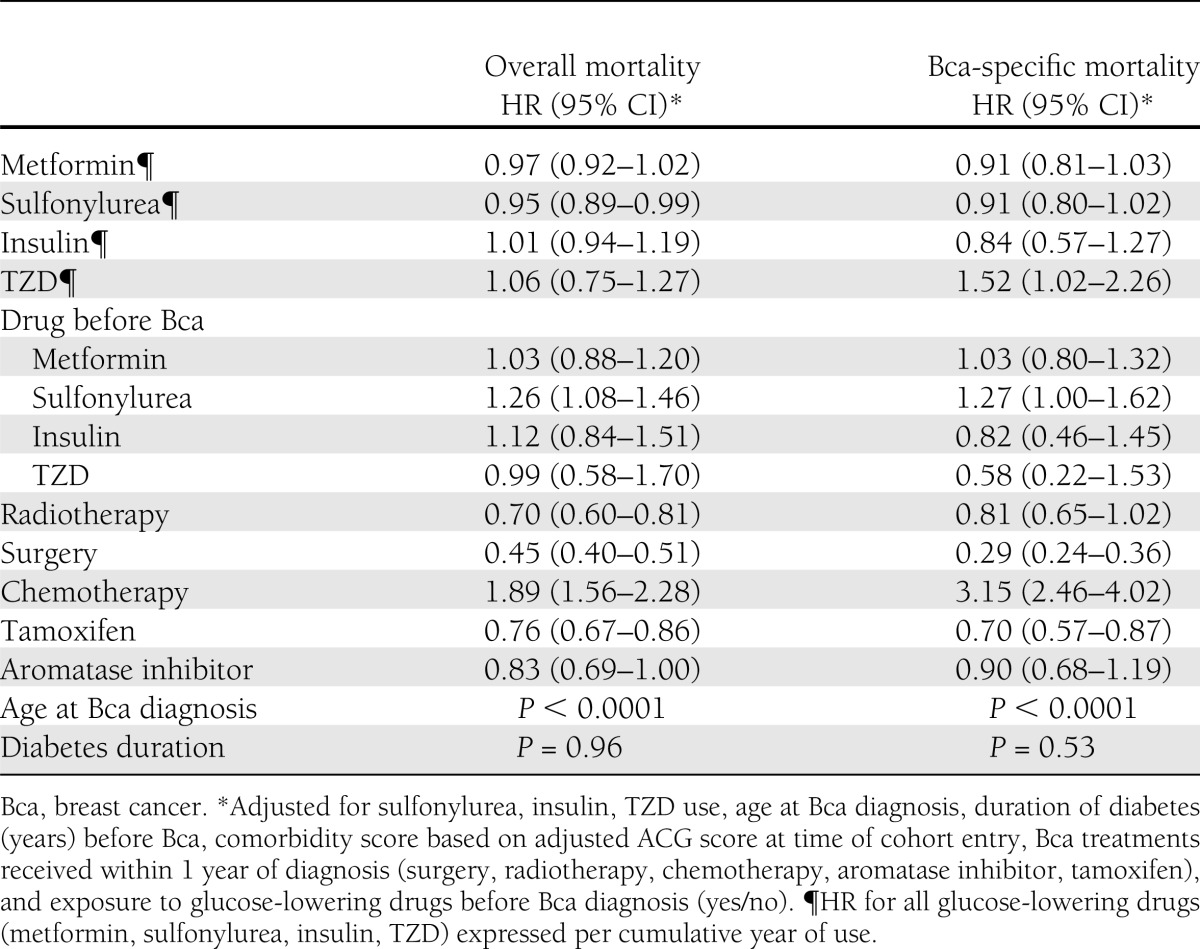
In regards to exposure to glucose-lowering therapy before the breast cancer diagnosis, only exposure to sulfonylureas was a significant independent predictor of all-cause (HR 1.35 [1.15–1.58]) and breast cancer–specific mortality (1.42 [1.10–1.82]) compared with never being exposed to sulfonylureas, but no association was found with prior use of other glucose-lowering agents (Table 2). As expected, surgery, radiotherapy, and aromatase inhibitor and tamoxifen use were all associated with an improvement in survival. However, receipt of chemotherapy was associated with an increase in all-cause (1.87 [1.53–2.28]) and breast cancer–specific mortality (3.91[2.89–5.27]) compared with no exposure to chemotherapy.
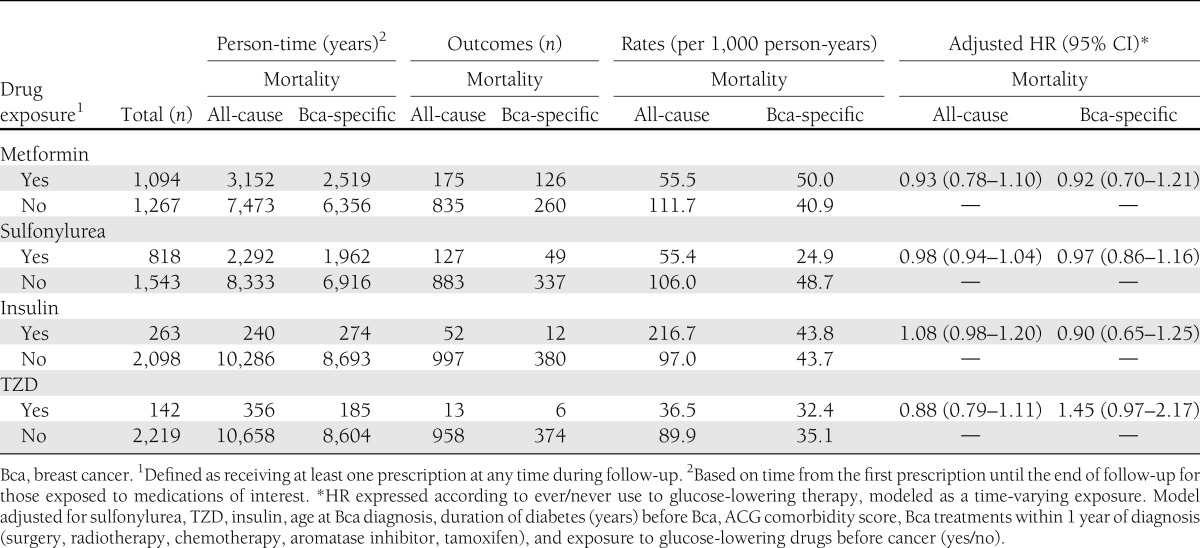
In our secondary time-varying ever-/never-exposure analysis, in which subjects were considered unexposed before their first metformin prescription and exposed after their first metformin prescription, the rate of all-cause mortality was 55.5 per 1,000 person-years of metformin exposure and 111.7 per 1,000 person-years unexposed to metformin. For breast cancer–specific mortality, the event rate was 50 per 1,000 person-years of metformin exposure and 40.9 per 1,000 person-years for metformin nonexposure (Table 3). On adjusted Cox regression analysis, the HR was 0.93 (0.78–1.10) for the association between any metformin during follow-up and all-cause mortality and 0.92 (0.70–1.21) for breast cancer–specific mortality (Table 3).
Table 3.
Event rate of all-cause and breast cancer–specific mortality among women exposed to glucose-lowering therapies with HRs from secondary analyses modeling glucose-lowering drugs as a time-varying never/ever exposure
Sensitivity analyses
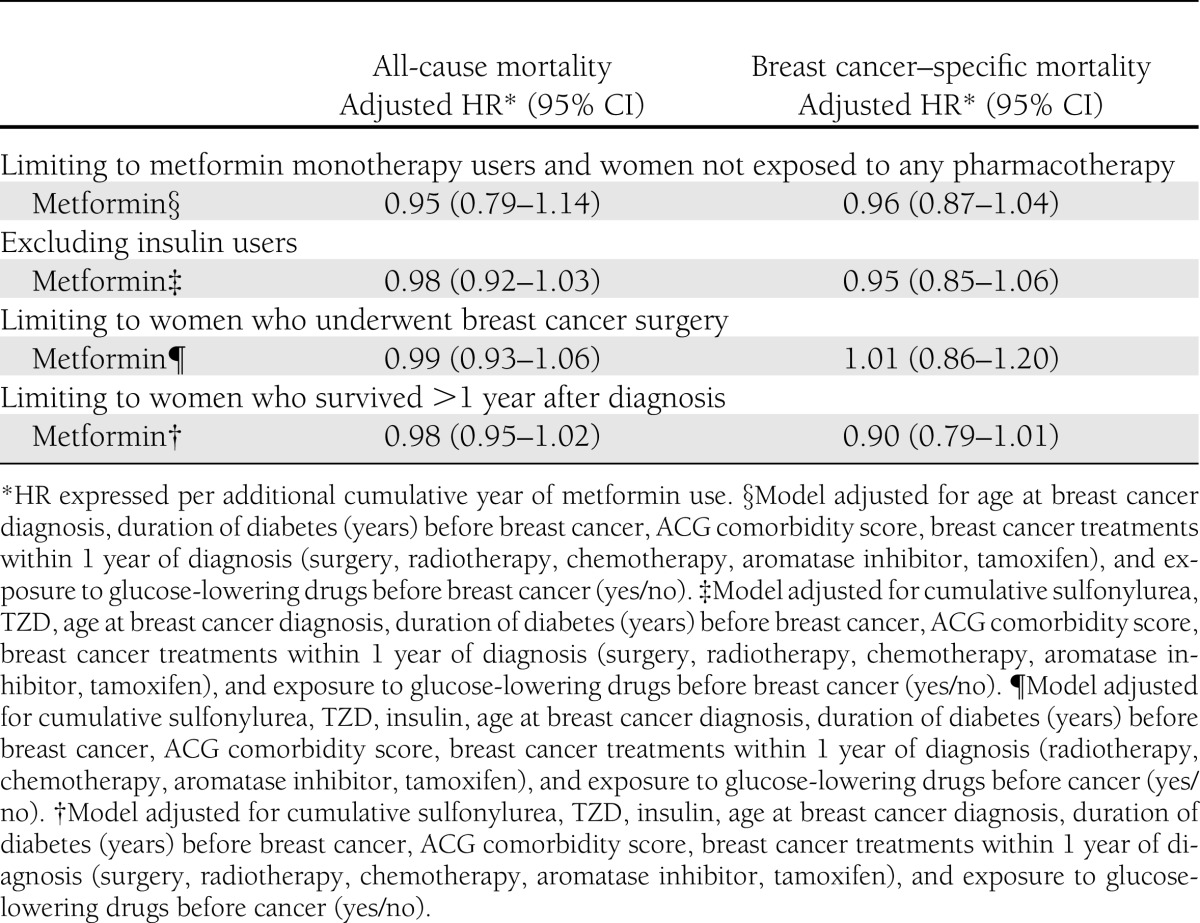
Sensitivity analyses found no association between cumulative duration of metformin use and all-cause or breast cancer–specific survival when our cohort was limited to women who had surgery, to only noninsulin users, and when women with metformin monotherapy were compared with diet-controlled patients or with women who survived at least the first year of follow-up (Table 4).
Table 4.
Summary of sensitivity analyses
CONCLUSIONS
This large, population-based study of older breast cancer patients with diabetes failed to show a significant association between metformin therapy and mortality. Our study is also the first to use a time-varying analytic approach to evaluate the effect of cumulative metformin use on mortality after breast cancer. Of note, our findings suggest a possible 9% reduction in breast cancer–specific mortality per additional year of cumulative metformin use that was not statistically significant, which translates into a potential 38% decrease over 5 years (HR 0.62 [95% CI 0.35–1.07]). Given the small number of women with long-term metformin exposure (N = 150) and the shorter follow-up for breast cancer–specific mortality of 3.7 years, lack of power cannot be excluded as a reason for our failure to detect statistical significance (type II error). Further studies in larger populations are needed.
Comparison with other studies
Two studies have evaluated the effect of metformin on mortality among breast cancer patients (13,14). However, direct comparison between these studies and ours is difficult given the differences in study population, lack of data on cancer subtypes in our study, and the methodology used. Bayraktar et al. (14) explored the association between metformin and mortality among women with triple-negative receptor breast cancer, whereas He et al. (13) studied the effect of metformin among women with HER2+ breast cancer. Although no effect of metformin was found for triple-negative receptor disease, metformin was associated with a 48% reduction in mortality in the HER2+ breast cancer population. Clinically, we know that triple-negative receptor breast cancer has a poorer prognosis and that these tumors may also have less response to metformin. In contrast, HER2+ breast cancer is not only receptive to antibody-based therapy, but metformin may directly reduce cell proliferation through the HER2 receptor (23). Further work will be necessary to explore whether the effects of metformin are limited to certain breast cancer subtypes. Another important difference between these studies and ours is that our cohort was considerably older at time of the breast cancer diagnosis, and the influence of metformin may differ in younger populations with breast cancer. These clinical differences between previous studies and our cohort raise the possibility that the benefits of metformin may depend on specific host or tumor factors.
Our main analysis differed from previous studies evaluating metformin’s effect in other cancers in two important ways (14,24–28). First, our cohort only included patients who had a relative homogenous duration of diabetes, whereas other studies used cohorts with prevalent diabetes in which the mean duration of diabetes ranged from 5.3 to 16.6 years. Given that metformin is first-line therapy for type 2 diabetes and is often used in patients with earlier diabetes (29), metformin users may be healthier and have an overall lower risk of mortality then patients in other comparator groups (30). By using a newly diagnosed group of diabetic patients, we minimized the risk of indication bias that may have affected the results of other studies.
Another important difference is how metformin exposure was defined. Whereas previous breast cancer studies dichotomized drug use as a never/ever variable, our study treated drug use in a cumulative time-varying fashion. Furthermore, some previous studies may have introduced an immortal time bias given their categorization of metformin use at baseline based on baseline exposure or exposure that occurred during the follow-up period (13,19). Defining an exposure that changes during the follow-up period as a fixed variable at baseline introduces bias because patients who die early in the follow-up period have less opportunity to be exposed (18,31,32). This “time-dependent” or “immortal time” bias was unaccounted for in the study by He et al., which further makes interpretation of findings from that study difficult (13). Suissa and Azoulay (33) recently highlighted the presence of immortal time bias in studies evaluating metformin and cancer mortality and concluded that the misclassification of metformin exposure has led to results that have overestimated the effect of metformin on mortality.
Our findings further contrast in vitro evidence supporting metformin’s anticancer effects. Direct and indirect pathways have both been proposed to explain this effect. Metformin may act directly on cancer cells through AMP-activated protein kinase pathways whereby it inhibits downstream signaling of mammalian target of rapamycin, a key growth factor, and causes a reduction in cellular growth and proliferation (34,35). Metformin may also act indirectly, through reduced insulin, leading to reduced activation of insulin/IGF-1 receptors on tumor cells and resulting in decreased stimulation of mitogenic pathways decreased cell proliferation, tumor formation, and metastasis (36,37).
Most recently, three window-of-opportunity studies have investigated the effect of administering metformin to nondiabetic breast cancer patients before surgery (10–12). All three studies showed a significant reduction in tumor proliferation markers among women exposed to metformin and thus support the ongoing evaluation of metformin in the clinical breast cancer setting. The postulated mechanisms for metformin are likely to mediate both the association with cancer incidence and mortality. Metformin’s growth-suppressing properties, which provide evidence to support its association with a reduction in mortality, have been most frequently described in preclinical and in in vitro studies. However, there is increasing evidence that metformin may also target cancer-initiating cells and suppress the initiation of tumor growth (38–40), thus affecting cancer incidence.
Strengths and limitations of study
Our study has multiple strengths. First, our study evaluated the effect of metformin on mortality using a cumulative time-varying approach that minimizes the biases present in many observational drug studies. Second, by using an incident diabetic cohort, we were able to study this question in a more homogeneous population and reduce selection biases based on differing diabetes severity. Third, we had access to data for a large population-based cohort of women, with validated definitions of diabetes and breast cancer. In addition, we had access to comprehensive drug and cancer treatment data with which we were able to derive and adjust for important diabetes- and cancer-related covariates.
However, findings from our study should be interpreted in the context of the following limitations. First, despite our careful methodologic approach, as in all observational studies, there remains risk of bias and misclassification.
Second, our cohort represented women of advanced age, and thus, our findings may not be generalizable to a younger breast cancer population or to patients with long-standing diabetes. Nearly 50% of breast cancers are diagnosed in women after age 65 years (41), but it is unclear whether metformin would be more effective for cancers that develop at a younger age. Although tumor characteristics have not been shown to differ greatly between younger (age <80 years) and older postmenopausal women, breast cancer treatments may differ quite substantially between these two age groups (41), with older women less likely to receive aggressive therapy and thus having a higher risk of dying compared with younger breast cancer patients (42,43). Furthermore, deaths after breast cancer are more likely to be related to cardiovascular and cerebrovascular disease (44). Therefore, if metformin indeed has specific antitumor properties, its effects on mortality may be more salient in younger breast cancer populations.
Third, our mean follow-up period of 4.5 years for overall survival and 3.7 years for breast cancer–specific survival was relatively short, and a longer duration may be required to better assess the effect of metformin on survival. However, the duration of our follow-up is comparable to other similar studies (13,14).
We did not have access to clinical data such as BMI, family history, and metabolic factors, nor was information available on tumor stage or recurrence. Specifically, our inability to adjust for the prognostic effects of BMI in our analyses represents an important limitation of our research (45,46). It is possible that obese diabetic patients were more likely to be prescribed metformin than nonobese subjects, thereby biasing our results toward the null. However, metformin users in our study tended to be younger and diabetes duration and cardiovascular disease rates were comparable with nonmetformin users, suggesting that this may not have been the case. Furthermore, no association of metformin use with level of obesity was identified in diabetic women enrolled into the Women’s Health Initiative (47). Lack of adjustment for cancer stage may have also underestimated the association between metformin and outcomes if metformin-treated women presented with a more advanced stage of disease. Future studies that control for BMI and cancer stage will be important for further elucidating the prognostic role of metformin in diabetic breast cancer patients.
Conclusion
In summary, we conducted a large population-based study to investigate the effects of metformin therapy on mortality after breast cancer in a diabetic population. Cumulative exposure of metformin was not significantly associated with all-cause or breast cancer–specific mortality in our incident diabetic cohort when modeled as a time-varying covariate. The lack of data on BMI and breast cancer stage, important predictors of survival after breast cancer, and the short follow-up for breast cancer–specific deaths, limits interpretation of these findings. Further work is required to examine the effect of metformin in a younger population of patients with breast cancer and diabetes as well as in a prevalent diabetic population, although careful attention will be needed to minimize indication bias in such a cohort and in nondiabetic populations. A better understanding of metformin’s effect on breast cancer is essential to help address the disparity in cancer outcomes between patients with and without diabetes, as well as to guide diabetes treatment strategies in this population. Questions regarding the benefit of metformin in nondiabetic patients will be answered in the ongoing National Cancer Institute of Canada Clinical Trials Group Phase III Randomized Trial of Metformin vs. Placebo in Early Stage Breast Cancer (NCIC CTG MA.32) that has randomized nondiabetic women with early-stage breast cancer to metformin (48).
Acknowledgments
I.C.L. is supported by a fellowship from the Canadian Breast Cancer Research Foundation. P.C.A. is partly supported by a Career Investigator award from the Heart and Stroke Foundation. L.L.L. was supported by a Canadian Diabetes Association/Canadian Institutes of Health Research (CIHR) Clinician Scientist Award and is currently supported by a CHIR New Investigator Award.
No potential conflicts of interest relevant to this article were reported.
This study was conducted with the support of the Ontario Institute for Cancer Research and Cancer Care Ontario (CCO) through funding provided by the Government of Ontario, and through provision of data by the Institute for Clinical Evaluative Sciences (ICES) and CCO. The opinions, results, and conclusions reported in this paper are those of the authors. No endorsement by ICES, CCO, or the Government of Ontario is intended or should be inferred.
I.C.L. researched the background, contributed to research design, performed the statistical analysis, and wrote and edited the manuscript. P.C.A. contributed to the research design, provided assistance with data analysis, and reviewed and edited the manuscript. A.G., P.J.G., and P.A.R. contributed to the research design and reviewed and edited the manuscript. L.L.L. contributed to the research design, contributed to discussion, and cowrote the manuscript. I.C.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
This study was presented as an abstract at the 15th Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism Professional (CSEM) Conference and Annual Meetings, Vancouver, BC, Canada, 10–13 October 2012.
The authors thank David Margel, MD, Princess Margaret Hospital, Toronto, for his useful comments and suggestions throughout this study, and Ying Liu, MSc, Institute for Clinical and Evaluative Sciences, for assistance with data access and preparation.
References
- 1.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 2008;300:2754–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol 2005;6:103–111 [DOI] [PubMed] [Google Scholar]
- 3.Schott S, Schneeweiss A, Sohn C. Breast cancer and diabetes mellitus. Exp Clin Endocrinol Diabetes 2010;118:673–677 [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya OK, Estey EA, Cheng AY, Canadian Diabetes Association 2008 Update on the Canadian Diabetes Association 2008 clinical practice guidelines. Can Fam Physician 2009;55:39–43 [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association Executive summary: Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS ONE 2012;7:e33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461 [DOI] [PubMed] [Google Scholar]
- 8.Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat 2012;135:639–646 [DOI] [PubMed] [Google Scholar]
- 9.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009;27:3297–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadad S, Iwamoto T, Jordan L, et al. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat 2011;128:783–794 [DOI] [PubMed] [Google Scholar]
- 11.Bonanni B, Puntoni M, Cazzaniga M, et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol 2012;30:2593–2600 [DOI] [PubMed]
- 12.Niraula S, Dowling RJ, Ennis M, et al. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat 2012;135:821–830 [DOI] [PubMed] [Google Scholar]
- 13.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol 2012;23:1771–1780 [DOI] [PMC free article] [PubMed]
- 14.Bayraktar S, Hernadez-Aya LF, Lei X, et al. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer 2012;118:1202–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–516 [DOI] [PubMed] [Google Scholar]
- 16.Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol 1988;41:495–501 [DOI] [PubMed] [Google Scholar]
- 17.Brenner DR, Tammemägi MC, Bull SB, Pinnaduwaje D, Andrulis IL. Using cancer registry data: agreement in cause-of-death data between the Ontario Cancer Registry and a longitudinal study of breast cancer patients. Chronic Dis Can 2009;30:16–19 [PubMed] [Google Scholar]
- 18.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008;167:492–499 [DOI] [PubMed] [Google Scholar]
- 19.He XX, Tu SM, Lee MH, Yeung SC. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann Oncol 2011;22:2640–2645 [DOI] [PMC free article] [PubMed]
- 20.Quan ML, Hodgson N, Schultz SE, et al. Surgery for breast cancer. In Surgery for Breast Cancer Toronto, Institute for Clinical Evaluative Sciences, 2008, p. 7–29 [Google Scholar]
- 21.Austin PC, Shah BR, Newman A, Anderson GM. Using the Johns Hopkins' Aggregated Diagnosis Groups (ADGs) to predict 1-year mortality in population-based cohorts of patients with diabetes in Ontario, Canada. Diabet Med 2012;29:1134–1141 [DOI] [PubMed]
- 22.Ake C, Carpenter C. Extending the Use of PROC PHREG in Survival Analysis Cary, NC, SAS Institute Inc. [Google Scholar]
- 23.Dean-Colomb W, Esteva FJ. Her2-positive breast cancer: herceptin and beyond. Eur J Cancer 2008;44:2806–2812 [DOI] [PubMed] [Google Scholar]
- 24.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012;35:299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrett CR, Hassabo HM, Bhadkamkar NA, et al. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer 2012;106:1374–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero IL, McCormick A, McEwen KA, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol 2012;119:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH, Kim WH. The effects of metformin on survival after diagnosis of colorectal cancer in diabetic patients. Int J Cancer 2012;131:752–759 [DOI] [PubMed]
- 28.Chen T-M, Lin C-C, Huang P-T, Wen C-F. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol 2011;26:858–865 [DOI] [PubMed]
- 29.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2008;32(Suppl. 1):S1–S201
- 30.Renehan AG, Yeh HC, Johnson JA, Wild SH, Gale EA, Møller H, Diabetes and Cancer Research Consortium Diabetes and cancer (2): evaluating the impact of diabetes on mortality in patients with cancer. Diabetologia 2012;55:1619–1632 [DOI] [PubMed] [Google Scholar]
- 31.van Walraven C, Austin P. Administrative database research has unique characteristics that can risk biased results. J Clin Epidemiol 2012;65:126–131 [DOI] [PubMed] [Google Scholar]
- 32.Beyersmann J, Wolkewitz M, Schumacher M. The impact of time-dependent bias in proportional hazards modelling. Stat Med 2008;27:6439–6454 [DOI] [PubMed] [Google Scholar]
- 33.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 2012;35:2665–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 2007;67:10804–10812 [DOI] [PubMed] [Google Scholar]
- 35.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006;66:10269–10273 [DOI] [PubMed] [Google Scholar]
- 36.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 2009;30:586–623 [DOI] [PubMed] [Google Scholar]
- 37.Frasca F, Pandini G, Sciacca L, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem 2008;114:23–37 [DOI] [PubMed] [Google Scholar]
- 38.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 2009;69:7507–7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Martin-Castillo B, Menendez JA. Metformin and energy metabolism in breast cancer: from insulin physiology to tumour-initiating stem cells. Curr Mol Med 2010;10:674–691 [DOI] [PubMed] [Google Scholar]
- 40.Anisimov VN, Berstein LM, Egormin PA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol 2005;40:685–693 [DOI] [PubMed] [Google Scholar]
- 41.Canadian Cancer Society's Steering Committee on Cancer Statistics. In Canadian Cancer Statistics 2011 Toronto, Canada, Canadian Cancer Society, 2011
- 42.Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol 2010;28:2038–2045 [DOI] [PMC free article] [PubMed]
- 43.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 2001;285:885–892 [DOI] [PubMed] [Google Scholar]
- 44.Colzani E, Liljegren A, Johansson AL, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol 2011;29:4014–4021 [DOI] [PubMed]
- 45.Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 2012;134:769–781 [DOI] [PubMed] [Google Scholar]
- 46.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010;123:627–635 [DOI] [PubMed] [Google Scholar]
- 47.Chlebowski RT, McTiernan A, Wactawski-Wende J, et al. Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol 2012;30:2844–2852 [DOI] [PMC free article] [PubMed]
- 48.Goodwin PJ, Stambolic V, Lemieux J, et al. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat 2011;126:215–220 [DOI] [PubMed] [Google Scholar]